Abstract
Background
Chemoresistance contributes to treatment failure of gastric cancer (GC) patients but the molecular mechanism of chemoresistance in GC is still unclear. Long-chain noncoding RNA (lncRNA) urothelial cancer associated 1 (UCA1) is associated with resistance to chemotherapy drugs.
Methods
We detected the expression of UCA1 in 53 pairs of GC tumor tissue and adjacent normal tissue, human normal gastric mucosa cells (GES-1) and human GC cells (HGC-27, SNU-5, AGS, SGC-7901, and NCI-N87) using RT-qPCR. Small RNA interference technology was used to knock down the expression of UCA1 in gastric cancer cells. CCK8 solution was used to detect cell viability. Flow cytometry was used to detect apoptosis, and Western blotting was used to detect protein expression.
Results
UCA1 was highly expressed in GC tissues and cells, and knockdown of UCA1 increased chemosensitivity to cisplatin by inducing cell apoptosis. Furthermore, UCA1 promoted CYP1B1 expression by binding to miR-513a-3p in human GC cells in vitro, and UCA1/CYP1B1 expression was negatively related to miR-513a-3p expression, while UCA1 expression was positively related to CYP1B1 expression in human GC tissues. Moreover, overexpression of miR-513a-3p or knockdown of CYP1B1 increased chemosensitivity to cisplatin, and knockdown of miR-513a-3p or overexpression of CYP1B1 decreased chemosensitivity to cisplatin by inducing cell apoptosis in human GC cells. Importantly, overexpression of CYP1B1 reduced chemosensitivity to cisplatin which increased by knockdown of UCA1, and knockdown of CYP1B1 increased chemosensitivity to cisplatin which decreased by knockdown of miR-513a-3p in human GC cells.
Conclusion
The lncRNA UCA1/miR-513a-3p/CYP1B1 axis regulates cisplatin resistance in human GC cells; hence, it is a potential target for treating chemoresistance in GC.
Keywords: gastric cancer, urothelial cancer associated 1, chemoresistance
Introduction
Gastric cancer (GC) is the fourth most common cancer in the world, accounting for an estimated 951,600 new GC cases and 738,000 deaths annually worldwide in 2012.1,2 In Europe, the mortality of GC patients ranks fourth among all cancer patients, and about 107,000 GC patients died in 2012.2,3 In China, the incidence of GC is the second highest compared to all malignant cancers, with approximately 679,000 new cases of GC and 498,000 deaths every year, almost half of the world.4,5 Unfortunately, most GC patients are diagnosed at a late stage due to the lack of effective techniques for early diagnosis.6 Chemotherapy is a common therapy used in the treatment of GC but the resistance to chemotherapy drugs reduces the efficacy of chemotherapy.7,8 Therefore, it is important to study the molecular mechanism of chemoresistance to prolong disease-free survival and the overall survival of GC patients.
Long-chain noncoding RNA (lncRNA) is a single-stranded noncoding RNA 200 nucleotides in length and was originally considered to be “junk” RNA, but in recent years, has been shown to be involved in chemoresistance, epigenetic regulation, cell cycle regulation, and cell differentiation regulation.9,10 Urothelial cancer associated 1 (UCA1), a lncRNA, is reported to be upregulated in bladder cancer, colorectal cancer, ovarian cancer, and breast cancer,11,12 and promotes cancer cell proliferation, invasion, migration, and chemoresistance.13,14 In recent studies, UCA1 was reported to promote the progression of paclitaxel resistance in ovarian cancer by regulating the miR-654-5p/SIK2 axis,15 also enhancing sensitivity to oncolytic vaccinia virus by sponging miR-18a/miR-182 and modulating the Cdc42/filopodia axis in colorectal cancer.16 Similar findings have also been reported in bladder cancer,17 breast cancer,18 ovarian cancer,19 and lung cancer,20 namely UCA1 enhanced chemoresistance in cancer.21 However, the effect of UCA1 on chemoresistance in GC is still unclear.
According to the sequencing data in the Gene Expression Omnibus (GEO) database (GSE53137),22 Gu et al identified differentially expressed lncRNAs by analyzing the expression profiles of lncRNAs and mRNAs in GC tissues and adjacent normal tissues using microarray, finding UCA1 was a highly expressed lncRNA in GC tissues.22 In the present study, we detected UCA1 expression in 53 pairs of GC tumor tissue and adjacent normal tissue and knocked down UCA1 to study the effect of UCA1 on the chemoresistance in GC cells. Our research confirms for the first time that UCA1 enhances cisplatin resistance by regulating CYP1B1-mediated apoptosis via miR-513a-3p in human GC.
Patients and Methods
Patients and Tissues
Samples of 53 pairs of GC tumor tissue and adjacent normal tissue were collected at the Affiliated Hospital of Inner Mongolia Medical University. The donor GC patients did not receive any treatment (chemotherapy, radiotherapy, immunotherapy or targeted therapy, etc) before donating the tissue and had not been diagnosed with other tumors, having completed three years of follow-up. All patients signed an informed consent, and this study was approved by the Affiliated Hospital of Inner Mongolia Medical University Ethics Committee (Approval No. YJ(2020001)).
Quantitative Real-Time PCR Analysis
Cells and tissues were lysed using RNAiso plus (9109, Takara, Japan), and phenol-chloroform/isopropanol was used to extract total RNA from the cells.19,20 After preparing the cDNA using a PrimeScript RT reagent kit with gDNA eraser (RR047A, Takara, Japan), 20 μL of the qPCR system was prepared and analyzed using of GoTaq qPCR Master Mix (A6001, Promega, USA) according to the manufacturer’s instructions. The relative gene expression was calculated by the 2–ΔΔCt method, with β-actin used as a control for mRNA, and U6 as a control for miRNA. The primers used are shown in Table 1.
Table 1.
Sequence of Primers for qPCR, siRNA and miRNA Regulation
| Primers Used in qPCR Analysis (5ʹ-3ʹ) | |
| UCA1 | Forward: CTCTCCATTGGGTTCACCATTC |
| Reverse: GCGGCAGGTCTTAAGAGATGAG | |
| miR-513a-3p | Forward: TAAATTTCACCTTTCTGAGAAGG |
| Reverse: GCGAGCACAGAATTAATACGAC | |
| CYP1B1 | Forward: ACGTACCGGCCACTATCACT |
| Reverse: CTCCCCACGACCTGATCCA | |
| U6 | Forward: AUAAAUCCCUUUACACCUCTT |
| Reverse: AAUAAAUCCCUUUACACCUCTT | |
| β-actin | Forward: AGCCCATCCTTCGAGTACAAA |
| Reverse: TCTTGGTGCGATAACTGGTGG | |
| si-RNA sequence (5ʹ-3ʹ) | |
| si-NC | CAGAUGGCCACCAGUAACUGU |
| si-UCA1-1 | AGUUAAAGGUGGUUAUCUGUU |
| si-UCA1-2 | ACAGUUAAAGGUGGUUAUCUG |
| si-UCA1-3 | UAUAGAAGACCACCUAAACAG |
| si-CYP1B1 | |
| miRNA regulate sequence (5ʹ-3ʹ) | |
| miR-513a-3p-NC | AUUCGGGUUAAGCUAGAAGGUUC |
| miR-513a-3p-mimic | UAAAUUUCACCUUUCUGAGAAGG |
| miR-513a-3p-inhibitor | UCGAAGUGUCCCUCCACAGUAGU |
Cell and Cell Culture
GES-1, a normal gastric mucosa cell, was purchased from Otwobiotech and SNU-5, AGS, and NCI-N87 cells were purchased from the American Type Culture Collection. HCG-27 and SGC-7901 were purchased from the Cell Bank of the Chinese Academy of Sciences. All cells were cultured in DMEM (61870044, Thermo Fisher, USA) with 10% fetal bovine serum (10437028, Thermo Fisher, USA) at 37°C with 5% CO2.
Cell Transfection
For miRNA and siRNA, 50 nmol/L of siRNA was directly transfected into 2.5×106 GC cells using Lipofectamine 2000 according to the protocol of manufacturer. After 72 h, the wild type (WT) or mutated (MUT) versions of the 3ʹ-UTR of UCA1 and CYP1B1 were cloned into pisCHECK2 (97157, Addgene, USA), then transfected into cells as si-RNA using the Dual-Lucy Assay kit (D00100, Solarbio, China) to detect luciferase activity following the protocol of manufacturer. After 72 h, the gene expression was determined by reverse transcription-quantitative qPCR.
For the overexpression of CYP1B1, we firstly cloned the gene sequence of CYP1B1 into pcDNA3.1 plasmid (VT1001, Youbao Biotechnology Co., Ltd, China), and transferred the recombinant plasmid into GC cells using Lipofectamine 2000. After 72 h, cells were cultured with 200 μg/mL G418 for seven days, puromycin resistant cells were set for experiments. The CYP1B1 protein expression was determined by immunoblotting.
Fluorescence in situ Hybridization
Cells (1×105) were seeded into Lab-Tek chambered (155411, Thermo Scientific, USA) and cultured for 24 h at 37°C with 5% CO2. The cell culture medium was removed and cells were fixed with 4% paraformaldehyde for 10 min at room temperature, then blocked with 5% BSA for one hour at room temperature. After fixing and blocking, cells were incubated with a fluorescent probe that binds to the human version of the UCA1 gene which was synthesized by Genomeditech Co., Ltd. The cell nuclei were counterstained with 5 μg/mL DAPI for five minutes at room temperature before analysis by confocal microscopy.
Cell Viability Assay
Cells (0.2×105) were seeded into a 96-well culture plate at 37°C with 5% CO2. Twelve hours later, the cell culture medium was changed to a medium containing cisplatin (BP809, Merck, Germany). After 24 h, 10 μL CCK8 solution (C0009, Beyotime Scientific, China) was added to 100 μL cell culture medium for one hour at 37°C with 5% CO2 before the absorbance was measured at 450 nm.
Cell Apoptosis Assay
Cells (2×106) were seeded into a six-well culture plate at 37°C with 5% CO2. Twelve hours later, the cell culture medium was changed to a medium containing cisplatin (BP809, Merck, German). After 24 h, the cells were harvested, washed with cold PBS, fixed with cold 70% ethanol at 4°C for one hour. The cells were resuspended in cold PBS after removal of the ethanol by centrifugation. As per the manufacturer’s instructions (E606336, Sangon Biotech), 15 min after addition of the labeling reagent, the cells were harvested and resuspended in PBS for detection.
Immunoblotting
The total protein was extracted from cells using the Nuclear/Cytosol Fractionation Kit (Biovision, USA) and the protein concentration was determined using a BCA kit (Thermo Fisher, USA). Then, 40 μg total protein was separated using 10% SDS-PAGE. After transfer, the PVDF membranes (Thermo Fisher) were blocked with 5% skimmed milk powder, then probed with primary antibodies against cleaved caspase-3, caspase-3, and CYP1B1. Proteins were visualized with ECL solution (Beijing Xinjingke Biotechnologies Co., Ltd, China), followed by densitometry analysis using Image J 3.0 (IBM, USA) and β-actin was loaded as a control. All antibodies used for immunoblotting testing were purchased from Abcam and diluted according to the manufacturer’s instructions.
Statistical Analysis
GraphPad prism 5 software was used to analyze the data, which was expressed as mean ±SD or percentage. One-way ANOVA with Turkey's post hoc test was used to compare the differences between multiple groups, and Student’s t-test was used to analyze the differences between the two groups. The log rank test was used to compare the differences in the three-year overall survival rate of different GC patients basing on UCA1 expression. A value of p<0.05 indicated a significant difference.
Results
UCA1 is Highly Expressed in Human GC Cells and Tissues
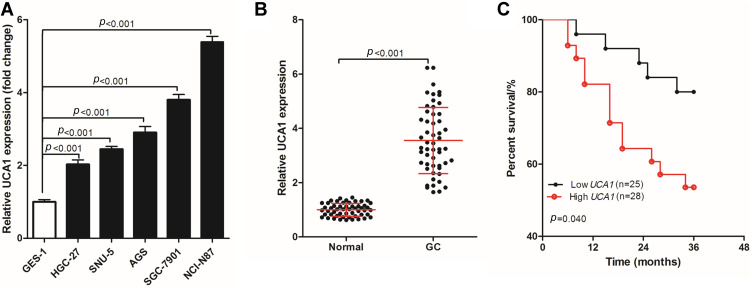
UCA1 was highly expressed in six cases of human GC tissues. To further verify the expression of UCA1 in GC tissues, UCA1 expression was detected in human normal gastric mucosa cells (GES-1) and human GC cells (HGC-27, SNU-5, AGS, SGC-7901, and NCI-N87). The expression of UCA1 in human GC cells was significantly higher than that in GES-1 cells (Figure 1A). Then, UCA1 expression was determined using qPCR in 53 pairs of GC tumor tissue and adjacent normal tissue samples (Figure 1B), showing that the expression of UCA1 in human GC tissues was significantly higher than in the adjacent normal tissue. In addition, the 53 GC patients were divided into two groups based on UCA1 expression, namely, low UCA1 expression group (n=25, UCA1 expression < the mean of UCA1 expression) and high UCA1 expression (n=25, UCA1 expression ≥ the mean of UCA1 expression). A comparison of the three-year overall survival rate of the two groups (Figure 1C) revealed that 80.0% (20/25) of GC patients with low UCA1 expression were still alive after three years, while only 53.57% (15/28) of GC patients with high UCA1 expression were still alive, and this difference in three-year survival was significant (p=0.040).
Figure 1.
UCA1 expression in human gastric cancer cells and tissues. (A-B) qPCR was used to detect the expression of UCA1 in human gastric mucosal cells (GES-1) and human gastric cancer cells (HGC-27, SNU-5, AGS, AGS and NCI-N87) (A), in human gastric cancer tissues and normal tissues (B, C) log rank test was used to compare the difference of the three-year overall survival rate of different gastric cancer patients basing on UCA1 expression. UCA1 expression was shown as mean ±SD, and three-year overall survival rate was shown as a percentage;. p-value was calculated by post hoc comparisons in (A and B) and by log rank test in (C).
UCA1 Knockdown Decreases Resistance to Cisplatin in Human GC Cells
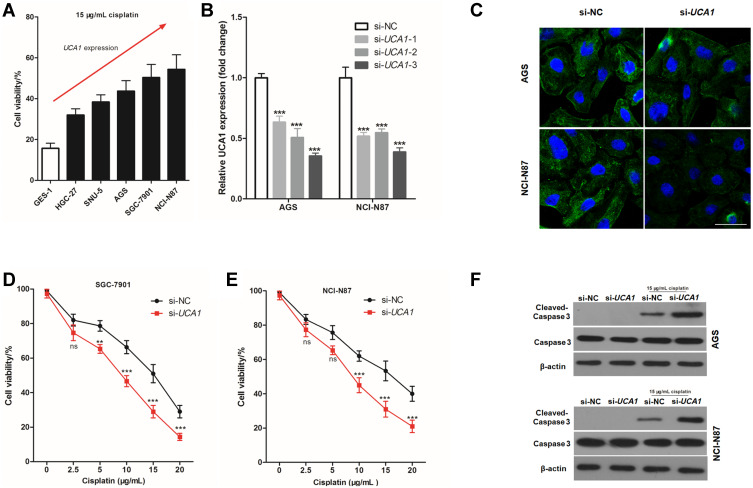
To further study the effect of UCA1 expression on the cisplatin resistance, 15 μg/mL cisplatin was used to stimulate human normal gastric mucosa cells, and human GC cells for 24 h. The cell viability increased with increased UAC1 expression (Figure 2A), hence, it was hypothesized that UAC1 enhances cisplatin resistance of GC cells. To test this hypothesis, small interfering RNA of UCA1 (si-UCA1) was transfected into GC cells to knock down the expression of UCA1. As shown in Figure 2B, the best interfering effect of UCA1 was si-UCA1-3, which reduced UCA1 expression to 35.3% in AGS cells and 38.7% in NCI-N87 cells. Hence, si-UCA1-3 was used to knock down UCA1 expression in AGS and NCI-N87 cells in the following experiments and abbreviated si-UCA1-3 as si-UCA1. FISH staining was performed to assess the expression of UCA1, showing that si-UCA1 si-UCA1 did knockdown UCA1 expression in AGS cells and NCI-N87 cells (Figure 2C).
Figure 2.
UCA1 knockdown enhances the toxicity of cisplatin to gastric cancer cells. (A) CCK8 kit was used to detect the cell viability after stimulating with 15 μg/mL cisplatin; (B–C) Small interfering RNA-UCA1 (si-UCA1) successfully knocked down UCA1 expression in gastric cancer cells verified by qPCR (B) and cellular immunofluorescence (C); (D–E) UCA1 knockdown by si-UCA1 increased the toxicity of cisplatin to AGS (D) and NCI-N87 (E, F) immunoblotting was used to detected the expression of caspase-3 and cleaved caspase-3 protein in human gastric cancer cell. Each experiment was repeated three times independently, ns was p>0.05, **p<0.01, ***p<0.001 vs si-NC group. The cell viability and UCA1 expression were shown as mean ±SD, and p-value was calculated by post hoc comparisons in (B) and by Student’s t-est in (D and E). Scale bar=50 μm.
