Abstract
Background
Heart failure is a prominent cardiovascular disease (CVD) manifestation in sub-Sarahan Africa. Myocardial fibrosis is a central feature of heart failure that we aimed to characterize among persons with human immunodeficiency virus (PWH) in South Africa.
Methods
Cardiovascular magnetic resonance (CMR) imaging was performed among PWH with viral suppression and uninfected controls, both free of known CVD. Plasma levels of N-terminal pro B-type natriuretic peptide (NT-proBNP) were measured. Comparisons by human immunodeficiency virus (HIV) status were made using linear and logistic regression, adjusted for age, sex, and hypertension.
Results
One hundred thirty-four PWH and 95 uninfected persons completed CMR imaging; age was 50 and 49 years, with 63% and 67% female, respectively. Compared with controls, PWH had greater myocardial fibrosis by extracellular volume fraction ([ECV] absolute difference, 1.2%; 95% confidence interval [CI], 0.1–2.3). In subgroup analyses, the effect of HIV status on ECV was more prominent among women. Women (vs controls) were also more likely to have elevated NT-proBNP levels (>125 pg/mL; odds ratio, 2.4; 95% CI, 1.0–6.0). Among all PWH, an elevated NT-proBNP level was associated with higher ECV (3.4% higher; 95% CI, 1.3–5.5).
Conclusions
Human immunodeficiency virus disease may contribute to myocardial fibrosis, with an effect more prominent among women. Research is needed to understand heart failure risk among PWH within sub-Saharan Africa.
Keywords: cardiovascular disease, HIV, myocardial fibrosis, South Africa
Among persons with human immunodeficiency virus (PWH), global efforts to increase access to effective antiretroviral therapy (ART) have reduced acquired immune deficiency syndrome (AIDS) progression and shifted the paradigm of morbidity and mortality towards noninfectious diseases, such as cardiovascular disease (CVD) [1, 2]. Data on human immunodeficiency virus (HIV)-associated CVD in the modern ART era have largely been drawn from high-income countries (HICs), where ischemic complications (ie, myocardial infarction) from atherosclerotic disease are the most common CVD manifestations [3, 4]. However, approximately 80% of the global CVD burden exists in low- to middle-income countries (LMICs) with 70% of the HIV epidemic existing in sub-Saharan Africa [5, 6]. There is reason to suspect that HIV-associated CVD may be different in this context.
South Africa (SA) has the largest population of PWH worldwide at >7 million, with >50% provided access to ART treatment [2, 6, 7]. In “The Heart of Soweto” study, a seminal epidemiologic study of CVD in SA, the most common “primary” CVD etiology among persons presenting for cardiology evaluation was heart failure ([HF] at 44%), followed by hypertension (19%), with ischemic coronary disease being infrequent (10%) [8]. Classically, HF associated with advanced untreated HIV involved a phenotype of cardiomyopathy with global systolic dysfunction [9, 10], but data among PWH on ART with higher CD4+ counts also demonstrate an excess burden of diastolic dysfunction [11–14]. When compared with uninfected controls, PWH from the US Veterans Aging Cohort Study had a higher incidence of HF, including both reduced and preserved ejection fraction phenotypes (HFrEF and HFpEF, respectively) [15, 16]. Contemporary data are needed to understand the pathogenesis of HIV-associated myocardial injury and HF risk, specifically in LMICs such as SA where CVD risk factor profiles differ from those in HICs.
Myocardial fibrosis represents a central underlying pathogenic feature of HF that is apparent before clinical presentation and can help identify potential factors contributing to disease risk [17–25]. Myocardial fibrosis is a complex process that may be triggered by injury, stress, and inflammation, resulting in extracellular matrix accumulation within the myocardium [26]. It is typically classified as either replacement or interstitial fibrosis, with the former resulting in focal patterns of fibrosis and the latter resulting in a diffuse pattern. Although causal mechanisms can overlap, focal scar is typically the result of ischemic injury, whereas the pathogenesis of diffuse fibrosis is often influenced by hypertension, ventricular hypertrophy, and aging [27–29].
Cardiac magnetic resonance imaging (CMR) permits noninvasive direct visualization and quantification of both replacement and interstitial fibrosis. Recent advancements in CMR have allowed for the assessment of myocardial extracellular matrix volume fraction (ECV). As a surrogate measure for early cardiac remodeling in multiple diseases that affect that heart, ECV is a powerful independent predictor of subsequent HF in people with apparently normal hearts, as defined by conventional functional and structural measures [30]. In the Multi-Ethnic Study of Atherosclerosis (MESA), both focal and diffuse patterns of myocardial fibrosis were associated with increased risk for a composite of CVD events [31].
The purpose of this study was to detect the frequency of myocardial fibrosis and characterize the associated patterns by CMR, which may be detectable before the development of clinical HF, among a healthy cohort of PWH in SA in the contemporary ART era.
METHODS
Study Population
Participants receiving routine care were recruited and enrolled at Site B health clinic within Khayelitsha township outside of Cape Town, SA. Eligibility criteria for PWH included the most recent HIV ribonucleic acid level <200 copies/mL (within prior year) and history of being on continuous ART for ≥1 year up until enrollment. Age criteria ranged between 30 and 70 years, with half of the study population prespecified to be ≥50 years old. Human immunodeficiency virus-uninfected participants were enrolled at the same Khayelitsha Site B health clinic. To facilitate prespecified subgroup comparisons, HIV-uninfected participants were frequency matched to PWH on the following: (1) age ≥50 years, (2) sex at birth, and (3) hypertension status (by clinical diagnosis or taking blood pressure [BP]-lowering therapy). Exclusion criteria included known heart disease (eg, HF, structural heart disease, coronary heart disease, or prior peri-/myocarditis), active treatment for tuberculosis or bacterial infection, an AIDS-defining illness within the prior year, or a body mass index (BMI) ≥45 kg/m2.
