Abstract
Purpose
Few data are available on the positive impact of photo-biomodulation (PBM) using low-level laser therapy as a complementary treatment for improving the cognitive function and optimizing the hemoglobin (Hb) level and oxygen carrying capacity in anemic elderly patients and consequently improving the quality-of-life. The present study aimed to evaluate a new, safe, and easy therapeutic approach to improve Alzheimer’s disease-related symptoms that interfere with the whole life activities and social interaction of elderly patients.
Patients and Methods
In this placebo-controlled clinical trial, 60 elderly patients suffering from anemia and mild cognitive dysfunction were randomly assigned into two equal groups to receive active or placebo low-level laser in addition to a moderate-intensity aerobic exercise over a 12-week period. Hb level as well as cognitive and functional tests were reassessed for any change after 12 weeks of intervention.
Results
By the end of this study, both groups showed significant improvements in Hb level, Montreal Cognitive Assessment Scale (MoCa – B basic), Quality-of-Life for Alzheimer’s Disease scale, and Berg Balance scale scores along with significant reduction in body mass index (BMI) and waist–hip ratio (WHR) (P<0.0001). The experimental group which received active low-level laser in addition to moderate-intensity aerobic exercise showed more significant results compared to the control group which received placebo low-level laser in addition to moderate-intensity aerobic exercise in all the measured outcomes (P<0.001).
Conclusion
Combined low-level laser therapy and moderate-intensity aerobic exercises are more effective in improving the cognitive function and quality-of-life of Alzheimer’s disease patients.
Clinical Trial Registration
www.ClinicalTrials.gov, identifier NCT04496778.
Keywords: Alzheimer’s disease, cognition, laser, exercise, quality of life
Introduction
Aging is linked to structural and functional changes which appear over time. The elderly have normal age-related changes in multiple body systems; the brain is one of these systems where neurons are damaged and neurological functions deteriorate. Alzheimer’s disease with mild cognitive impairment is the border line between the normal decline of cognition by aging and serious condition of dementia1 which is characterized by a slowly decline in memory and thinking that accordingly evolves into language, orientation, mood, and self-care problems.2 Over 25 million people are suffering from this disease worldwide.3
There is no clear single cause of Alzheimer’s disease. Anemia can be counted as an independent risk factor of poor cognitive function,4,5 with high incidence of Alzheimer’s disease (about two-fold among anemic patients).6,7
Low hemoglobin level in the elderly affects red blood cells (RBCs) oxygen carrying capacity, leading to the inability of this capacity to meet the physical needs and changes of different body parts which in turn compromises the cognitive function.8
The limited effectivness of pharmacological therapy to treat an Alzheimer-related cognitive impairment course9 is what prompted researchers to develop more effective alternatives that delay the deterioration of the disease and alleviate its symptoms.10 Physical exercise has proved its protective role as a non-pharmacological treatment for dementia and cognitive impairment. Also, it has been proved that aerobic exercises strengthen cognitive functions by increasing brain vascularization, releasing neurotrophic substances,11,12 and improving hematologic indices reflected by the increase in RBCs mass and Hb.13,14
Photo-biomodulation (PBM) or low-level laser therapy (LLLT) is a spectacular safe technology which proved its beneficial effect in treating several conditions.15 It could play a critical role in treating neurodegenerative diseases such as Alzheimer's and psychiatric disorders16 with significant improvement in cognition tests.17
PBM via laser watch governs combined ways of non-invasive blood irradiation, wrist laser acupuncture, and nasal laser irradiation, which has a clear imprint as a neuroprotective approach in mild cognitive impairment, Alzheimer’s disease, cerebrovascular diseases, and depression through enhancing the cerebral metabolic activity and blood flow via anti-inflammatory and antioxidant pathways.18–20
Therefore, this study aimed to determine the effectiveness of PBM therapy and moderate aerobic exercises on the cognitive function in mild Alzheimer's anemic elderly patients.
Methods and Materials
Study Design and Sampling Setting
In this parallel, a two-group, randomized controlled trial which was conducted and reported according to CONSORT guidelines, 60 Alzheimer's anemic elderly patients (30 men and 30 women) aged from 65–75 years old were recruited from the outpatient clinics of the internal medicine department of Cairo University Hospital. The included patients wereanemic (with hemoglobin concentration <12 g/dL and 13 g/dL in women and men, respectively),21 patients with mild cognitive dysfunction defined by Montreal Cognitive Assessment scores (MoCa- B basic) (with score ranging from 19–25),22 body mass index (BMI) ranging from 30–34.9 kg/m2, as well as patients with disease duration not less than 6 months and time between diagnosis and participation in the study ranging from 6–24 months. Also, patients with a low level of physical activity defined by the categorical scoring of International Physical Activity Questionnaire (IPAQ)23 and those who completed their elementary education were included.
On the other hand, patients with moderate-to-severe cognitive impairment, history of neurological or cardiovascular diseases, history of diabetes, history of nasal bleeding or fracture, known photo-sensitivity disorders, concurrent active cancer, or within 1 year of cancer treatment or remission, and those with serious mental health illness precluding them from participation were excluded from the study. Moreover, patients taking medications that affect the hemoglobin level or psychological condition and those with active infection in addition to wound or other external trauma on the target area of laser therapy application were excluded. We also excluded current regular exercisers within the past month and participants in a clinical study or another type of research in the past 30 days.
A written informed consent was taken from each participant. The trial protocol was treated according to the principles of the Declaration of Helsinki. It was pre-registered (NCT04496778) and approved by the Faculty of Physical Therapy Ethics Committee for Scientific Research (P.T.REC/012/0027772).
Randomization
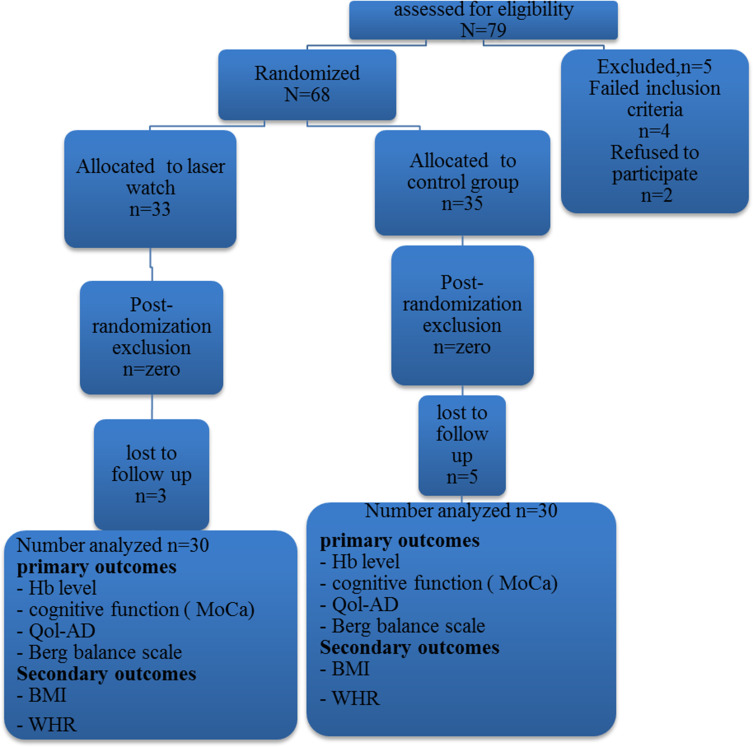
For randomization of this controlled study, all participants were divided into two equal groups; a control group and experimental group (Figure 1). A random table was used for randomization. A series of random numbers was selected from this table (from the first line, left to right) to assign each subject based on an odd or even number. An odd number assigned a subject into the control group (15 men), while an even number assigned a subject into the experimental group (15 men). The same procedure was applied for women participants, with a total number of 60 patients.
Figure 1.
Flow chart for the participants through the study showing out of 79, only 60 patients accomplished the procedures of the study.
Methods in Details
Before any intervention, the baseline data were collected for each participant eligible for this study; these data are (summarized in Table 1) including the general characteristics of the 60 included anemic elderly patients with Alzheimer's disease.
Table 1.
Basic Characteristics and Demographic Data of the Study Participants
| Characteristics | Control Group (N=30) | Study Group (N=30) | P-value* | ||
|---|---|---|---|---|---|
| Age (years) | Median (IQR) | 70.00 (67.00–72.00) | 69.50 (72.00–68.00) | 0.676 | |
| Range | 65.00–73.00 | 65.00–73.00 | |||
| BMI (kg/m2) | Median (IQR) | 32.04 (33.27–31.05) | 32.40 (34.13–31.48) | 0.115 | |
| Range | 30.17–34.21 | 30.40–34.70 | |||
| SBP (mmHg) | Median (IQR) | 148.00(153.00–141.00) | 145.00(148.00–141.00) | 0.241 | |
| Range | 140.00–156.00 | 140.00–156.00 | |||
| DBP (mmHg) | Median (IQR) | 94.00 (96.00–92.00) | 93.50 (95.00–92.00) | 0.686 | |
| Range | 90.00–98.00 | 90.00–98.00 | |||
| MoCA | Median (IQR) | 23.42 (23.93–22.78) | 23.96 (24.12–22.09) | 0.853 | |
| Range | 22.01–24.53 | 22.01–24.70 | |||
| Berg Balance Scale | Median (IQR) | 51.35 (52.07–50.62) | 51.42 (51.84–51.04) | 0.790 | |
| Range | 50.01–52.50 | 50.23–52.90 | |||
| Qol-AD | Median (IQR) | 37.30 (38.95–35.75) | 38.11(39.75–36.55) | 0.107 | |
| Range | 34.40–40.40 | 35.20–41.10 | |||
| Hb | Male | Median (IQR) | 10.70 (11.30–10.40) | 11.10 (11.70–10.70) | 0.105 |
| Range | 10.00–12.60 | 10.50–12.00 | |||
| Female | Median (IQR) | 9.80 (10.40–9.30) | 10.10 (10.60–9.70) | 0.085 | |
| Range | 9.00–10.70 | 9.40–11.00 | |||
| WHR | Male | Median (IQR) | 0.89 (0.90–0.88) | 0.90 (0.91–0.89) | 0.072 |
| Range | 0.87–0.91 | 0.89–0.91 | |||
| Female | Median (IQR) | 0.85 (0.90–0.81) | 0.84 (0.88–0.80) | 0.467 | |
| Range | 0.78–0.93 | 0.78–0.91 | |||
Notes: Data represented as median (IQR). * Statistically significant at P≤0.05 according to Mann–Whitney U-test.
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MoCA–B basic, Montreal Cognitive Assessment test for dementia; QOL-AD, Quality-of-Life in Alzheimer’s Disease; Hb, hemoglobin; WHR, waist-to-hip ratio; IQR, interquartile range (the difference between 3rd quartile (Q3) and 1st quartile (Q1)..
Primary outcomes involved the cognitive function and quality-of-life, while secondary outcomes involved hemoglobin and anthropometric measures.
Cognitive testing, quality-of-life assessment, and blood sample collection took place at baseline and after completion of the study (3 months).
