Abstract
Objectives
To describe the epidemiology, clinical and laboratory features, and outcome of children hospitalized with coronavirus disease 2019 (COVID-19) in the Middle East.
Methods
A multicenter retrospective study of children hospitalized with COVID-19 in 7 centers across Oman between February and July 2020.
Results
In total, 56 children <14 years old required hospitalization in 7 Omani centers over 5 months (February – July 2020). Thirty-seven (68%) children were admitted with uncomplicated COVID-19, 13 (23%) with pneumonia and 5 (9%) with multisystem inflammatory syndrome in children. Infants constituted 41% of cases (23/56), approximately half of whom (12/23, 52%) were <2-months old. Fever was the most common symptom (46, 82%), followed by respiratory symptoms (33, 59%), and gastrointestinal symptoms (31, 55%). Twenty-two (39%) children had underlying medical conditions: sickle cell disease (7, 13%), chronic respiratory disease (4, 7%) and severe neurological impairment (4, 7%). Leukocytosis, elevated inflammatory markers and anemia were independently associated with intensive care admission. There were no mortalities related to admission with COVID-19 in this cohort.
Conclusion
Most of the children hospitalized with COVID-19 had a mild course and a satisfactory outcome. Sickle cell disease is the most common comorbidity associated with pediatric admission of COVID-19 in Oman.
Keywords: COVID-19, Children, Hospitalized, Outcome, Oman
Introduction
Children tend to have mild COVID-19 and less mortality compared to adults (Chan et al., 2020). There is limited literature on the epidemiology and effect of COVID-19 in children from Middle Eastern countries. COVID-19 was reported in Oman for the first time on the 24 February 2020 (WHO, 2020a). From the 1 February to 31 July 2020, there were 68 400 confirmed COVID-19 cases in Oman of whom 4379 (6.6%) were children (WHO, 2020a). This study describes the epidemiology, clinical and laboratory features, and outcome of children hospitalized with COVID-19 in Oman. It is one of the first such studies in the Middle East and the gulf region.
Methods
We performed a multicenter, retrospective study of children <14 years old with laboratory-confirmed COVID-19 and admitted to any of 7 hospitals across Oman between February and July 2020. The centers involved in this study were the Royal Hospital, Sultan Qaboos University Hospital (SQUH), Al Nahda Hospital, Al Rustaq Hospital, Suhar Hospital, Nizwa Hospital, and Ibri Hospital. Ethical approval was obtained through the central research committee at the Ministry of Health and the SQUH medical research ethics committee.
In all these centers, patients with mild to moderate illness were admitted to the general ward. Those requiring close monitoring or noninvasive ventilation (CPAP and Bi-PAP) were placed in the high dependency unit (HDU). Children with hemodynamic instability, or those requiring invasive ventilation, were admitted to the pediatric intensive care unit (PICU).
Data concerning patient demographics, clinical characteristics, laboratory and radiological investigations, management, and patient outcomes were collected in an MS Excel spreadsheet using the hospitals’ electronic health care systems. In our study, uncomplicated COVID-19 was defined as patients who required admission to the general ward and had no sign of multi-organ involvement. Severe COVID-19 was defined as patients who required supplemental oxygen as per the World Health Organization (WHO) definition. We followed the WHO definition for multisystem inflammatory syndrome in children (MIS-C) (Varghese et al., 2020, WHO, 2020b). Confirmed fever was defined as a measured temperature of > = 38.0 °C. Leukocytosis, leukopenia, neutrophilia, neutropenia, lymphocytosis, lymphopenia, anemia for age, thrombocytopenia, and thrombocytosis were determined based on normal values for age as described in Nathan and Oski's Hematology of Infancy and Childhood, 6th Edition.
Cepheid’s GeneXpert SARS-CoV-2 RT-PCR (Egene, Ngene) and SARS-CoV-2 RT-PCR through TIB MOLBIOL, Liferiver and Sansure were used to confirm the diagnosis of COVID-19. Respiratory samples (nasopharyngeal swabs, throat swabs, or nasopharyngeal aspirates) were sent in a viral transport medium for virus detection to the central public health lab (CPHL) or SQUH laboratory. The remaining samples were processed locally. Trained laboratory technicians prepared the swab sample tubes for processing. Each tube had a unique barcode for identification and traceability. Samples were loaded into the molecular testing system for extraction, amplification and detection. All test results were analyzed and approved by experienced laboratory technicians or microbiologists before entry to laboratory reporting systems.
Non-parametric two-tailed Mann-Whitney U tests were used to compare continuous variables, all of which were found to be not normally distributed. χ² or Fisher’s exact tests were used to compare categorical variables, as appropriate. Normality of data distribution was assessed with the Shapiro-Wilk test. The clinical endpoint was the need for admission to an intensive care unit (ICU; either neonatal or pediatric intensive care). The association of baseline characteristics and clinical findings with ICU admission was initially evaluated using univariable logistic regression. Subsequently, multivariable logistic regression analysis with the backward stepwise method was used to explore variables that were independently associated with ICU admission. Only variables that were significant in univariable analyses were introduced into the model. Factors related to drug treatment for COVID-19 were also explored with univariable analysis. All probabilities were two-tailed. P < 0.05 was considered statistically significant. All calculations were done with Prism (version 8.0; GraphPad, La Jolla, CA, USA) and SPSS (version 23.0; IBM, Armonk, NY, USA).
Results
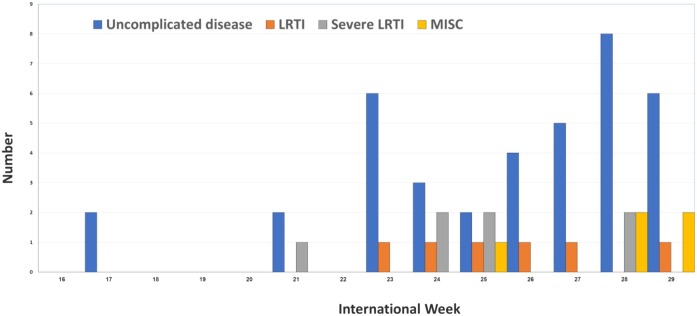
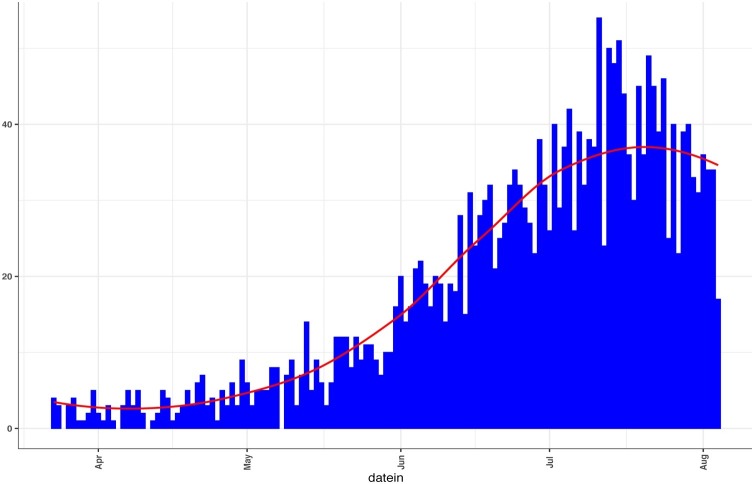
Fifty-six children were hospitalized with COVID-19 in the 7 hospitals during the study period. Weekly hospitalization among children increased steadily during the study period (Figure 1) coinciding with the national trend for all age groups (Figure 2 ). The highest number of admissions was in the Muscat Governorate (Table 1 ).
Figure 1.
The distribution of pediatric COVID-19 admission along international weeks to the final diagnosis
LRTI: Lower respiratory tract infection, MISC: Multisystem inflammatory diseases in children
Figure 2.
The national COVID-19 admission curve up to August 2020 including all age groups and nationalities*.
Table 1.
Demographics, baseline clinical characteristics, and outcomes of pediatric patients admitted with laboratory-confirmed COVID-19.
| Number of patients | 56 |
|---|---|
| Age, years, median (IQR) | 1.8 (0.2-6.9) |
| Age groups | |
| <1 year | 23 (41%) |
| <2 months | 12 (21%) |
| 1–4 years | 15 (27%) |
| 5–9 years | 10 (18%) |
| 10–13 years | 8 (14%) |
| Male gender | 36 (64%) |
| Omani nationality | 55 (98%) |
| Governorate | |
| Muscat | 31 (55%) |
| North Batinah | 10 (18%) |
| Dakhiliyah | 9 (16%) |
| South Batinah | 5 (9%) |
| Sharqiyah | 1 (2%) |
| Dhahirah | 0 |
| Buraimi | 0 |
| Wusta | 0 |
| Dhofar | 0 |
| History of travel | 0 |
| History of contact with a known case of COVID-19 | 38 (68%) |
| Immediate vs extended family members | 29 vs 5a |
| Underlying medical condition | 22 (39%) |
| Sickle cell disease | 7 (13%) |
| Severe neurologic impairment | 4 (7%) |
| Asthma | 2 (4%) |
| Immunocompromised | 2 (4%) |
| Cystic fibrosis | 2 (4%) |
| Congenital heart disease | 1 (2%) |
| Prematurity | 1 (2%) |
| Other | 5 (9%) |
| COVID-19 illness classification | |
| Uncomplicated disease | 38 (68%) |
| Pneumonia | 13 (23%) |
| Severe pneumonia | 5 (9%) |
| MIS-C | 5 (9%) |
| Level of care required | 38 (68%) |
| Regular ward bed | 45 (80%) |
| High dependency bed | 4 (7%) |
| Intensive care | 7 (13%) |
| Days in intensive care, median (IQR) | 3 (2-8) |
| Hospital length of stay, days, median (IQR) | 2 (1-4) |
| Complications | 15 (27%) |
| Sickle cell disease-related complications2 | 7 (13%) |
| Seizure | 3 (5%) |
| Acute kidney injury | 2 (4%) |
| Myocardial dysfunction | 1 (2%) |
| Shock | 1 (2%) |
| Acute respiratory distress syndrome | 1 (2%) |
| Subdural hematoma (associated with trauma) | 1 (2%) |
| Acute appendicitis | 2 (4%) |
| Death or chronic morbidity | 0 (0%) |
Degree of relatedness of the known contact was not specified in 4 cases. For 18 cases, contact with a known case of COVID-19 was not mentioned. 2Three patients’ course was complicated by acute chest syndrome, 2 with splenic sequestration, while vaso-occlusive pain crisis and acute anemia requiring transfusion were observed in 1 patient each.
Among the study's cohort, 55 (98%) children were Omani, 36 (64%) were male, and the median (IQR) age was 1.8 (0.2-6.9) years. Infants constituted 23 (41%) of the cases, approximately half of whom (12/23, 52%) were <2-months old. There was a history of contact with a positive case of COVID-19 in 38 (68%) cases; contact was with an immediate family member in 29 (76%), an extended family member in 5 (13%), and unspecified in 4 (10%). Whether the contact was an adult or a child was not specified in most cases.
There were 22 (39%) cases with an underlying medical condition. The most prevalent condition was sickle cell disease (SCD) (7, 13%) followed by chronic respiratory conditions (4, 7%: 2 Asthma, 2 cystic fibrosis) and severe neurological impairment (4, 7%) (Table 1).
Fever was the most common symptom (46, 82%), followed by respiratory symptoms (33, 59%) and gastrointestinal symptoms (31, 55%) (Table 2 ). Skin rash, loss of smell, and loss of taste were not documented in any of the cases.
Table 2.
Reported signs and symptoms among pediatric patients admitted with laboratory-confirmed COVID-19.
| Constitutional | 49 (88%) |
|---|---|
| Reported fever (by history) | 46 (82%) |
| Confirmed fever (by measurement) | 32 (57%) |
| Fatigue or decreased activity | 28 (50%) |
| Body aches | 4 (7%) |
| Respiratory tract | 33 (59%) |
| Cough | 26 (46%) |
| Nasal congestion | 16 (29%) |
| Tachypnea | 11 (20%) |
| Hypoxia | 10 (18%) |
| Crackles | 9 (16%) |
| Shortness of breath | 8 (14%) |
| Sore throat | 6 (11%) |
| Chest retractions | 5 (9%) |
| Wheezing | 4 (7%) |
| Cyanosis | 2 (4%) |
| Stridor | 0 |
| Apnea | 0 |
| GI | 31 (55%) |
| Vomiting | 20 (36%) |
| Diarrhea | 16 (29%) |
| Abdominal pain | 14 (25%) |
| Neurologic | 5 (9%) |
| Seizure | 4 (7%) |
| Headache | 1 (2%) |
| Cardiac | |
| Tachycardia | 28 (50%) |
| Decreased peripheral perfusion | 14 (25%) |
| Hypotension | 1 (2%) |
| Other | |
| Lymphadenopathy | 4 (7%) |
| Oedema of hands and feet | 3 (5%) |
| Oral mucosal changes | 2 (4%) |
| Conjunctival injection | 2 (4%) |
| Loss of smell or taste | 0 |
| Rash | 0 |
Most of the children were admitted to a regular care unit (45, 80%), whereas 4 (7%) needed intermediate level care (HDU), and 7 (13%) required intensive care. Invasive mechanical ventilation was needed for only 1 (2%), noninvasive ventilation was needed for 2 (4%), and 8 (14%) needed oxygen support. The median (IQR) length of stay in the hospital was 2 (1-4) days (Table 1). Among patients requiring intensive care, the median (IQR) length of stay in the critical care unit was 3 (2-8) days.
The COVID-19 disease classification in this cohort was as follows: uncomplicated illness in 38 (68%), pneumonia in 13 (23%; severe in 7, 13%), and MIS-C in 5 (9%). Fifteen (27%) patients were suffering from various complications at admission. Most common SCD-related complications, observed in 7 (13%) patients. These included acute chest syndrome (n = 3), splenic sequestration (n = 2), pain crisis (n = 1), and acute anemia (n = 1). Two children required blood transfusion and the 3 patients with acute chest syndrome required exchange transfusion. Seizures were observed in 3 patients who were known to have underlying seizure disorders. Acute appendicitis was diagnosed in 2 patients who underwent an appendectomy and were subsequently diagnosed with MIS-C. Acute kidney injury was noted in 2 (4%) critically-ill patients. Other complications in critically-ill patients included myocardial dysfunction, shock, and acute respiratory distress syndrome. A subdural hematoma was noted in 1 (2%) patient and was attributed to trauma. No mortalities or chronic complications related to admission with COVID-19 were recorded (Table 1).
Laboratory investigations revealed that lymphopenia and anemia for age were the most common hematologic abnormalities, occurring in 22 (39%) and 21 (38%) of patients, respectively (Table 3 ). Serum C-reactive protein (CRP), sodium, creatinine, alanine aminotransferase, total bilirubin, and albumin were normal in the majority of patients. All the children diagnosed with MIS-C had positive COVID-19 PCR and 3/4 had positive COVID-19 IgG.
Table 3.
Comparing the clinical and laboratory findings of patients who required intensive care with those who did not, among pediatric patients admitted with laboratory-confirmed COVID-19.a
| Investigation | All patients N = 56 | Non-ICU N = 49 | ICU N = 7 | P | Missing |
|---|---|---|---|---|---|
| Age | 1.8 (0.2-6.9) | 1.4 (0.2-6.4) | 4.9 (3.8-9.5) | 0.055 | 0 |
| Male gender | 36 (64%) | 29 (59%) | 7 (100%) | 0.035 | 0 |
| Underlying medical condition | 22 (39%) | 18 (37%) | 4 (57%) | 0.415 | 0 |
| Reported fever (by history) | 46 (82%) | 40 (82%) | 6 (86%) | 0.792 | 0 |
| Measured fever | 32 (57%) | 27 (55%) | 5 (71%) | 0.414 | 0 |
| Any respiratory tract signs or symptoms | 33 (59%) | 27 (55%) | 6 (86%) | 0.220 | 0 |
| Lower respiratory signs | 15 (27%) | 10 (20%) | 5 (71%) | 0.012 | 0 |
| Gastrointestinal symptoms | 31 (55%) | 27 (55%) | 4 (57%) | 1 | 0 |
| Tachycardia | 28 (50%) | 23 (47%) | 5 (71%) | 0.422 | 0 |
| Signs of Kawasaki diseaseb | 5 (9%) | 3 (6%) | 2 (29%) | 0.113 | 0 |
| WBC, median (IQR) | 9.4 (5.8-12.9) | 8.3 (5.4-12.3) | 17.3 (8.5-25.6) | 0.012 | 0 |
| Leukocytosis | 9 (16%) | 5 (10%) | 4 (57%) | 0.009 | 0 |
| Leukopenia | 12 (21%) | 12 (25%) | 0 (0%) | 0.326 | 0 |
| ANC, median (IQR) | 3.8 (1.7-7.5) | 3.1 (1.6-5.3) | 11.9 (5.4-17.1) | <0.001 | 0 |
| Neutrophilia | 9 (16%) | 5 (10%) | 4 (57%) | 0.009 | 0 |
| Neutropenia | 7 (13%) | 7 (14%) | 0 (0%) | 0.578 | 0 |
| ALC, median (IQR) | 2.7 (1.7-5.0) | 2.7 (1.6-5.1) | 2.5 (2.4-3.4) | 0.734 | 0 |
| Lymphocytosis | 3 (5%) | 3 (6%) | 0 (0%) | 1 | 0 |
| Lymphopenia | 22 (39%) | 19 (39%) | 3 (43%) | 1 | 0 |
| Hemoglobin, median (IQR) | 10.9 (9.3-12.4) | 11.3 (9.4-12.6) | 9.5 (9.3-10.6) | 0.093 | 0 |
| Anemia for age | 21 (38%) | 15 (31%) | 6 (86%) | 0.009 | 0 |
| Platelets, mean (standard deviation) | 328 (153) | 336 (156) | 276 (124) | 0.339 | 0 |
| Thrombocytosis | 9 (16%) | 9 (18%) | 0 (0%) | 0.583 | 0 |
| Thrombocytopenia | 6 (11%) | 4 (8%) | 2 (29%) | 0.158 | 0 |
| CRP, median (IQR) | 4 (0-40) | 2 (0-32) | 116 (25-171) | <0.001 | 1 |
| Sodium, mean (standard deviation) | 136 (3) | 136 (3) | 134 (4) | 0.043 | 2 |
| Creatinine, median (IQR) | 27 (19-38) | 27 (19-39) | 25 (17-33) | 0.801 | 2 |
| ALT, median (IQR) | 18 (12-28) | 18 (13-28) | 15 (12-30) | 0.614 | 23 |
| Total bilirubin, median (IQR) | 10 (4-20) | 9 (4-19) | 11 (5-26) | 0.647 | 22 |
| Albumin, mean (standard deviation) | 41 (6) | 42 (5) | 36 (6) | 0.010 | 23 |
| Chest x-ray infiltrates | 27 (63%) | 24 (67%) | 3 (43%) | 0.394 | 13 |
Nathan and Oski's Hematology of Infancy and Childhood, 6th Edition (used as a reference for normal hematological values).
Rash, conjunctivitis, oral mucosal changes, edema of the hands or feet, or lymphadenopathy.
Among 43 children who had a chest radiograph, 27 (63%) radiographs showed an infiltrate or consolidation, this was bilateral in 12 (28%) cases. Five patients underwent an echocardiogram, which was normal for 2 patients, 1 showed coronary ectasia but no aneurysm, and 2 showed small pericardial effusions with 1 having a reduced left ventricular ejection fraction of 49%. Blood cultures were obtained for 48 (86%) patients, all of which showed no growth. Urine cultures were obtained for 29 (52%) patients, 4 had significant growth (Enterobacter cloacae, 1, Enterococcus faecalis, 1, and Burkholderia cepacia, 2).
COVID-19 investigational treatments were administered to some patients, including, Lopinavir/ritonavir (1, 2%), convalescent plasma (1, 2%), Favipiravir (3, 5%), Tocilizumab (3, 5%), Hydroxychloroquine (4, 7%), steroids (7, 13%), and intravenous immunoglobulin (4, 7%). Anticoagulants were given to 6 (11%) patients. Antimicrobials were used in 38 (68%) patients; beta-lactams (34, 61%), Macrolides (6, 11%), and Oseltamivir (9, 16%).
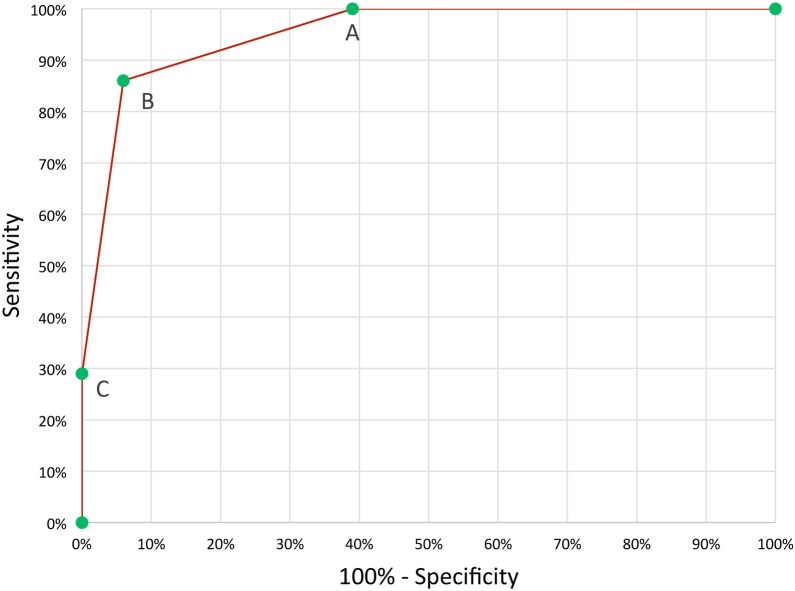
Patients who required ICU care were more likely to be male, have signs of lower respiratory disease, leukocytosis, neutrophilia, anemia for age, higher CRP levels, and lower serum sodium and albumin levels (Table 3). After multivariable logistic regression analysis, leukocytosis, elevated CRP (optimal cut-off was >100 mg/L), and anemia for age emerged as independently associated with ICU requirement (Table 3). The presence of all 3 risk factors was 100% predictive of ICU requirement. The presence of 2/3 factors was 86% sensitive and 94% specific in predicting ICU requirement (Figure 3). Serum IL-6, Lactate Dehydrogenase D, D-dimer, and ferritin were only measured in a small number of patients and were not included in the analysis.
Figure 3.
Receiver operating characteristics curve for the 3 predictors of intensive care requirement among pediatric patients admitted with laboratory-confirmed COVID-19. The 3 predictors were leukocytosis, CRP ≥ 100 mg/L, and anemia for age. A, ≥one predictor present, 100% sensitivity, 61% specificity. B, ≥two predictors present, 86% sensitivity, 94% specificity. C, ≥three predictors present, 29% sensitivity, 100% specificity. Area under the curve = 0.95 (95% CI 0.88-1.00).
Discussion
COVID-19 has a higher burden among adults with pediatric cases appearing to be less frequent and less severe (DeBiasi et al., 2020). According to the national data in Oman, the total number of pediatric confirmed COVID-19 cases during the study period was 4379 (6.6% of total cases) (WHO, 2020a).
Overall, we found that hospitalized and critically ill patients were more likely to have underlying conditions (22, 39%), similar to what has been described in other studies (e.g., DeBiasi et al., 2020). SCD was the most common condition in this study. COVID-19 triggered acute chest syndrome (ACS) in 3 children with SCD in our cohort, a major cause of morbidity and mortality in this population. Since COVID-19 mainly affects the respiratory tract, this complication is to be expected (De Luna et al., 2020, Panepintoa et al., 2020). An SCD registry in the United States reported 178 SCD patients with COVID-19 over 2 months (March-May 2020). In that cohort, 69% required hospital admission, 11% required intensive care, and 7% died; more than half developed vaso-occlusive crisis and 32% were diagnosed with ACS (Panepintoa et al., 2020). Heilbronner at al reported 4 children with SCD and COVID-19 who required intensive care management of ACS (Heilbronner et al., 2020). This suggests that children with SCD are at risk for severe COVID-19 and ACS seems to be the lead driver of this severity.
The clinical features at the time of presentation in our cohort were similar to what has been described in the literature (DeBiasi et al., 2020, Wang et al., 2020a). COVID-19 with cardiac manifestations is rare in children compared to adults, and it has been reported more in children with MIS-C. Belhadjer Z.et al. reported a ventricular ejection fraction of <30% in a third of their cases diagnosed with MIS-C (Belhadjer et al., 2020). Two patients in our study with MIS-C had minimal pericardial effusion and 1 had reduced ventricular function, findings that were likely related to COVID-19 and MIS-C.
The diagnosis of MIS-C in Oman started a few weeks after the peak of COVID-19 cases among adults; a finding that was also observed internationally (DeBiasi et al., 2020). MIS-C is a severe manifestation of COVID-19 and its pathogenesis is not yet fully understood. Gastrointestinal symptoms have been reported in more than 80% of children with MIS-C in 3 recently reported series (Belhadjer et al., 2020, Gonzalez Jimenez et al., 2020, Miller et al., 2020). In 2/5 cases of MIS-C described in our cohort, the initial diagnosis was appendicitis and the patients underwent an appendectomy. This phenomenon has been observed in other studies. Lishman et al reported 4 children with COVID-19 who presented with acute appendicitis of whom 3 were subsequently diagnosed with MIS-C (Lishman et al., 2020). The mechanism of acute appendicitis in these children remains unclear but has been hypothesized to be related to either vasculitis of the appendicular artery, reactive lymphoid hypertrophy, or inflammation resulting from viral invasion of the cells (Lishman et al., 2020). Surgeons, emergency physicians and pediatricians should keep an index of suspicion for COVID-19 and MIS-C when managing children with fever and acute abdomen during this pandemic.
In our study, 38 children (68%) had at least 1 family member with confirmed COVID-19, which is similar to studies in China and Saudi Arabia where a contact history was present in 70% of patients (Chan et al., 2020, Raba et al., 2020). This highlights the importance of infection prevention measures within households to prevent COVID-19 infection in children.
Data from adult studies showed very high rates of lymphopenia (Guan et al) among severely ill patients. However, the complete blood count has been reported to be normal in the majority of pediatric cases of COVID-19, which is consistent with our findings (Guan et al., 2020, Henry et al., 2020). Low hemoglobin level has been reported in infants with COVID-19 (Henry et al., 2020). In our cohort, anemia was present in 38% of cases and was more frequent among cases of MIS-C.
Liguoro et al in his systematic review found that elevated inflammatory markers in children with COVID-19 is uncommon and this is similar to what we have seen in our study (Liguoro et al., 2020). Elevated inflammatory markers has been associated with severe disease in children (Kainth et al., 2020, Zachariah et al., 2020). In this study, an increase in CRP, anemia for age, and leukocytosis were strongly associated with ICU admission.
The majority of patients in our study had normal findings on chest imaging. A systematic review showed that approximately 50% of children with confirmed COVID-19 had chest X-ray abnormalities, which is slightly higher than the rate in our study (37%) (Liguoro et al., 2020).
More than half of the patients in our cohort were treated empirically with antibiotics, and 16% were treated with oseltamivir despite a lack of evidence for the efficacy of these agents in cases of COVID-19 (Ibrahim & Olasinde, n.d.). We suspect this may reflect concerns among physicians for the potential severity of COVID-19 and concerns about co-infection with other viruses or bacteria. Hoang et al. reported co-infection with bacterial or viral organisms in only 5.6% of patients with COVID-19; only 4 patients in our study had co-infection (urinary tract infection) (Hoang et al., 2020). Reports of antibiotic use in children with COVID-19 have ranged from 19.4% to 100.0% (Wang et al., 2020b). Our study suggests that the majority of children admitted with COVID-19 do not require antimicrobials.
Children with COVID-19 tend to have milder disease manifestations and lower mortality compared to adults (Chan et al., 2020). Only 13% of our patients required intensive care and these cases were mostly children with MIS-C or underlying co-morbidities (Götzinger et al., 2020). However, the presence of an underlying condition was not significantly associated with ICU admission in our study. We suspect that this is mainly accounted for by the presence of critical illness among otherwise healthy children with MIS-C. Among children without MIS-C who required intensive care, sickle cell disease and cystic fibrosis were the main underlying conditions. The association between underlying comorbidities and severe COVID-19 has been suggested by other studies (Harman et al., 2020). The prognosis for children with underlying comorbid conditions and severe COVID-19 remained excellent in our study, with no reported mortality.
The limitation of this study is that it is retrospective and all data were collected from hospital medical records. Therefore, important information might have been omitted or not documented. As such, validation of our results with prospective studies is needed. Additionally, the sample size was relatively small in our study.
Conclusions
Most children hospitalized with confirmed COVID-19 have a mild course and favorable outcome. Sickle cell disease is the commonest comorbidity associated with COVID-19 admissions among Omani children. Children with sickle cell disease and COVID-19 are at increased risk for complications such as ACS. MIS-C is a serious complication of COVID-19 which can affect otherwise healthy children and can be misdiagnosed initially as acute appendicitis. Leukocytosis, elevated CRP, and anemia for age are independently associated with critical cases of COVID-19 among Omani children.
Declaration of interests
The authors declare that they have no competing financial interests or personal relationships that have appeared to influence the work reported in this paper.
Conflicts of interest
The authors declare that they have no competing financial interests or personal relationships that have appeared to influence the work reported in this paper.
Sources of support/fund
None
Ethical approval
This study was approved by the medical research and ethics committee of the College of Medicine and Health Sciences at Sultan Qaboos University, Oman and the central research committee of the Ministry of Health.
All authors made substantial contributions to this multicenter study based on the ICMJE criteria
Laila S Al Yazidi; Conceptualized, designed and registered the study, interpreted the data, drafted the data, drafted and revised the manuscript, and takes the responsibility for the integrity of the work.
Zaid Al Hinai: Conceptualized, designed the study and interpreted the data, drafted the data, and revised the manuscript.
Badriya Al Waili, Hilal Al Hasami, Nawal Al Maskari, Ammal Al Maani and Abdullah Al Qayoudhi: Conceptualized, designed the study, interpreted the data, and drafted and revised the manuscript.
Mohammed Al Reesi, Farhana Al Othmani, Bushra Al Jabri, Ibrahim Al Busaidi, Lamya Al Barwani, Nagi Elsidig, Nuha Al Tahir and Balqees Al Noobi: Conceptualized, designed the study, collected and summarized the data, and revised the manuscript.
Acknowledgements
We thank all the medical staff who worked hard in the emergency department, outpatient, intensive care, and pediatric isolation wards to manage these children. We also thank Dr Adil Al Wehaibi for helping with some statistical analysis.
References
- Belhadjer Z., Méot M., Bajolle F., Khraiche D., Legendre A., Abakka S., Auriau J., Grimaud M., Oualha M., Beghetti M., Wacker J., Ovaert C., Hascoet S., Selegny M., Malekzadeh-Milani S., Maltret A., Bosser G., Giroux N., Bonnemains L., Bonnet D. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luna G., Habibi A., Deux J.F., Colard M., Pham Hung d’Alexandry d’Orengiani A.L., Schlemmer F. Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol. 2020 doi: 10.1002/ajh.25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBiasi R.L., Song X., Delaney M., Bell M., Smith K., Pershad J. Severe Coronavirus Disease-2019 in Children and Young Adults in the Washington, DC, Metropolitan Region. J Pediatr. 2020;223 doi: 10.1016/j.jpeds.2020.05.007. 199-203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Jimenez D., Velasco Rodríguez-Belvís M., Ferrer Gonzalez P., Domínguez Ortega G., Segarra O., Medina Benitez E. COVID-19 Gastrointestinal Manifestations Are Independent Predictors of PICU Admission in Hospitalized Pediatric Patients. Pediat Infect Dis J. 2020 doi: 10.1097/INF.0000000000002935. [DOI] [PubMed] [Google Scholar]
- Götzinger F., Santiago-García B., Noguera-Julián A., Lanaspa M., Lancella L., Calò Carducci F.I. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman K., Verma A., Cook J., Radia T., Zuckerman M., Deep A. Ethnicity and COVID-19 in children with comorbidities. Lancet Child Adolesc Health. 2020;4(7):e24–e25. doi: 10.1016/S2352-4642(20)30167-X. Elsevier B.V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner C., Berteloot L., Tremolieres P., Dupic L., de Saint Blanquat L., Lesage F. Patients with sickle cell disease and suspected COVID-19 in a paediatric intensive care unit. Br J Haematol. 2020 doi: 10.1111/bjh.16802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Benoit S.W., de Oliveira M.H.S., Hsieh W.C., Benoit J., Ballout R.A. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): A pooled analysis and review. Clin Biochem. 2020 doi: 10.1016/j.clinbiochem.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang A., Chorath K., Moreira A., Evans M., Burmeister-Morton F., Burmeister F. COVID-19 in 7780 pediatric patients: A systematic review. E Clin Med. 2020;24 doi: 10.1016/j.eclinm.2020.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainth M.K., Goenka P.K., Williamson K.A., Fishbein J.S. Early Experience of COVID-19 in a US Children’s Hospital. Pediatrics. 2020;146(4) doi: 10.1542/peds.2020-003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguoro I., Pilotto C., Bonanni M., Ferrari M.E., Pusiol A., Nocerino A. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179(7):1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishman J., Kohler C., de Vos C., van der Zalm M.M., Itana J., Redfern A. Acute Appendicitis in Multisystem Inflammatory Syndrome in Children with COVID-19. Pediatr Infect Dis J. 2020;39(12):e472–e473. doi: 10.1097/INF.0000000000002900. [DOI] [PubMed] [Google Scholar]
- Miller J., Cantor A., Zachariah P., Ahn D., Martinez M., Margolis K.G. Gastrointestinal Symptoms as a Major Presentation Component of a Novel Multisystem Inflammatory Syndrome in Children That Is Related to Coronavirus Disease 2019: A Single Center Experience of 44 Cases. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepintoa J.A., Brandow A., Mucalo L., Yusuf F., Singh A., Taylor B. Coronavirus Disease among Persons with Sickle Cell Disease, United States, March 20-May 21, 2020. Emerging Infect Dis. 2020 doi: 10.3201/EID2610.202792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raba A.A., Abobaker A., Elgenaidi I.S., Daoud A. Novel coronavirus infection (COVID-19) in children younger than one year: A systematic review of symptoms, management and outcomes. Acta Paediatrica, Int J Paediatr. 2020;109(10):1948–1955. doi: 10.1111/apa.15422. Blackwell Publishing Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese G., John R., Manesh A., Karthik R., Abraham O. Clinical management of COVID-19. Indian J Med Res. 2020;151(5):401–410. doi: 10.4103/ijmr.IJMR_957_20. https://doi.org/10.4103/ijmr.IJMR_957_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhou