Transcatheter aortic valve replacement (TAVR) can cause postimplantation conduction disturbance.1 Balloon-expandable valves exert higher radial forces than self-expanding (SE) transcatheter heart valves (THVs),2 but permanent pacemaker implantation (PPI) rates are higher after SE TAVR, for example, 17.4% for the Medtronic CoreValve.3 The His bundle surfaces at the base of the membranous septum. Implant depth greater than the length of the membranous septum is an independent predictor of PPI.4 CoreValve implantation 3 to 5 mm below the aortic annulus in a projection coaxial to the device is recommended. This is rarely in the annular plane of the valve. In light of differential pacing rates reported between balloon-expandable and SE TAVR platforms, we retrospectively analyzed PPI outcomes following routine implantation of THVs in the annular plane.
The data that support the findings of this study are available from the corresponding author upon reasonable request. All successful TAVR implants from January 2013 to August 2019 were analyzed (n=527). The retrospective analysis of routinely collected data in this service evaluation did not require formal ethics approval. All patients were consented for the procedure and data collection for use in an anonymized registry. The Edwards balloon-expandable THV platform (n=434; Edwards Lifesciences, Irvine, CA) and the Medtronic SEV platforms (n=93; Medtronic LLC, Minneapolis, MN)—Evolut R (n=50) and Evolut PRO (n=43)—were used exclusively. Annular plane projection, routinely predicted from multidetector computed tomography images using 3mensio software (Pie Medical Imaging, Maastricht, the Netherlands), was used in all cases. For CoreValves, correction of parallax in the device was performed by adding right anterior oblique caudal angulation, preserving the anatomic annular plane of the native valve5 and producing a cusp overlap (right coronary cusp/left coronary cusp) view. This contrasts to the manufacturer’s recommendation to use left anterior oblique angulation, which produces a shallower apparent device depth of ≈1 to 2 mm (Figure). Thirty-day PPI rates were determined for CoreValve and Sapien recipients after excluding those with prior pacemaker implantation (37 Sapien and 6 CoreValve; this included preemptive PPI for the right bundle branch block in 1 CoreValve patient) and valve-in-valve cases (2 Sapien, 4 CoreValve). We hypothesized that Evolut valves would have comparable pacing rates to Sapien valves when implanted in a right anterior oblique caudal annular plane projection. Statistical analysis was performed using SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY). Means were compared using the unpaired t test for parametric data and otherwise the Mann-Whitney U test. Comparisons in pacing rates between valve types were adjusted for covariates using ANCOVA. Significance was set at α=0.05.
Figure.
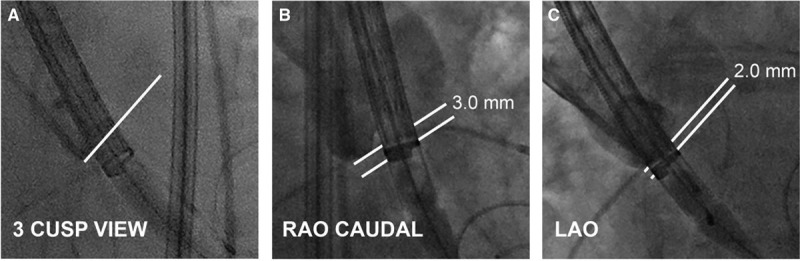
Correction for parallax in a Medtronic Evolut device. A, The sheathed device across the native aortic valve in the 3-cusp view. The line indicates the bottom of the noncoronary cusp. B, Correction of device parallax by adding right anterior oblique (RAO) caudal angulation maintains an annular plane projection and a true device depth of 3.0 mm. C, Correction for device parallax with left anterior oblique (LAO) angulation results in a perceived device depth of 2 mm.
The final cohorts were demographically similar. Two hundred thirty-six of 400 (59.0%) Sapien recipients and 45 of 87 CoreValve recipients (51.7%) were men. The mean age (years) was 78.9±8.9 (SD) years for Sapien and 79.6±8.5 years for CoreValve, respectively (P=0.55 for the difference between groups). Baseline electrocardiographic findings for the SE THV cohort were as follows: Evolut R (n=50): 31 sinus rhythm (SR) only, 2 SR with first-degree heart block, 2 SR with left bundle branch block, 10 atrial fibrillation (AF) only, 1 AF/left bundle branch block, and 4 paced; Evolut PRO (n=43): 29 SR only, 2 paced, 2 SR/right bundle branch block, 7 AF only, 2 AF/left bundle branch block, and 1 AF/right bundle branch block. Post-procedure, 32 of 400 Sapien recipients (8.0%) and 4 of 87 CoreValve recipients (4.6%, 3 Evolut R, 1 Evolut PRO) had PPI by 30 days following TAVR (P=0.13 for difference, adjusted for age, sex, diabetes status, creatinine, baseline conduction disturbances, and depth of implantation). The indication for pacing was complete heart block or slow AF during admission. In the SE THV cohort, 10 developed new left bundle branch block (11.5%).
To rationalize the biological plausibility of equivalent pacing rates between the two platforms, which conflicts with differential PPI rates in published trial data, depth of implantation was determined where possible from archived aortography, measured as the distance of the lowest part of the device in millimeters below the noncoronary cusp in an annular plane projection calibrated to the pigtail catheter. For 60 of 87 CoreValve cases, the mean implant depth was 4.27±1.59 mm below the noncoronary cusp, confirming that the mean implant depth was in the recommended range. A penalty for higher implantation may be paravalvular regurgitation or embolization. Two CoreValve recipients (2.3%) had moderate AR on echocardiography but otherwise no or mild AR. There were no cases of valve embolization.
An annular plane projection is required to accurately measure the depth of implantation below the aortic annulus. A permanent pacemaker rate of <5% was achieved in this single-center case series for the self-expanding Medtronic CoreValve by implanting 3 to 5 mm below the aortic annulus, using a radiographic projection chosen for both the valve and the device. This implantation strategy was not associated with valve embolization, although 2 CoreValve recipients had moderate AR post-procedure. The extent to which these single-center, nonrandomized, and unblinded findings made without core laboratory adjudication are generalizable is uncertain. The effect of this method of implantation for self-expanding THVs could be evaluated in a multicenter registry and may be particularly relevant in the low-risk TAVR population.
Sources of Funding
None.
Disclosures
None.
Footnotes
For Sources of Funding and Disclosures, see page 114.
Contributor Information
Anthony D. Pisaniello, Email: a1076875@adelaide.edu.au.
Haytham B.E. Makki, Email: haytham.makki@mft.nhs.uk.
Saleem Jahangeer, Email: saleem.jahangeer@mft.nhs.uk.
Matthew J. Daniels, Email: matthew.daniels@mft.nhs.uk.
Ragheb Hasan, Email: ragheb.hasan@mft.nhs.uk.
References
- 1.Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, Rodés-Cabau J. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation. 2017;136:1049–1069. doi: 10.1161/CIRCULATIONAHA.117.028352 [DOI] [PubMed] [Google Scholar]
- 2.Egron S, Fujita B, Gullón L, Pott D, Schmitz-Rode T, Ensminger S, Steinseifer U. Radial force: an underestimated parameter in oversizing transcatheter aortic valve replacement prostheses: in vitro analysis with five commercialized valves. ASAIO J. 2018;64:536–543. doi: 10.1097/MAT.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 3.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. ; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 4.Jilaihawi H, Zhao Z, Du R, Staniloae C, Saric M, Neuburger PJ, Querijero M, Vainrib A, Hisamoto K, Ibrahim H, et al. Minimizing permanent pacemaker following repositionable self-expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:1796–1807. doi: 10.1016/j.jcin.2019.05.056 [DOI] [PubMed] [Google Scholar]
- 5.Gurvitch R, Wood DA, Leipsic J, Tay E, Johnson M, Ye J, Nietlispach F, Wijesinghe N, Cheung A, Webb JG. Multislice computed tomography for prediction of optimal angiographic deployment projections during transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:1157–1165. doi: 10.1016/j.jcin.2010.09.010 [DOI] [PubMed] [Google Scholar]