Cognitive function assessment (via Montreal Cognitive Assessment scale- Model B): It is a valid tool for screening different cognitive domains among low educated elderly patients ranging from 0– 30. A score of 26 or above is normal.
- Quality of life assessment:
- Quality of Life for Alzheimer’s Disease scale: It comprises 13 items of a total score of 13–52, with higher scores indicating better quality-of-life.
- Berg Balance Assessment scale: It is used to determine the patient’s ability to maintain balance during a series of tasks suitable for elderly subjects, including a 14-item list; each item consists of a 5-point ordinal scale ranging from 0– 4, with 0 indicating the lowest level of function and 4 indicating the highest level of function.
Hemoglobin level (Hb): A blood sample was drawn by venipuncture from the right arm of each participant in a test tube containing anticoagulant (EDTA) using Biochemistry analyzer (Robonik Prietest Touch Biochemistry, India).
Body mass index (BMI): Body mass index was calculated by this equation (weight by kilogram/height per m2), . The results of BMI indicate: -less than 20=the patient is underweight; 20–25=the patient has desirable weight; 25–30=the patient is overweight; 30–35=the patient is obese; and more than 35=the patient is very obese.
Waist-to-hip ratio (WHR): WHR was measured through dividing the waist circumference by hip circumference. The patient is asked to stand up straight and breathe out. For measuring waist circumference, a tape was used to measure the distance around the smallest part of the waist, just above the belly button, while hip circumference, was measured at the widest parts of the buttocks.
Intervention Protocol
Patients in both groups carried out a moderate-intensity aerobic exercise using a treadmill 3 days/week for 45–60 minutes per session for 3 months. The moderate aerobic exercise intensity started by 40–50% HRR (determined by using a walking treadmill exercise test) during the first 6 weeks, then was increased gradually up to 50–70% HRR24 and maintained along the remaining duration of the study. The first 12 sessions were supervised closely, then a group supervision was carried out (one session per week). A simple warm-up and cooling down stretching of shoulders, arms, trunk, and hips was performed for 5–10 minutes.
Regarding PBM, both groups received low level laser irradiation via a laser watch (650 nm)- LASPOT, Wuhan, China). The experimental group received active radiation, while the control group received placebo. A PBM device25 radiates non-thermal infra-red laser therapy via nasal probe for simultaneous laser blood irradiation and wrist laser watch acupuncture which irradiates radial artery acupoints (Lieque Lu 7, Jingqu Lu 8, Taiyuan Lu 9), unlar artery acupoints (Lingdao He 4, Tongli He 5, Yinxi He 6, Shenmen He 7), and middle wrist acupoints (Neiguan PC 6, Daling PC 7) which were applied at maximal power for 30 minutes per session, 2-times/day, 3 days/week for 3 months (Figure 2).
Figure 2.

A study participant who received PBM via laser watch.
Sample Size Calculation
The sample size was estimated prior to the study using G*POWER statistical software (version 3.1.9.2; Franz Faul, Universitat Kiel, Germany) [t tests]. The means (difference between two independent means (two groups) was based on the d (effect size) of 0.73, α error prob=0.05, and 1-β error prob=0.8. The test revealed that the appropriate required sample size for this study is 60.
Statistical Analysis
After collecting data and checking for completeness and logical consistency, all analyses were performed with SPSS statistical software, version 25. Normal distribution of the measured data was examined by the Shapiro–Wilk test. Continuous data were described as median (IQR) for non-normally distributed variables (P<0.05). Baseline characteristics were compared between the two groups using Mann–Whitney U-test. Both Wilcoxon Signed Ranks (WSR) and Mann–Whitney U-tests were used to investigate the changes in variables before and after intervention as well as the differences between control and experimental groups, respectively. P-values less than or equal to 0.05 were considered statistically significant.
Results
The present study included a total of 60 Alzheimer's anemic elderly patients with mild cognitive impairment (50% males and 50% females); their median age was 70 (72–67.25) years and median BMI was 32.30 (33.58–31.22) kg/m2. Thirty patients served as the control group and received placebo low-level laser in addition to moderate-intensity aerobic exercise, while the remaining 30 patients were enrolled in the experimental group and received active low-level laser in addition to the same exercise regimen.
Baseline characteristics of both groups, including demographic data, anthropometric measurements, blood tests for Hb level, health-related quality-of-life, and cognitive function components were not significantly different (all P>0.05; Table 1).
In this study, statistically significant differences were observed between the values of BMI, MoCA–B basic score, Berg Balance scale score, QOL-AD, WHR, and Hb blood levels in both groups post-intervention compared to pre-intervention according to Wilcoxon signed-ranks test (WSR) (P<0.000).
Outcomes
Montreal Cognitive Assessment Scale (MoCA- B Basic) for Cognitive Function Assessment
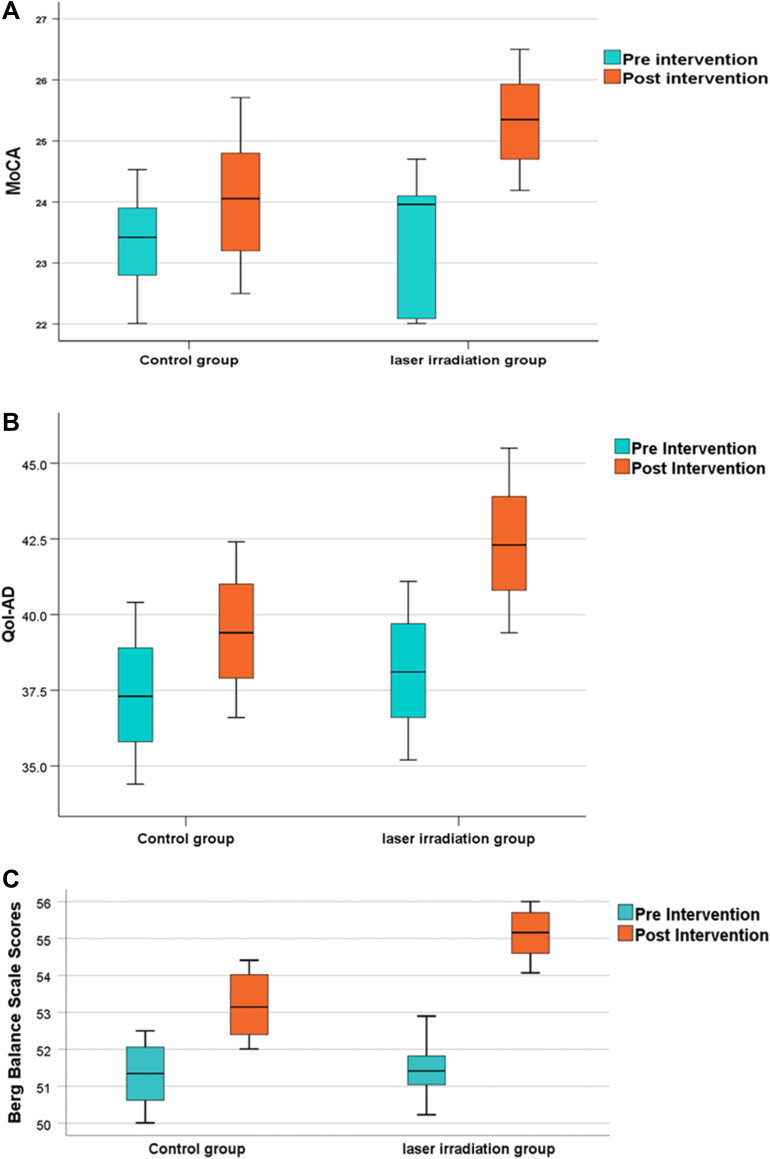
The analysis showed a significant increase in the scale of median scores in both groups (P<0.000) with 95% CI (−2.27, −1.74) and (−0.88, −0.54) in experimental and control groups, respectively
However, comparing the results of both groups revealed a significant increase in the experimental group compared to the control group (P<0.001), with 95% CI (−1.73, −0.86) (Figure 3A).
Figure 3.
(A) Boxplot chartshowing the post-Intervention change (improvement) in the median of Montreal Cognitive Assessment test score (MoCA) compared to the pre-intervention score within both,study and control groups Significant change occurred in study group more than the control group (P<0.001). (B) Boxplot chart showing the post-Intervention change (improvement) in the median of quality-of- life Qol-AD scores compared to the preintervention score within both study and control groups. Significant change occurred in study group more than the control group (P<0.001). (C) Boxplot chart showing the post-Intervention change (improvement) in the median of Berg balance scale scores compared to the pre-intervention score within both, study and control groups. The study group showed a significantly higher score than the control group (P<0.001).
Quality-of-Life Assessment
Quality-of-Life for Alzheimer’s Disease scale (QoL-AD) The data showed a significant increase in scale scores in both groups (P<0.0001), with 95% CI (−4.22, −4.20) and (−2.11, −2.08) in experimental and control groups respectively. Moreover, there was a statistically significant increase in the median scores of the experimental group compared to the control group (P<0.001), with 95% CI (−3.84, −1.97) (Figure 3B).
Berg Balance scale The scores of two groups were significantly elevated after intervention (P<0.0001), with 95% CI (−3.99, −3.47) and (−1.96, −1.81) in the experimental and control groups, respectively. Yet, the experimental group showed a significantly higher score than the control group (P<0.001), with 95% CI (−2.32- −1.55) (Figure 3C).
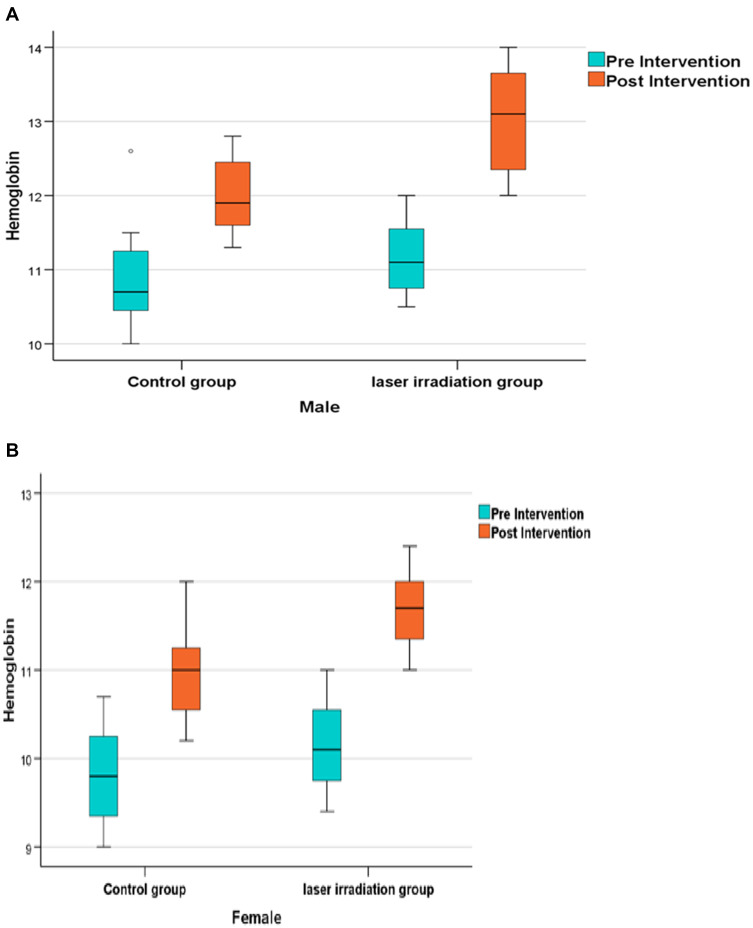
Blood Hb Level
There was a significant increase in blood Hb concentration in both elderly men and women patients after intervention in each group (P<0.0001), where significantly higher Hb levels were observed in all men (median=13.10 (13.70–12.30) (Figure 4A) and women (Figure 4B) (median=11.70 (12.00–11.30) within the experimental group compared to the men (median=11.90 (12.51–11.50)) and women (median=11.00 (11.30–10.50)) within the control group (P<0.001).
Figure 4.
(A) Boxplot chart showing the post-Intervention change (improvement) in the median of Hemoglobin (Hb) concentration compared to the pre-intervention concentration in males within both, studyand control groups. Significant change occurred in study group more than the control group (P<0.001). (B) Boxplot chart showing the post-Intervention change (improvement) in the median of Hemoglobin (Hb) concentration compared to the pre-intervention concentration in females within both study and control groups with a significant increase in HB concentration occurring in the study group compared to the control group (P<0.001).
(0.88, 2.38)
Anthropometric Measures
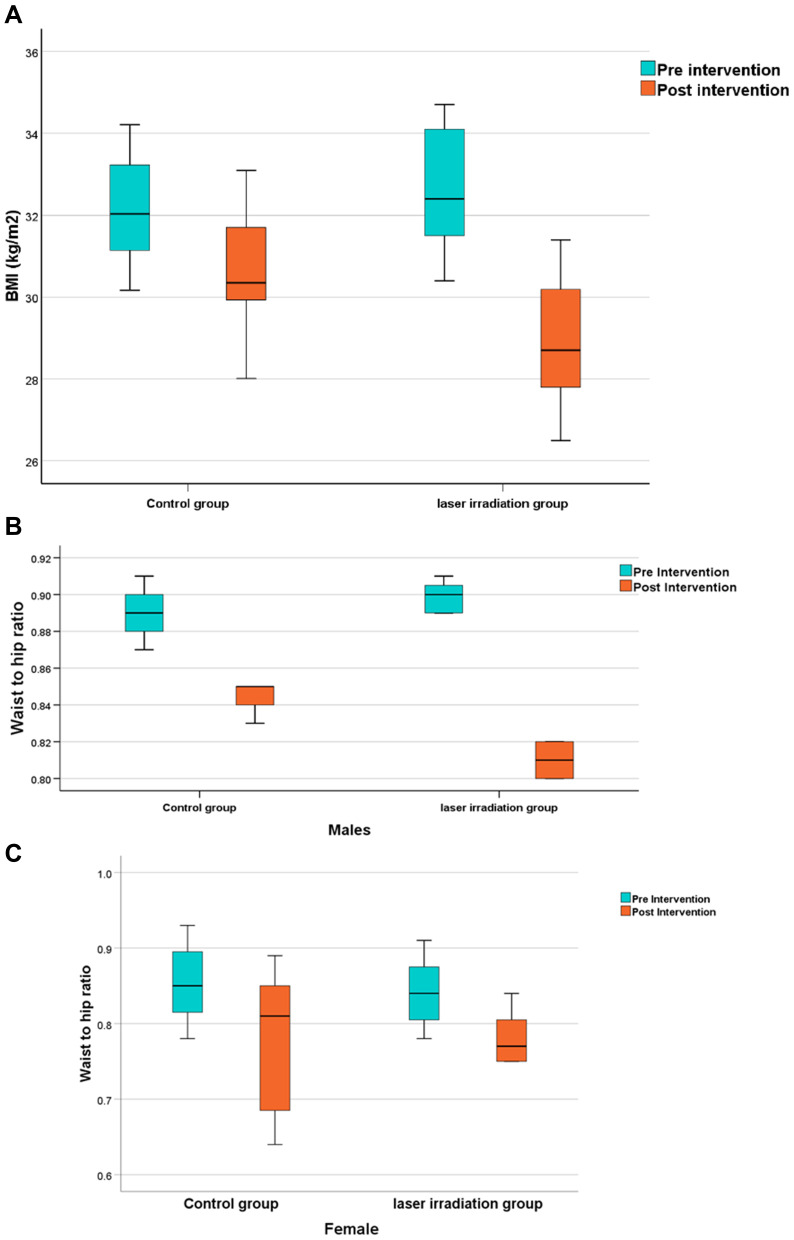
According to Mann–Whitney U-test, both BMI (Figure 5A) and WHR showed a significant reduction in both men (Median (IQR)=0.81 (0.82–0.80), Figure 5B) and women (median=0.77 (0.81–0.75), Figure 5C) after intervention in the experimental and control groups (P<0.0001). When comparing the two groups, a significant decrease was observed in BMI and WHR in the experimental group (men: median=0.85 (0.85–0.84)) compared to the control group (P<0.001), with 95% CI (0.88–2.38) and (0.03–0.04), respectively.
Figure 5.
(A) Boxplot chart showing the post-Intervention change (reduction) in the median of Body mass index (BMI) compared to the pre-intervention BMI level within both study and control groups. The study group showed a significantly lower BMI than the control group (P<0.001). (B) Boxplot chart showing the post-intervention change (reduction) in the median of waist-to-hip ratio (WHR) compared to the pre-intervention WHR in males level within both study and control groups, the study group exhibits a significant decrease compared to the control group (P<0.001). (C) Boxplot chart showing the post-intervention change (reduction) in the median of waist-to-hip ratio (WHR) compared to the pre-intervention WHR in females level within both study and control groups (P<0.001).
Interpretation
The current study concluded that photo-biomodulation low laser therapy and physical exercise offer a safe alternative to improve cognition among anemic Alzheimer elderly patients compared to traditional approaches.
Egypt, as one of the developing countries, has a remarkable growth in the number of older adults, representing around 94.8 million people in the 2017 census.26 As known, older adult subjects have high risk for mental and psychological disorders. Concerning the mental health professionals in Arab countries, Egypt only has approximately 70% of the total mentally ill Arab population.27
Searching for new non-pharmacological and safe treatments for cognitive impairment in anemic Alzheimer's elderly patients was our target in this study to improve their quality-of-life, which in turn lowers healthcare costs and delays the deterioration of disease symptoms. It is well established that pharmacological treatment outcomes for treating Alzheimer’s disease are still limited. Yet, aerobic exercise and PBM stand as a savior for better life and cognitive function among those patients. This is an initial study that examined the effect of combined PBM application in the form of acupuncture and nasal radiation on the cognitive function.
By the end of this study, we found marked improvements in the cognitive function (reflected by MoCA–B basic scores) and overall quality-of-life in the studied subjects after combined PBM application and moderate-intensity aerobic exercise. Similarly, other studies reported better cognition using different types of interventions. Christina et al28 employed a 12-week aerobic exercise program combined with music therapy and memory exercises, while Venturelli et al29 evaluated the effect of a walking program in a retirement home. On the other hand, multiple studies utilized different exercise modes for improving cognitive impairment30,31 in which improvements are observed in the cognitive and functional abilities of the included patients.
Also, Yu et al,32 Requena et al,33 and Yang et al34 noticed marked improvements in the cognitive function and AD symptoms after an aerobic exercise program. Aerobic exercises are also considered as a potent non-pharmacological intervention that delays the cognitive decline in high-risk subjects through increased total Hb and RBCs oxygen-carrying capacity. The results of this study are supported by many studies in literature which confirm the positive impact of exercise in enhancing cognitive and psychological functions.35,36
PBM, as a source of non-harmful methods for treating and preventing uncountable conditions, has become an acceptable approach, either for healthcare providers or patients themselves, to improve Alzheimer’s disease-related symptoms.
In addition to Javad et al,37 who concluded that PBM application had a significant role in neurorehabilitation, Gregory Hipskind 38 commented that pulsed transcranial photo-biomodulation therapy showed promise in enhancing both the cognitive function and cerebral blood flow several years after traumatic brain injury.
Considerable studies demonstrated the effectiveness of PBM therapy by different ways of application and wavelengths, either in human or animal models, on several conditions; however, the studies related to how laser therapy can improve the cognitive function and prevent depression and anxiety are still limited.39
PBM therapeutic effects are not fully covered, several data supported the idea of its effect on the mitochondrial function; either the direct or indirect effect. The direct effect is represented in increasing the activity of mitochondrial electron transport chain, leading to the increase of adenosine triphosphate (ATP)40 production which may enhance gene transcription and protein synthesis, while the indirect effect is represented in oxidating and activating the mitochondrial permeability transition pore which contributes to the displacement of nitric oxide (NO) from the cytochrome c oxidase (COX) molecule which in turn increases local blood flow.41
Our results revealed remarkable improvements in the cognitive function and health-related quality-of- life in anemic elderly patients suffering from Alzheimer’s disease after PBM application which is consistent with previous reports. This possible improvement may relay on the previously mentioned mechanism of PBM on cellular and molecular levels. Marvin et al42 revealed that PBM with near infrared light improves neuroplasticity; this was proved by EEG amplitude and improvement of connectivity measures.
Agnes et al's43 findings were in the line of our outcomes, showing that PBM may be considered as a supplementary intervention for improving or maintaining the cognitive function in older adults compared to the behavioral training that requires multiple sessions to show a significant effect and it may not be suitable for illiterate elderly subjects.44
Our results are consistent with the previous studies of Karger et al45 and Qiu et al,46 who demonstrated that low-level laser altered RBCs membrane permeability, enhancing ATP production, playing a critical role in RBCs deformability, and increased the viability of anemic RBCs.
LLLT has been proved to decrease the body fat and body mass index through various mechanisms by lysing of fat cells and lipid peroxidation.47 This has also been proved by many studies which were conducted to determine the potential improvements in body anthropometric measurements by low laser therapy.48,49 In the current study, BMI and WHR were noticed to be significantly decreased in both men and women after intervention.
This study is focusing on low-educated Alzheimer's elderly patients with low Hb level who exhibit cognitive impairment-related symptoms influencing their quality-of-life. Previous studies evaluated the effect of physical exercises or only one approach of PBM on cognitive impairment. Moreover, we examined the effect of combined approaches of low-level laser applied at the same time (nasal laser irradiation and wrist acupuncture) via laser watch in addition to a moderate-intensity aerobic exercise on these patients. This kind of intervention is the first one to be applied for improving cognitive function impairment in Alzheimer's elderly anemic patients to achieve healthy aging with optimal psychological condition.
It is worthy to point to the shortcomings of the current study. The first shortcoming is the lack of screening the effect of our approach on different grades of cognitive affection or the influences of the outcomes on various age groups. The second shortcoming is that other cognitive domains were not examined. Nevertheless, our study explores an area of inquiry which has not been widely investigated in Alzheimer's patients, particularly those with anemia. We recommend further studies on larger scales and to use other exercise intensities and different types of laser wavelengths, energy intensities, as well as irradiation modes, and durations for more affirmative results. Moreover, the benefits of this treatment protocol suggest that much wider use could be made in cases of both brain diseases and injuries.
Conclusion
Combined nasal laser irradiation and wrist acupuncture in addition to moderate-intensity aerobic exercise may exhibit an alternative, safe, and easy way to improve cognition among anemic mild Alzheimer's elderly patients compared with the control group using the same line of exercise only. More research should be conducted to determine the exact role of PBM in improving cognitive functions.
Acknowledgments
The authors thank Ebtehal Mohamed for her assistance in data analysis and interpretation. We would like to thank all participants who joined in this study.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
No potential conflict of interest was reported by the authors.Financial disclosure: There was no financial support for the research and publication of this article.
References
- 1.Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158 [DOI] [PubMed] [Google Scholar]
- 2.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9 [DOI] [PubMed] [Google Scholar]
- 3.McPherson S, Schoephoester G. Screening for dementia in a primary care practice. Minn Med. 2012;95(1):36–40. [PubMed] [Google Scholar]
- 4.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atti AR, Palmer K, Volpato S, Zuliani G, Winblad B, Fratiglioni L. Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiol Aging. 2006;27(2):278–284. doi: 10.1016/j.neurobiolaging.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 6.Beghe CWA, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(7):35–109. doi: 10.1016/j.amjmed.2003.12.009 [DOI] [PubMed] [Google Scholar]
- 7.Vinay K, Abul K. Robbins Basic Pathology. 8th ed. Philadelphia.Saunders; 2007:16. [Google Scholar]
- 8.Argyriadou S, Vlachonikolis I, Melisopoulou H, Katachanakis K, Lionis C. In what extent anemia coexists with cognitive impairment in elderly: A cross-sectional study in Greece. BMC Fam Pract. 2001;2(1):5. doi: 10.1186/1471-2296-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson CA, Spilsbury K, Hall J, Birks Y, Barnes C, Adamson J. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatrics. 2007;7(1):18. doi: 10.1186/1471-2318-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med. 2009;43:22–24. doi: 10.1136/bjsm.2008.052498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol. 2010;30(4):493–503. doi: 10.1007/s10571-009-9488-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009;28(Suppl. 6):690. doi: 10.1037/a0016136 [DOI] [PubMed] [Google Scholar]
- 13.Otto JM, Montgomery HE, Richards T. Haemoglobin concentration and mass as determinants of exercise performance and of surgical outcome. Extrem Physiol Med. 2013;2(1):33. doi: 10.1186/2046-7648-2-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azarbayjani MA, Fathi R, Daloii AA, Abdi A, Fatolahi H. Acute hematological profile response to one session of aerobic and anaerobic exercise among young male kickboxers. Türk Fiz Tip Rehab Derg. 2014;60:92–97. [Google Scholar]
- 15.Gupta A, Avci P, Sadasivam M, et al. Shining light on nanotechnology to help repair and regeneration. Biotechnol Adv. 2013;31(5):607–631. doi: 10.1016/j.biotechadv.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang J, Ren Y, Wang R, Li C, Wang Y, Ping Chu X. Transcranial low-level laser therapy for depression and Alzheimer’s disease. Neuropsychiatry. 2018;8:477–483. [Google Scholar]
- 17.Gonzalez-Lima F, Barrett DW. Augmentation of cognitive brain functions with transcranial lasers. Front Syst Neurosci. 2014;8:36. doi: 10.3389/fnsys.2014.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashmi JT, Huang -Y-Y, Osmani BZ, Sharma SK, Naeser MA, Hamblin MR. Role of low-level laser therapy in neurorehabilitation. PM R. 2010;2(Suppl 12):S292–305. doi: 10.1016/j.pmrj.2010.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma SK, Kharkwal GB, Sajo M, et al. Dose response effects of 810 nm laser light on mouse primary cortical neurons. Lasers Surg Med. 2011;43(8):851–859. doi: 10.1002/lsm.21100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma W-J, Li X-R, Li Y-X, Xue Z-X, Yin H-J, Ma H. Antiinflammatory effect of low-level laser therapy on Staphylococcus epidermidis endophthalmitis in rabbits. Lasers Med Sci. 2012;27(3):585–591. doi: 10.1007/s10103-011-0991-1 [DOI] [PubMed] [Google Scholar]
- 21.WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. [Google Scholar]
- 22.Ihle-Hansen H, Vigen T, Berg T, et al. Montreal Cognitive Assessment in a 63- to 65-year-old Norwegian Cohort from the General Population: data from the Akershus Cardiac Examination 1950 Study. Dementia Geriatric Cognitive Dis Extra. 2017;7(3):318–327. doi: 10.1159/000480496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.M??der U, Martin BW, Schutz Y, Marti B. Validity of four short physical activity questionnaires in middle-aged persons. Med Sci Sports Exerc. 2006;38(7):1255–1266. doi: 10.1249/01.mss.0000227310.18902.28 [DOI] [PubMed] [Google Scholar]
- 24.Hagberg JM. Exercise assessment of arthritic and elderly individuals. Baillieres Clin Rheumatol. 1994;8(1):29–52. doi: 10.1016/S0950-3579(05)80223-7 [DOI] [PubMed] [Google Scholar]
- 25.Litscher G, Litscher D. A laser watch for simultaneous laser blood irradiation and laser acupuncture at the wrist. Integrative Med Int. 2016;3(1–2):75–81. doi: 10.1159/000448099 [DOI] [Google Scholar]
- 26.Hermez J, Khattabi H, Sabry A, Riedner G, Hajjeh R. Achieving the Sustainable Development Goal 3: challenges in HIV testing in the Eastern Mediterranean Region. East Mediterr Health J. 2017;23(10):647–648. doi: 10.26719/2017.23.10.647 [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim A. Arab World Psychology In: Keith KD, editor The Encyclopedia of Cross‐Cultural Psychology. 2013. [Google Scholar]
- 28.Christina K, Paris I, Eftychia K. Eleftherios K Effects of a 12-week aerobic exercise program combined with music therapy and memory exercises on cognitive and functional ability in people with middle type of Alzheimer’s disease. Int J Physiother. 2017;4(5):262–268. [Google Scholar]
- 29.Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen. 2011;26(5):381–388. doi: 10.1177/1533317511418956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolland Y, Rival L, Pillard F, et al. Feasibility [corrected] of regular physical exercise for patients with moderate to severe Alzheimer disease. J Nutr Health Aging. 2000;4(2):109–113. [PubMed] [Google Scholar]
- 31.Requena C, López Ibor MI, Maestú F, Campo P, López Ibor JJ, Ortiz T. Effects of cholinergic drugs and cognitive training on dementia. Dementia Geriatric Cognitive Dis. 2004;18(1):50–54. doi: 10.1159/000077735 [DOI] [PubMed] [Google Scholar]
- 32.Yu F, Bronas UG, Konety S, et al. Effects of aerobic exercise on cognition and hippocampal volume in Alzheimer’s disease: study protocol of a randomized controlled trial (The FIT-AD trial). Trials. 2014;15:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuzono K, Hishikawa N, Takao Y. Combination benefit of cognitive rehabilitation plus donepezil for Alzheimer’s disease patients. Geriatrics Gerontology Int. 2016;16(2):200–204. doi: 10.1111/ggi.12455 [DOI] [PubMed] [Google Scholar]
- 34.Yang S-Y, Shan C-L, Qing H, et al. The Effects of aerobic exercise on cognitive function of Alzheimer’s disease patients. CNS Neurol Disord Drug Targets. 2015;14(10):1292–1297. doi: 10.2174/1871527315666151111123319 [DOI] [PubMed] [Google Scholar]
- 35.Stimpson NJ, Davison G, Javadi A-H. Joggin’ the noggin: towards a physiological understanding of exercise-induced cognitive benefits.Neurosci Biobehavioral Rev. 2018;88:177–186. doi: 10.1016/j.neubiorev.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 36.Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52(3):154–160. doi: 10.1136/bjsports-2016-096587 [DOI] [PubMed] [Google Scholar]
- 37.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gregory Hipskind S, Grover FL, Fort TR. Pulsed Transcranial Red/Near-Infrared Light Therapy Using Light-Emitting Diodes Improves Cerebral Blood Flow and Cognitive Function in Veterans with Chronic Traumatic Brain Injury: A Case Series. Photomed Laser Surg. 2019;37(2):77–84. doi: 10.1089/photob.2018.4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naeser MA, Zafonte R, Krengel MH, et al. Significant Improvements in Cognitive Performance Post-Transcranial, Red/Near-Infrared Light-Emitting Diode Treatments in Chronic, Mild Traumatic Brain Injury: open-Protocol Study. J Neurotrauma. 2014;31(11):1008–1017. doi: 10.1089/neu.2013.3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Lasers Med Sci. 2014;5(2):58–62. [PMC free article] [PubMed] [Google Scholar]
- 41.Leung MCP, Lo SCL, Siu FKW, So K-F. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg Med. 2002;31(4):283–288. doi: 10.1002/lsm.10096 [DOI] [PubMed] [Google Scholar]
- 42.Berman MH, Halper JP, Nichols TW, H J, Lundy A, Huang JH. Photobiomodulation with Near Infrared Light Helmet in a Pilot, Placebo Controlled Clinical Trial in Dementia Patients Testing Memory and Cognition. J Neurol Neurosci. 2017;8(1):176. doi: 10.21767/2171-6625.1000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan AS, Lee TL, Yeung MK, Hamblin MR. Photobiomodulation improves the frontal cognitive function of older adults. Int J Geriatr Psychiatry. 2019;34(2):369–377. doi: 10.1002/gps.5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anguera JA, Boccanfuso J, Rintoul JL, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97‐101. doi: 10.1038/nature12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karger R, Lukow C, Kretschmer V. Deformability of Red Blood Cells and Correlation with ATP Content during Storage as Leukocyte-Depleted Whole Blood. Transfusion Med Hemotherapy. 2012;39(4):277–282. doi: 10.1159/000339809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu X, Huang H, Huang Z, Zhuang Z, Guo Z, Liu S. Effect of Red Light-Emitting Diodes Irradiation on Hemoglobin for Potential Hypertension Treatment Based on Confocal Micro-Raman Spectroscopy. Scanning. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avci P, Nyame TT, Gupta GK, Sadasivam M, Hamblin MR. Low-level laser therapy for fat layer reduction: a comprehensive review. Lasers Surg Med. 2013;45(6):349–357. doi: 10.1002/lsm.22153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Croghan IT, Ebbert JO, Schroeder DR, Hurt RT, Hagstrom V, Clark MM. A randomized, open-label pilot of the combination of low-level laser therapy and lorcaserin for weight loss. BMC. 2016;3(1):42. doi: 10.1186/s40608-016-0122-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Croghan IT, Hurt RT, Schroeder DR, et al. Low-level laser therapy for weight reduction: a randomized pilot study. Lasers Med Sci. 2020;35(3):663–675. doi: 10.1007/s10103-019-02867-5 [DOI] [PubMed] [Google Scholar]