Abstract
A 26-year-old male driver presented with a history of pain in the neck for the past 1 week following trauma due to a road traffic accident. The patient had no neurological deficit. He had type 1 diabetes and was on regular oral hypoglycemics. After radiological investigations, the patient was diagnosed to have traumatic AO Spine Classification type C translational injury involving anterolisthesis of C6 over C7. After a detailed preoperative assessment, the patient was taken up for surgery. The patient underwent posterior stabilisation with instrumentation from C5 to T2. On extubation from anaesthesia, he immediately complained of complete painless loss of this vision in his left eye. Ophthalmological investigations attributed the cause to be due to central retinal artery occlusion. The patient was discharged with reassurance on the 20th postoperative day with minimal improvement in his vision and at 6-month follow-up, his vision improved to 1/60 and was advised for close follow-up.
Keywords: eye, trauma, trauma CNS /PNS, ophthalmology, orthopaedic and trauma surgery
Background
Although there is an expectant risk of impairment in vision following ocular surgeries, postoperative vision loss (POVL) following non-ocular surgeries is a catastrophic complication for both the patient as well as for the operating surgeon. Neither the patient nor the surgeon is prepared to handle the scenario. The frequency of POVL observed was 0.03% (3.09 per 10 000) in spine surgeries and 0.086% (8.64 per 10 000) among cardiac surgeries.1 However, the incidence of POVL following spine surgery varies between 0.028% and 0.2%.2 The majority of these POVL were due to ischaemic optic neuropathy (ION)3 followed by central retinal artery occlusion (CRAO) and cortical blindness.4 The potential risk factors for this ischaemic insult to the eye include pre-existing peripheral vascular disease, arterial hypertension, anaemia, organic mass lesions and intraoperative factors such as positioning and hypotensive anaesthesia.5 6
We present a case of a 26-year-old male patient operated for cervical spine trauma by posterior stabilisation with instrumentation and fusion who had painless complete vision loss in his left eye immediately after surgery due to CRAO.
Case presentation
A 26-year-old male driver presented with a history of pain in the neck for the past 1 week following trauma due to a road traffic accident which involved toppling of his heavy vehicle. The patient had no difficulty in using his limbs. He did not have any comorbidities except for type 1 diabetes for which he was on regular oral hypoglycemic drugs.
On examination, posterior midline tenderness was noted in the cervicothoracic junction. The patient had no neurological deficit and reflexes were normal.
Investigations
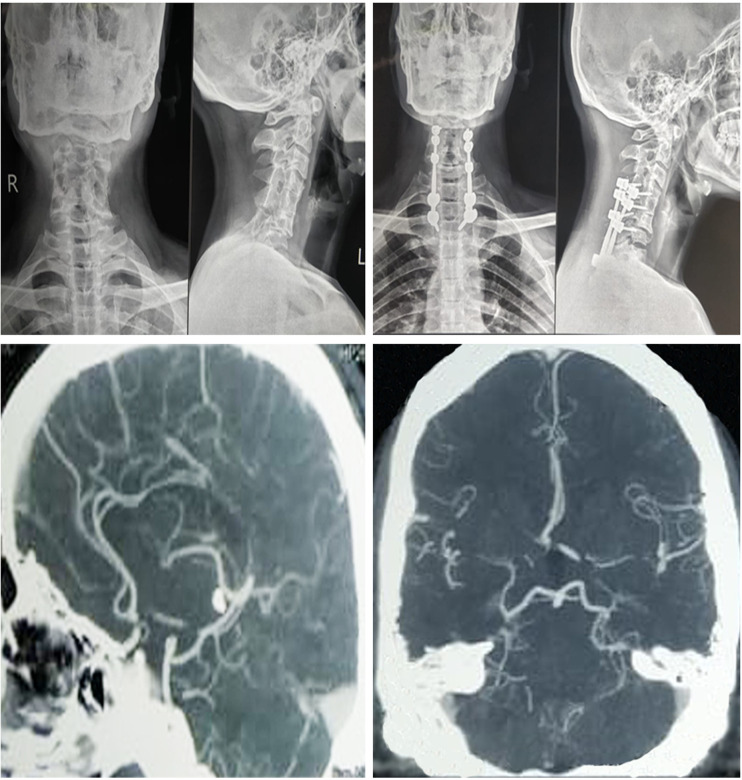
On radiological examination, the patient was diagnosed to have traumatic AO Spine Classification type C translational injury involving anterolisthesis of C6 over C7 as shown in figure 1. All the baseline blood investigations such as blood counts and liver and renal function tests were within normal limits. He was switched over to insulin from oral hypoglycemics as per the diabetologist’s opinion.
Figure 1.
Preoperative and postoperative radiographs of the spine showing the spinal stabilisation for the translational injury and postoperative CT angiogram showing hypoplasia of the A1 segment of the right anterior cerebral artery.