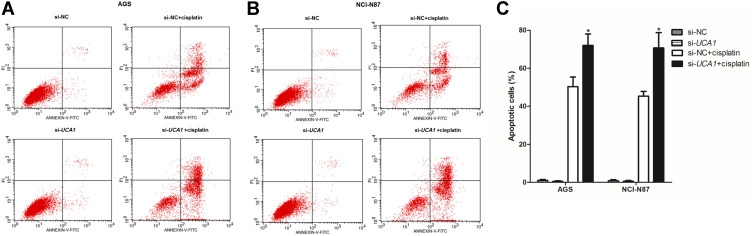
Next, we assessed the effect of UCA1 expression on the toxicity of cisplatin to human GC cells in vitro, showing that UCA1 knockdown not only decreased the cell viability of AGS and NCI-N87 cells after stimulation with 15 μg/mL cisplatin (Figure 2D and E), but also increased the expression of cleaved caspase-3 protein (Figure 2F). Importantly, UCA1 knockdown increased cisplatin-induced apoptosis in AGS and NCI-N87 cells (Figure 3).
Figure 3.
UCA1 knockdown enhances cisplatin induced apoptosis in human gastric cancer cells. After transferring the si-UCA1, human gastric cancer cells were stimulated with or without 15 μg/mL cisplatin, and then cells were harvested to detect apoptosis by flow cytometer, and the representative images were shown in (A and B), and the rates of apoptosis cells were statistically compared (C). Each experiment was repeated three times independently. The rates of apoptosis cell were shown as mean ±SD, *p<0.001 vs si-NC+cisplatin group and the p-value was calculated by Student’s t-test.
UCA1 Promotes CYP1B1 Expression by Binding to miR-513a-3p
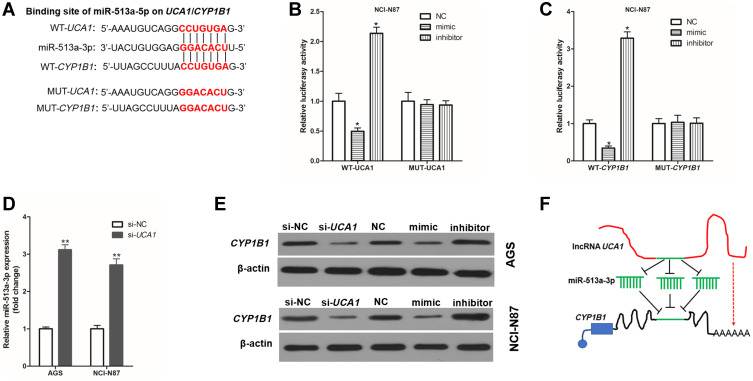
To investigate the molecular mechanism by which UCA1 regulates the resistance of cisplatin in human GC cells, the public databases (USCS, miRbase, and BiBiserv2) were searched. Interestingly, we found that miR-513a-3p had the same binding sites to UCA1` and CYP1B1 (Figure 4A). The luciferase gene report results showed that overexpression of miR-513a-3p by transfection into miR-513a-3p-mimic (mimic) could decrease and knockdown of miR-513a-3p by transfection into miR-513a-3p-inhibitor (inhibitor) could increase the relative luciferase activity in NCI-N87 cells, which were transferred into WT-UCA1 (Figure 4B) or WT-CYP1B1 (Figure 4C), but could not affect the relative luciferase activity in NCI-N87 cells which were transfected into MUT-UCA1 or MUT-CYP1B1. This suggested miR-513a-3p targeted inhibition of UCA1 and CYP1B1 expression in human GC cells. Interestingly, we also found that UCA1 knockdown could significantly decrease the expression of miR-513a-3p in human GC cells (Figure 4D). Furthermore, UCA1 knockdown or overexpression of miR-513a-3p could significantly decrease the expression of CYP1B1 protein in human GC cells, while knockdown of miR-513a-3p could significantly increase the expression of CYP1B1 protein in human GC cells (Figure 4E). Taken together, these results indicated that UCA1 and miR-513a-3p inhibited each other’s expression in GC cells, and UCA1 promoted the expression of CYP1B1, while miR-513a-3p inhibited the expression of CYP1B1 protein in human GC cells (Figure 4F).
Figure 4.
UCA1 and CYP1B1 bind to miR-513a-3p in human gastric cancer cell. (A) Wild-type (WT) or mutant (MUT) sequence of UCA1 and CYP1B1 bound to miR-513a-3p; B-C, WT or MUT sequence of UCA1 (B) and CYP1B1 (C) bound to miR-513a-3p were cotransferred into AGS cells with miR-513a-3p-NC (NC), miR-513a-3p-mimic (mimic) or miR-513a-3p-inhibitor (inhibitor), and then measured the luciferase activity; (D), UCA1 knockdown increased the expression of miR-513a-3p in AGS and NCI-N87 cells; (E) immunoblotting was used to detected the expression of CYP1B1 protein in human gastric cancer cells; (F) schematic diagram of UCA1 promoting the expression of CYP1B1 by binding to miR-513a-3p. Each experiment was repeated three times independently. The relative luciferase activity was shown as mean ±SD, *p<0.001 vs NC group and **p<0.001 and the p-value was calculated by post hoc comparisons in (B and C) and by Student’s t-test in (D).
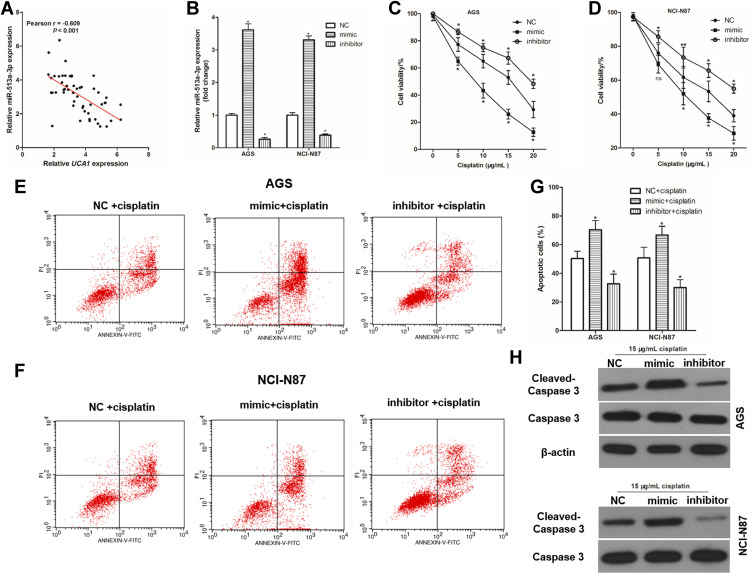
miR-513a-3p Decreases Resistance to Cisplatin in Human GC Cells
To investigate the effect of miR-513a-3p on the resistance of cisplatin in human GC cells, the correlation between miR-513a-3p expression and UCA1 expression in human GC tissues was analyzed, showing a negative correlation between miR-513a-3p expression and UCA1 expression in human GC tissues (Figure 5A). Then, we established miR-513a-3p overexpression and miR-513a-3p knockdown GC cells by transferring mimic and inhibitor, respectively (Figure 5B). Similarly, miR-513a-3p knockdown could increase the cell viability of AGS and NCI-N87 cells after stimulation with 15 μg/mL cisplatin, while miR-513a-3p overexpression decreased the cell viability of AGS (Figure 5C) and NCI-N87 (Figure 5D) cells after stimulation with 15 μg/mL cisplatin. Importantly, miR-513a-3p knockdown decreased cisplatin-induced apoptosis in AGS and NCI-N87 cells, while miR-513a-3p overexpression increased cisplatin-induced apoptosis in AGS and NCI-N87 cells (Figure 5E-G). For apoptosis-related protein expression, miR-513a-3p knockdown decreased and miR-513a-3p overexpression increased the expression of cleaved caspase-3 protein in human GC cells (Figure 5H).
Figure 5.
miR-513a-3p inhibits cisplatin induced apoptosis in human gastric cancer cells. (A) There was a negative correlation between UCA1 expression and miR-513a-3p expression in human gastric cancer tissues; (B) qPCR was used to detect the expression of miR-513a-3p in human gastric cancer cells; (C and D), miR-513a-3p regulated the toxicity of cisplatin to AGS (C) and NCI-N87 (D); (E–G) after transferring the miRNA, human gastric cancer cells were stimulated with or without 15 μg/mL cisplatin, and then cells were harvested to detect apoptosis by flow cytometer, and the representative images were shown in (E and F), and the rates of apoptosis cells were statistically compared (G); (H) immunoblotting was used to detected the expression of caspase-3 and cleaved caspase-3 protein in human gastric cancer cell. Each experiment was repeated three times independently. Data was expressed as mean ±SD, *p<0.001 vs NC group or NC+cisplatin group, and the p-value was calculated by post hoc comparisons.
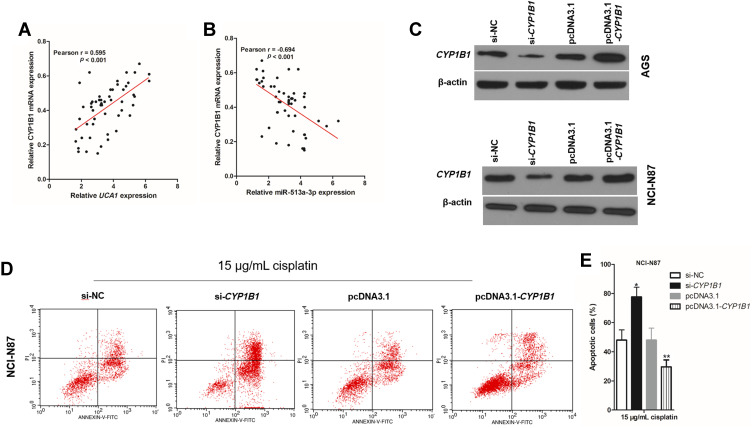
CYP1B1 Executes UCA1/miR-513a-3p to Regulate the Cisplatin Resistance of GC Cells
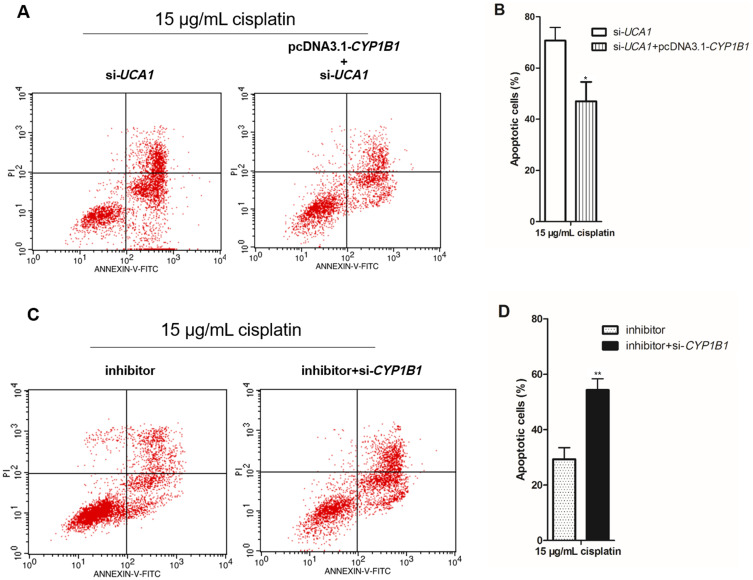
In the UCA1/miR-513a-3p/CYP1B1 axis, CYP1B1 is the only protein and regulates the biological characteristics of cells. There was a positive correlation between CYP1B1 mRNA expression and UCA1 expression in human GC tissues (Figure 6A), whereas there was a negative correlation between CYP1B1 mRNA expression and miR-513a-3p expression in human GC tissues (Figure 6B). To investigate the effect of CYP1B1 on the resistance to cisplatin in human GC cells, we established CYP1B1 knockdown GC cells by transfection into si-CYP1B1 and established CYP1B1 overexpression GC cells by transfection into pcDNA3.1-CYP1B1 (Figure 6C). Then knockdown of CYP1B1 increased cisplatin-induced apoptosis of GC cells, while overexpression of CYP1B1 decreased cisplatin-induced apoptosis of GC cells (Figure 6D and 6E). More importantly, cisplatin induced less apoptosis in GC cells cotransfected with pcDNA3.1-CYP1B1 and si-UCA1 compared to the transfection of si-UCA1 alone (Figure 7A and 7B). At the same time, cisplatin induced more apoptosis in GC cells cotransfected with miR-513a-3p-mimic and si-CYP1B1 compared to transfection of the miR-513a-3p-mimic alone (Figure 7C and 7D).
Figure 6.
CYP1B1 promotes cisplatin induced apoptosis in human gastric cancer cells. (A) There was a negative correlation between UCA1 expression and CYP1B1 mRNA expression in human gastric cancer tissues; (B) there was a negative correlation between CYP1B1 expression and miR-513a-3p expression in human gastric cancer tissues; (C) immunoblotting was used to detected the expression of CYP1B1 protein in human gastric cancer cells; (D and E) CYP1B1 knockdown increased cisplatin induced apoptosis, while CYP1B1 overexpression decreased cisplatin induced apoptosis in NCI-N87 cells (D), and apoptotic cells were statistically compared (E). Each experiment was repeated three times independently. The rate of apoptosis cells was shown as mean ±SD, *p<0.001 vs si-NC group, and **p<0.001 vs pcDNA3.1 group, and the p-value was calculated by Student’s t-test.
Figure 7.
CYP1B1 reverses the regulation of cisplatin induced apoptosis by UCA1 or miR-513a-3p in human gastric cancer cells. (A–B) CYP1B1 overexpression decreased cisplatin induced apoptosis in UCA1 knockdown NCI-N87 cells using detection of flow cytometer (A), and apoptotic cells were statistically compared (B); (C and D) CYP1B1 knockdown increased cisplatin induced apoptosis in miR-513a-3p knockdown NCI-N87 cells using detection of flow cytometer (C), and apoptotic cells were statistically compared (D). Each experiment was repeated three times independently. The rate of apoptosis cells was shown as mean ±SD, *p<0.001 vs si-UCA1 group, **p<0.001 vs inhibitor group, and the p-value was calculated by Student’s t-test.
Discussion
Surgical removal of tumors can clear visible tumors from the surgical area but tumor cells may remain outside the surgical area, which are the main cause of recurrence and metastasis. The purpose of postoperative adjuvant chemotherapy is to clear these tumor cells, reduce the probability of recurrence and prolong patient survival.7,8 Cisplatin, one of the most commonly used chemotherapeutic drugs for GC patients, is used in combination with 5-fluorouracil, oxaliplatin, and calcium folinate as an adjuvant treatment for GC patients.23,24 Unfortunately, many GC patients are insensitive to cisplatin over time, eventually influencing the efficacy of chemotherapy.25,26 In recent years, efforts have been devoted to understanding the mechanism of chemoresistance and investigating the genes/pathways involved.9,10 Recent research has documented that post-transcriptional and translational controls have key roles in the mechanism of cellular-mediated resistance to anticancer drug treatment, involving numerous noncoding RNA.21
LncRNA is more than 200 nucleotides in length and although it does not encode a protein, it can become a platform for integration with other DNA and RNA due to its long primary structure.27,28 Moreover, it can also form a diverse and complex secondary space structure and can interact with protein molecules to exert biological functions.27,28 Compared with the mRNA encoding protein, most lncRNAs are also produced by RNA polymerase catalytic transcription II but the sequence of lncRNAs is less conservative and the expression abundance is lower, showing a strong specificity in tissues and cells.27,28 LncRNA was originally regarded as “garbage” for gene transcription, a by-product of RNA polymerase transcription, and had no biological effect.27,28 However, in recent years, lncRNA has been found to be involved in X chromosome silencing, genomic imprinting, chromatin modification, activation of chromatin transcription, infection of chromatin transcription and intracellular transport, and the regulatory effect shown by lncRNA has been valued by researchers.9 In cancer, many lncRNAs are abnormally expressed in malignant tumor tissues, which are involved in regulating tumor progression and are related to the efficacy and prognosis of chemotherapy.29 In addition, lncRNAs are involved in regulating tumor cell proliferation, invasion, migration, and sensitivity to chemotherapy drugs in vitro.30
UCA1 is located on human chromosome 19p13.12, and is reportedly upregulated in bladder cancer, colorectal cancer, ovarian cancer, and breast cancer,11,12 and promotes cancer cell proliferation, invasion, migration, and chemoresistance.13,14 In this study, we found that UCA1 expression in 53 cases of human GC tissues was significantly higher than in the adjacent normal tissue, and knockdown of UCA1 increased chemosensitivity to cisplatin by inducing cell apoptosis. Previous studies have found that UCA1 functions as an oncogene, enhancing the resistance of cancer cells to chemotherapy drugs by regulating downstream gene expression.21 In this study, we found that miR-513a-3p had the same binding sites to UCA1 and CYP1B1, UCA1, and miR-513a-3p inhibited each other’s expression in GC cells, and UCA1 promoted the expression of CYP1B1, while miR-513a-3p inhibited the expression of CYP1B1 protein in human GC cells. MicroRNA (miRNA), another type of noncoding RNA regulates the translation of one or more mRNA molecules by binding to their 3ʹ-UTRs, leading to translational inhibition or mRNA degradation. At the same time, miRNA is also a bridge for lncRNA to regulate protein expression, as lncRNA indirectly reduces miRNA’s regulation of target gene expression by combining with miRNA.27,28
MiR-513a-3p can bind to both UCA1 and CYP1B1 in the same site and has been found to play an important role in the regulation of chemoresistance, for example, miR-513a-3p sensitizes human lung adenocarcinoma cells to chemotherapy by targeting GSTP1.31 In this study, we found that overexpression of miR-513a-3p increased cisplatin-induced apoptosis, while knockdown of miR-513a-3p increased cisplatin-induced apoptosis in human GC cells. This might be related to miR-513a-3p promoting cell apoptosis by inhibiting the expression of Bcl2.32 Herein, we found that miR-513a-3p targeted inhibition of CYP1B1 expression.
The cytochrome P450 family 1 subfamily B member 1 (CYP1B1) acts as an executor of the UCA1/miR-513a-3p/CYP1B1 molecular axis regulating GC cell resistance to cisplatin and has been found to play an important role in the regulation of apoptosis and chemoresistance.33,34 CYP1B1 is a member of the cytochrome P450 supergene family, and involved in the regulation of multiple important transcription factors, helping cancer cells reduce the toxicity of chemical drugs by oxidizing and metabolizing a variety of pre-carcinogens and anticancer drugs, such as the activation of polycyclic aromatic hydrocarbons, heterocyclic amines, aromatic amines, nitrates and mediating C4-hydroxylation of 17-estradiol.35,36 Furthermore, CYP1B1 metabolite 4-hydroxylated estrogen can significantly increase the level of 8-hydroxyguanine causing DNA single-strand breaks, has a DNA damaging effect, and potential carcinogenicity.37,38 For chemoresistance, previous studies have found that CYP1B1 enhances the resistance of epithelial ovarian cancer cells to paclitaxel in vivo and in vitro,39 and suppression of CYP1B1 synergizes with anticancer drugs.40 CYP1B1 enhanced chemoresistance may be related to CYP1 inhibiting cancer cell apoptosis through sp1-mediated suppression of trail expression.41
To further confirm that CYP1B1 is the executor of the UCA1/miR-513a-3p/CYP1B1 molecular axis regulating GC cell apoptosis induced by cisplatin, we simultaneously knocked down UCA1 and overexpressed CYP1B1 in GC cells, or knocked down both CYP1B1 and miR-513a-3p, finding that overexpression of CYP1B1 reduced chemosensitivity to cisplatin increased by knockdown of UCA1, and knockdown of CYP1B1 increased chemosensitivity to cisplatin decreased by knockdown of miR-513a-3p in human GC cells. Therefore, in conclusion, lncRNA UCA1 enhances cisplatin resistance by regulating CYP1B1-mediated apoptosis via miR-513a-3p in human GC.
Acknowledgment
Co-first authors: Haidong Cheng and Gaowa Sharen. Co-correspondence authors: Zhaoyang Wang and Jing Zhou.
Funding Statement
This work was supported by the Doctoral Program of Inner Mongolia Natural Science Foundation (No. 2018BS08004), Inner Mongolia Natural Science Foundation (2019LH08028) and Inner Mongolia Medical University Science and Technology Million Project joint project (No. YKD2017KJBW(LH)002).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Peleteiro B, Padr P, Castro C, Ferro A, Morais S, Lunet N. Worldwide burden of gastric cancer in 2012 that could have been prevented by increasing fruit and vegetable intake and predictions for 2025. Br J Nutri. 2016;115(5):851–859. doi: 10.1017/S000711451500522X [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3.Kamiya S, Rouvelas I, Lindblad M, Nilsson M. Current trends in gastric cancer treatment in Europe. J Cancer Metastasis Treat. 2018;4:35–46. doi: 10.20517/2394-4722.2017.76 [DOI] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. doi: 10.1016/j.canlet.2017.04.024 [DOI] [PubMed] [Google Scholar]
- 6.An Y, Zhang Z, Shang Y, et al. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015;6(5):e1766. doi: 10.1038/cddis.2015.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sastre J, Garcia-Saenz JA, Diaz-Rubio E. Chemotherapy for gastric cancer. World J Gastroenterol. 2006;12(2):204–213. doi: 10.3748/wjg.v12.i2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Shen H, Kapesa L, Zeng S. Lauren classification and individualized chemotherapy in gastric cancer (Review). Oncol Lett. 2016;11(5):2959–2964. doi: 10.3892/ol.2016.4337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi: 10.1038/nrg2521 [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Li H, Fang S, et al. NONCODE 2016: an informative and valuable data source of long non-coding RNAs. Nucleic Acids Res. 2016;44(D1):D203–D208. doi: 10.1093/nar/gkv1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan D, Cao H, Feng J. Advances of long non-coding RNA UCA1 in cancers. J Int Oncol. 2017;44(2):108–111. [Google Scholar]
- 12.Wang X, Peng F, Cheng L, et al. Prognostic and clinicopathological role of long non-coding RNA UCA1 in various carcinomas. Oncotarget. 2017;8(17):28373. doi: 10.18632/oncotarget.16059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Wang G, Yang L, Qu J, Yang Z, Zhou X. Knockdown of long non-coding RNA UCA1 increases the tamoxifen sensitivity of breast cancer cells through inhibition of Wnt/β-catenin pathway. PLoS One. 2016;11(12):e0168406. doi: 10.1371/journal.pone.0168406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han Y, Yang Y-N, Yuan H-H, et al. UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology. 2014;46(5):396–401. doi: 10.1097/PAT.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Wang X, Dang Y, et al. Long non-coding RNA UCA1 promotes the progression of paclitaxel resistance in ovarian cancer by regulating the miR-654-5p/SIK2 axis. Eur Rev Med Pharmacol Sci. 2020;24:591–603. [DOI] [PubMed] [Google Scholar]
- 16.Horita K, Kurosaki H, Nakatake M, Ito M, Kono H, Nakamura T. Long noncoding RNA UCA1 enhances sensitivity to oncolytic vaccinia virus by sponging miR-18a/miR-182 and modulating the Cdc42/filopodia axis in colorectal cancer. Biochem Biophys Res Commun. 2019;516(3):831–838. doi: 10.1016/j.bbrc.2019.06.125 [DOI] [PubMed] [Google Scholar]
