Supplemental Digital Content is available in the text.
Keywords: axillary artery, brachial plexus, early ambulation, left ventricular dysfunction, percutaneous coronary intervention, registries
Abstract
Background:
There has been increasing utilization of short-term mechanical circulatory support devices for a variety of clinical indications. Many patients have suboptimal iliofemoral access options or reasons why early mobilization is desirable. Axillary artery access is an option for these patients, but little is known about the utility of this approach to facilitate short-term use for circulatory support with microaxial pump devices.
Methods:
The Axillary Access Registry to Monitor Safety (ARMS) was a prospective, observational multicenter registry to study the feasibility and acute safety of mechanical circulatory support via percutaneous upper-extremity access.
Results:
One hundred and two patients were collected from 10 participating centers. Successful device implantation was 98% (100 of 102). Devices were implanted for a median of 2 days (interquartile range, 0–5 days; range, 0–35 days). Procedural complications included 10 bleeding events and 1 stroke. There were 3 patients with brachial plexus–related symptoms all consisting of C8 tingling and all arising after multiple days of support. Postprocedural access site hematoma or bleeding was noted in 9 patients. Device explantation utilized closure devices alone in 61%, stent grafts in 17%, balloon tamponade facilitated closure in 15%, and planned surgical explant in 5%. Duration of support appeared to be independently associated with a 1.1% increased odds of vascular complication per day ([95% CI, 0.0%–2.3%] P=0.05).
Conclusions:
Percutaneous axillary access for use with microaxial support pumps appears feasible with acceptable rates of bleeding despite early experience. Larger studies are necessary to confirm the pilot data presented here.
What Is Known
Large-bore arterial access is not always feasible from, or best suited to, the femoral artery.
Percutaneous axillary access has been reported in case reports and limited series, but systematic evaluation of this access technique has not been performed.
What the Study Adds
Large-bore percutaneous axillary access for insertion of microaxial pumps may be feasible among centers facile with this technology.
The feasibility data presented here can serve as the basis for developing a larger, more robust analyses of this novel access technique.
There has been increasing utilization of mechanical circulatory support devices for short-term support to facilitate increasingly complex percutaneous coronary intervention (PCI) or as an acute bridge to cardiac recovery, durable assist devices, or transplant in the setting of cardiogenic shock or cardiac arrest.1–3 These devices are most commonly inserted using the common femoral artery and a percutaneous modified Seldinger technique. However, this strategy has limitations; most notably, these devices all require relatively large-bore catheters for arterial access, and thus patients with significant lower-extremity peripheral vascular occlusive disease may experience ischemic complications or may be unable to receive support altogether. Additionally, femoral access requires persistent bed rest among patients who may otherwise greatly benefit from in-hospital conditioning programs and physical therapy.4
For placement via the upper-extremity vessels, the standard technique is to perform surgical exposure of the axillary artery and attach a conduit graft. Though not a challenging surgical procedure, this process typically requires the availability of an anesthesiologist, a surgeon, and, ideally, a hybrid operating room with fluoroscopic imaging capabilities. Conversely, a fully percutaneous approach may facilitate more ready and rapid placement in the standard catheterization laboratory and avoid the need for surgical cutdown or anesthesia while retaining the benefits of earlier patient rehabilitation. Historically, percutaneous axillary access has not been pursued based on differences in anatomic complexity, perceived inability to perform manual hemostasis, and luminal caliber relative to the femoral artery.5
The most commonly utilized mechanical support devices include intraaortic balloon pumps and the Impella family of ventricular assist devices (Abiomed, Danvers, MS). An intraaortic balloon pump typically requires a 7F or 8F arterial access point, whereas the Impella 2.5 and CP devices require 13F and 14F sheaths, respectively. Axillary access for intraaortic balloon pumps is relatively well described,6,7 and there is a growing body of literature regarding percutaneous access of the axillary artery for Impella support,8–10 but the global experience, particularly for the Impella devices, remains small. We, therefore, developed the Axillary Access Registry to Monitor Safety (ARMS)—a prospective multicenter registry—to study the feasibility and acute safety of mechanical circulatory support via percutaneous upper-extremity access.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. ARMS was a multicenter registry across 10 participating US centers (see Appendix I in the Data Supplement for a list of participating centers and primary operators). Anatomic and proposed technique considerations were standardized across participating centers through an in-person meeting before registry initiation. A standardized data collection instrument was designed to capture implant technique and choices, procedural efficacy, and in-hospital safety (Data Supplement). The goal was to collect data on 100 consecutive patients who underwent attempted implantation of a percutaneous axillary microaxial pump support device (the Impella family of devices). There were no roll-in patients, and thus the total experience of each center was captured. Central case selection screening was not performed and was at the discretion of the participating centers. A waiver of consent was requested based on the deidentified and observational nature of the data collected and critical illness severity of the patients undergoing mechanical circulatory support. All participating centers obtained local institutional review board approval before participation.
