Abstract
A previously healthy 37-year-old man presented with fevers and myalgias for a week with a minimal dry cough. Initial SARS-CoV-2 nasopharyngeal testing was negative, but in light of high community prevalence, he was diagnosed with COVID-19, treated with supportive care and self-quarantined at home. Three days after resolution of all symptoms, he developed sudden onset chest pain. Chest imaging revealed a large right-sided pneumothorax and patchy subpleural ground glass opacities. IgM and IgG antibodies for SARS-CoV-2 were positive. His pneumothorax resolved after placement of a small-bore chest tube, which was removed after 2 days.
This case demonstrates that patients with COVID-19 can develop a significant pulmonary complication, a large pneumothorax, despite only minimal lower respiratory tract symptoms and after resolution of the original illness. Medical professionals should consider development of a pneumothorax in patients who have recovered from COVID-19 and present with new respiratory symptoms.
Keywords: pneumothorax, pneumonia (infectious disease), adult intensive care, pneumonia (respiratory medicine)
Background
Development of a spontaneous pneumothorax and pneumomediastinum is one of the emerging respiratory complications of COVID-19 viral pneumonitis. This complication has been described in mechanically ventilated patients with COVID-19, as well as patients on non-invasive ventilation or high flow nasal cannula during the acute phase of COVID-19 infection. Potential causes include the high airway pressures delivered by these modalities of respiratory support, as well as spontaneous rupture of fragile small airways infected by the virus.
Only a few cases of spontaneous pneumothorax after mild cases of COVID-19 have been described in the literature so far. In these cases, the development of the pneumothorax was preceded by significant respiratory symptoms and accompanied by significant pulmonary infiltrates on chest imaging.
We describe the case of a patient who had negligible respiratory symptoms including a minimal dry cough without associated shortness of breath or hypoxaemia, who then developed a spontaneous pneumothorax after having recovered from the acute phase of COVID-19 viral pneumonitis.
Case presentation
A previously healthy 37-year-old man developed fevers and myalgias, with a minimal dry cough without associated chest pain or shortness of breath. Initial SARS-CoV-2 RT-PCR nasopharyngeal testing was negative, but in light of the high community prevalence of COVID-19 and typical symptoms, he was diagnosed with COVID-19. He was instructed to self-quarantine at home and treated with supportive care with antipyretics. His symptoms resolved after 1 week. Three days after resolution of his fevers and myalgias, he developed sudden onset right-sided chest pain. He sought care at his primary care physician’s office and was treated with prednisone and levofloxacin for presumed community-acquired pneumonia. Chest imaging was not performed. When his chest pain persisted and he subsequently developed shortness of breath with exertion, he presented to an outside emergency room. He had no significant medical or surgical history and no recent trauma or falls. He did have a habit of smoking hookah (tobacco) a couple of times each month, though he had not smoked since he was diagnosed with COVID-19. He did not consume any alcohol or use illicit drugs. He had not travelled outside the local area. His family history was significant for both father and mother with hypertension.
Initial vital signs included blood pressure of 150/97 mm Hg, pulse 124/min, temperature 99.3 °F (37.4°C), respiratory rate 25/min, weight 72.1 kg, height 158 cm, body mass index 28.9 kg/m2. His oxygen saturation on room air was 99%. On physical exam, he appeared to be uncomfortable and in pain, and he had mild respiratory distress. His pupils were round and reactive to light. Heart rate was regular, and cardiac exam revealed no murmurs, rubs or gallop. His pulmonary exam was significant for diminished breath sounds on the right, without crackles or wheezes. His abdomen was soft, non-tender, non-distended with normoactive bowel sounds. He had no peripheral oedema and had strong peripheral pulses bilaterally. His neurological and skin exam were normal.
Investigations
The initial labs showed a normal haemoglobin of 14.3 gm/dL and haematocrit of 44%; white cell count was elevated at 13 200/µL, the lymphocyte count was 1270/µL (normal range: 1320–3570/µL) or 10% and platelets were elevated at 791000/mm3. His sodium was 141 meq/L, potassium was slightly low at 3.4 meq/L, bicarbonate was 27 meq/L, chloride was 103 meq/L, glucose was 96 mg/dL, calcium was 10.1 mg/dL, BUN was 13 mg/dL, creatinine was 0.9 mg/dL, total bilirubin was 0.6 mg/dL, alkaline phosphatase was 74 U/L, alanine aminotransferase was 61 U/L (normal <55 U/L), aspartase aminotransferase was 41 U/L and albumin was 3.7 g/dL.