After verbal and written informed consent procedures (in English and/or Xhosa as indicated), participants underwent a medical history and chart review to ascertain clinical diagnoses, medications, and historic laboratory monitoring. A blood draw was obtained on the day of enrollment, and clinical laboratory measures were performed at Groote Schuur Hospital. Transportation was then arranged for participants to attend a second study visit at the University of Cape Town for CMR procedures.
Cardiovascular Magnetic Resonance
Participants underwent a standardized contrast-utilization CMR protocol in a large bore 3T Skyra MR system (Siemens Healthcare). T1 mapping was performed using the shortened Modified Look-Locker Inversion Recovery (MOLLI) sequence, T2 mapping using SSFP imaging, and T2-weighted imaging was performed with the black-blood short-Tau inversion recovery (STIR) sequence, as previously published [32, 33]. Late-gadolinium enhancement (LGE) imaging and typical imaging parameters were the same as previously published [32, 34]. The CMR images were analyzed by 3 independent reviewers, who were each blinded to clinical factors (eg, HIV status) as well as the analysis of the other reviewers.
Postcontrast T1 mapping to quantify diffuse fibrosis within the extracellular matrix was performed by MOLLI technique, [35] at 2 time points between 10 and 25 minutes after contrast injection. Global extracellular volume (ECV) fraction was calculated as [partition coefficient] × [1 − HCT], where the partition coefficient was determined by the slope of the linear relationship of 1/T1 times of myocardium against the blood pool, assessed pre- and postcontrast. Myocardial edema was estimated by a T2 mapping and T2 signal intensity (SI) ratio using STIR imaging of myocardium and remote skeletal muscle.
Late-gadolinium enhancement imaging, based on a T1-weighted phase-sensitive inversion recovery sequence, was performed after administration of gadolinium [36]. The inversion time was adjusted for optimal nulling of normal myocardium. Images were evaluated qualitatively for the presence or absence of LGE. Semiquantitative analysis of LGE volume fraction was estimated by manually contouring endocardial and epicardial region of interest. Focal areas of LGE were defined by an SI ≥2.0 standard deviations (SDs) above the mean SI of normal myocardium.
Analysis of left ventricular (LV) volumes, mass, and ejection fraction was performed using CVI42 (v5.9; Circle Cardiovascular Imaging, Calgary, Canada). Myocardial function was assessed by calculating circumferential, radial, and longitudinal strain parameters during both diastole and systole. Strain and strain rates were assessed using feature tracking, and semiautomated analysis was performed using CVI42.
Cardiac Biomarkers
Plasma was processed from blood samples on the day of collection and stored at −80°C until biomarker analyses were performed. N-terminal pro B-type natriuretic peptide (NT-proBNP) was measured with a Roche Diagnostics sandwich electrochemiluminescence assay (Elecsys ProBNP), and high-sensitivity cardiac Troponin-T (cTnT) was measured utilizing Roche Generation 5 STAT assay.
Statistical Methods
Participant characteristics, CMR parameters, and laboratory measures were summarized by mean (SD) for continuous variables and proportion (percentage) for categorical variables. Complete case analysis was performed to determine the effect of HIV status (primary input) on clinical characteristics as well as CMR parameters of cardiac structure and function (response variables). Inference was made using linear and logistic regression models for continuous and dichotomous characteristics, respectively, adjusting for continuous age, sex at birth, and hypertension diagnosis as the factors that were frequency matched between groups a priori. Subgroup analyses were also performed by sex, dichotomous age (≥50 years), dichotomous BMI (>30 kg/m2), hypertension status, current smoking status, and prior tuberculosis infection. To aid interpretation of continuous parameter comparisons by HIV status, percentage differences and 95% CIs comparing adjusted means were calculated using the delta method [37]. All analyses were conducted using SAS version 9.4 with a 2-sided Type I error probability of 0.05.
RESULTS
Study Participants
Among 261 participants screened, 255 were enrolled, and 250 attended a second CMR study visit. Among these, 229 (n = 134 PWH and n = 95 uninfected controls) completed the full CMR protocol including pre- and postcontrast images and were thus included in our analysis sample. Table 1 presents participant characteristics by HIV status. All participants were residents of Khayelitsha township and 99% were black African race; 1 PWH and 2 controls identified as mixed race. The PWH and uninfected participants were similar with respect to age and cardiometabolic risk factors including hypertension, smoking, and diabetes. The proportion of female sex at birth was similar between PWH and controls, 63% and 67%, respectively; however, PWH had a more frequent history of prior active tuberculosis infection (55% vs 16%).
Table 1.
Participant Characteristics by HIV Status (n = 229)
| Mean (SD) or % (n) | |||
|---|---|---|---|
| Characteristics | PWH (n = 134) | Uninfected (n = 95) | P Valuea |
| Demographics | |||
| Age, years | 49.5 (9.4) | 48.7 (9.5) | .54 |
| Sex at birth, female | 63% (85) | 67% (64) | .58 |
| Clinical Characteristics | |||
| Body mass index, kg/m2 | 27.3 (6.7) | 29.7 (7.8) | .01 |
| Systolic blood pressure, mmHg | 134 (19) | 135 (16) | .45 |
| Diastolic blood pressure, mmHg | 81 (12) | 81 (12) | .82 |
| Heart rate, beats per minute | 67 (11) | 67 (12) | .98 |
| Total cholesterol, mg/dL | 178 (34) | 184 (45) | .28 |
| High-density lipoprotein cholesterol, mg/dL | 64 (22) | 68 (30) | .29 |
| Low-density lipoprotein cholesterol, mg/dL | 87 (29) | 93 (44) | .23 |
| Hemoglobin, g/dL | 13.0 (1.4) | 13.4 (1.2) | .02 |
| eGFR (CKD-EPI), mL/min/1.73 m2 | 115 (18) | 118 (15) | .15 |
| Current smoker | 30% (40) | 34% (32) | .57 |
| Hypertension diagnosis | 34% (46) | 35% (33) | 1.00 |
| Diabetes diagnosis | 7% (9) | 9% (9) | .46 |
| Hepatitis B infection | 5% (7) | 2% (2) | .31 |
| Hepatitis C infection | 0% (0) | 0% (0) | -- |
| Prior active tuberculosis infection | 55% (74) | 16% (15) | <.001 |
| HIV-Related Characteristics | |||
| CD4+ count, cells/μL | 534 (270) | -- | -- |
| CD4+ nadir count, cells/μL | 271 (213) | -- | -- |
| HIV viral load <200 copies/mL | 94% (126) | -- | -- |
| HIV diagnosis duration, years | 9.5 (5.8) | -- | -- |
| Years receiving antiretroviral therapy | 7.0 (4.3) | -- | -- |
| Antiretroviral regimen contains | -- | -- | -- |
| Nucleoside reverse-transcriptase inhibitor | 98% (131) | -- | -- |
| Nonnucleoside reverse-transcriptase inhibitor | 88% (118) | -- | -- |
| Protease inhibitor | 6% (8) | -- | -- |
| HIV suppression duration 4+ years | 31% (42) | -- | -- |
| Prior AIDS | 43% (58) | -- | -- |
| Cardiac Biomarkers | |||
| NT-proBNP levels, pg/mL | 92 (131) | 92 (136) | .99 |
| TnT levels, %detectable (>6.00 ng/L) | 26% (35) | 28% (27) | .76 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; NT-proBNP, N-terminal pro B-type natriuretic peptide; PWH, persons with human immunodeficiency virus; SD, standard deviation; Tnt, Troponin-T.
a P value computed for between-group difference in mean or proportion using 2-sample t test or Fisher’s exact χ 2 test, respectively.
Among PWH, mean (SD) time since HIV diagnosis was 10 (6) years, current CD4+ count was 534 (270) cells/µL, nadir CD4+ count was 271 (213) cells/µL, and 43% had prior AIDS diagnosis. Duration of viral suppression was >4 years among 31% of participants. With regard to therapy, 98% (n = 131) took a nucleoside reverse-transcriptase inhibitor (NRTI), 85% of which was tenofovir disoproxil fumarate and emtricitabine in combination, whereas 88% (n = 118) took a non-NRTI, 94% of which was efavirenz.
For comparisons restricted to female sex at birth, PWH and controls had mean (SD) age of 49 (9) and 49 (10), BMI of 29.6 (6.2) and 33.0 (7.0), and the frequency of hypertension of 31% and 39%, respectively. Corresponding characteristics for men, respectively, were age of 50 (10) and 49 (9), BMI of 23.4 (5.6) and 23.0 (4.5), and hypertension of 41% and 26%.
Cardiovascular Magnetic Resonance Measurements
Table 2 presents participant CMR parameters for PWH and uninfected controls, as well as HIV effect estimates—adjusted mean differences for continuous parameters and odds ratios (ORs) for dichotomous parameters, comparing PWH with uninfected controls. There was no evidence of myocardial dysfunction by systolic or diastolic parameters in either group. The LV ejection fraction and global systolic circumferential strain were significantly higher among PWH compared with controls after adjustment (P = .02 and P = .05, respectively), although mean values were within normal ranges for both groups. The frequency of LV hypertrophy was low at 10% and 7%, respectively, and there were no differences in LV mass index between PWH and controls, respectively. There were no differences in end-diastolic or end-systolic LV volumes between groups. The difference in LA area index among PWH versus controls was 12.8 vs 12.3 cm2/m2, respectively (P = .05), although both groups were within normal range and there was no corresponding difference in LV mass index. However, among PWH, greater LV mass index was associated with lower CD4 count (0.48 g/m2 higher per 100 cells/µL lower; 95% CI, 0.07–0.89) and prior AIDS (4.94 g/m2 higher with vs without prior AIDS diagnosis).
Table 2.
CMR Measures by HIV Status (n = 229)
| Mean (SD) or % (n) | ||||
|---|---|---|---|---|
| Characteristics | PWH (n = 134) | Uninfected (n = 95) | Adjusted Mean Difference or Odds Ratio (95% CI)a | P Valuea |
| Functional Characteristics | ||||
| LV ejection fraction, % | 59.3 (6.7) | 57.4 (7.1) | 2.0 (0.3–3.7) | .02 |
| LV ejection fraction, <50%b | 7% (10) | 11% (11) | 0.59 (0.24–1.49) | .26 |
| Global systolic circumferential strain, % | −21.9 (3.1) | −20.8 (5.5) | −1.1 (−2.2 to 0.0) | .05 |
| Peak diastolic circumferential strain rate, %s−1 | 1.36 (0.33) | 1.30 (0.30) | 0.07 (−0.01 to 0.15) | .09 |
| Myocardial Chamber Characteristics | ||||
| LV end diastolic volume, mL | 138 (31) | 142 (25) | −3 (−11 to 4) | .35 |
| LV end systolic volume, mL | 56.9 (19.4) | 60.8 (17.3) | −4.3 (−8.7 to 0.2) | .06 |
| LA dilation | 30% (40) | 25% (24) | 1.24 (0.67–2.28) | .50 |
| LA area index, cm2/m2 | 12.8 (2.2) | 12.3 (1.7) | 0.54 (0.01–1.07) | .05 |
| LV hypertrophy | 10% (14) | 7% (7) | 1.36 (0.51–3.63) | .55 |
| LV mass index, g/m2 | 55.4 (13.2) | 55.0 (11.0) | −0.2 (−2.8 to 2.4) | .88 |
| LV mass/volume ratio | 0.72 (0.12) | 0.73 (0.12) | −0.02 (−0.05 to 0.01) | .28 |
| LV septal wall thickness, mm | 10.11 (1.94) | 9.88 (1.62) | −0.15 (−0.58 to 0.28) | .50 |
| LV anterior wall thickness, mm | 7.44 (1.52) | 7.53 (1.51) | 0.15 (−0.22 to 0.53) | .42 |
| LV lateral wall thickness, mm | 7.69 (1.73) | 7.52 (1.65) | −0.11 (−0.53 to 0.31) | .60 |
| LV anterior wall thickness, mm | 8.23 (1.68) | 8.05 (1.59) | −0.13 (−0.54 to 0.28) | .54 |
| Myocardial Tissue Characteristics | ||||
| LV LGE presence | 72% (93) | 72% (65) | 0.98 (0.54–1.80) | .95 |
| Native T1, ms | 1247 (44) | 1242 (40) | 6 (−5 to 16) | .29 |
| Native T2, ms | 39.2 (2.5) | 39.2 (2.4) | 0.0 (−0.7 to 0.6) | .92 |
| T1W imaging, signal intensity ratio | 0.90 (0.15) | 0.87 (0.13) | 0.04 (0.00–0.07) | .07 |
| T2W imaging, signal intensity ratio | 1.46 (0.24) | 1.44 (0.21) | 0.02 (−0.03 to 0.08) | .50 |
| Extracellular volume fraction, % | 30.4 (5.1) | 29.3 (2.7) | 1.2 (0.1–2.3) | .04 |
| Pericardial effusion | 13% (18) | 9% (9) | 1.56 (0.66–3.68) | .31 |
Abbreviations: CI, confidence interval; CMR, cardiac magnetic resonance; HIV, human immunodeficiency virus; LGE, late-gadolinium enhancement; LA, left atrial; LV, left ventricular; PWH, persons with HIV; SD, standard deviation.
aComputed via linear or logistic regression for continuous and dichotomous parameters, respectively, adjusted for age, sex, and diagnosis of hypertension.
bTwo participants (1 person with HIV and 1 uninfected) had a LV ejection fraction <40%.
Among both PWH and controls, there was a high frequency of scarring or fibrosis within the LV myocardium as indicated by LGE (72% in each group). Precontrast T1 time did not significantly differ between PWH (1247 ms) and uninfected controls (1242 ms). However, CMR estimates of ECV, considering pre- and postcontrast T1 images, demonstrated greater degree of myocardial fibrosis among PWH compared with controls (30.4% and 29.3%, respectively; adjusted P = .04).
Subgroup Analyses
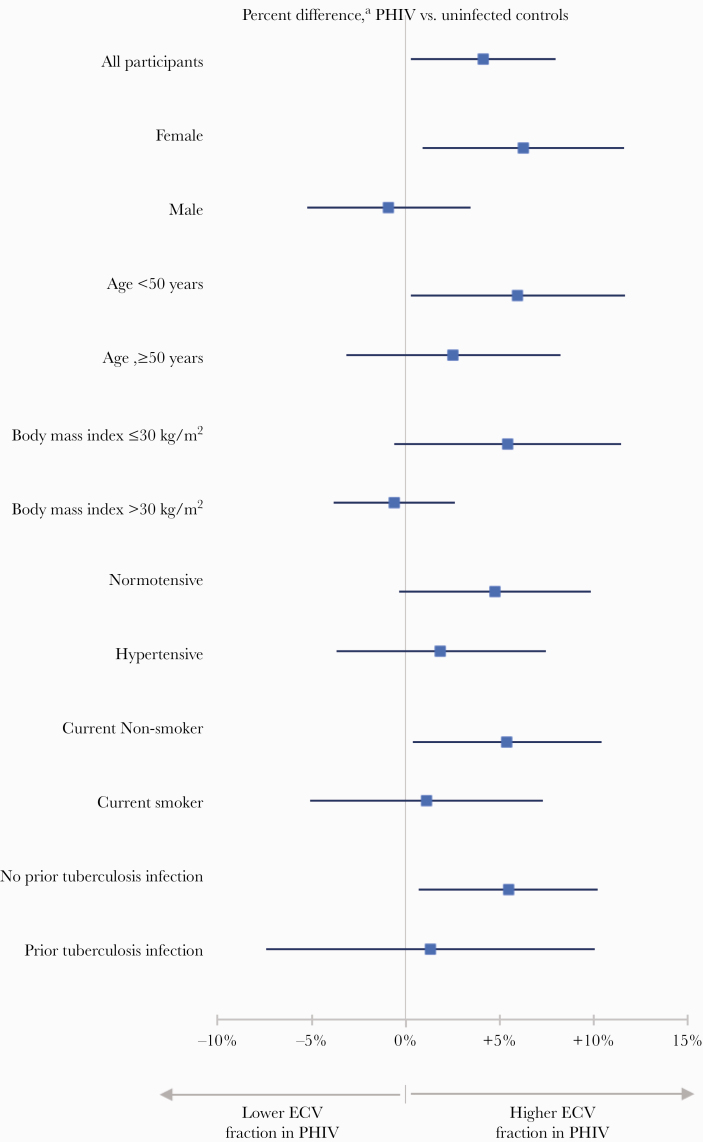
The effect of HIV infection on ECV fraction was further analyzed by a priori-defined subgroups (Figure 1). The effect of HIV infection on ECV fraction observed in the total study population was present among females but not among males (P = .12 for interaction). Likewise, a pattern was present where the association between HIV status and higher ECV fraction was more apparent among participants <50 years, those with a BMI ≤30 kg/m2, those who were normotensive, those who were current nonsmokers, and those with no history of prior tuberculosis infection. Tests for heterogeneity did not reveal any significant interactions.
Figure 1.
Percentage difference in myocardial fibrosis (extracellular volume [ECV]) by human immunodeficiency virus (HIV) status overall and among subgroups defined by risk factor (n = 229). aComputed via linear regression, adjusted for age, sex, and hypertension. PHIV, persons with HIV.
Cardiac Biomarkers
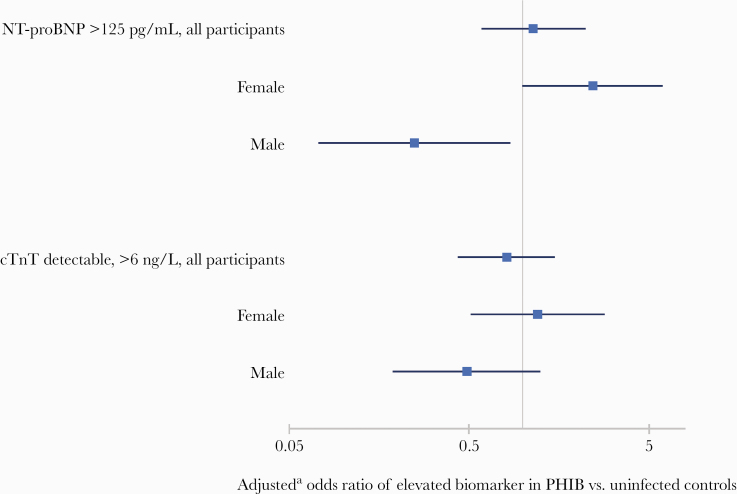
Among all participants, median NT-proBNP levels were 47.8 pg/mL (interquartile range, 18.9 to 93.8), and 21% had elevated levels >125 pg/mL and 7% had levels >300 pg/mL. Cardiac TnT levels were detectable (>6.00 ng/L) among 27% of participants. There were no differences between PWH and controls in absolute NT-proBNP level, proportion with elevated NT-proBNP (>125 pg/mL), or proportion with detectable cTnT level (Figure 2). However, when compared with controls, women with HIV were more likely to have elevated NT-proBNP (OR, 2.4; 95% CI, 1.0 to 6.0), whereas men showed the opposite effect (OR, 0.2; 95% CI, 0.1 to 0.8). The presence of an elevated NT-proBNP was also associated with significantly higher CMR estimates of myocardial fibrosis in ECV (3.4% higher; 95% CI, 1.3 to 5.5; P = .002) and native T1 mapping (14.7 ms higher; 95% CI, −2.7 to 32.2; P = .10) among PWH, after adjustment for age, sex, and hypertension status. However, no associations were present between elevated NT-proBNP and ECV (0.9; 95% CI, −0.6 to 2.4; P = .24) or native T1 mapping (−1.5; 95% CI, −22.5 to 19.6; P = .89) among uninfected controls.
Figure 2.
Odds ratioa of elevated cardiac biomarkers by human immunodeficiency virus (HIV) status overall and among subgroups defined by sex (n = 229). aComputed via logistic regression, adjusted for age, sex, and hypertension status. cTnT, cardiac Troponin-T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PHIV, persons with HIV.
Comparisons of Persons With Human Immunodeficiency Virus and Uninfected Controls From High-Income Countries
Table 3 presents clinical characteristics and CMR imaging parameters for studies that compared PWH and uninfected controls within the United Kingdom and the United States [38–41]. When compared with these cohorts from HICs, our study had higher proportion of women (except for Zanni et al [41] who studied only women) and higher prevalence of hypertension. Differences in magnetic resonance imaging systems between the studies limit direct comparisons of T1 mapping parameters used to inform ECV. However, in HICs, HIV status had a large effect on the presence of LGE and a consistent effect on ECV that was greatest among the cohort that only studied women (Zanni et al [41]). The difference in ECV in an LMIC in our study is consistent with these data from HICs, but they did not detect a difference in LGE due to the higher proportion of uninfected persons with LGE in SA when compared with the United Kingdom.
Table 3.
Characteristics of PWH and Controls Studied With CMR in High-Income Settings [38–41]
| United Kingdom | United States | |||||||
|---|---|---|---|---|---|---|---|---|
| Ntusi et al [38] | Luetkens et al [39] | Thiara et al [40] | Zanni et al [41] | |||||
| Clinical Characteristics | PWH (n = 103) | Controls (n = 92) | PWH (n = 28) | Controls (n = 22) | PWH (n = 95) | Controls (n = 30) | PWH (n = 20) | Controls (n = 14) |
| Age, mean (SD) years | 45 (10) | 44 (10) | 49 (9) | 45 (16) | 49 (10) | 46 (8) | 52 (4) | 53 (6) |
| Sex at birth, female | 23% | 42% | 21% | 32% | 25% | 27% | 100% | 100% |
| Current smoker | 39% | 13% | 25% | 27% | 19% | 7% | 50% | 29% |
| BMI, mean (SD) kg/m2 | 26 (4) | 25 (4) | 25 (4) | 25 (3) | 28 (5) | 30 (4) | 32 (7) | 32 (7) |
| Hypertension diagnosis | 5% | 15% | 11% | 23% | NA | NA | 25% | 29% |
| Diabetes diagnosis | 0% | 1% | 0% | 0% | 10% | 0% | NA | NA |
| ART treatment | 87% | -- | 100% | 100% | 93% | -- | 100% | 100% |
| CMR Parameters (T1 Mapping Technique) | 1.5-T MR (Avanto) (MOLLI) | 3.0-T MR (Ingenia) (MOLLI) | 3.0-T MR (Verio) (MOLLI) | 3.0-T MR; (Skyra) (LL Sequence) | ||||
| LV EF, mean (SD) % | 68 (6) | 72 (5) | 61 (7) | 65 (6) | 62 (6) | 63 (4) | 58 (4) | 60 (5) |
| LV MI mean (SD) g/m2 | 58 (11) | 54 (11) | NA | NA | NA | NA | 48 | 42 |
| LV LGE presence | 83% | 16% | 82% | 27% | NA | NA | NA | NA |
| ECV, mean (SD) % | NA | NA | 28 (5) | 26 (3) | 28 (4) | 26 (3) | 34 (6) | 29 (4) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CMR, cardiac magnetic resonance; ECV, extracellular volume; EF, ejection fraction; LGE, late-gadolinium enhancement; LV, left ventricular; MI, mass index; NA, not applicable; PWH, persons with human immunodeficiency virus; SD, standard deviation.
DISCUSSION
We describe the frequency and characteristics of myocardial fibrosis by CMR among PWH without clinical HF or known CVD living in Khayelitsha, SA, and we assess the potential influence of demographics and clinical characteristics. A number of key findings emerged from this study. First, the prevalence of myocardial fibrosis by LGE (72%) was high among both persons with and without HIV infection. However, in the absence of HIV among uninfected controls, the frequency of any LGE in our study in SA was 3- to 4-fold higher when compared with that reported in HICs [38, 39]. Second, despite the higher-than-expected prevalence of fibrosis in the control population, ART-treated PWH had evidence of greater myocardial fibrosis by ECV. Third, the effect of HIV status on myocardial fibrosis was more prominent among women, among younger patients, and in the absence of several key risk factors. Fourth, women with HIV who had myocardial fibrosis by ECV were also more likely to have elevated levels of NT-proBNP (>125 pg/mL).
Our findings add to data from recent CMR studies describing higher frequencies of myocardial fibrosis and inflammation among PWH, when compared with uninfected controls [32, 38–41]. In a US study, 95 PWH on ART demonstrated higher CMR estimates of ECV at an absolute degree of approximately 2%, when compared with 30 uninfected persons [40]. Likewise, 2 cross-sectional studies conducted in the United Kingdom reported higher estimates of myocardial fibrosis among PWH, as estimated by native T1 mapping and prevalence of LGE [38, 39]. In these 3 studies, approximately three quarters of participants were men, limiting comparisons among women. In a recent report including only women with and without HIV infection from the United States, women with HIV had significantly greater ECV measures by CMR with a higher absolute difference of approximately 5% (34% vs 29%, respectively), which is similar to the magnitude we describe in subgroup analyses by sex (Figure 1) [41]. In the US study of women with HIV, measures of monocyte activation (eg, plasma soluble CD163 levels) were correlated with ECV [41]. Research is needed to better understand the pathogenesis of myocardial fibrosis among PWH in sub-Saharan Africa, the influence of ongoing systemic inflammation despite plasma viral suppression with ART, and potential differences in this pathogenesis among women with HIV.
In addition to differences by sex, subgroup analyses in our study demonstrated a greater effect of HIV status on myocardial fibrosis among those that would be categorized as having lower CVD by traditional cardiometabolic factors (Figure 2). Reasons for this are unclear but may be influenced by the additive effects of HIV factors in the presence of traditional cardiometabolic risk factors and/or by the eligibility criteria in our study. Specifically, the combination of risk from HIV and cardiometabolic factors could be additive, subadditive, or synergistic for myocardial fibrosis in an SA setting. If subadditive, as the risk from HIV disease is combined with other risk factors, the absolute increase in myocardial fibrosis would be less than the sum of each individual effect, consistent with a “ceiling effect.” Such a relationship could explain the pattern we observed in subgroup comparisons (Figure 2), where the isolated effect of HIV status was greater in the absence of traditional risk factors. Conversely, if additive or synergistic, the effect on myocardial fibrosis might increase substantially among PWH and traditional cardiometabolic risk factors. However, the exclusion of persons with known CVD in our study target population could have systematically reduced the ability to detect an additive or synergistic effect from HIV disease when in the presence of hypertension, obesity, or smoking. These cardiometabolic risk factors are associated with both myocardial fibrosis [22, 28, 42] as well as clinical CVD, [43] which, if present, would have excluded the participant.