Data Capture and Analysis
Clinical outcomes of interest were predefined and entered into case report forms by each participating center. Outcome definitions adhered to the Valve Academic Research Consortium (VARC) criteria.11 All care was clinically indicated, and there were no obligate laboratory or imaging data requested pre- or post-procedure. Imaging results and outcomes were reported and adjudicated by the individual sites based on the exploratory nature of this study. Data elements of interest sought to explore procedural techniques, as well as immediate and in-hospital outcomes. Intraprocedural complications were defined as those noted during the procedure or within 6 hours of completion of the procedure. In-hospital complications were defined as arising between 6 hours post-procedure and hospital discharge or death. Successful implantation was defined as percutaneous access of the axillary artery followed by delivery of the intended device across the aortic valve without the need for surgical assistance or remediation. Patients were not censored for in-hospital outcomes if they escalated support or transitioned to a durable support device, and thus complications arising from those procedures or surgeries were attributed to the axillary pump implant. For the purposes of regression analyses, composite vascular complications were defined as a combination of access site blood loss requiring transfusion, hematoma >4 cm, pseudoaneurysm, dissection, ischemic limb, unplanned access site, or chest surgery of the subtended limb anytime during the hospitalization.
Secondary analyses were performed by category of MCS device indication. Shock with PCI, ischemic shock without PCI, nonischemic shock, and decompensated heart failure were considered as an acute heart failure (AHF) indication, whereas stable high-risk PCI, unstable high-risk PCI, electrophysiology procedures, and other indications were categorized as non-AHF.
The University of Washington served as the data collection center, and all data were stored via REDCap (Vanderbilt University, Nashville, TN). This study was approved by the institutional review boards of all participating centers. Based on the deidentified data submitted and observational nature of the data, patient consent was not required. Data are reported as mean±SD or median (25th to 75th percentile) as appropriate. Continuous and integer data were compared using Student t tests or Fisher exact test, respectively. To identify features associated with vascular complications, univariate and multiple regression models were designed a priori with covariates of interest including age, sex, laterality, patient weight, and duration of support. Data were analyzed using Stata, version 15 (StataCorp, LLC, College Station, TX).
Results
Ultimately, 105 patients were submitted to the registry following attempted percutaneous axillary MCS device implantation. Three cases were excluded for missing >50% data, resulting in a cohort of 102 patients. Most implanted devices were Impella CPs (84), whereas Impella 2.5 (14) and Impella 5.0 (4) were less common. Successful device implantation was 98% (100 of 102), with both unsuccessful attempts occurring with the Impella 5.0 device.
Table 1 describes the patient population. Sixty-two percent of patients had inhospitable femoral artery access as the rationale for axillary artery use, whereas the other 38% underwent axillary artery access irrespective of femoral artery caliber and health. As demonstrated in Table 2, multiple concomitant procedures were typically performed during the index implantation. Most patients had at least 1 additional arterial access point obtained during the index procedure including from the femoral artery in 93 patients and from the radial or brachial in 25. Only 2 patients did not have a secondary arterial access point.
Table 1.
Demographics

Table 2.