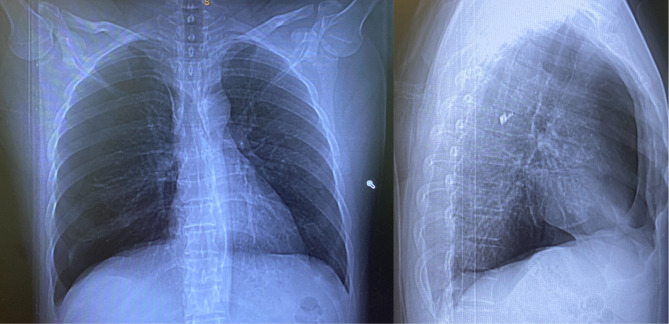
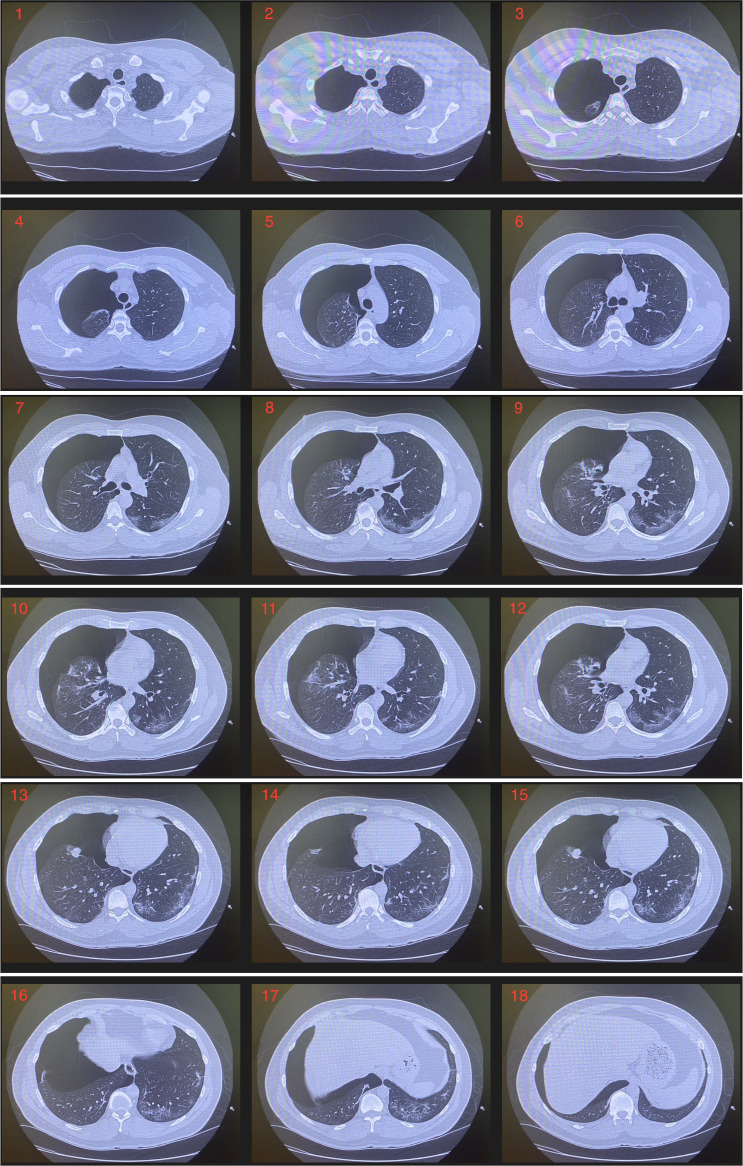
A chest X-ray and subsequently a CT chest were obtained in the emergency room. This revealed a large right-sided pneumothorax as well as patchy subpleural ground glass opacities (figures 1 and 2, video 1).
Figure 1.
Chest X-ray PA/lateral on presentation. PA, posterioranterior.