Treatment
The patient underwent posterior stabilisation under general anaesthesia from C5 to T2 in the prone position on the Mayfield frame with proper padding over bony prominences. A foam headrest with padding was used for the head and face. He was induced with propofol (3 mg/kg), fentanyl (160 mg) and glycopyrrolate. Succinylcholine (100 mg) was used for neuromuscular relaxation. Anaesthesia was maintained on sevoflurane. Preoperative haemoglobin was 14.5 g%. His blood pressure was maintained at around 130/80 mm Hg during the surgery. Total surgical time was 2.5 hours with intraoperative blood loss of 300 mL. Intraoperatively, 1500 mL of crystalloids was infused. Extubation was uneventful and neurology was immediately examined postoperatively and was found to be intact.
Outcome and follow-up
In the immediate postoperative period, he noticed painless vision loss in his left eye without any local signs of swelling or chemosis. Emergency ophthalmology consultation was sought. The patient was found to have no perception of light in the left eye with fundus examination, optical coherence tomography and fundus fluorescein angiography showing features of CRAO with disc pallor, narrowing arterioles and cherry-red spot. Visual evoked potentials (VEPs) and electroretinogram were performed showing a relative afferent pupillary defect (RAPD) and loss of VEPs on the left side. The patient was immediately started on oral acetazolamide 250 mg along with aspirin 150 mg, clopidogrel 75 mg and atorvastatin 20 mg together with subcutaneous enoxaparin in view to prevent further complications considering a thromboembolic aetiology to the POVL. Postoperative radiological evaluation for implant position was normal as shown in figure 1. Colour Doppler for the carotids turned out to be a normal study, whereas the CT angiogram of the carotids showed C4-C6 segmentation anomaly of the vertebrae associated with hypoplasia of the A1 segment of the right anterior cerebral artery as shown in figure 1. The patient was evaluated by the cardiologists with an echocardiogram which did not reveal any significant changes in the cardiovascular system. The patient was discharged with reassurance on the 20th postoperative day with minimal improvement in his vision and advised for close follow-up.
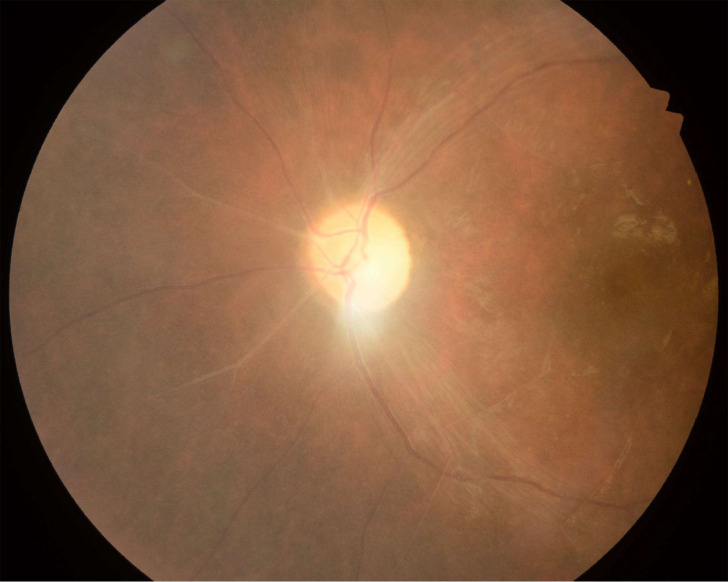
On review at 6 months after discharge, his vision improved to 1/60 in the left eye. Fundus examination showed minimal disc pallor, resolving cherry-red spot with attenuation and sheathing of the arteries as shown in figure 2. The patient is on close follow-up to monitor the progress of improvement in his vision and for the functional outcome of the posterior stabilisation.
Figure 2.
Fundus examination showing minimal disc pallor, resolving cherry-red spot with attenuation and sheathing of the arteries.
Discussion
Although uncommon, POVL is a devastating complication with dire consequences.7 Along with the sharp increase in the number of spinal instrumentation surgeries in the recent past, there is also an ominous increase in serious complications.8 One in one hundred spine surgeons will face a similar scenario annually.1 Spine surgeries have taken over the place of cardiac surgery to be the leading cause of POVL.9
POVL from spine surgery may be due to four major reasons:
External ocular injury.
Cortical blindness.
ION.
CRAO.
External ocular injury may result in chemosis, abrasion and corneal lacerations. It is usually preventable by routine eye preparation before positioning with lubricants and eye bandages. Cortical blindness occurs secondary to ischaemic insult to the visual tracts or visual cortex which might be due to cardiovascular thromboembolic events.10 In addition to blindness, patients with cortical involvement, although rare, may experience confusion and visual hallucinations. This is a reversible condition if perfusion is re-established.11
ION is the more common cause of POVL after spine surgery.12 It may be anterior or posterior depending on the site of the lesion in the optic nerve. It is more common in degenerative vaso-occlusive diseases and is common among smokers.13 Intraoperative factors such as severe blood loss and intraoperative hypotension may be a contributing factor.5
In a retrospective case–control analysis by Myers et al,14 duration of surgery and intraoperative blood loss were the significant factors contributing to POVL. In a recent analysis by Lee et al15 from the American Society of Anaesthesiologists POVL Registry, 71% of POVL was due to elective spinal surgery among which 89% was attributed to ION. It is also noted from their analysis that 94% of cases with POVL had undergone surgery ≥6 hours and 82% with blood loss >1 L. They also mentioned that fluid overload may also have a possible role in the pathogenesis of the ischaemic insult.16
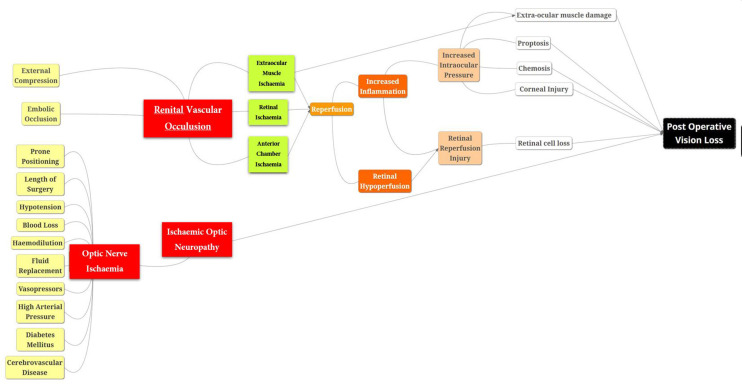
CRAO is usually due to direct external pressure on the globe resulting in unilateral loss of vision with periorbital swelling or ecchymosis.17 18 The pathophysiology of CRAO involves occlusion to the first intraorbital branch of the ophthalmic artery resulting in ischaemic injury to the retina and eventually vision loss leading to necrosis as shown in figure 3. A pale retinal picture with a cherry-red spot over the macula and an RAPD, as in our case, is pathognomonic of CRAO. It may be due to improper positioning or embolic episode or raised intraocular pressure.15 Increased intraocular pressure in an environment of mild ischaemic insult from intraoperative hypotension or anaemia may be more than sufficient in some individuals to get vision loss following spine surgeries in a prone position. When the occlusion is incomplete, vision may be regained after 8–24 hours with the macular collateral circulation from the cilioretinal artery which is found in 15% of the population.19 With this anatomical variant, patients have less severe presentation along with a better long-term prognosis. In our case, there was no such collateral circulation noted in angiography and the head was in a slightly dependant position to get adequate exposure which might be a contributing factor to raise the intraocular pressure to cause an ischaemic insult to the eye.
Figure 3.
Pathophysiology of central retinal artery occlusion and ischaemic optic neuropathy resulting in postoperative vision loss.
The following measures could avoid a conducive environment for such untoward complications in spine surgery.20 21
Preoperative measures
Risk of POVL could be explained preoperatively in complex spine surgeries especially when prolonged operating time and blood loss are expected. It is a much necessary precaution in a setting of comorbidities such as diabetes and hypertension when risk factors such as obesity and smoking are co-existent.
Intraoperative measures
Optimal positioning with orbit above the level of the heart with adequate padding. Overhydration should be avoided. When prolonged surgical time is expected, a resting stop to elevate the patient’s head to minimise periorbital oedema and an ischaemic insult to the optic nerve would be optimal to prevent disastrous complications such as POVL postoperatively.
Postoperative measures
Avoid flat positioning on recovery from anaesthesia, instead a head-up position is preferred. Perioperative blood pressure monitoring and avoiding fluid overload.
Having mentioned the preventive measures, one must understand that there is a paucity of definitive evidence to show a causal relationship for their etiopathogenesis in spine surgeries.20 21 However, in case of POVL despite following the above preventive measures, urgent and complete ophthalmological evaluation is mandatory for prompt treatment which is critical in the possible recovery of the patient’s vision.
Learning points.
The incidence of postoperative vision loss (POVL) is increasing in proportion to the number of prone spinal surgeries.
It must not be overlooked while obtaining informed consent in high-risk individuals to avoid litigations following devastating postoperative consequences if it occurs.
Adequate perioperative preventive measures should be followed to avoid POVL due to a preventable risk factor.
In the event of POVL despite following the preventive measures, urgent and complete ophthalmological evaluation is mandatory for prompt treatment which is critical in recovering the vision of the patient.
Footnotes
Twitter: @drsathishmuthu
Correction notice: This article has been corrected since it has been published online. The order of the authors has been changed.
