Abstract
Background:
As the use of left atrial appendage closure (LAAC) becomes more widespread, improvements in resource utilization and cost-effectiveness are necessary. Currently, there are limited data on same-day discharge (SDD) after LAAC. We aimed to evaluate the safety and feasibility of SDD versus non-SDD in patients with nonvalvular atrial fibrillation who underwent LAAC.
Methods:
We retrospectively studied 211 patients who underwent the WATCHMAN procedure in a tertiary hospital (June 2016 to June 2019). The primary safety outcome was the composite of stroke, systemic embolism, major bleeding requiring transfusion, vascular complications requiring endovascular intervention, or death through 7 days (periprocedural) and 45 days post-procedure. The secondary outcomes were the individual components of the primary outcome and all-cause readmission. We compared the clinical outcomes of patients who had SDD and non-SDD post-procedure.
Results:
Patients with procedure-related complications on the day of LAAC and patients who were admitted for acute clinical events before LAAC were excluded. One hundred ninety patients were included in the final analysis. Seventy-two of 190 (38%) patients had SDD, and 118 of 190 (62%) had non-SDD. There were no statistically significant differences in the primary safety outcome through 7 days (1.4% versus 5.9%; P=0.26) and 45 days post-procedure (2.8% versus 9.3%; P=0.14) between the two groups. The secondary outcomes were similar in both groups. No patients had device-related thrombus on transesophageal echocardiography at 45 days. Only 1 patient from the non-SDD group had clinically significant peri-device flow (>5 mm) at 45 days.
Conclusions:
In a selected cohort of patients who underwent successful elective LAAC with WATCHMAN without same-day procedure-related complications, the primary safety outcome and secondary outcomes through 7 and 45 days post-procedure were similar in the SDD and non-SDD groups. Our findings are hypothesis generating and warrant further investigation in prospective trials.
Keywords: atrial appendage, atrial fibrillation, humans, patient discharge, thrombosis
What Is Known
Currently, standard practice after percutaneous left atrial appendage closure involves monitoring patients overnight and discharging the following day. There are limited data on same-day discharge after elective left atrial appendage closure.
What the Study Adds
In a selected cohort of patients who underwent elective left atrial appendage closure with the WATCHMAN device without same-day procedure-related complications, same-day discharge appears to be safe.
Same-day discharge reduces length of stay, thereby improving resource utilization and overall costs to the health care system.
Our findings are considered hypothesis generating and warrant further investigation in prospective trials.
Atrial fibrillation (AF) has a prevalence of 1% to 2% in the adult population.1 The majority of AF patients receive oral anticoagulation (OAC) to reduce the risk of stroke. Previous studies showed that ≈13% of AF patients have a contraindication to OAC,2 and 2% have an absolute contraindication, most frequently due to a history of intracranial hemorrhage.3 Percutaneous left atrial appendage (LAA) closure (LAAC) has emerged as a feasible option for stroke prevention in patients with nonvalvular AF.4
The rate of procedure-related complications with the WATCHMAN device at 7 days was 8.7% and 4.2% in the PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) and PREVAIL (Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy) trials, respectively.5,6 However, the more recent EWOLUTION study (Evaluating Real-World Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology) has shown a considerably lower 7-day procedure-related complication rate (2.7%).7 The complication rate is not negligible but improves with higher annual hospital volume and operator experience.8 The current real-world in-hospital adverse event rate is low (2.2%), as reported in the National Cardiovascular Data Registry LAA Occlusion Registry.9 In contemporary practice, patients are hospitalized overnight after LAAC and typically discharged the following day.10 Currently, there are limited data on same-day discharge (SDD) for LAAC.11,12 SDD following LAAC has the potential to reduce hospital costs and improve patient satisfaction.
In this retrospective analysis of a single center, we report the safety and outcomes of SDD compared with non-SDD following WATCHMAN implantation.
Methods
The data that support the findings of this study may be made available from the corresponding author upon reasonable request.
Patient Selection
This was a retrospective study of patients who underwent LAAC with WATCHMAN in a tertiary hospital (United States) between June 2016 and June 2019.13 Institutional review board approval was obtained. Institutional review board waived patient informed consent for this study. The following patients were excluded from the analysis for the primary safety and secondary outcomes: (1) patients with unsuccessful procedures, (2) patients with same-day procedure-related complications (ie, occurring on the day of LAAC), and (3) patients admitted for acute clinical events before LAAC.
Procedure
Three experienced operators (J.P.D., D.C., and A.S.) performed LAAC in our center. All procedures were performed under general anesthesia with transesophageal echocardiography (TEE) and fluoroscopy guidance. All patients were fully anticoagulated with heparin to maintain an activated clotting time >250 s. A single Perclose was performed on all patients post-procedure to achieve hemostasis.
Postprocedural Care
Post-procedure, all patients were extubated in the catheterization laboratory and recovered in the procedural recovery area. They were ambulated 2 hours post-procedure to assess the integrity of the vascular access site. Depending on the patient’s and family’s preference, patients were discharged within 3 to 4 hours post-procedure (ie, SDD) or the following day (non-SDD). SDD patients were discharged directly from the procedural recovery area, while non-SDD patients were admitted to and discharged from a cardiac floor (Figure 1). OAC was started the evening of the procedure on all patients without a bleeding event. The use of predischarge transthoracic echocardiography (TTE) was at the discretion of the operator. Patients were typically maintained on aspirin and OAC (warfarin or direct OAC) for 45 days following successful LAAC.
Figure 1.