- 17.Pan J, Li X, Wu W, et al. Long non-coding RNA UCA1 promotes cisplatin/gemcitabine resistance through CREB modulating miR-196a-5p in bladder cancer cells. Cancer Lett. 2016;382(1):64–76. doi: 10.1016/j.canlet.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Jiang F, Zhang X, Xu X. Long Non-Coding RNA UCA1 modulates paclitaxel resistance in breast cancer via miR-613/CDK12 axis. Cancer Manag Res. 2020;12:2777. doi: 10.2147/CMAR.S241969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Ye C, Liu J, Hu Y. UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem Biophys Res Commun. 2018;501(4):1034–1040. doi: 10.1016/j.bbrc.2018.05.104 [DOI] [PubMed] [Google Scholar]
- 20.Luo Z, Kang G. Long non-coding RNA UCA1 enhances drug resistance of lung cancer cells via activating mTOR signaling. Int J Clin Exp Pathol. 2016;9(2):1408–1415. [Google Scholar]
- 21.Wang H, Guan Z, He K, Qian J, Cao J, Teng L. LncRNA UCA1 in anti-cancer drug resistance. Oncotarget. 2017;8(38):64638. doi: 10.18632/oncotarget.18344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu W, Ren JH, Zheng X, Hu XY, Hu MJ. Comprehensive analysis of expression profiles of long non‑coding RNAs with associated ceRNA network involved in gastric cancer progression. Mol Med Rep. 2019;20(3):2209–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shitara K, Doi T, Nagano O, et al. Phase 1 study of sulfasalazine and cisplatin for patients with CD44v-positive gastric cancer refractory to cisplatin (EPOC1407). Gastric Cancer. 2017;20(6):1004–1009. doi: 10.1007/s10120-017-0720-y [DOI] [PubMed] [Google Scholar]
- 24.Yamada Y, Boku N, Mizusawa J, et al. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, Phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4(7):501–510. doi: 10.1016/S2468-1253(19)30083-4 [DOI] [PubMed] [Google Scholar]
- 25.Zheng P, Chen L, Yuan X, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):53. doi: 10.1186/s13046-017-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai J, Shen J, Xie G, et al. Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett. 2019;454:37–43. doi: 10.1016/j.canlet.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 27.Uszczynska-Ratajczak B, Lagarde J, Frankish A, Guigó R, Johnson R. Towards a complete map of the human long non-coding RNA transcriptome. Nat Rev Genet. 2018;19(9):535–548. doi: 10.1038/s41576-018-0017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. [DOI] [PubMed] [Google Scholar]
- 29.Hanly D, Esteller M, Berdasco M. Altered Long Non-coding RNA Expression in Cancer: potential Biomarkers and Therapeutic Targets? 2019.
- 30.Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T. Emerging roles of long non‐codingRNAin cancer. Cancer Sci. 2018;109(7):2093–2100. doi: 10.1111/cas.13642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Zhu J, Xing R, et al. miR-513a-3p sensitizes human lung adenocarcinoma cells to chemotherapy by targeting GSTP1. Lung Cancer. 2012;77(3):488–494. doi: 10.1016/j.lungcan.2012.05.107 [DOI] [PubMed] [Google Scholar]
- 32.Li S, Xu Y-N, Niu X, Li Z, Wang J-F. miR-513a-5p targets Bcl-2 to promote dichlorvos induced apoptosis in HK-2 cells. Biomed Pharmacother. 2018;108:876–882. doi: 10.1016/j.biopha.2018.09.101 [DOI] [PubMed] [Google Scholar]
- 33.Morvan VL, Laroche-Clary A, Robert J. Genetic polymorphisms of cytochrome P450 1B1 (CYP1B1) are linked to the response to chemotherapy: studies of in vitro models. Bull Cancer. 2010;97:S47–S47. [Google Scholar]
- 34.Morvan VL, Auzanneau C, Pasquies A, Brault M, Robert J, Lansiaux A. Abstract 2200: relationship between cytochrome P450 1B1 (CYP1B1) and head and neck cancer cells proliferation, dissemination and chemosensitivity to anticancer drugs. Cancer Res. 2013;73(8 Supplement):2200. [Google Scholar]
- 35.Nishida CR, Everett S, Ortiz dM, PR. Specificity determinants of CYP1B1 estradiol hydroxylation. Mol Pharmacol. 2013;84(3):451–458. doi: 10.1124/mol.113.087700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacob A, Potin S, Chapy H, et al. Aryl hydrocarbon receptor regulates CYP1B1 but not ABCB1 and ABCG2 in hCMEC/D3 human cerebral microvascular endothelial cells after TCDD exposure. Brain Res. 2015;1613:27–36. doi: 10.1016/j.brainres.2015.03.049 [DOI] [PubMed] [Google Scholar]
- 37.Gajjar K, Martin-Hirsch PL, Martin FL. CYP1B1 and hormone-induced cancer. Cancer Lett. 2012;324(1):13–30. doi: 10.1016/j.canlet.2012.04.021 [DOI] [PubMed] [Google Scholar]
- 38.Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol. 2001;41(1):297–316. doi: 10.1146/annurev.pharmtox.41.1.297 [DOI] [PubMed] [Google Scholar]
- 39.Zhu Z, Mu Y, Qi C, Wang J, Zhao F. CYP1B1 enhances the resistance of epithelial ovarian cancer cells to paclitaxel in vivo and in vitro. Int J Mol Med. 2014;35(2):340–348. doi: 10.3892/ijmm.2014.2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mu W, Hu C, Zhang H, et al. miR-27b synergizes with anticancer drugs via p53 activation and CYP1B1 suppression. Cell Res. 2015;25(4):477–495. doi: 10.1038/cr.2015.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon YJ, Baek HS, Ye DJ, Shin S, Chun YJ. CYP1B1 inhibits cancer cell apoptosis through sp1-mediated suppression of trail expression. Drug Metab Pharmacokinet. 2017;32(1):S39. doi: 10.1016/j.dmpk.2016.10.166 [DOI] [Google Scholar]