Abstract
Background
Coronavirus disease 2019 (COVID-19) is highly contagious and deadly and is associated with coagulopathy. Pentraxin-3(PTX3) participates in innate resistance to infections and plays a role in thrombogenesis.
Purpose
The present study aimed to investigate the role of PTX3 in coagulopathy in patients with COVID-19.
Methods
A retrospective study, including thirty-nine COVID-19 patients, enrolled in Hunan, China, were performed. The patients were classified into the D-dimer_L (D-dimer <1mg/L) and D-dimer_H (D-dimer≥1mg/L) groups basing on the plasma D-dimer levels on admission. Serum PTX3 levels were detected by enzyme-linked immunosorbent assays and compared between those two groups, then receiver operating characteristic (ROC) curve analysis, correlation analysis, and linear regression models were performed to analyze the association between PTX3 and D-dimer.
Results
Our results showed that serum PTX3 levels (median values, 10.21 vs. 3.36, P<0.001), computerized chest tomography (C.T.) scores (median values, 10.0 vs. 9.0, P<0.05), and length of stay (LOS) (mean values, 16.0 vs. 10.7, P=0.001) in the D-dimer_H group were significantly higher than that in D-dimer_L group. ROC curve analysis revealed that the AUC of white blood corpuscle counts, C-reaction protein, erythrocyte sedimentation rate, and PTX3 for COVID-19 were 0.685, 0.863, 0.846, and 0.985, respectively. Correlation analysis showed that there was a positive relationship between PTX3 and D-dimer (r=0.721, P<0.001), chest CT imaging score (r=0.418, P=0.008), and LOS (r=0.486, P=0.002). Multiple linear regression analysis showed that the coefficient of determination was 0.657 (P < 0.001).
Conclusion
Serum level of PTX3 was positively correlated with disease severity and coagulopathy. Detection of serum PTX3 level could help identify severer patients on admission and may be a potential therapeutic target for coagulopathy in patients with COVID-19.
Keywords: Coronavirus disease 2019, Pentraxin-3, D-dimer, Coagulopathy, Disease Severity
Introduction
In December 2019, a cluster of severe pneumonia cases of unknown cause, lately named Coronavirus disease 2019 (COVID-19), was reported in Wuhan, China. A novel strain of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the pathogen.1 COVID-19 can be asymptomatic or mild to severe symptomatic. As of September 1, 2020, more than 25 million cases of COVID-19 have been reported worldwide, with over 854 000 deaths, and the number is overgrowing.2
Pentraxin-3 (PTX3) is a pentraxin superfamily member and is involved in acute and chronic inflammation and innate immunity.3 The level of PTX3 fluctuates with the intensity of the immune-inflammatory response.4 PTX3 is also involved in endothelial dysfunction through various mechanisms and is correlated with acute pulmonary embolism-related deaths,5 while endothelial dysfunction has been reported in severe COVID-19 and plays a vital role in coagulopathy.6 As an indirect marker of coagulation activation, the D-dimer level greater than 1μg/mL (i.e. 1mg/L) on admission has been correlated with an increased likelihood of in-hospital death in COVID-19 patients.7 The present study aimed to detect the serum level of PTX3 in different groups according to the serum level of D-dimer and to analyze the correlation of serum PTX3 levels with disease severity and coagulopathy.
Materials and Methods
A retrospective study was conducted. From February 1 to March 10, 2020, thirty-nine adult patients (age ≥18 years) tested positive with SARS-CoV2 of throat-swab samples were admitted into the Infectious Disease Ward in the Fourth People’s Hospital of Yiyang in Hunan, China, and they were all recruited into the study. The clinical characteristics and laboratory findings of COVID-19 patients were extracted from the medical records. Upon admission, the patients underwent blood routine tests, biochemical and immunological routine tests, quantifications of plasma C-reaction protein(CRP), erythrocyte sedimentation rate(ESR) and D-dimer, and computerized chest tomography(C.T.) scanning to assess the severity of COVID-19. Diagnostic criteria for COVID-19 severity were based on the guidelines of the National Health Commission of China.8 The patients were treated with inhaled interferon α-2b and oral lopinavir-ritonavir as antiviral therapies and supportive treatments. In our setting, no patients died during the observation period.
The patients were discharged at the following conditions: the absence of fever for at least three days; significant improvement in both lungs on chest C.T. scanning; clinical remission of respiratory symptoms; repeated negative in RT-PCR test of throat-swab samples at least 24-hours interval.