Figure 2.
CT chest without intravenous contrast: large right pneumothorax. Patchy bilateral subpleural ground glass opacities.
Video 1.
The electrocardiogram (EKG) showed sinus rhythm of 84 beats per minute, QTc (corrected QT time) 420 ms and T wave inversions in II, III, aVF and V3-V6 without ST elevations or depressions (figure 3).
Figure 3.
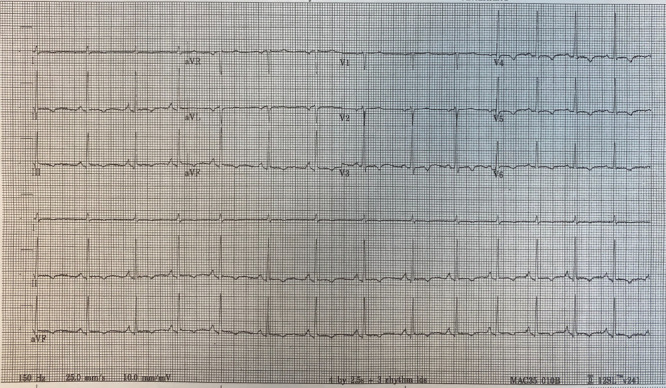
EKG on presentation. Sinus rhythm with sinus arrhythmia at 84 bpm; PR interval 136 ms, QRS duration 74 ms, QT/QTc 356/420 ms. T wave inversions in leads II, III, aVF, V3-V6. No ST elevations or depressions.
Repeat SARS-CoV-2 RT-PCR nasopharyngeal testing was negative both in the emergency room and after admission to the hospital, but IgM and IgG antibodies for SARS-CoV-2 resulted positive.
A viral respiratory panel (testing for human metapneumovirus, influenza A and B, parainfluenza virus 1, 2, 3, rhinovirus, adenovirus, Bordetella pertussis, Chlamydophila pneumoniae, Coronavirus 229E, HKU1, NL63, OC43, Mycoplasma pneumoniae and parainfluenza virus 4), the urine legionella antigen and urine streptococcus pneumoniae antigen were negative.
Differential diagnosis
Since this patient presented at the height of the COVID-19 pandemic, the initial differential diagnosis of the patient’s chest pain and shortness of breath included acute respiratory distress due to COVID-19 viral pneumonia, as well as a secondary bacterial or fungal pneumonia. Pulmonary embolism was a consideration since thromboembolic complications have been described in COVID-19 with increased frequency. A myocardial ischaemic event was unlikely given the patient’s young age and lack of significant risk factors. Costochondritis was also less likely, as the chest pain was not reproducible with palpation, and the patient was in mild respiratory distress and had an abnormal pulmonary exam. Gastro-oesophageal reflux disease, oesophageal spasm, aortic dissection, lung abscess, myocarditis and pericarditis were on the differential as well. The physical exam with diminished breath sounds was suspicious for a pneumothorax, pleural effusion or atelectasis. Subsequent chest imaging quickly revealed the large pneumothorax without evidence of tension pneumothorax.
Treatment
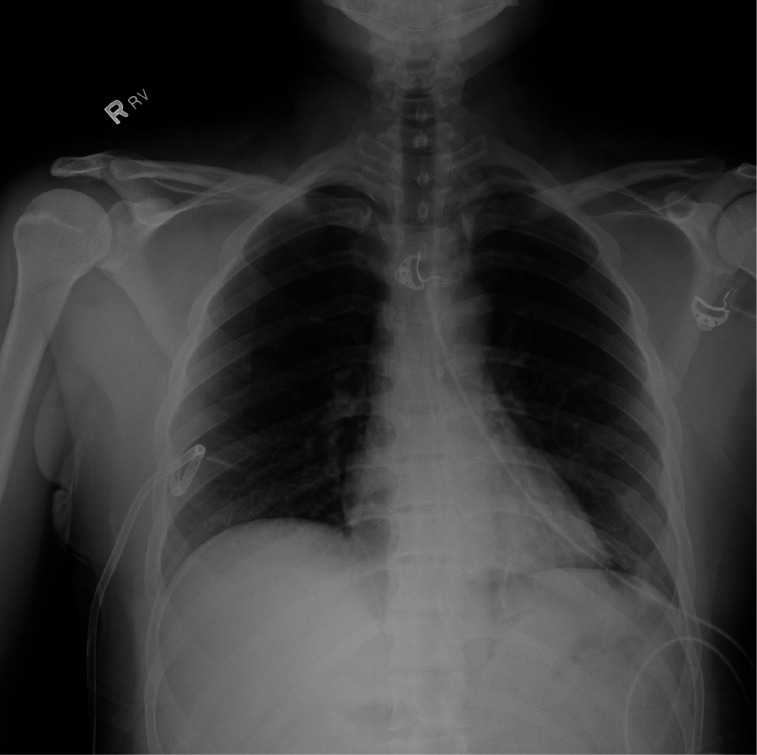
The patient was transferred from the small outside hospital emergency room to our tertiary care facility for further care. On arrival, the critical care physician on duty placed a small-bore 14-French chest tube under ultrasound guidance into the right pleural space. A repeat chest X-ray showed resolution of the large right pneumothorax with a small residual right apical pneumothorax (figure 4). The chest tube was initially placed on –20 cm H2O suction. There was no evidence of an air leak that would have raised the suspicion for a bronchopleural fistula. On the next day, the chest tube was placed on water seal, and the patient remained without shortness of breath. Less than 48 hours after insertion, the chest tube was clamped for 4 hours, a repeat chest X-ray was obtained, and since it showed only a tiny residual right apical pneumothorax, the chest tube was removed without complication.
Figure 4.
Chest X-ray after placement of small-bore right chest tube. Small residual right apical pneumothorax.
The patient’s chest pain was controlled with oral acetaminophen. He received low molecular weight heparin for deep venous thrombosis (DVT) prophylaxis. The patient was initially placed in droplet and contact isolation with face shield (COVID-19 precautions), until repeat SARS-CoV-2 testing resulted negative twice, which was the hospital protocol at the time.
Outcome and follow-up
The patient’s shortness of breath resolved after placement of the chest tube. The chest tube was removed within 48 hours, the patient stayed symptom free and a repeat chest X-ray demonstrated no residual pneumothorax after chest tube removal, and he was discharged home.
Discussion
A pneumothorax can develop spontaneously, either as a primary spontaneous pneumothorax or a secondary spontaneous pneumothorax due to an underlying pulmonary disorder. It can be caused by trauma to the chest, or it can be iatrogenic, for example, as a complication of central venous catheter placement. Risk factors for a spontaneous pneumothorax include cigarette and cannabis smoking, male gender, tall stature, thin body habitus, chronic obstructive pulmonary disease, alpha-1 antitrypsin deficiency, cystic fibrosis, other cystic lung disorders, malignancy, pulmonary infections or architectural abnormalities such as Marfan syndrome, Ehlers-Danlos syndrome or homocystinuria.1 2
A literature search for spontaneous pneumothorax in COVID-19 yielded 25 cases reported at the time this manuscript was submitted. These cases include a 37-year-old man without medical history who was hospitalised for COVID-19 pneumonia and acute hypoxaemic respiratory failure requiring high-flow nasal cannula, then developed a right-sided pneumothorax requiring chest tube placement 2 weeks after disease onset and 2 days after discharge from the hospital.3 A 62-year-old Chinese man in Wuhan developed a right-sided pneumothorax, pneumothorax and pneumomediastinum 20 days after hospitalisation, which resolved without invasive interventions; he was treated with high-flow nasal cannula.4 A 49-year-old man without medical history treated with a 100% non-rebreather oxygen mask at 15 L/min flow developed a right-sided pneumothorax requiring chest tube placement on day 12 of admission.5 A 38-year-old man from Wuhan was treated with high-flow nasal cannula and non-invasive ventilation and developed pneumomediastinum on day 11 and a small left pneumothorax not requiring intervention on day 34 after admission.6 A 38-year-old man developed cystic pulmonary lesions and a small left pneumothorax 32 days after disease onset; he received oxygen only at a low flow of 5 L/min.7 A 78-year-old woman with diabetes and hypertension presented with pneumomediastinum at the time of COVID-19 diagnosis, and a 41-year-old man presented with subcutaneous emphysema, pneumomediastinum and a small left pneumothorax.8 In another case, a 50-year-old man developed a loculated right posterior pneumothorax over a month after his initial presentation with COVID-19 pneumonia.9 A 38-year-old male smoker treated with high-flow nasal cannula developed a pneumothorax 23 days after admission.10 A case series described men aged 55, 33 and 50 years with COVID-19 developing a pneumothorax on non-invasive ventilation 3 days after admission, the second patient 15 days after extubation and the third patient 7 days post admission, respectively.11 In another case report by Rohailla, a 26-year-old man presented with a large