Concomitant Procedures Performed With Microaxial Pump Insertion

Table 3 describes the procedure planning details including the rationale for laterality of device placement and the methodology used for vessel assessment. Devices were evenly distributed between the right and left axillary arteries (52% right sided), and the most common reason for choosing left versus right axillary artery was operator preference (63%). The most common method of preimplantation vessel assessment was angiography during the index procedure (76%) though many patients were screened with multiple imaging modalities but only 2 patients underwent assessment before their index procedure (1 computed tomographic angiography and 1 duplex ultrasound).
Table 3.
Procedural Planning
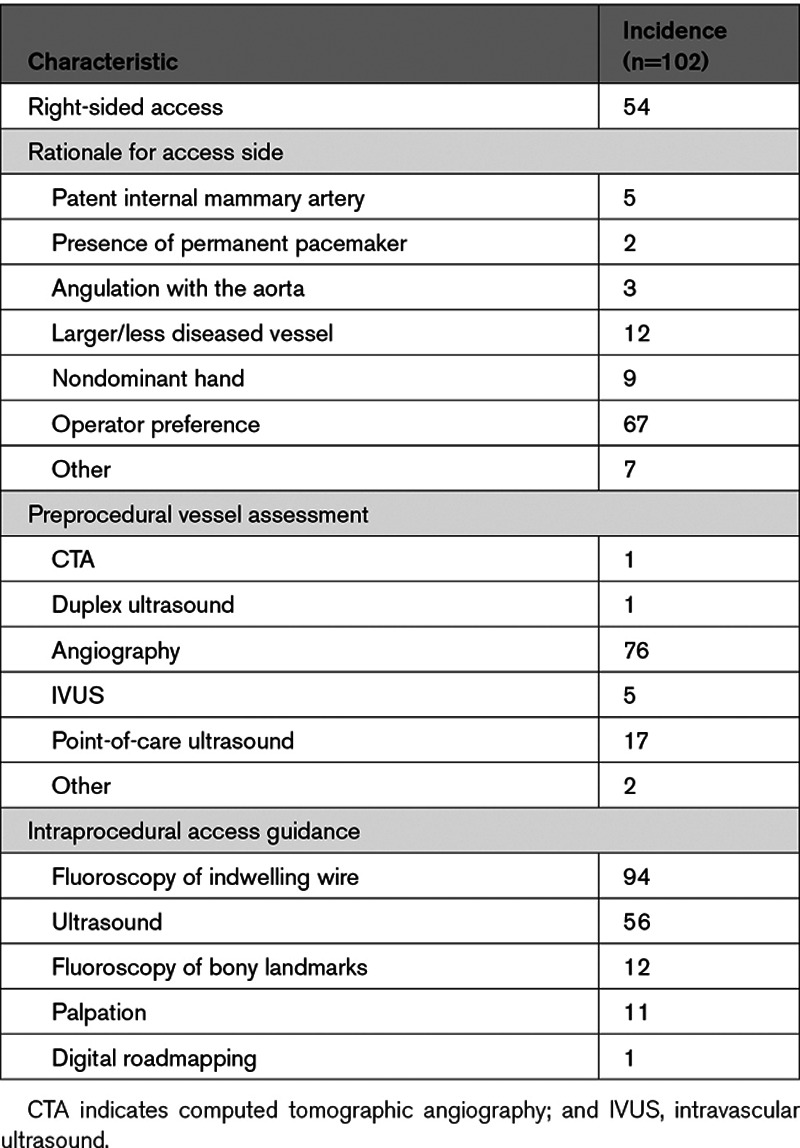
Implantation technique varied slightly between operators and over time. Nineteen patients underwent primary access via an infrapectoral needle approach (within the true axilla), whereas the remainder underwent a transpectoral approach (Figure). The transpectoral approach (with access roughly akin to the positioning of a permanent pacemaker pocket) was exclusively used at 8 of the 10 implanting centers and was ultimately adopted at the remaining 2 enrolling centers. Intraprocedural access guidance is listed in Table 3. Devices were implanted for a median of 2 days (interquartile range, 0–5 days; range, 0–35 days). There were significant differences in the duration of implantation by indication. The 51 patients receiving devices for AHF had a median implant time of 4 days (interquartile range, 2–7), whereas the 51 patients with non-AHF indications had a median implant duration of 0 days (interquartile range, 0–1).
Figure.

Illustration of options regarding access point for percutaneous axillary artery access. Catheter A traverses the pectoralis minor muscle from the anterior chest before entering the vessel. Catheter B runs lateral and inferior to the pectoralis muscles from the anterior axilla.
Procedural complications are listed in Table 4. Of all attempted implants, 85 had no reported intraprocedural complications. There were no cases of access-related deaths, lost vascular access, pneumothorax, or acute distal vessel occlusion/ischemic limb. One patient had an embolic stroke confirmed by brain imaging. The most common complication involved VARC minor bleeding: 10 cases (10%) reported hematoma, track bleeding, ecchymoses, or bleeding requiring transfusion. There were no cases of VARC major or life-threatening bleeding. There were no significant differences in rates of procedural complications by access site (infrapectoral versus transpectoral, P=0.59) or by AHF indication (AHF versus non-AHF, P=0.43).
Table 4.
Procedural Complications

Of the 100 successfully implanted devices, 75 were explanted. Among patients with explanted devices, 60 recovered native function, while 8 went on to a durable left ventricular assist device, 6 transitioned to a different circulatory support device, and 1 received a total artificial heart. Techniques for explant included use of a primary closure device in 46 patients (61%) but also included stent graft implantation in 13 patients (17%), balloon tamponade with closure device or manual compression in 11 patients (15%), and planned surgery in 4 patients (5%). One device was explanted following mechanical failure with a second device reimplanted through the same access. There were no unplanned surgical explants. There was high variance between centers in stent graft use ranging from 0% to 47% among their respective explanted patients. Excluding the highest frequency stent graft center (9 of 19 explants), stent grafts were used in 7% of explanted patients (4 of 56). Among the vascular closure device population, most scenarios involved 2 Perclose ProGlide sutures (Abbott Vascular, Santa Clara, CA). Eight patients received Angioseal devices (Terumo, Somerset, NJ) in combination with Perclose(s), and 1 received a ProStar XL device (Abbott Vascular, Santa Clara, CA).
Table 5 describes in-hospital outcomes. Forty-six patients (44%) had an in-hospital complication including in-hospital mortality in 30 patients (30%). There were 9 patients who had late access site bleeding or hematomas consistent with VARC minor bleeding. Three patients had complaints attributable to the brachial plexus (all were sensation changes in the C8 dermatome arising late in the hospitalization; 2 resolved at follow-up and 1 patient was lost to follow-up). Four patients had a stroke during the index hospitalization, and 1 had a transient ischemic attack; 1 patient had a clinically silent pseudoaneurysm of the axillary artery following explant (which underwent thrombin injection), and 1 had occlusion of the axillary artery and ischemic hand symptoms during explant requiring balloon angioplasty of the distal axillary artery. Significant differences in in-hospital complications were noted by indication. All 4 nonprocedural strokes were in the AHF cohort (8% versus 0%; P=0.04), and rates of in-hospital mortality were also significantly greater in the AHF cohort (43% versus 16%; P<0.01). Bleeding and plexopathy complaints were not significantly different between the two cohorts. The proportion of right- versus left-sided access was not different between the AHF and non-AHF populations (49% versus 51%; P=0.69), and there were no differences in overall procedural complications, vascular complications specifically, or in-hospital adverse events by access side (P=0.24, P=0.95, and P=0.43 for right versus left arm, respectively).
Table 5.
In-Hospital Complications

Regression analyses were performed to evaluate patient and procedural factors associated with vascular complications. In univariate analysis, only duration of implant was significantly associated with in-hospital vascular complications (1.2% increase per day; P=0.03). After adjusting for patient age, sex, weight, and access laterality, duration of implant was associated with a 1.1% increased odds of vascular complications per day, though it was of borderline significance ([95% CI, 0.0%–2.3%] P=0.05).
Discussion
The ARMS was a prospective, observational, multicenter registry designed to explore the feasibility and safety of percutaneous axillary access for short-term mechanical circulatory support with microaxial pump devices. Our data represent the largest real-world registry to date and suggest that (1) among centers with robust preexisting knowledge of large-bore access, percutaneous implantation and explantation maybe feasible with high rates of technical success despite reflecting the initial learning experience in this vascular territory and (2) rates of vascular complications and bleeding may be acceptable within the context of previously published large-bore percutaneous access complication rates.12,13
Vascular injury and bleeding are the most frequent complications and the principal concerns inherent to short-term circulatory support based on the large sheath sizes required, indwelling nature of the catheters, requisite anticoagulants, and relatively high incidence of concomitant peripheral arterial disease. Prior analyses of all nondurable circulatory support systems have suggested that the incidence of vascular complications and bleeding may be as high as 25.8% of all patients12 while rates of vascular complications following large-bore femoral access for structural heart indications have consistently been 5% to 16%.13,14 In this context the 10% rate of VARC minor bleeding reported in this article may be considered reasonable, particularly given the early experiences captured here. The use of stent grafts to establish hemostasis, however, was higher than anticipated. There were significant differences in rates of stent graft use among centers, and lower thresholds for use can be seen in the axillary artery compared with femoral access since the first segment of the axillary does not cross a true joint space. Nevertheless, given the unclear durability of stent grafts in the axillary artery, they should likely be considered only as a bailout strategy. It is unclear whether more routine adoption of ultrasound-guided access, which was used in only 55% of the cases reported here but is generally considered best practice, might further reduce vascular complication rates or stent graft usage in this population.
Much remains unknown about percutaneous axillary access and that was reflected in our registry. There are no available data to guide the choice or left- versus right-sided access. As such, more often than not the decision regarding laterality was driven by operator preference. Practically speaking, the access site choice may dictate changes in room setup to facilitate operator ergonomics. It is thus interesting that so few patients underwent a preprocedural vascular assessment (1 patient underwent computed tomographic angiography and 1 underwent duplex ultrasound), which may reflect the urgent or potentially unexpected nature of these cases. Questions regarding the benefits of right versus left axillary access remain germane since prior data regarding transcatheter aortic valve replacement suggest the incidence of stroke following any axillary access (surgical or percutaneous) may be elevated,15 and there are obvious differences in the respective conduit great vessels (eg, the innominate also provides the right common carotid artery and right vertebral, whereas the left subclavian provides only the vertebral, but the device needs to traverse more of the aortic arch). Within our cohort, there was 1 reported stroke within 6 hours of the index procedure and 5 in-hospital cerebral vascular events in total, though the complex nature of these patients’ hospitalizations makes interpretation of the in-hospital events difficult.
Of course, the indwelling nature of microaxial pumps creates specific challenges in regard to vascular access when compared with transcatheter aortic valve replacement. Within our dataset, the median duration of device implantation was 2 days with the longest device in place for 35 days. And, in fact, only duration of implantation appeared to be associated with in-hospital vascular complications or bleeding; however, these data should be considered hypothesis generating and based on a limited number of patients and events. It is also important to recognize that the timing of the vascular complication was not recorded, only that it occurred. Thus, it is possible that vascular complications could lead to longer device dwell times rather than vice versa. Nevertheless, prior single-center data have also drawn a link between the duration of implantation and the formation of laminar thrombus in the axillary artery.16 Taken together, there may be a correlation between adverse events and the duration of axillary device dwell time though causality can not be inferred.