Contributors: URN: Conceptualisation, data curation, formal analysis, investigations, methodology, administration, resources and supervision. SM: Resources, supervision, validation, visualisation, writing original drafts and reviewing drafts. BA: Conceptualisation, data curation, formal analysis, investigations and methodology. ER: Investigations, methodology and review and editing of the draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Roth S. Perioperative visual loss: what do we know, what can we do? Br J Anaesth 2009;103 Suppl 1:i31–40. 10.1093/bja/aep295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop 2014;5:100–6. 10.5312/wjo.v5.i2.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho VT-G, Newman NJ, Song S, et al. Ischemic optic neuropathy following spine surgery. J Neurosurg Anesthesiol 2005;17:38–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman W, Ward WT. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest. Case report and literature review. Spine 1993;18:1226–8. 10.1097/00007632-199307000-00017 [DOI] [PubMed] [Google Scholar]
- 5.Brown RH, Schauble JF, Miller NR. Anemia and hypotension as contributors to perioperative loss of vision. Anesthesiology 1994;80:222–6. 10.1097/00000542-199401000-00033 [DOI] [PubMed] [Google Scholar]
- 6.Chalam KV, Shah VA. Severe bilateral posterior ischemic optic neuropathy as a complication of spinal surgery. Eye 2005;19:367–8. 10.1038/sj.eye.6701482 [DOI] [PubMed] [Google Scholar]
- 7.Gabel BC, Lam A, Chapman JR, et al. Perioperative vision loss in cervical spinal surgery. Global Spine J 2017;7:91S–5. 10.1177/2192568216688196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan K, Rawall S, Nene A. Visual loss after spine surgery. Indian J Orthop 2012;46:106–8. 10.4103/0019-5413.91645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth S, Thisted RA, Erickson JP, et al. Eye injuries after nonocular surgery. A study of 60,965 anesthetics from 1988 to 1992. Anesthesiology 1996;85:1020–7. 10.1097/00000542-199611000-00009 [DOI] [PubMed] [Google Scholar]
- 10.Aldrich MS, Alessi AG, Beck RW, et al. Cortical blindness: etiology, diagnosis, and prognosis. Ann Neurol 1987;21:149–58. 10.1002/ana.410210207 [DOI] [PubMed] [Google Scholar]
- 11.Dalman JE, Verhagen WI, Huygen PL. Cortical blindness. Clin Neurol Neurosurg 1997;99:282–6. 10.1016/S0303-8467(97)00100-5 [DOI] [PubMed] [Google Scholar]
- 12.Goyal A, Elminawy M, Alvi MA, et al. Ischemic optic neuropathy following spine surgery: case control analysis and systematic review of the literature. Spine 2019;44:1087–96. 10.1097/BRS.0000000000003010 [DOI] [PubMed] [Google Scholar]
- 13.Roth S, Moss HE. Update on perioperative ischemic optic neuropathy associated with Non-ophthalmic surgery. Front Neurol 2018;9:557. 10.3389/fneur.2018.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers MA, Hamilton SR, Bogosian AJ, et al. Visual loss as a complication of spine surgery. A review of 37 cases. Spine 1997;22:1325–9. 10.1097/00007632-199706150-00009 [DOI] [PubMed] [Google Scholar]
- 15.Jaben SL, Glaser JS, Daily M. Ischemic optic neuropathy following general surgical procedures. J Clin Neuroophthalmol 1983;3:239–44. 10.3109/01658108308997311 [DOI] [PubMed] [Google Scholar]
- 16.Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists postoperative visual loss registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology 2006;105:652–9. 10.1097/00000542-200610000-00007 [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery - the case for restraint. N Engl J Med 2004;350:722–6. 10.1056/NEJMsb031771 [DOI] [PubMed] [Google Scholar]
- 18.Kamming D, Clarke S. Postoperative visual loss following prone spinal surgery. Br J Anaesth 2005;95:257–60. 10.1093/bja/aei173 [DOI] [PubMed] [Google Scholar]
- 19.Farris W, Waymack JR. Central Retinal Artery Occlusion. In: StatPearls [Internet. Treasure Island (FL: StatPearls Publishing, 2020. http://www.ncbi.nlm.nih.gov/books/NBK470354/ [Google Scholar]
- 20.Baig MN, Lubow M, Immesoete P, et al. Vision loss after spine surgery: review of the literature and recommendations. Neurosurg Focus 2007;23:E15. 10.3171/FOC-07/11/15 [DOI] [PubMed] [Google Scholar]
- 21.American Society of Anesthesiologists Task Force on Perioperative Visual Loss . Practice Advisory for perioperative visual loss associated with spine surgery: an updated report by the American Society of Anesthesiologists Task force on perioperative visual loss. Anesthesiology 2012;116:274–85. 10.1097/ALN.0b013e31823c104d [DOI] [PubMed] [Google Scholar]