right pneumothorax without associated COVID-19 symptoms but was found to be positive for COVID-19 on RT-PCR testing.12 A 87-year-old male smoker presented with COVID-19 and a left pneumothorax.13 Eperjesiova et al 14 reported that out of 976 COVID-19 patients, five developed spontaneous pneumomediastinum and two isolated pneumothoraxes; only one was a smoker, three had emphysema or asthma and five had been intubated before. Another case series from Spain described an 84-year-old woman and two men ages 67 and 73 years with COVID-19 associated spontaneous pneumothorax; all three patients passed away from COVID-19.15 A right-sided tension pneumothorax developed in a 47-year-old man requiring non-invasive ventilation for COVID-19 11 days after initial presentation and 4 days after discharge from the hospital.16 A 36-year-old man had a left tension pneumothorax on presentation with COVID-19 disease.17 In another case, an 82-year-old woman was found to have pneumomediastinum, a large left-sided pneumothorax and subcutaneous emphysema on presentation with COVID-19.18 A more unusual case was an 80-year-old woman who had both a right pneumothorax and extensive pneumoperitoneum at the time of presentation for COVID-19.19 In a case report from Turkey, a 24-year-old non-smoking man presented with COVID-19 pneumonia and a large left pneumothorax.20 A 61-year-old man in Germany, again a non-smoker, presented 17 days after hospitalisation for COVID-19 pneumonia with a right-sided tension pneumothorax, and then 20 days later, he developed a left-sided tension pneumothorax after a coughing fit, without evidence of any new infection, supporting the theory that increased intrathoracic pressure caused by frequent and heavy coughing could be one of the explanations for the development of barotrauma complications in these patients.21 In a review of 20 case reports of COVID-19 patients with pneumothorax, a male preponderance of 88.8% was observed.22 This is consistent with the gender distribution in non-COVID-19 associated spontaneous pneumothorax patients.23 What becomes clear from reviewing these reported cases is that most of these patients were quite ill from COVID-19, many required ICU care, most developed a pneumothorax during their hospitalisation, though some presented with a pneumothorax at the same time that they had symptomatic COVID-19 infection. Data on oxygen requirement prior to the development of a pneumothorax were reported in 21 patients, of which 19 patients required some form of oxygen therapy. Only 2 of those 19 patients were on non-invasive ventilation, while the rest were on high flow nasal cannula, non-rebreather or face mask prior to pneumothorax development. Our patient never experienced shortness of breath, and he reported having had a minimal cough during his acute illness only after he was specifically asked about this symptom. He never required supplemental oxygen until he presented with right-sided pleuritic chest pain and was diagnosed with the pneumothorax. Rohailla et al12 reported a similar case, where the patient was completely asymptomatic and was not aware of his COVID-19 diagnosis until he developed a pneumothorax and presented with sudden onset right-sided pleuritic chest pain, though the positive PCR test for SARS-CoV-2 in that case suggests active infection rather than a postinfectious phenomenon. Therefore, it seems that patients with COVID-19 can develop a spontaneous pneumothorax, despite only minimal to no lower respiratory tract symptoms, even after resolution of the original symptoms. Our patient is different from the other cases reported in the literature, because he did not require hospitalisation at the time of COVID-19 infection. Abnormal CT findings consistent with COVID-19 pneumonitis have been described in asymptomatic patients. Medical professionals should consider the development of a pneumothorax as a cause of respiratory symptoms in patients with active COVID-19 viral pneumonitis and in patients who have recovered from their COVID-19 illness and present with new respiratory symptoms, irrespective of the severity of their disease prior to resolution of the original illness. The patient’s occasional tobacco use in the form of hookah smoking might have increased his risk of developing a pneumothorax in the setting of a viral pneumonia. Among the cases of COVID-19 associated spontaneous pneumothorax published so far, 13 out of 16 patients for which information on smoking status was reported were non-smokers.