Our overall rates of brachial plexus complaints were <3%. Interestingly, these findings are contrary to a previous report in the setting of endovascular procedures that cited neurovascular complication rates of up to 24% in cases when percutaneous axillary access was obtained. At the time, these data resulted in the near-complete abandonment of percutaneous axillary artery access.17 The difference in rates of neurovascular complications may be explained by our concerted effort to move more proximal with our arteriotomy site, in line with the first or second portion of the artery. Previously, the predominant method used for percutaneous axillary access was via the true axilla with the arm abducted and access site targeted along the deltopectoral grove, which often resulted in access in the third portion of the vessel, and in some instances may have in fact been in the proximal brachial artery itself, both of which are encompassed by the brachial fascial sheath and as such much less tolerant of any extravasation. It is also important to note the location of the brachial plexus with respect to the axillary artery changes along the course of the artery. The artery is surrounded by brachial plexus structures in the third portion of the artery, while the first and second portions of the vessel are completely devoid of any neural structures along its anterior surface. Furthermore, it is possible that secular changes in best practices for large-bore access, including the routine use of ultrasound guidance and more uniform use of vascular closure devices, may have lead to more optimal access and effective hemostasis.
The axillary artery is known to have a number of histological differences when compared with the femoral artery; specifically, it has more elastic lamina in its media as opposed to smooth muscle cells and lacks a fibrous adventitia.18 The instances of brachial plexus complaints in the registry may be related to these differences insofar as they may contribute to distention and subclinical extravasation from the arteriotomy site. Subclinical hematoma within the brachial fascial sheath—a structure that encompasses the artery, vein, and brachial plexus—could lead to compression of the nerve, which would be in keeping with our findings of late brachial plexus complaints that seem to resolve over time. Alternatively, the C8 nerve complaints could have occurred from inadvertent through and through arterial punctures since the posterior cord of the brachial plexus supplies the median nerve and frequently runs directly underneath the axillary artery. However, the timing of the plexus complaints was not consistent with injury during implantation.
This analysis needs to be considered within the context of several limitations. The ARMS registry was a pilot analysis of the feasibility of percutaneous axillary access as such we did not perform independent adjudication of procedural success or adverse outcomes, which were self-reported by each center. Furthermore, angiographic core laboratory review and postprocedural axillary artery imaging were not required. Additionally, since our focus was on the immediate technical success and safety of percutaneous axillary access and closure, we did not collect outcomes beyond the index hospitalization. We also did not collect detailed information regarding demographics, medical history, or associated procedures (eg, target revascularization vessels). And, though these data represent the early learning curve at each center, all participating centers were well versed in large-bore access and had robust mechanical circulatory support programs. Thus, our data should not be generalized to all institutions and operators. Finally, as this was an exploratory pilot study, the decision to enroll 100 patients was based on historical precedent19 rather than power calculations around a particular outcome.
Conclusions
Percutaneous large-bore access for indwelling support devices such as the Impella family of pumps is a complex and evolving field. As demonstrated in this ARMS dataset, the complexity of such patients is often exceedingly high, and devices may be left in place for prolonged durations. We have demonstrated that percutaneous large-bore axillary access has a feasibility and safety profile that is consistent with standard femoral access and can be considered as an option to facilitate support when required. However, larger and more rigorous studies will be necessary to build upon the data presented here.
Sources of Funding
Funding for this registry was provided through a grant from Abiomed Corporation (Danvers, MA). Abiomed did not participate in the data acquisition, statistical analysis, writing, or editing of the manuscript.
Disclosures
Dr McCabe reports grant support from Abiomed and consulting at Edwards LifeSciences, Boston Scientific, Cardiovascular Systems Inc, and Teleflex. Dr Kaki reports consulting at Abbott Vascular, Abiomed, Cardiovascular Systems Inc, and Terumo Medical. Dr Kirtane reports institutional funding to the Columbia University or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, Cardiovascular Systems Inc, CathWorks, Siemens, Philips, and ReCor Medical. In addition to research grants, institutional funding includes fees paid to the Columbia University or Cardiovascular Research Foundation for speaking engagements and consulting; no speaking/consulting fees were personally received. Dr Kirtane reports reports personal travel expenses/meals from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, Cardiovascular Systems Inc, CathWorks, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. Dr Grantham reports speaking fees, honoraria, and travel reimbursement from Abbott Vascular, Boston Scientific, Asahi-Intecc, and Abiomed; institutional research grants from Boston Scientific; and consulting fees from Corindus A Siemens Healthineers Company. Dr Nicholson reports consulting from Abbott Vascular, Abiomed, Medtronic, and Boston Scientific. Dr Kapur reports speaker/consulting honoraria and institutional research grants from Abbott, Abiomed, Boston Scientific, Getinge, Medtronic, MDStart, LivaNova, and precardiac. Dr Wyman reports consulting at Boston Scientific, and Abbott Vascular. Dr Lombardi is a consultant at Asahi, Boston Scientific, Medtronic, Teleflex, Abiomed, and Abbott Vascular and reports salary from Phillips (wife). Dr Tayal reports consulting at Abiomed. The other authors report no conflicts.