Consistent with our eligibility criteria, the majority of participants in our study had normal LV systolic and diastolic function by CMR strain parameters as well as NT-proBNP levels. This is in contrast to other western cohorts that have described HIV-associated reductions in systolic function even during treated HIV infection [16]. One potential explanation is that systolic dysfunction among PWH on ART in the United States and Europe may be influenced by increased risk for ischemic heart disease, whereas this remains less common in sub-Saharan Africa. In addition, the mean nadir CD4 count of 271 reflects that many participants in our study had not previously progressed to advanced AIDS where HIV-associated cardiomyopathy with systolic dysfunction would be more common. However, among women, HIV status was associated with a greater likelihood of having elevated NT-proBNP levels but not cTnT levels. Elevations in NT-proBNP were associated with greater myocardial fibrosis by ECV and native T1 mapping among PWH but not among controls. Within SA, rates of several cardiometabolic risk factors also differ between women and men, respectively, including for hypertension (21% vs 12%) and obesity (39% vs 10%) [44]. Prospective studies are needed in LMIC settings such as SA that focus on characterizing the prevalence of clinical HF in the current era of ART, along with understanding the combined and individual effects of HIV infection, sex, as well as traditional cardiometabolic risk factors on HF risk.
In contrast to HICs that have seen recent declines in CVD mortality, the percentage of deaths due to CVD are increasing in LMICs [5]. In SA and other areas in sub-Saharan Africa, hypertensive heart disease and risk for HF is more prominent than in other global regions [5, 8]. We describe a high prevalence of myocardial fibrosis among both PWH and uninfected persons in the absence of known CVD, which was also severalfold higher than the prevalence of myocardial fibrosis reported among uninfected persons in HICs [32, 38–40]. The high prevalence of myocardial fibrosis estimated by LGE among patients in SA may be an important mechanism of CVD in this setting, contributing to a phenotype of CVD characterized by an increased risk for HF and sudden cardiac death [22, 31, 42, 45].
This study provides important novel descriptions of myocardial fibrosis among PWH in SA in the current era of ART but includes several limitations. The CMR measures are an indirect assessment of tissue fibrosis, the clinical signficance of absolute differences in ECV are not clearly defined, and differences by HIV status were not robust overall. The sample also had limited power to study interactions and mediation analyses with clinical factors. The design approach to frequency match groups on hypertension status also limited our ability to explore the degree to which HIV-associated changes in myocardial fibrosis were accounted for by BP. The cross-sectional design has inherent potential for unmeasured confounding. We lack objective assessments of mental health and substance abuse that may contribute to risk, and clinical data available through usual care were limited to HIV measures and did not include metabolic assessments (ie, to characterize the metabolic syndrome). Men without HIV enrolled as controls in our study also had more frequent CVD risk factors than men with HIV in our sample, which may have actually mitigated differences in CMR measures by HIV status overall.
CONCLUSIONS
The study findings support that HIV disease may increase myocardial fibrosis, an effect that was more prominent among women and may have important implications for understanding HF risk. The manifestations of CVD among PWH in SA differ in important ways, in part, due to the unique profile and combined influence of cardiometabolic and HIV or other infectious factors. Further research is needed to continue to evaluate this hypothesis, particularly among women with HIV, to better understand the clinical consequences of myocardial fibrosis among PWH, specifically within LMIC countries such as SA.
Acknowledgments
We thank all the study participants for their commitment and support of the project. In addition, we thank Rene Goliath and research staff who assisted with recruitment at Site B, as well as CIDRI-Africa.
Disclaimer. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The opinions, findings and conclusions expressed in this manuscript reflect those of the authors alone.
Financial support. This study was funded by the National Heart Lung and Blood Institute, National Institutes of Health (R21 HL137435) and the American Heart Association (17IRG33350064). This work was also supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. G. M. was supported by the Wellcome Trust (098316, 214321/Z/18/Z, and 203135/Z/16/Z) and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant Number 64787).
Potential conflicts of interest. S. R. S. and L.-Y. W. are Doris Duke International Clinical Research Fellows. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mayosi BM, Flisher AJ, Lalloo UG, et al. The burden of non-communicable diseases in South Africa. Lancet 2009; 374:934–47. [DOI] [PubMed] [Google Scholar]
- 2. Osler M, Hilderbrand K, Goemaere E, et al. The continuing burden of advanced HIV disease over 10 years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis 2018; 66:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation 2019; 140:e98–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roth GA, Huffman MD, Moran AE, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015; 132:1667–78. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. Progress Report 2016: Prevent HIV, Test and Treat All. Geneva, Switzerland: The WHO Document Production Services; 2016. [Google Scholar]
- 7. Huerga H, Shiferie F, Grebe E, et al. A comparison of self-report and antiretroviral detection to inform estimates of antiretroviral therapy coverage, viral load suppression and HIV incidence in Kwazulu-Natal, South Africa. BMC Infect Dis 2017; 17:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sliwa K, Wilkinson D, Hansen C, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet 2008; 371:915–22. [DOI] [PubMed] [Google Scholar]
- 9. Himelman RB, Chung WS, Chernoff DN, et al. Cardiac manifestations of human immunodeficiency virus infection: a two-dimensional echocardiographic study. J Am Coll Cardiol 1989; 13:1030–6. [DOI] [PubMed] [Google Scholar]
- 10. Twagirumukiza M, Nkeramihigo E, Seminega B, et al. Prevalence of dilated cardiomyopathy in HIV-infected African patients not receiving HAART: a multicenter, observational, prospective, cohort study in Rwanda. Curr HIV Res 2007; 5:129–37. [DOI] [PubMed] [Google Scholar]
- 11. Hsue PY, Hunt PW, Ho JE, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail 2010; 3:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mondy KE, Gottdiener J, Overton ET, et al. ; SUN Study Investigators. High prevalence of echocardiographic abnormalities among HIV-infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis 2011; 52:378–86. [DOI] [PubMed] [Google Scholar]
- 13. Schuster I, Thöni GJ, Edérhy S, et al. Subclinical cardiac abnormalities in human immunodeficiency virus-infected men receiving antiretroviral therapy. Am J Cardiol 2008; 101:1213–7. [DOI] [PubMed] [Google Scholar]
- 14. Reinsch N, Neuhaus K, Esser S, et al. ; German Competence Network for Heart Failure; German Competence Network for HIV AIDS. Prevalence of cardiac diastolic dysfunction in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials 2010; 11:156–62. [DOI] [PubMed] [Google Scholar]
- 15. Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011; 171:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the veterans aging cohort study. JAMA Cardiol 2017; 2:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006; 48:1977–85. [DOI] [PubMed] [Google Scholar]
- 18. Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res 2014; 115:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Margolis JR, Kannel WS, Feinleib M, et al. Clinical features of unrecognized myocardial infarction–silent and symptomatic. Eighteen year follow-up: the Framingham study. Am J Cardiol 1973; 32:1–7. [DOI] [PubMed] [Google Scholar]
- 20. Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 2006; 113:2733–43. [DOI] [PubMed] [Google Scholar]
- 21. Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol 2008; 51:2414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62:263–71. [DOI] [PubMed] [Google Scholar]
- 23. Tschöpe C, Van Linthout S. New insights in (inter)cellular mechanisms by heart failure with preserved ejection fraction. Curr Heart Fail Rep 2014; 11:436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roman MJ, Ganau A, Saba PS, et al. Impact of arterial stiffening on left ventricular structure. Hypertension 2000; 36:489–94. [DOI] [PubMed] [Google Scholar]
- 25. Müller-Brunotte R, Kahan T, López B, et al. Myocardial fibrosis and diastolic dysfunction in patients with hypertension: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA). J Hypertens 2007; 25:1958–66. [DOI] [PubMed] [Google Scholar]
- 26. Liu T, Song D, Dong J, et al. Current understanding of the pathophysiology of myocardial fibrosis and its quantitative assessment in heart failure. Front Physiol 2017; 8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature 2002; 415:240–3. [DOI] [PubMed] [Google Scholar]
- 28. Liu CY, Liu YC, Wu C, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013; 62:1280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ambale Venkatesh B, Volpe GJ, Donekal S, et al. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: the Multi-Ethnic Study of Atherosclerosis study. Hypertension 2014; 64:508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang EY, Ghosn MG, Khan MA, et al. Myocardial extracellular volume fraction adds prognostic information beyond myocardial replacement fibrosis. Circ Cardiovasc Imaging 2019; 12:e009535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ambale-Venkatesh B, Liu CY, Liu YC, et al. Association of myocardial fibrosis and cardiovascular events: the multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging 2019; 20:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 2013; 128:814–22. [DOI] [PubMed] [Google Scholar]
- 33. Piechnik SK, Ferreira VM, Dall’Armellina E, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010; 12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Young AA, Imai H, Chang CN, Axel L. Two-dimensional left ventricular deformation during systole using magnetic resonance imaging with spatial modulation of magnetization. Circulation 1994; 89:740–52. [DOI] [PubMed] [Google Scholar]
- 35. Messroghli DR, Radjenovic A, Kozerke S, et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004; 52:141–6. [DOI] [PubMed] [Google Scholar]
- 36. Wu E, Judd RM, Vargas JD, et al. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet 2001; 357:21–8. [DOI] [PubMed] [Google Scholar]
- 37. Doob JL. The limiting distributions of certain statistics. Ann Math Statist 1935; 6:160–9. [Google Scholar]
- 38. Ntusi N, O’Dwyer E, Dorrell L, et al. HIV-1-related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging 2016; 9:e004430. [DOI] [PubMed] [Google Scholar]
- 39. Luetkens JA, Doerner J, Schwarze-Zander C, et al. Cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV-infected patients. Circ Cardiovasc Imaging 2016; 9:e004091. [DOI] [PubMed] [Google Scholar]
- 40. Thiara DK, Liu CY, Raman F, et al. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis 2015; 212:1544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zanni MV, Awadalla M, Toribio M, et al. Immune correlates of diffuse myocardial fibrosis and diastolic dysfunction among aging women with human immunodeficiency virus. J Infect Dis 2020; 221:1315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kai H, Kuwahara F, Tokuda K, Imaizumi T. Diastolic dysfunction in hypertensive hearts: roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res 2005; 28:483–90. [DOI] [PubMed] [Google Scholar]
- 43. Bozkurt B, Aguilar D, Deswal A, et al. ; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular and Stroke Nursing; Council on Hypertension; and Council on Quality and Outcomes Research. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the american heart association. Circulation 2016; 134:e535–78. [DOI] [PubMed] [Google Scholar]
- 44. Shisana O, Labadarios D, Rehle T, et al. South African National Health and Nutrition Examination Survey (SANHANES-1). Cape Town: HSRC Press; 2013. [Google Scholar]
- 45. Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013; 309:896–908. [DOI] [PubMed] [Google Scholar]