Many bacterial, viral, fungal and mycobacterial infections of the lung can also be complicated by development of a spontaneous pneumothorax. The mechanism of injury for the development of a secondary spontaneous pneumothorax in COVID-19 infection, although not established, could be due to damage predominantly to the subpleural alveoli, leading to spontaneous alveolar rupture into the pleural space. Other potential mechanisms of injury include increased intrathoracic pressures in the setting of frequent coughing. Despite minimal respiratory symptoms during his active COVID-19 illness, our patient had typical CT chest findings of COVID-19 viral pneumonitis with bilateral patchy subpleural and peripheral ground glass opacities.24 These CT findings, together with the positive SARS-CoV-2 antibody test and the epidemiological situation with high community prevalence of COVID-19 and typical symptoms, confirm the clinical diagnosis of COVID-19 viral pneumonitis despite the initial negative SARS-CoV-2 nasopharyngeal PCR test. False negative PCR test rates of 10%–40% have been reported and are likely dependent on how the specimen is collected and the timing of the test in the disease course.25
Notably, among the 25 cases reported so far, the time to development of pneumothorax varied from as short as 3 days to as long as 39 days from the time of onset of COVID-19 symptoms, and the median time to development of pneumothorax was 19 days across 15 cases for which the data were reported. This is in most cases later in the disease course and supports the argument that pneumothorax must be on the differential in a patient with new onset or worsening of respiratory distress or chest pain even after complete resolution of COVID-19 symptoms. One of the interesting questions this case raises is whether the development of the spontaneous pneumothorax was related to the COVID-19 viral pneumonia or not. Since the CT chest at the time of pneumothorax development still showed significant predominantly subpleural infiltrates, which put the patient at risk of alveolar rupture leading to air in the pleural space, and since it is well known that other pulmonary infections are associated with the development of a pneumothorax, it is highly likely that his pneumothorax was a direct consequence of the COVID-19 viral pneumonitis.
Learning points.
Patients with COVID-19 who have minimal or no respiratory symptoms can still have significant pulmonary changes on CT chest.
A spontaneous pneumothorax can develop as a complication of COVID-19 infection in patients with a mild disease course treated as outpatients, and clinicians should consider this in their differential diagnosis.
COVID-19 patients without a smoking history can develop a spontaneous pneumothorax as a complication of the disease.
The mean time from onset of COVID-19 symptoms to development of pneumothorax in non-mechanically ventilated patients was 19 days among the cases reported so far. Therefore, pneumothorax should be on the differential diagnosis if a patient presents with sudden onset of chest pain or respiratory distress even after resolution of the initial symptoms.
Footnotes
Contributors: ABB and KN equally contributed to the drafting and writing of the case report and provided final approval of the manuscript. Both agree to be accountable for all aspects of the work. ABB was the attending physician for the patient, and KN was the critical care fellow who admitted the patient.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666–71. 10.1136/thorax.55.8.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868–74. 10.1056/NEJM200003233421207 [DOI] [PubMed] [Google Scholar]
- 3.Yasukawa K, Vamadevan A, Rollins R. Bulla formation and tension pneumothorax in a patient with COVID-19. Am J Trop Med Hyg 2020;103:943–4. 10.4269/ajtmh.20-0736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Gao R, Zheng Y, et al. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med 2020;27 10.1093/jtm/taaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhakeem A, Khan MM, Al Soub H, Soub HA, et al. Case report: COVID-19-Associated bilateral spontaneous Pneumothorax-A literature review. Am J Trop Med Hyg 2020;103:1162–5. 10.4269/ajtmh.20-0680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol 2020;21:541–4. 