Supplementary Material
Footnotes
Nonstandard Abbreviations and Acronyms
- AHF
- acute heart failure
- ARMS
- Axillary Access Registry to Monitor Safety
- PCI
- percutaneous coronary intervention
- VARC
- Valve Academic Research Consortium
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.120.009657.
For Sources of Funding and Disclosures, see page 48.
Contributor Information
Amir A. Kaki, Email: amirkaki@hotmail.com.
Duane S. Pinto, Email: dpinto@bidmc.harvard.edu.
Ajay J. Kirtane, Email: ak189@cumc.columbia.edu.
William J. Nicholson, Email: lombaw@uw.edu.
J. Aaron Grantham, Email: jgrantham@saint-lukes.org.
R. Michael Wyman, Email: rmwcor@gmail.com.
Jeffery W. Moses, Email: jm2456@mail.cumc.columbia.edu.
Theodore Schreiber, Email: tlschreiber16@gmail.com.
Alexis K. Okoh, Email: disciple951@gmail.com.
Ranjith Shetty, Email: ranjith.shetty@carondelet.org.
Kapildeo Lotun, Email: klotun@yahoo.com.
William Lombardi, Email: lombaw@uw.edu.
Navin K. Kapur, Email: nkapur@tuftsmedicalcenter.org.
Raj Tayal, Email: dr.tayal@gmail.com.
References
- 1.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory supportincidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–1415. doi: 10.1016/j.jacc.2014.07.958 [DOI] [PubMed] [Google Scholar]
- 2.Dixon SR, Henriques JP, Mauri L, Sjauw K, Civitello A, Kar B, Loyalka P, Resnic FS, Teirstein P, Makkar R, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (the PROTECT I Trial): initial U.S. experience. JACC Cardiovasc Interv. 2009;2:91–96. doi: 10.1016/j.jcin.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 3.O’Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, Palacios I, Maini B, Mulukutla S, Dzavík V, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126:1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Hu W, Cai Z, Liu J, Wu J, Deng Y, Yu K, Chen X, Zhu L, Ma J, et al. Early mobilization of critically ill patients in the intensive care unit: a systematic review and meta-analysis. PLoS One. 2019;14:e0223185 doi: 10.1371/journal.pone.0223185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tayal R, Iftikhar H, LeSar B, Patel R, Tyagi N, Cohen M, Wasty N. CT angiography analysis of axillary artery diameter versus common femoral artery diameter: implications for axillary approach for transcatheter aortic valve replacement in patients with hostile aortoiliac segment and advanced lung disease. Int J Vasc Med. 2016;2016:3610705 doi: 10.1155/2016/3610705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estep JD, Cordero-Reyes AM, Bhimaraj A, Trachtenberg B, Khalil N, Loebe M, Bruckner B, Orrego CM, Bismuth J, Kleiman NS, et al. Percutaneous placement of an intra-aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long-term support as bridge to heart transplantation. JACC Heart Fail. 2013;1:382–388. doi: 10.1016/j.jchf.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 7.Nishida H, Ota T, Koda Y, Onsager D, Nguyen A, Chung B, Smith B, Kalantari S, Sarswat N, Kim G, et al. Ten year experience of axillary intra aortic balloon pump support for heart failure patients. J Am Coll Cardiol. 2020;75:803. [Google Scholar]
- 8.Lotun K, Shetty R, Patel M, Arain SA. Percutaneous left axillary artery approach for Impella 2.5 liter circulatory support for patients with severe aortoiliac arterial disease undergoing high-risk percutaneous coronary intervention. J Interv Cardiol. 2012;25:210–213. doi: 10.1111/j.1540-8183.2011.00696.x [DOI] [PubMed] [Google Scholar]