The study was approved by the ethics committee of the indicated hospital by the Code of Ethics of the World Medical Association Declaration of Helsinki. Written informed consent was waived due to the nature of our retrospective study.
Blood sample collection
PTX3 detections were routinely performed for the patients who had been suspected of systemic infection in our hospital. In the cohort, vein blood samples for PTX3 detection were collected at admission in a fasting state, 6 a.m. the next morning after admission, and immediately centrifuged at 1500×g and stored at −80°C until thawed once and analyzed.
Chest computerized tomography
The chest C.T. image analysis and grading were performed by two radiologists with extensive thoracic radiology experience. The final scores and grading were determined using a scoring system described in the previous study;9 the details are shown in Table 1.
Table 1.
C.T. imaging performance and corresponding score system.
| Number | CT Imaging performance | Score |
|---|---|---|
| 1 | unilateral patchy shadows or ground glass opacity | 5 |
| 2 | bilateral patchy shadows or ground glass opacity | 7 |
| 3 | diffuse changes for (1) or (2) | 2 |
| 4 | unilateral solid shadow or strip shadow | 2 |
| 5 | bilateral solid shadow or strip shadow | 4 |
| 6 | unilateral pleural effusion | 2 |
| 7 | bilateral pleural effusion | 4 |
| 8 | increased or enlarged mediastinal lymph nodes | 1 |
Enzyme-linked immunosorbent assay (ELISA)
Strictly according to the manufacturer’s instructions, quantitative determination of the serum PTX3 level of one batch was performed weekly using commercially available ELISA kits.
Statistical Analysis
Categorical variables were reported as the counts and percentages, and significance was detected by the chi-square test. The continuous variables were described using mean and standard deviation if they were normally distributed, or median and interquartile range (IQR) value if they were not normally distributed. Continuous variables were compared using independent group t-tests when they were normally distributed; otherwise, the Mann-Whitney U-test was used. Correlation analysis was performed by Pearson’s correlation coefficient. Statistical analysis was performed by SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). A two-sided P-value <0.05 was considered statistically significant.
Results
Patient Characteristics
According to the plasma D-dimer level on admission, the patients were divided into the D-dimer_L group (<1mg/L) and the D-dimer_H group (≥1mg/L). 48.7% (19 cases) were male, and there was no difference in sex ratio between the D-dimer_L group and the D-dimer_H group. The patients’ average age was 49.0 years old, and the median age was 49.0 and 54.0 years old in D-dimer_L and D-dimer_H groups, respectively (P=0.03). There were no significant differences in smoking, cardiovascular disease, diabetes between both groups, while significant differences in length of stay were observed (mean values, 10.7 vs. 16.0, P=0.001, Table 2).
Table 2.
Clinical and laboratory findings in patients with COVID-19.
| All patients(n=39) | D-dimer_L(n=30) | D-dimer_H(n=9) | p-value | |
|---|---|---|---|---|
| Characteristics | ||||
| Age(years) | 49.0(31.0–56.0) | 49.0(24.5–55.3) | 54.0(47.0–74.5) | 0.030 |
| Gender | ||||
| Male(n[%]) | 19[48.7] | 15[50.0] | 4[44.4] | 1.000 |
| Female(n[%]) | 20[51.3] | 15[50.0] | 5[55.6] | 1.000 |
| Chronic medical illness | ||||
| CVD(n[%]) | 3[7.7] | 1[3.3] | 2[22.2] | 0.127 |
| Current Smokers(n[%]) | 4[10.3] | 3[10.0] | 1[11.1] | 1.000 |
| Diabetes(n[%]) | 4[10.3] | 2[6.7] | 2[22.2] | 0.223 |
| length of stay(days) | 12.0±4.3 | 10.7±3.6 | 16.0±4.2 | 0.001 |
| CT scores on admission | 9(5–11) | 9(5–11) | 10(9–15) | 0.022 |
| Laboratory Findings | ||||
| Leucocytes(×109 /L) | 6.27(4.64–7.82) | 5.70(4.34–7.42) | 7.55(5.47–8.80) | 0.096 |
| Lymphocytes(×109 /L) | 1.16(0.84–1.68) | 1.28(1.02–1.74) | 1.04(0.59–1.42) | 0.194 |
| Neutrophils(×109 /L) | 3.52(2.91–4.44) | 3.23(1.92–4.17) | 5.20(3.66–7.19) | 0.002 |
| NLR | 2.59 (1.86–4.28) | 2.35(1.72–3.71) | 6.19(2.79–9.79) | 0.014 |
| Monocytes(×109 /L) | 0.46(0.33–0.52) | 0.46(0.35–0.51) | 0.51(0.27–0.58) | 0.920 |
| LDL-C(mmol/L) | 1.88(1.55–2.34) | 1.85(1.55–2.35) | 1.95(1.46–2.33) | 0.764 |
| Triglycerides (mmol/L) | 1.09(0.93–1.58) | 1.24(0.95–1.60) | 0.95(0.83–1.07) | 0.072 |
| Total-cholesterol(mmol/L) | 3.87±0.95 | 3.82±0.90 | 4.04±1.00 | 0.546 |
| C-reaction protein(mg/L) | 3.50(0.50–16.50) | 1.59(0.50–5.66) | 38.88(13.60–66.60) | 0.001 |
| ESR(mm/h) | 22.0(12.0–39.5) | 19.5(10.6–29.4) | 41.7(27.0–76.8) | 0.002 |
| D-dimer(mg/L) | 0.43(0.19–0.88) | 0.35(0.15–0.52) | 4.49(1.55–7.00) | 0.000 |
Data are expressed as median (IQR), mean ± standard deviation or number[%]. Between group comparisons of continuous variables were analyzed by independent sample t-tests or Mann-Whitney U-tests, and comparisons of categorical variables by chi-square tests.
Abbreviations: COVID-19=coronavirus disease 2019; CVD=cardiovascular disease; NLR= neutrophil-lymphocyte ratio; LDL-C=low-density lipoprotein cholesterol; ESR=erythrocyte sedimentation rate; NLR: neutrophil-lymphocyte ratio.
For laboratory findings, there were no significant differences in white blood corpuscle (WBC) counts, lymphocyte counts, monocyte counts, plasma levels of low-density lipoprotein-cholesterol (LDL-C), triglycerides, and total cholesterol between both groups. In contrast, significant differences in neutrophil counts (P=0.002), neutrophil-lymphocyte ratio(NLR) (P=0.014), plasma levels of CRP, and ESR (P<0.01) were observed. D-dimer’s median values in D-dimer_L and D-dimer_H groups were 0.35 vs. 4.49mg/L (P<0.001, Table 2).
On admission, abnormalities in chest C.T. images were observed for all patients. The representative chest C.T. findings were bilateral ground-glass opacity and sub-segmental areas of consolidation. The CT imaging score was higher in the D-dimer_H group than the D-dimer_L group (median values, 10.0 vs. 9.0, P =0.022; Table 2).
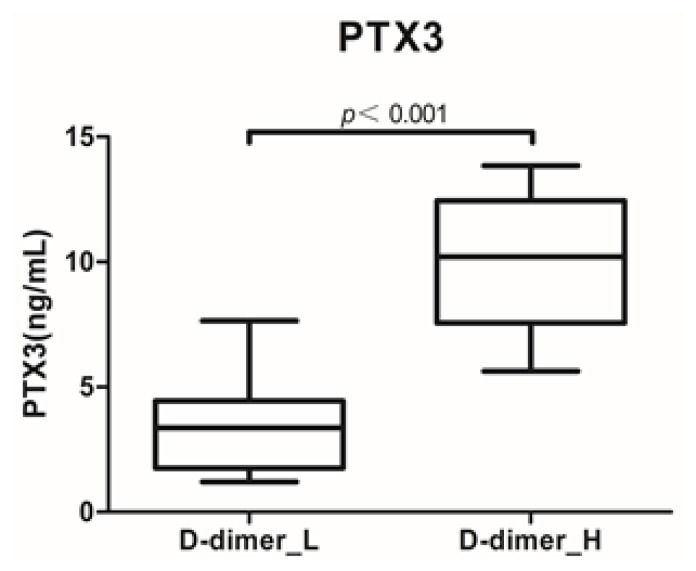
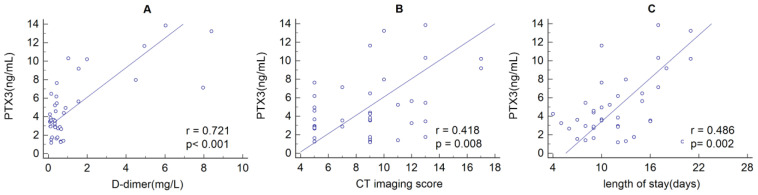
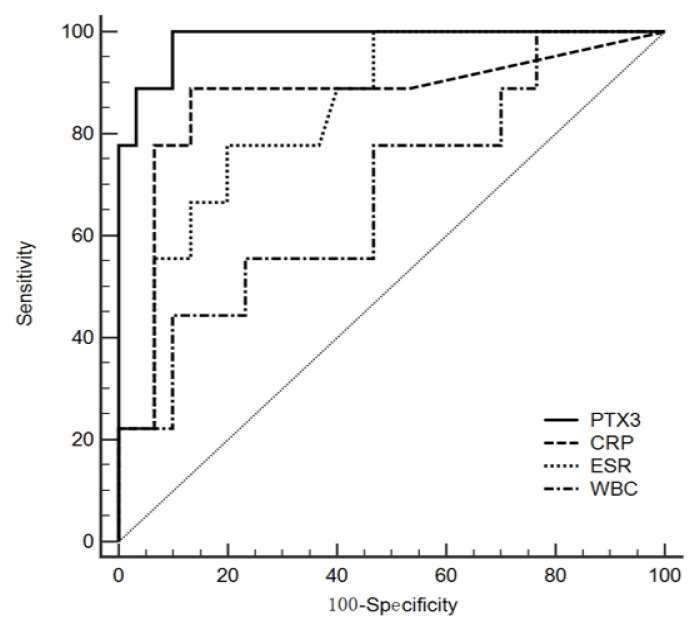
The serum level of PTX3 in the D-dimer_H group was significantly higher than that in the D-dimer_L group (median values, 10.21 vs. 3.36; P<0.001; Figure 1). Correlation analysis showed that there was a positive relationship between PTX3 and D-dimer (r=0.721, P<0.001), chest CT imaging score (r=0.418, P=0.008), and LOS (r=0.486, P=0.002) (Figure 2). ROC curve analysis revealed that the area under the curve (AUC) of WBC was 0.685 (95% confidence interval (CI) 0.517 – 0.824, P=0.010), and the optimum cutoff was 8.57×109/L (sensitivity 44.4%, specificity 90.0%). The AUC of CRP was 0.863 (95%CI 0.715 – 0.952, P =0.001), and the optimum cutoff was 9.05 (sensitivity 88.9%, specificity 86.7%). The AUC of ESR was 0.846 (95%CI 0.695 – 0.942, P =0.002), and the optimum cutoff was 20.17 (sensitivity 100%, specificity 53.3%). The AUC of PTX3 was 0.985 (95%CI 0.883 – 1.000, P<0.001), and the optimum cutoff was 5.54 (sensitivity 100%, specificity 90.0%) (Figure 3).
Figure 1.
Serum PTX3 levels in D-dimer_L (D-dimer<1mg/L) and D-dimer_H (D-dimer≥1mg/L) groups. (P<0.001).
Figure 2.
The relationship between serum PTX3 levels and plasma D-dimer(A), chest CT imaging scores(B) and length of stay(C). There was a positive relationship between PTX3 and D-dimer (r=0.461, P=0.003), chest CT imaging scores(r=0.418, P=0.008) and length of stay(r=0.486, P=0.002).
Figure 3.
ROC curve for WBC, CRP, ESR, and PTX3 in COVID-19 patients. ROC curve analysis revealed that the AUC of WBC, CRP, ESR, and PTX3 for COVID-19 were 0.685, 0.863, 0.846, and 0.985, respectively.
Univariate analysis of categorical variables
Univariate analysis of different categorical variables showed no significant group differences in plasma D-dimer levels based on stratification of gender, smokers, diabetes, and cardiovascular diseases (Table 3).
Table 3.
Univariate analysis of categorical variables.
| Variable | Category | D-dimer | Z | P-value |
|---|---|---|---|---|
| gender | male | 0.35(0.15,0.88) | −0.9 | 0.368 |
| female | 0.45(0.23,1.37) | |||
| smokers | no | 0.42(0.15,0.77) | −1.437 | 0.151 |
| yes | 0.84(0.46,6.19) | |||
| diabetes mellitus | no | 0.43(0.15,0.8) | −1.205 | 0.228 |
| yes | 2.45(0.36,7.42) | |||
| cardiovascular disease | no | 0.43(0.23,0.79) | −1.293 | 0.196 |
| yes | 7.96(4.06,8.18) |
There were no significant differences in plasma D-dimer levels between different groups based on the stratification of gender, smokers, diabetes mellitus, and cardiovascular disease by nonparametric Mann Whitney U-test.
Correlation analysis between continuous variables and plasma D-dimer levels
Pearson’s correlation coefficient analysis showed that there were significant correlations between WBC (r=0.339, P=0.035), NEU (r=0.565, P<0.001) , NLR (r=0.567, P<0.001), CRP (r=0.625, P<0.001), ESR (r=0.623, P<0.001), PTX3 (r=0.721, P<0.001) and D-dimer, while no significant correlations between age, lymphocyte counts, monocyte counts, LDL-C, total cholesterol, triglycerides and D-dimer were observed (Table 4).
Table 4.
Correlation analysis between continuous variables and plasma D-dimer levels
| Variables | r | p-value |
|---|---|---|
| Age(years) | 0.305 | 0.059 |
| WBC(×109/L) | 0.339 | 0.035 |
| LYM(×109/L) | −0.204 | 0.213 |
| NEU(×109/L) | 0.565 | 0.000 |
| NLR | 0.567 | 0.000 |
| MON(×109/L) | −0.118 | 0.475 |
| CRP(mg/L) | 0.625 | 0.000 |
| ESR(mm/h) | 0.623 | 0.000 |
| LDL-C(mmol/L) | −0.124 | 0.450 |
| Total cholesterol(mmol/L) | −0.129 | 0.435 |
| Triglycerides(mmol/L) | −0.254 | 0.118 |
| PTX3(ng/mL) | 0.721 | 0.000 |
There were significant correlations between WBC, CRP, ESR, PTX3 and D-dimer by Spearman correlation coefficiency analysis.
Abbreviations: WBC=White blood corpuscle counts; LYM=lymphocyte counts; NEU=neutrophil counts; NLR=neutrophil-lymphocyte ratios; MON=monocyte counts; CRP=C-reaction protein; ESR=erythrocyte sedimentation rate; LDL-C=low-density lipoprotein cholesterol; PTX3=pentraxin-3.
Linear regression analysis of PTX3 and D-dimer
D-dimer’s influencing factors were analyzed with multiple linear regression models, and the results showed that PTX3 levels were found to be independently positively associated with D-dimer levels (β = 0.451, P=0.006) (Table 5).
Table 5.
Multiple linear regression analysis of the relationship between laboratory findings and plasma D-dimer levels.
| B | SE | β | t | P-value | |
|---|---|---|---|---|---|
| (Constant) | −0.938 | 0.671 | −1.397 | 0.172 | |
| WBC | −0.094 | 0.166 | −0.116 | −0.565 | 0.576 |
| NEU | 0.170 | 0.222 | 0.192 | 0.768 | 0.448 |
| CRP | 0.014 | 0.012 | 0.197 | 1.191 | 0.242 |
| ESR | 0.018 | 0.013 | 0.223 | 1.324 | 0.195 |
| PTX3 | 0.282 | 0.095 | 0.451 | 2.969 | 0.006 |
| R2 | 0.657 | ||||
| F | 12.647 | ||||
| p-value | 0.000 |
Abbreviations: WBC=White blood corpuscle counts; NEU=neutrophil counts; CRP=C-reaction protein; ESR=erythrocyte sedimentation rate; PTX3=pentraxin-3.
Discussion
To our knowledge, this is the first study on the role of PTX3 in coagulopathy in patients with COVID-19. In this study, we divided 39 COVID-19 patients into two groups based on the plasma D-dimer levels on admission, and ELISA was performed to detect the serum levels of PTX3 in both groups. Our results proved that higher plasma concentrations of D-dimer were positively associated with higher serum levels of PTX3, which were also positively associated with higher plasma levels of CRP and ESR, higher C.T. imaging scores, and longer durations of in-hospital stay, indicating that the serum levels of PTX3 may contribute to disease severity and coagulopathy in patients with COVID-19.
The spectrum of COVID-19 ranges from asymptomatic, fever and dry cough, gastrointestinal symptoms, coagulation dysfunction, to multiple organ dysfunction, and it has been described as a process of systemic inflammation and immune dysfunction, with a large amount of interleukin (I.L.)-1β, interferon(IFN)-γ, tumor necrosis factor(TNF)-α, and other cytokines present in the system.10 PTX3 is upregulated and released by hematopoietic and stromal cells in response to pro-inflammatory stimuli, such as IL-1β and TNF-α.11 It is an essential component of innate immunity’s humoral arm, participating in innate resistance to infections of fungal, bacterial, and viral pathogens.12 Besides, PTX3 is an opsonin for pathogens, facilitating recognition and phagocytosis by neutrophils in a Complement- and FcγR-dependent manner and by neutralizing virus infectivity.13 Moreover, PTX3 exerts its regulatory function on the inflammatory response by modulating complement activity, recruiting inflammatory cells through interacting with the adhesion molecule P-selectin, or affecting apoptotic cells’ engulfment.12 Thus, PTX3 plays a vital role in innate immunity and inflammation.
Acute-phase proteins, such as CRP, serum amyloid A, and ferritin have been well investigated in patients with COVID-19,14 while the role of PTX3, which is frequently used to diagnose, predict, and evaluate many inflammatory diseases,3,15 has not been reported in COVID-19. Elevated PTX3 has been reported to be associated with disease severity and outcome in infectious diseases. Studies proved that PTX3 elevated significantly in hospitalized adult patients with community-acquired pneumonia,16 and the admission levels of PTX3 were useful for predicting the severity of community-acquired pneumonia, independent of possible pathogens,17 suggesting it could be used as a biomarker to assess disease severity and the detection of PTX3 on admission might be useful for clinical judgment. Although whether PTX3 plays a compensatory protective role or a detrimental role in COVID-19 patients is unclear, in a mouse model with a severe acute respiratory syndrome (SARS), PTX3 has been demonstrated to play a protective role in coronavirus-induced acute lung injury.18 Due to the close similarity of viral genome and pathogenicity for SARS-CoV and SARS-CoV2,19 it is reasonable to envisage that PTX3 may play a similar protective role in the process of immune responses, although further research is needed.
Since the outbreak of COVID-19, it was characterized as highly contagious and deadly. Coagulopathy has been reported repeatedly in recent studies, and the high incidence of massive pulmonary embolism and deep venous thrombosis suggests a pivotal role of coagulopathy in the deaths of COVID-19.20 As the smallest fibrinolysis-specific degradation product found in the circulation, D-dimer is a very sensitive biomarker for intravascular thrombus and is markedly elevated in disseminated intravascular coagulation and pulmonary embolus. It has been reported that higher concentrations of D-dimer are independently associated with in-hospital mortality in COVID-19 patients,21 and the value greater than 1 mg/L helps to identify patients with poor prognosis at an early stage.7 In our study cohort, serum PTX3 levels were associated with higher plasma D-dimer levels, independent of diabetes mellitus, current smoking, cardiovascular diseases, aging, and dyslipidemia, which implies that PTX3 may contribute to coagulopathy in patients with COVID-19.
Endothelial cells express angiotensin-converting enzyme 2 (ACE2), the receptor for SARS-CoV-2, and the interaction of SARS-CoV-2 and ACE2 possibly mediates endothelial activation, which has also been confirmed pathologically in patients with COVID-19.22 At present, it is believed that PTX3 is dramatically related to endothelial dysfunction, and several pathogenic pathways, such as inhibition of nitric oxide (NO) and P-selectin, have been identified. NO signaling pathway plays a central role in maintaining endothelial cell functions, regulating platelet aggregation, adhesion, and clot formation.23 The inhibition of PTX3 on NO synthesis leads to endothelial dysfunction, resulting in an imbalance in vascular homeostasis and a prothrombotic state.24 P-selectin is a pivotal factor in the initiation of leukocyte and endothelial cell adhesion,25 while the dysregulated expression of P-selectin contributes to pathological inflammation and deep vein thrombosis.26 Through interacting with P-selectin, PTX3 promotes vascular inflammatory response and endothelial dysfunction.27 Besides, PTX3 may influence coagulation through activating tissue factors (T.F.). T.F. is the high-affinity receptor and cofactor for FVII/VIIa. The TF-FVIIa complex is the primary initiator of blood coagulation and plays a crucial role in hemostasis. By increasing T.F. expression in endothelial cells, activated monocytes, and monocyte-derived dendritic cells, PTX3 potentially has a thrombophilic activity and plays a role in thrombogenesis.28–30 In our cohort study, although apparent thrombosis formation was excluded by Doppler ultrasound in deep veins in the lower extremities and repeated chest C.T. scans, plasma D-dimer levels were elevated in nine patients with COVID-19, we speculate that the relationship between pre-thrombosis levels of D-dimer and thrombotic disease is likely attributable to microvascular thrombosis formation.31
Considering that PTX3 is produced and released rapidly by damaged tissue cells and inflammatory cells, it could rapidly respond to the systemic inflammation at an early stage. Meanwhile, PTX3 was found to be significantly elevated in severe COVID-19 patients and independently predicted coagulation abnormalities in our study. Therefore, it is of great urgency to evaluate the potential therapeutic effects of inducers or inhibitors of PTX3, anti-PTX3 antibodies,32 simvastatin,33 and small interfering RNA (siRNA)34 in patients with COVID-19.
Limitations should be noted when interpreting the results of this study. First, the number of patients was too small (only 39 cases), leading to statistical deviation. Second, since we did not measure the coagulation system’s direct biomarkers and endothelial dysfunction, the specific disturbed pathways and mechanisms are still unknown, which deserves further study. Third, the lack of data on longitudinal samples limited the evaluation of the prognostic value of PTX3. Fourth, the relationships between PTX3 and other inflammatory markers (such as IL-6 and fibrinogen) are unclear since those markers were untested in the study.
Conclusions
In conclusion, although PTX3 is likely to participate in coagulation and may serve as a therapeutic target in this direction, the role of PTX3 in COVID-19 is still unclear, and further studies are needed to determine its reliability.
Acknowledgments
On behalf of the authors, we sincerely thank medical staffs in The Fourth People’s Hospital of Yiyang, Hunan, China.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University. Coronavirus Resource Center. [Accessed September 1, 2020]. https://coronavirus.jhu.edu/map.html.
- 3.Porte R, Davoudian S, Asgari F, Parente R, Mantovani A, Garlanda C, Bottazzi B. The Long Pentraxin PTX3 as a Humoral Innate Immunity Functional Player and Biomarker of Infections and Sepsis. Front Immunol. 2019 Apr 12;10:794. doi: 10.3389/fimmu.2019.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zlibut A, Bocsan IC, Agoston-Coldea L. Pentraxin-3 and endothelial dysfunction. Adv Clin Chem. 2019;91:163–179. doi: 10.1016/bs.acc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Zhang J, Huan Y, Xu Y, Guo R. Pentraxin-3 Levels Relate to the Wells Score and Prognosis in Patients with Acute Pulmonary Embolism. Dis Markers. 2019 Mar 12;2019 doi: 10.1155/2019/2324515. 2324515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escher R, Breakey N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020 Jun;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health Commission of the People’s Republic of China home page. http://www.nhc.gov.cn(published March 3, 2020). [DOI] [PMC free article] [PubMed]
- 9.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 Mar;31:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottazzi B, Inforzato A, Messa M, Barbagallo M, Magrini E, Garlanda C, Mantovani A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J Hepatol. 2016 Jun;64(6):1416–27. doi: 10.1016/j.jhep.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garlanda C, Bottazzi B, Magrini E, Inforzato A, Mantovani A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol Rev. 2018 Apr 1;98(2):623–639. doi: 10.1152/physrev.00016.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reading PC, Bozza S, Gilbertson B, Tate M, Moretti S, Job ER, Crouch EC, Brooks AG, Brown LE, Bottazzi B, Romani L, Mantovani A. Antiviral activity of the long chain pentraxin PTX3 against influenza viruses. J Immunol. 2008 Mar 1;180(5):3391–8. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 14.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, Psaltopoulou T, Gerotziafas G, Dimopoulos MA. Hematological findings and complications of COVID-19. Am J Hematol. 2020 Jul;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perea L, Coll M, Sanjurjo L, Blaya D, Taghdouini AE, Rodrigo-Torres D, Altamirano J, Graupera I, Aguilar-Bravo B, Llopis M, Vallverdú J, Caballeria J, van Grunsven LA, Sarrias MR, Ginès P, Sancho-Bru P. Pentraxin-3 modulates lipopolysaccharide-induced inflammatory response and attenuates liver injury. Hepatology. 2017 Sep;66(3):953–968. doi: 10.1002/hep.29215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao SJ, Yang HW, Tsao SM, Cheng CW, Bien MY, Yu MC, Bai KJ, Yang SF, Chien MH. Plasma long pentraxin 3 (PTX3) concentration is a novel marker of disease activity in patients with community-acquired pneumonia. Clin Chem Lab Med. 2013 Apr;51(4):907–13. doi: 10.1515/cclm-2012-0459. [DOI] [PubMed] [Google Scholar]
- 17.Luo Q, He X, Ning P, Zheng Y, Yang D, Xu Y, Shang Y, Gao Z. Admission Pentraxin-3 Level Predicts Severity of Community-Acquired Pneumonia Independently of Etiology. Proteomics Clin Appl. 2019 Jul;13(4):e1800117. doi: 10.1002/prca.201800117. [DOI] [PubMed] [Google Scholar]
- 18.Han B, Ma X, Zhang J, Zhang Y, Bai X, Hwang DM, Keshavjee S, Levy GA, McGilvray I, Liu M. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab Invest. 2012 Sep;92(9):1285–96. doi: 10.1038/labinvest.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naqvi AAT, Fatima K, Mohammad T, Fatima U, Singh IK, Singh A, Atif SM, Hariprasad G, Hasan GM, Hassan MI. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 2020 Oct 1;1866(10):165878. doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann Intern Med. 2020 Aug 18;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, Hochman BR, Salazar-Schicchi J, Yip NH, Brodie D, O‘Donnell MR. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020 Jun 6;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gresele P, Momi S, Guglielmini G. Nitric oxide-enhancing or -releasing agents as antithrombotic drugs. Biochem Pharmacol. 2019 Aug;166:300–312. doi: 10.1016/j.bcp.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Incalza MA, D‘Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018 Jan;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Lam FW, Da Q, Guillory B, Cruz MA. Recombinant Human Vimentin Binds to P-Selectin and Blocks Neutrophil Capture and Rolling on Platelets and Endothelium. J Immunol. 2018 Mar 1;200(5):1718–1726. doi: 10.4049/jimmunol.1700784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015 Aug 1;107(3):331–9. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrizzo A, Lenzi P, Procaccini C, Damato A, Biagioni F, Ambrosio M, Amodio G, Remondelli P, Del Giudice C, Izzo R, Malovini A, Formisano L, Gigantino V, Madonna M, Puca AA, Trimarco B, Matarese G, Fornai F, Vecchione C. Pentraxin 3 Induces Vascular Endothelial Dysfunction Through a P-selectin/Matrix Metalloproteinase-1 Pathway. Circulation. 2015 Apr 28;131(17):1495–505. doi: 10.1161/CIRCULATIONAHA.114.014822. discussion 1505. [DOI] [PubMed] [Google Scholar]
- 28.Napoleone E, Di Santo A, Bastone A, Peri G, Mantovani A, de Gaetano G, Donati MB, Lorenzet R. Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: a novel link between vascular inflammation and clotting activation. Arterioscler Thromb Vasc Biol. 2002 May 1;22(5):782–7. doi: 10.1161/01.ATV.0000012282.39306.64. [DOI] [PubMed] [Google Scholar]
- 29.Napoleone E, di Santo A, Peri G, Mantovani A, de Gaetano G, Donati MB, Lorenzet R. The long pentraxin PTX3 upregulates tissue factor in activated monocytes: another link between inflammation and clotting activation. J Leukoc Biol. 2004 Jul;76(1):203–9. doi: 10.1189/jlb.1003528. [DOI] [PubMed] [Google Scholar]
- 30.Kasuda S, Sakurai Y, Tatsumi K, Takeda T, Kudo R, Yuui K, Hatake K. Enhancement of Tissue Factor Expression in Monocyte-Derived Dendritic Cells by Pentraxin 3 and Its Modulation by C1 Esterase Inhibitor. Int Arch Allergy Immunol. 2019;179(2):158–164. doi: 10.1159/000496744. [DOI] [PubMed] [Google Scholar]
- 31.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020 Jun;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatto M, Ghirardello A, Luisetto R, Bassi N, Fedrigo M, Valente M, Valentino S, Del Prete D, Punzi L, Doria A. Immunization with pentraxin 3 (PTX3) leads to anti-PTX3 antibody production and delayed lupus-like nephritis in NZB/NZW F1 mice. Journal of autoimmunity. 2016;74:208–216. doi: 10.1016/j.jaut.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Yokota K, Miyoshi F, Sato K, Asanuma Y, Akiyama Y, Mimura T. Geranylgeranyl-pyrophosphate regulates secretion of pentraxin 3 and monocyte chemoattractant protein-1 from rheumatoid fibroblast-like synoviocytes in distinct manners. Clinical and experimental rheumatology. 2011;29(1):43–49. [PubMed] [Google Scholar]
- 34.Margheri F, Serratì S, Lapucci A, Chillà A, Bazzichi L, Bombardieri S, Kahaleh B, Calorini L, Bianchini F, Fibbi G, Del Rosso M. Modulation of the angiogenic phenotype of normal and systemic sclerosis endothelial cells by gain-loss of function of pentraxin 3 and matrix metalloproteinase 12. Arthritis and rheumatism. 2010;62(8):2488–2498. doi: 10.1002/art.27522. [DOI] [PubMed] [Google Scholar]