10.3348/kjr.2020.0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, Zeng Y, Xie P, et al. COVID-19 with cystic features on computed tomography: a case report. Medicine 2020;99:e20175. 10.1097/MD.0000000000020175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brogna B, Bignardi E, Salvatore P, et al. Unusual presentations of COVID-19 pneumonia on CT scans with spontaneous pneumomediastinum and loculated pneumothorax: a report of two cases and a review of the literature. Heart Lung 2020;49:864–8. 10.1016/j.hrtlng.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollingshead C, Hanrahan J. Spontaneous pneumothorax following COVID-19 pneumonia. IDCases 2020;21:e00868. 10.1016/j.idcr.2020.e00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruibing Lyu, MD, Xin Li, MD. Diagnosis and treatment of severe COVID-19 complicated with spontaneous pneumothorax: a case report. AUDT 2020;4:142–6. 10.37015/AUDT.2020.200019 [DOI] [Google Scholar]
- 11.Al-Shokri SD, Ahmed AOE, Saleh AO, et al. Case report: COVID-19–Related Pneumothorax—Case series highlighting a significant complication. Am J Trop Med Hyg 2020;103:1166–9. 10.4269/ajtmh.20-0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohailla S, Ahmed N, Gough K. SARS-CoV-2 infection associated with spontaneous pneumothorax. Can Med Assoc J 2020;192:E510 10.1503/cmaj.200609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poggiali E, Vercelli A, Iannicelli T, et al. COVID-19, chronic obstructive pulmonary disease and pneumothorax: a frightening triad. Eur J Case Rep Intern Med 2020;7:001742. 10.12890/2020_001742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eperjesiova B, Hart E, Shokr M, et al. Spontaneous Pneumomediastinum/Pneumothorax in patients with COVID-19. Cureus 2020;12:e8996. 10.7759/cureus.8996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López Vega JM, Parra Gordo ML, Diez Tascón A, et al. Pneumomediastinum and spontaneous pneumothorax as an extrapulmonary complication of COVID-19 disease. Emerg Radiol 2020;27:727–30. 10.1007/s10140-020-01806-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiro JE, Sisovic S, Ockert B, et al. Secondary tension pneumothorax in a COVID-19 pneumonia patient: a case report. Infection 2020;48:941–4. 10.1007/s15010-020-01457-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flower L, Carter J-PL, Rosales Lopez J, et al. Tension pneumothorax in a patient with COVID-19. BMJ Case Rep 2020;13. 10.1136/bcr-2020-235861. [Epub ahead of print: 17 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ucpinar BA, Sahin C, Yanc U. Spontaneous pneumothorax and subcutaneous emphysema in COVID-19 patient: case report. J Infect Public Health 2020;13:887–9. 10.1016/j.jiph.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrêa Neto IJF, Viana KF, da SMBS. Perforated acute abdomen in a patient with COVID-19: an atypical manifestation of the disease. J Coloproctology 2020. 10.1016/j.jcol.2020.05.011 [DOI] [Google Scholar]
- 20.Aydin S, Öz G, Dumanli A, et al. A case of spontaneous pneumothorax in Covid-19 pneumonia. J Surg Res 2020;03:96–101. 10.26502/jsr.10020060 [DOI] [Google Scholar]
- 21.Büttner R, Heiligensetzer A, Fürst A. Pneumothorax nACh COVID-19-Pneumonie. MMW Fortschr Med 2020;162:11 10.1007/s15006-020-0667-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quincho-Lopez A, Quincho-Lopez DL, Hurtado-Medina FD. Case report: pneumothorax and pneumomediastinum as uncommon complications of COVID-19 Pneumonia-Literature review. Am J Trop Med Hyg 2020;103:1170–6. 10.4269/ajtmh.20-0815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bobbio A, Dechartres A, Bouam S, et al. Epidemiology of spontaneous pneumothorax: gender-related differences. Thorax 2015;70:653–8. 10.1136/thoraxjnl-2014-206577 [DOI] [PubMed] [Google Scholar]
- 24.Simpson S, Kay FU, Abbara S, et al. Radiological society of North America expert consensus statement on reporting chest CT findings related to COVID-19. endorsed by the society of thoracic radiology, the American College of radiology, and RSNA - secondary publication. J Thorac Imaging 2020;35:219–27. 10.1097/RTI.0000000000000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissleder R, Lee H, Ko J, et al. COVID-19 diagnostics in context. Sci Transl Med 2020;12. 10.1126/scitranslmed.abc1931. [Epub ahead of print: 03 Jun 2020]. [DOI] [PubMed] [Google Scholar]