- 9.Tayal R, Barvalia M, Rana Z, LeSar B, Iftikhar H, Kotev S, Cohen M, Wasty N. Totally percutaneous insertion and removal of impella device using axillary artery in the setting of advanced peripheral artery disease. J Invasive Cardiol. 2016;28:374–380. [PubMed] [Google Scholar]
- 10.Mathur M, Hira RS, Smith BM, Lombardi WL, McCabe JM. Fully percutaneous technique for transaxillary implantation of the impella CP. JACC Cardiovasc Interv. 2016;9:1196–1198. doi: 10.1016/j.jcin.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 11.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. Eur Heart J. 2011;32:205–217. doi: 10.1093/eurheartj/ehq406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redfors B, Watson BM, McAndrew T, Palisaitis E, Francese DP, Razavi M, Safirstein J, Mehran R, Kirtane AJ, Généreux P. Mortality, length of stay, and cost implications of procedural bleeding after percutaneous interventions using large-bore catheters. JAMA Cardiol. 2017;2:798–802. doi: 10.1001/jamacardio.2017.0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Généreux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, Davidson CJ, Eisenhauer AC, Makkar RR, Bergman GW, et al. ; PARTNER Trial Investigators. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of Aortic Transcatheter Valve) trial. J Am Coll Cardiol. 2012;60:1043–1052. doi: 10.1016/j.jacc.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 14.Liang P, O’Donnell TFX, Swerdlow NJ, Li C, Lee A, Wyers MC, Hamdan AD, Schermerhorn ML. Preoperative risk score for access site failure in ultrasound-guided percutaneous aortic procedures. J Vasc Surg. 2019;70:1254–1262.e1. doi: 10.1016/j.jvs.2018.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahle TG, Kaneko T, McCabe JM. Outcomes following subclavian and axillary artery access for transcatheter aortic valve replacement: Society of the Thoracic Surgeons/American College of Cardiology TVT Registry Report. JACC Cardiovasc Interv. 2019;12:662–669. doi: 10.1016/j.jcin.2019.01.219 [DOI] [PubMed] [Google Scholar]
- 16.Jones TL, Kearney KE, McCabe JM. Prevalence and predictors of vascular thrombus formation after percutaneous axillary artery impella insertion. Circ Cardiovasc Interv. 2019;12:e008046 doi: 10.1161/CIRCINTERVENTIONS.119.008046 [DOI] [PubMed] [Google Scholar]
- 17.AbuRahma AF, Robinson PA, Boland JP, Umstot RK, Clubb EA, Grandia RA, Kennard W, Bastug DF. Complications of arteriography in a recent series of 707 cases: factors affecting outcome. Ann Vasc Surg. 1993;7:122–129. doi: 10.1007/BF02001005 [DOI] [PubMed] [Google Scholar]
- 18.Schäfer U, Ho Y, Frerker C, Schewel D, Sanchez-Quintana D, Schofer J, Bijuklic K, Meincke F, Thielsen T, Kreidel F, et al. Direct percutaneous access technique for transaxillary transcatheter aortic valve implantation: “the Hamburg Sankt Georg approach”. JACC Cardiovasc Interv. 2012;5:477–486. doi: 10.1016/j.jcin.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 19.Greenbaum AB, Babaliaros VC, Chen MY, Stine AM, Rogers T, O’Neill WW, Paone G, Thourani VH, Muhammad KI, Leonardi RA, et al. Transcaval access and closure for transcatheter aortic valve replacement: a prospective investigation. J Am Coll Cardiol. 2017;69:511–521. doi: 10.1016/j.jacc.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