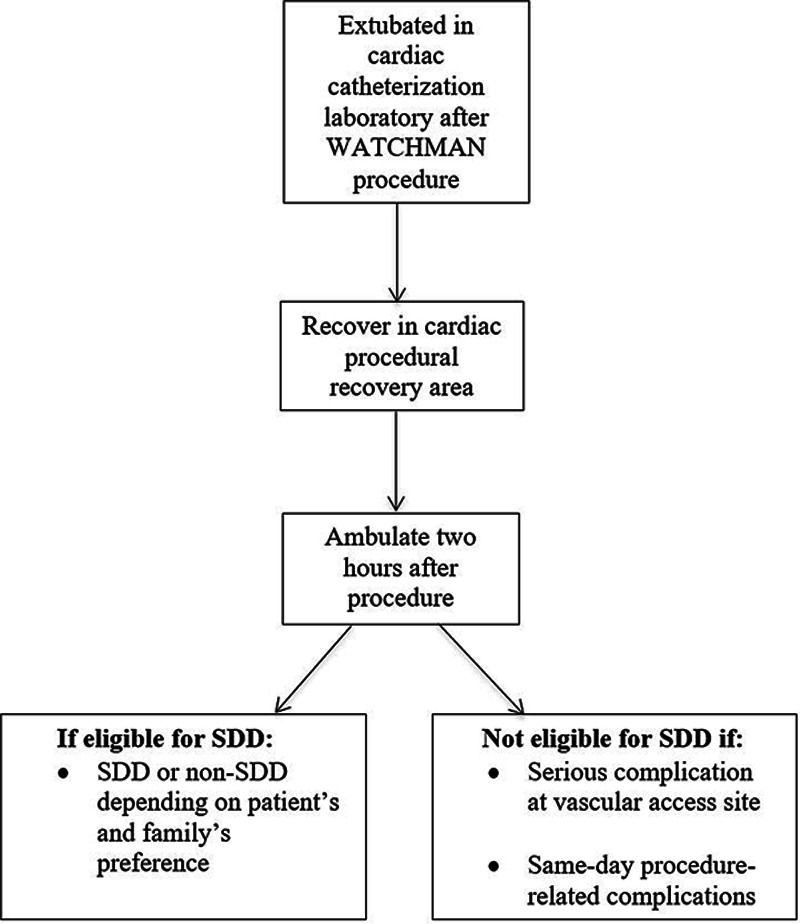
Flowchart of postprocedural care after left atrial appendage closure. SDD indicates same-day discharge.
Seven-Day Procedure/Device-Related Complications
We assessed 7-day procedure/device-related complications in all patients, including those excluded in the analysis of the primary safety outcome and secondary outcomes. Using identical definitions from previous trials, 7-day procedure/device-related complications were defined as a composite of periprocedural stroke, systemic embolism, pericardial tamponade, cardiac perforation, device embolization, serious vascular complications, or death through 7 days post-LAAC.6,7
Primary and Secondary Outcomes
The primary safety outcome was a composite of stroke, systemic embolism, major bleeding requiring transfusion, vascular complications requiring endovascular intervention, or death through 7 (periprocedural) and 45 days. The secondary outcomes were the individual components of the primary safety outcome and all-cause readmission. Peri-device flow and device-related thrombus were assessed on 45-day TEE.
Statistical Analysis
Categorical variables were presented as counts and percentages. Continuous variables were presented as mean±SD. Pearson χ2 test was performed to compare categorical variables between SDD and non-SDD groups; if any cells in a 2×2 table contained a value <5, then Fisher exact test was performed. For continuous variables, independent samples t test was performed to compare the means between SDD and non-SDD groups. Analyses were performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp, Armonk, NY). P values of <0.05 were deemed statistically significant.
Results
Population
Between June 2016 and June 2019, 211 patients underwent LAAC using WATCHMAN. Preoperative imaging for LAA evaluation was performed in 80.1% (169 of 211) of patients. Among them, 62.7% (106 of 169) had TEE and 37.3% (63 of 169) had computed tomography.
Device deployment was not attempted in 3 patients (2 had difficult vascular access and 1 had unsuitable LAA anatomy; Figure 2). Among procedures in which a device was deployed, 96.2% (200 of 208) were successfully implanted. We excluded 8 aborted procedures, which included 6 patients who did not meet the PASS device release criteria, 1 LAA perforation requiring emergent surgical repair, and 1 death due to hemothorax and suspected cardiac perforation of the left atrium (Figure 2). Of the 200 patients with a device successfully deployed, 4 patients were excluded for procedure-related complications that occurred on the same day of the procedure (Figure 2), where 1 patient developed a pericardial effusion during the procedure requiring percutaneous drainage and 3 patients developed hypotension in the first 2 hours after the procedure secondary to pericardial effusion requiring either percutaneous drainage (n=2) or open surgical drainage (n=1). Of the 196 patients with a successful procedure without complication, we excluded 6 patients admitted to the hospital for acute clinical events before nonelective LAAC (3 gastrointestinal bleeds and 3 mechanical falls). The final analysis of the primary safety and secondary outcomes included 190 patients (Figure 2).
Figure 2.
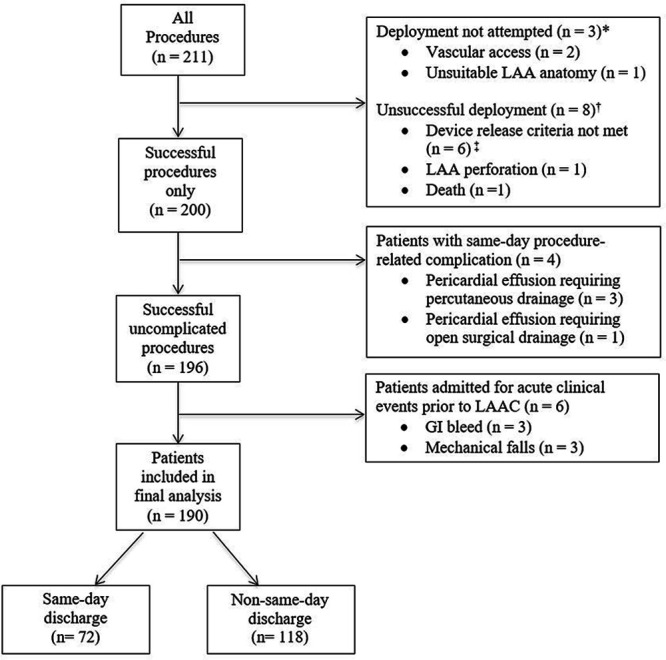
Flowchart of patients included in the final analysis of periprocedural and 45-d outcomes post-WATCHMAN procedure. GI indicates gastrointestinal; LAA, left atrial appendage; and LAAC, left atrial appendage closure. *Procedure aborted before device deployment. †WATCHMAN deployed but unsuccessful. ‡All device release criteria, position, anchor, size, and seal, must be met for device (PASS) release.
The 7-day procedure/device-related complication rate was 4.3% (9 of 211). In addition to the 6 patients who had same-day events (4 pericardial effusion requiring drainage, 1 LAA perforation, and 1 death as stated in the previous paragraph), 1 patient had an event the day following the procedure before discharge (significant groin hematoma requiring transfusion and epinephrine injection), and 2 patients had events after discharge through 7 days post-procedure (1 ischemic stroke and 1 femoral pseudoaneurysm requiring endovascular surgery).
Population in the Analysis for Primary and Secondary Outcomes
All the patients in the final analysis (n=190) had complete follow-up during the 45-day period post-procedure. All patients were ambulated 2 hours after the procedure, and none had immediate issues with vascular access site. Based on operator and patient/family preference, 72 patients had SDD and 118 had non-SDD. The SDD rate during the first year (June 2016 to May 2017), second year (June 2017 to May 2018), and third year (June 2018 to June 2019) of the study was 0% (0 of 26), 32.4% (24 of 74), and 53.3% (48 of 90), respectively (Figure 3).
Figure 3.
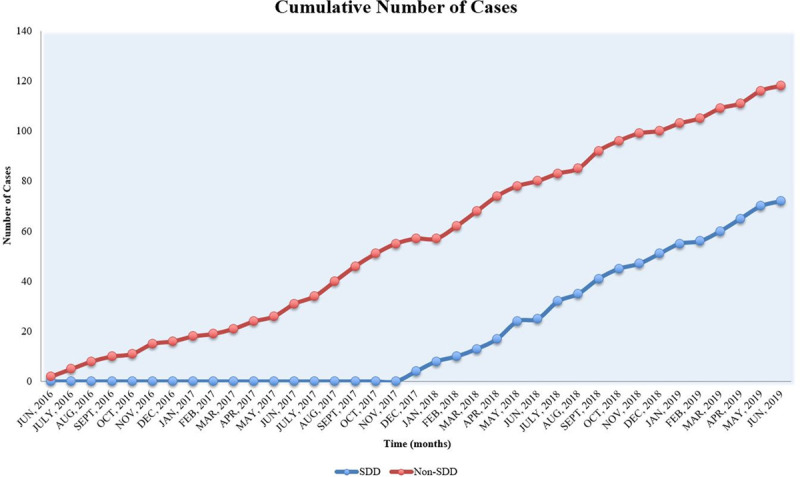
Temporal trend of same-day discharge (SDD) and non-SDD cases.
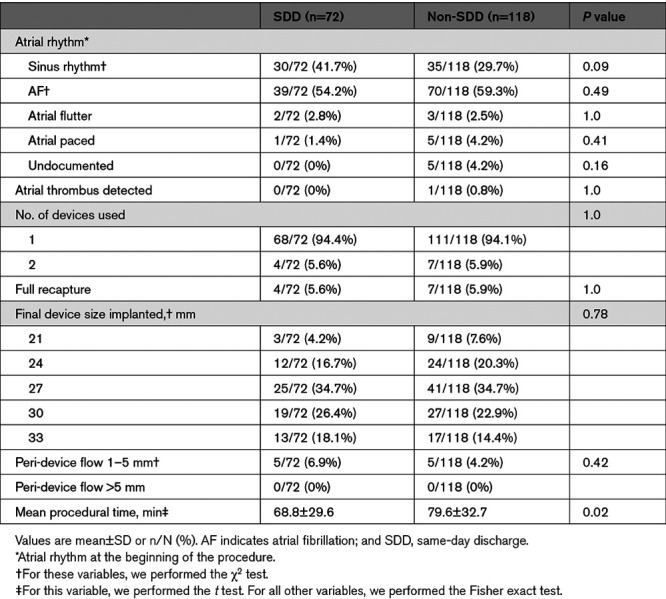
Age, sex, CHA2DS2-VASc score, and comorbidities were similar between the two groups, but the mean HAS-BLED score (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly) in the SDD group was lower than the non-SDD group (2.7 versus 3.0; P=0.04; Table 1). A history of stroke/TIA/systemic embolism was less prevalent in the SDD group (23.6% versus 39.8%; P=0.02). More patients from SDD group were on OAC at referral for LAAC (56.9% versus 41.5%; P=0.04). All procedural characteristics (Table 2) were similar except for a shorter mean procedural time in the SDD group compared with the non-SDD group (68.8 versus 79.6 minutes; P=0.02). One patient from the non-SDD group had persistent LAA thrombus despite OAC and underwent successful, uncomplicated LAAC with off-label use of transcatheter cerebral embolic protection.14
Table 1.
Baseline and Discharge Characteristics
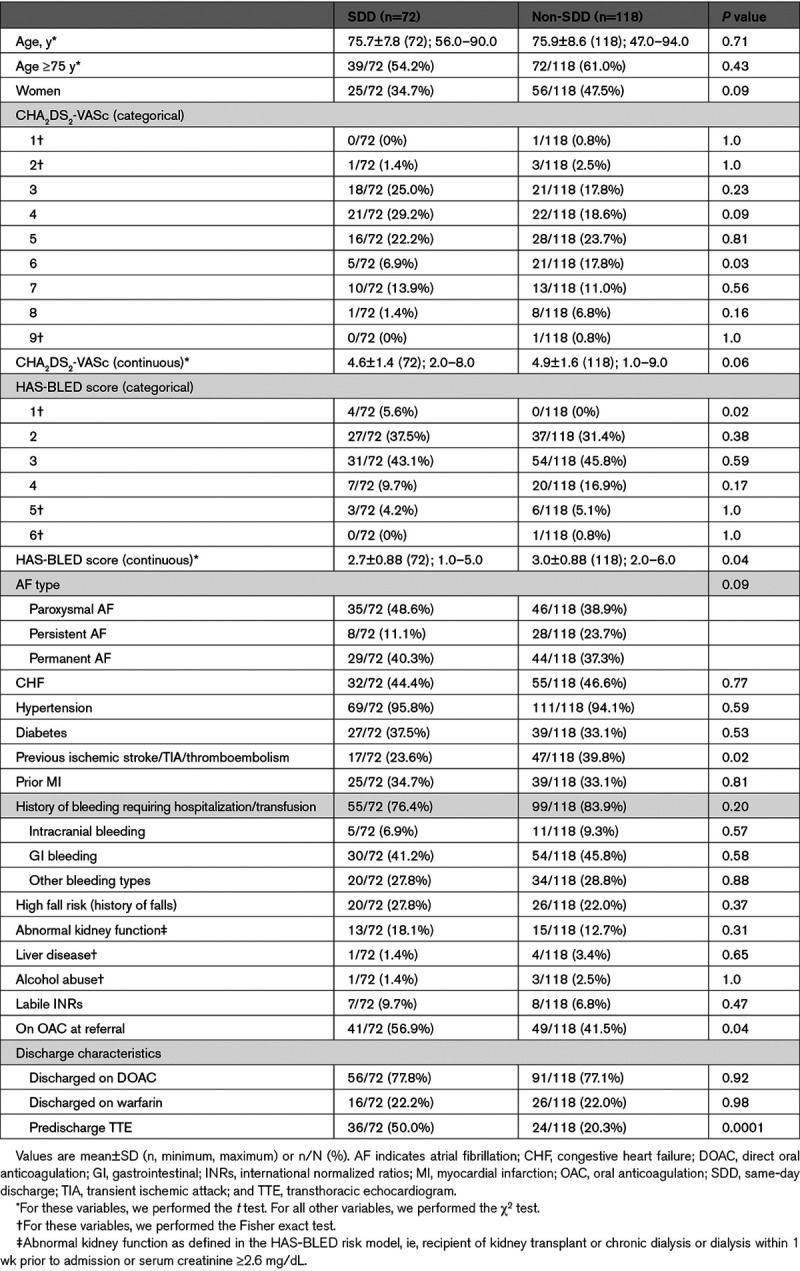
Table 2.
Procedural Characteristics
TTE was performed in 31.6% (60 of 190) of patients before discharge. Patients were discharged more frequently on direct OACs (77.4%) as opposed to warfarin (22.1%). Of the patients discharged on OAC (n=189), antiplatelet therapy was also prescribed in 90.5% of patients (171 of 189) and included aspirin (168 of 171) and clopidogrel (3 of 171).
Length of stay ranged from 0 (SDD) to 4 days, where 38% of patients had SDD, 58% were discharged the following day post-procedure, and 4% had an extended length of stay ≥2 days. Two-day length of stay occurred in 2 patients (1 rapid AF and 1 patient preference). Three-day length of stay occurred in 4 patients: 2 had vascular access complications (1 significant groin hematoma requiring epinephrine injection and transfusion and 1 groin hematoma managed conservatively), 1 had digoxin toxicity, and 1 was related to social issues. Four-day length of stay occurred in 1 patient (right adductor hematoma requiring transfusion).
Periprocedural Outcomes
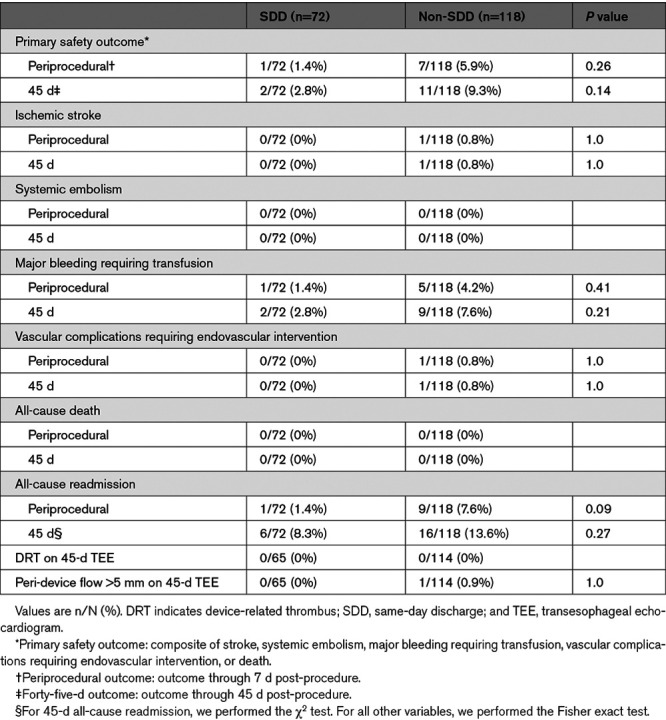
At 7 days post-procedure, the primary safety outcome was similar in the SDD compared with non-SDD groups (1.4% versus 5.9%; P=0.26; Table 3). There were no significant differences in the individual components of the primary safety outcome and all-cause readmission.
Table 3.
Periprocedural and 45-Day Outcomes
Forty-Five–Day Outcomes
At 45 days, there were no statistically significant differences in primary safety outcome between the two groups (SDD, 2.8% versus non-SDD, 9.3%; P=0.14; Table 3). Major bleeding requiring transfusion occurred in 2.8% (2 of 72) of SDD patients and 7.6% (9 of 118) of non-SDD patients (P=0.21). These include the following: SDD group: 2 gastrointestinal bleeds; non-SDD group: 7 gastrointestinal bleeds, 1 right adductor hematoma (during hospital stay), and 1 groin hematoma (during hospital stay).
At 45 days, ischemic stroke occurred in 0% (0 of 72) of SDD patients and 0.8% (1 of 118) of non-SDD patients (P=1.0). One patient had an ischemic stroke on OAC through 7 days post-procedure (found to have iatrogenic atrial septal defect and lower-extremity proximal deep vein thrombosis). Vascular complications requiring endovascular intervention occurred in 0% (0 of 72) of SDD patients and 0.8% (1 of 118) of non-SDD patients (P=1.0). One patient was readmitted for femoral pseudoaneurysm and arteriovenous fistula requiring endovascular repair.
The 45-day all-cause readmission rate was 8.3% (6 of 72) in the SDD group and 13.6% (16 of 118) in the non-SDD group (P=0.27). In the SDD group, 2 patients were readmitted for gastrointestinal bleed requiring transfusion, 3 cardiac-related (1 heart failure exacerbation and 2 rapid AF hospitalizations), and 1 mechanical fall. Within the non-SDD group, there were 20 readmissions in 16 patients. These included 1 ischemic stroke (as stated in the previous paragraph), 9 gastrointestinal bleeds requiring transfusion, 1 vascular complication requiring endovascular repair, 6 cardiac-related (3 heart failure exacerbations and 3 rapid AF hospitalizations), and 3 noncardiac related (1 pneumonia, 1 transient acute kidney injury, and 1 severe migraine).
TEE at 45 days was performed in 90% (65 of 72) in the SDD group and 97% (114 of 118) in the non-SDD group. There were no differences in peri-device flow >5 mm between the two groups (SDD, 0% versus non-SDD, 0.9%; P=1.0). No patients had device-related thrombus, systemic embolism, or death through 45 days post-procedure.
Discussion
This study demonstrates the potential safety and feasibility of SDD in patients undergoing elective LAAC with the WATCHMAN device in a real-world clinical setting.
The mean age of our population is higher than the pivotal trials5,6 and the EWOLUTION trial7 and is similar to the National Cardiovascular Data Registry LAA Occlusion registry.9 Our mean CHA2DS2-VASc score of 4.8 indicates a higher stroke risk compared with the PROTECT-AF (CHA2DS2-VASc of 3.4), PREVAIL (CHA2DS2-VASc of 4.0), and EWOLUTION (CHA2DS2-VASc of 4.5) trials.5–7 In our study, 62% of patients had a HAS-BLED score ≥3, compared with 20% in PROTECT-AF, 30% in PREVAIL, and 40% in EWOLUTION.5–7 Additionally, 81.1% of patients had a history of bleeding requiring hospitalization or transfusion compared with 38.7% in the EWOLUTION trial.7 It is worth noting that 53% of our study population was not on OAC at the time of LAAC referral, likely due to an elevated risk for or history of bleeding. The National Cardiovascular Data Registry LAA Occlusion registry is the largest analysis of LAAC (>38 000 patients enrolled from January 2016 to December 2018) in the post-FDA-approval era of WATCHMAN.9 The mean CHA2DS2-VASc and HAS-BLED score was 4.6 and 3.0, respectively, and 69.4% of subjects in this registry had a clinically relevant bleeding history.9 Similar to the National Cardiovascular Data Registry LAA Occlusion Registry, our study comprised an older and sicker patient population with a higher risk of stroke and bleeding compared with previous trials.5–7 Thus, our study findings should be applicable to patients currently undergoing LAAC in the United States.
Our implantation success rate (96.2%) is comparable to previous studies (Figure 4).5–7,9,15 Our 7-day procedure/device-related complication rate was 4.3%, lower than PROTECT-AF (8.7%)5 and compared favorably to the subsequent WATCHMAN studies.6,7,15 (Figure 5). In our study, 38% of patients had SDD and 62% had non-SDD. The trend of SDD, comparing the first year and final year of the study, increased significantly from 0% to 53.3%, reflecting our center/operator experience in postprocedural care and improved comfort level for SDD.
Figure 4.
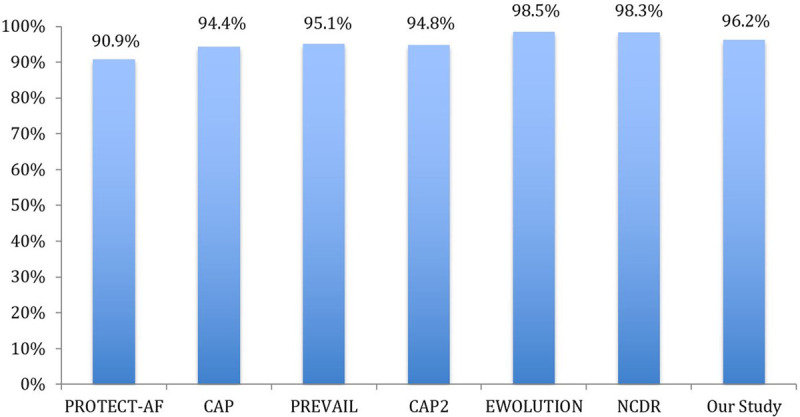
Implant success of our study compared with prior WATCHMAN studies. CAP indicates Continued Access to PROTECT-AF; CAP2, Continued Access to PREVAIL; EWOLUTION, Evaluating Real-World Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology; NCDR, National Cardiovascular Data Registry; PREVAIL, Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy; and PROTECT-AF, WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation.
Figure 5.
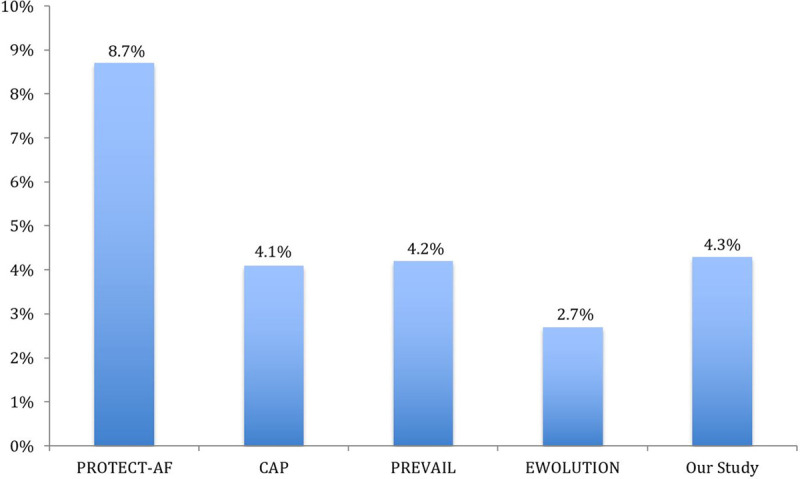
Seven-day procedure/device-related complications of our study compared with prior WATCHMAN studies. CAP indicates Continued Access to PROTECT-AF; EWOLUTION, Evaluating Real-World Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology; PREVAIL, Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy; and PROTECT-AF, WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation.
A retrospective single-center study (United Kingdom) reported SDD in 66% of patients after LAAC with acceptable in-hospital adverse event rates.11 In that study, 50.4% of patients had LAAC with the Amplatzer Cardiac Plug, 41% with the Amulet Occluder, and 2.5% with WATCHMAN.11 That study did not report the clinical outcomes during follow-up post-discharge. Moreover, the patients in that study were discharged on dual antiplatelet therapy for 4 weeks and a single antiplatelet thereafter for at least 6 months,11 which is consistent with the current European recommendations for patients not suitable for OAC.16 In North America, patients are typically maintained on OAC and aspirin for 45 days and transitioned to dual antiplatelet therapy for 6 months if the 45-day TEE shows an absence of peri-device flow >5 mm or device-related thrombus. This practice is consistent with the study protocol of previous trials, where warfarin was used exclusively.5,6 Direct OACs may be a preferable alternative to warfarin for short-term anticoagulation.17 In our study, 77% of patients were discharged on a direct OAC post-procedure.
Evaluating the primary safety outcome and secondary outcomes during the periprocedural period is important as patients are prone to clinical events within the first 7 days post-procedure.7 In EWOLUTION, the adverse event rate was 7.9% at 30 days, with close to half of the events (4.1%) occurring by 7 days post-procedure.7 In our study, 53% of patients were not on OAC at LAAC referral and started OAC postprocedurally, which has the potential to increase bleeding risk. Although the differences were not statistically significant, more patients from the non-SDD group experienced the primary safety outcome, which was mainly driven by major bleeding requiring transfusion both during the periprocedural period and at 45 days. This was likely related to the higher mean HAS-BLED score in the non-SDD group (2.7 versus 3.0; P=0.04).
The average length of stay for LAAC was 4.6 days in the pre-FDA approval era of WATCHMAN.8 Currently, standard practice post-procedure typically involves monitoring patients overnight and discharging the following day.10 According to a recent study utilizing the National Inpatient Sample database, the median length of stay is 1 day.10 At present, SDD has been used for other cardiac procedures, including elective percutaneous coronary intervention,18 radiofrequency catheter ablation,19 and patent foramen ovale closure without increased risk of complications compared with overnight monitoring.20 With increasing experience, elective LAAC should be included among these procedures. SDD in uncomplicated elective LAAC can reduce the length of stay, thereby improving resource utilization and reducing overall health care costs. The average cost savings have been estimated at $2500 per patient,21 which should translate into better use of hospital resources. Furthermore, SDD may improve patient satisfaction, as shown in previous literature on elective percutaneous coronary intervention.22
Limitations
The main limitation of this study is its observational nature with several inherent limitations, including risk for selection bias, confounding bias, and the inability to attribute causation. However, the inherent selection bias associated with SDD patients reinforces that the operator and patient/family are appropriately selecting those who may be suitable for SDD.
Second, the small sample size may lead to the study being underpowered to detect a statistically significant difference in clinical outcomes. However, the complication rates with the WATCHMAN procedure were relatively low in recent studies, likely due to increased hospital and operator experience.6–8 As such, a study powered to detect a statistically significant difference in clinical outcomes between the SDD and non-SDD groups would require a much larger sample size.
Finally, routine predischarge TTE was not part of the institutional protocol unless the patient developed symptoms/hypotension. Predischarge TTE was at the discretion of the operators. Variability in its use may not capture all periprocedural device-related complications (eg, pericardial effusion). However, in our study, only 2 patients were readmitted through 7 days post-procedure (1 femoral pseudoaneurysm and 1 periprocedural stroke); none could have been prevented with a predischarge TTE. In the EWOLUTION trial, of 1019 patients who underwent LAAC, 0.4% (5 of 1019) had procedure-related pericardial effusion (including 1 cardiac tamponade) within 24 hours of LAAC.7 The risk of late pericardial effusion within 24 hours of LAAC cannot be adequately assessed by intraprocedural TEE. Thus, routine predischarge TTE for all patients may be warranted in future trials and clinical practice, especially for patients considered for SDD.
Conclusions
In a selected cohort of patients who underwent successful elective LAAC with the WATCHMAN device without same-day procedure-related complications, the primary safety outcome and secondary outcomes were similar in patients with SDD compared with non-SDD. At 7 and 45 days post-procedure, there were no statistically significant differences in stroke, systemic embolism, major bleeding requiring transfusion, vascular complications requiring endovascular intervention, death, all-cause readmission, and significant peri-device flow or device-related thrombus on 45-day TEE between the two groups. SDD has the potential to minimize the unnecessary use of medical resources and improve patient satisfaction without compromising patient safety. Due to the retrospective nature of this study, our findings are considered hypothesis generating. Prospective trials with postprocedural randomization of SDD and non-SDD are warranted to confirm the findings of our study.
Acknowledgments
Drs Depta and Tan conceived and designed the study; Dr Tan performed the statistical analyses; Drs Tan, Boppana, and Depta wrote the manuscript; and all authors acquired, analyzed, or interpreted the data and critically revised the manuscript.
Sources of Funding
None.
Disclosures
Dr Depta is a consultant/belongs to the Advisory Board at Edwards Lifesciences, Boston Scientific, and W.L. Gore and Associates. Dr Bhatt belongs to the Advisory Board at Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, and Regado Biosciences; belongs to the Board of Directors at the Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; is a chair at the American Heart Association Quality Oversight Committee; belongs to the Data Monitoring Committees at the Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial [Portico Re-Sheathable Transcatheter Aortic Valve System US IDE Trial], funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial [A Prospective, Single-Arm, Controlled, Multicenter Study to Establish the Safety and Effectiveness of the CENTERA THV System in Intermediate Risk Patients Who Have Symptomatic, Severe, Calcific, Aortic Stenosis Requiring Aortic Valve Replacement], funded by Edwards), Contego Medical (Chair, PERFORMANCE 2 [Protection Against Emboli During Carotid Artery Stenting Using the Neuroguard IEP System]), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial [Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation], funded by Daiichi Sankyo), and Population Health Research Institute; reports honoraria from the American College of Cardiology (ACC; Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI Clinical Trial [Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting] Steering Committee funded by Boehringer Ingelheim; AEGIS-II [Study to Investigate CSL112 in Subjects With Acute Coronary Syndrome] Executive Committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial [A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease], funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (cochair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME Steering Committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA National Co-Leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), and WebMD (CME Steering Committees); other from Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (chair), and VA CART Research and Publications Committee (chair); research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, and The Medicines Company; royalties from Elsevier (Editor, Cardiovascular Intervention: a Companion to Braunwald’s Heart Disease); site coinvestigator at Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; trustee at the American College of Cardiology; and unfunded research at FlowCo, Merck, Novo Nordisk, and Takeda. The other authors report no conflicts.
Footnotes
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- LAA
- left atrial appendage
- LAAC
- left atrial appendage closure
- OAC
- oral anticoagulation
- SDD
- same-day discharge
- TEE
- transesophageal echocardiography
- TTE
- transthoracic echocardiography
For Sources of Funding and Disclosures, see page 82.
Contributor Information
Bryan E-Xin Tan, Email: bryantan.exin@gmail.com.
Leela Krishna Teja Boppana, Email: LeelaKrishnaTeja.Boppana@rochesterregional.org.
Abdullah S. Abdullah, Email: Abdullah.Abdullah@rochesterregional.org.
Dmitry Chuprun, Email: Dmitry.Chuprun@rochesterregional.org.
Abrar Shah, Email: Abrar.Shah@rochesterregional.org.
Mohan Rao, Email: Mohan.Rao@rochesterregional.org.
Jeremiah P. Depta, Email: Jeremiah.Depta@rochesterregional.org.
References
- 1.Alkhouli M, Alqahtani F, Aljohani S, Alvi M, Holmes DR. Burden of atrial fibrillation-associated ischemic stroke in the United States. JACC Clin Electrophysiol. 2018;4:618–625. doi: 10.1016/j.jacep.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 2.O’Brien EC, Holmes DN, Ansell JE, Allen LA, Hylek E, Kowey PR, Gersh BJ, Fonarow GC, Koller CR, Ezekowitz MD, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2014;167:601–609.e1. doi: 10.1016/j.ahj.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 3.Steinberg BA, Greiner MA, Hammill BG, Curtis LH, Benjamin EJ, Heckbert SR, Piccini JP. Contraindications to anticoagulation therapy and eligibility for novel anticoagulants in older patients with atrial fibrillation. Cardiovasc Ther. 2015;33:177–183. doi: 10.1111/1755-5922.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS Focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 5.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X [DOI] [PubMed] [Google Scholar]
- 6.Holmes DR, Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the WATCHMAN left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 7.Boersma LV, Schmidt B, Betts TR, Sievert H, Tamburino C, Teiger E, Pokushalov E, Kische S, Schmitz T, Stein KM, et al. ; EWOLUTION Investigators. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. 2016;37:2465–2474. doi: 10.1093/eurheartj/ehv730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badheka AO, Chothani A, Mehta K, Patel NJ, Deshmukh A, Hoosien M, Shah N, Singh V, Grover P, Savani GT, et al. Utilization and adverse outcomes of percutaneous left atrial appendage closure for stroke prevention in atrial fibrillation in the United States: influence of hospital volume. Circ Arrhythm Electrophysiol. 2015;8:42–48. doi: 10.1161/CIRCEP.114.001413 [DOI] [PubMed] [Google Scholar]
- 9.Freeman JV, Varosy P, Price MJ, Slotwiner D, Kusumoto FM, Rammohan C, Kavinsky CJ, Turi ZG, Akar J, Koutras C, et al. The NCDR left atrial appendage occlusion registry. J Am Coll Cardiol. 2020;75:1503–1518. doi: 10.1016/j.jacc.2019.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuddanda VLK, Turagam MK, Umale NA, Shah Z, Lakkireddy DR, Bartus K, McCausland FR, Velagapudi P, Mansour M, Heist EK. Incidence and causes of in-hospital outcomes and 30-day readmissions after percutaneous left atrial appendage closure: a US nationwide retrospective cohort study using claims data. Heart Rhythm. 2020;17:374–382. doi: 10.1016/j.hrthm.2019.09.018 [DOI] [PubMed] [Google Scholar]
- 11.Williams T, Alsanjari O, Parker J, Gannaway A, Thomson C, Gomes A, Hildick-Smith D. Day-case percutaneous left atrial appendage occlusion-safety and efficacy. Catheter Cardiovasc Interv. 2018;92:1439–1443. doi: 10.1002/ccd.27791 [DOI] [PubMed] [Google Scholar]
- 12.Nietlispach F, Gloekler S, Krause R, Shakir S, Schmid M, Khattab AA, Wenaweser P, Windecker S, Meier B. Amplatzer left atrial appendage occlusion: single center 10-year experience. Catheter Cardiovasc Interv. 2013;82:283–289. doi: 10.1002/ccd.24872 [DOI] [PubMed] [Google Scholar]
- 13.Tan BE, Depta JP, Baibhav B, Bhatt DL. Necessity of 45-Day transesophageal echocardiography after the WATCHMAN procedure amid the COVID-19 pandemic. JACC Cardiovasc Imaging. 2020;13:2461–2462. doi: 10.1016/j.jcmg.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan BE, Depta JP. Transcatheter cerebral embolic protection during WATCHMAN procedure in two patients with persistent left atrial appendage thrombus: case report with review of the literature [published online ahead of print June 15, 2020]. Catheter Cardiovasc Interv. 2020. 10.1002/ccd.29060 [DOI] [PubMed] [Google Scholar]
- 15.Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the WATCHMAN left atrial appendage system for embolic Protection in Patients With AF (PROTECT AF) clinical trial and the continued access registry. Circulation. 2011;123:417–424. doi: 10.1161/CIRCULATIONAHA.110.976449 [DOI] [PubMed] [Google Scholar]
- 16.Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYH, Fauchier L, Betts TR, Lewalter T, Saw J, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion - an update. EuroIntervention. 2020;15:1133–1180. doi: 10.4244/EIJY19M08_01 [DOI] [PubMed] [Google Scholar]
- 17.Enomoto Y, Gadiyaram VK, Gianni C, Horton RP, Trivedi C, Mohanty S, Di Biase L, Al-Ahmad A, Burkhardt JD, Narula A, et al. Use of non-warfarin oral anticoagulants instead of warfarin during left atrial appendage closure with the Watchman device. Heart Rhythm. 2017;14:19–24. doi: 10.1016/j.hrthm.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 18.Shroff A, Kupfer J, Gilchrist IC, Caputo R, Speiser B, Bertrand OF, Pancholy SB, Rao SV. Same-day discharge after percutaneous coronary intervention: current perspectives and strategies for implementation. JAMA Cardiol. 2016;1:216–223. doi: 10.1001/jamacardio.2016.0148 [DOI] [PubMed] [Google Scholar]
- 19.Marijon E, Albenque JP, Boveda S, Jacob S, Schmutz M, Bortone A, Combes N, Zimmermann M; RETAC Group. Feasibility and safety of same-day home discharge after radiofrequency catheter ablation. Am J Cardiol. 2009;104:254–258. doi: 10.1016/j.amjcard.2009.03.024 [DOI] [PubMed] [Google Scholar]
- 20.Ponnuthurai FA, van Gaal WJ, Burchell A, Mitchell AR, Wilson N, Ormerod OJ. Safety and feasibility of day case patent foramen ovale (PFO) closure facilitated by intracardiac echocardiography. Int J Cardiol. 2009;131:438–440. doi: 10.1016/j.ijcard.2007.07.141 [DOI] [PubMed] [Google Scholar]
- 21.Hospital Adjusted Expenses per Inpatient Day 2018. Accessed June 9, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day/
- 22.Knopf WD, Cohen-Bernstein C, Ryan J, Heselov K, Yarbrough N, Steahr G. Outpatient PTCA with same day discharge is safe and produces high patient satisfaction level. J Invasive Cardiol. 1999;11:290–295. [PubMed] [Google Scholar]


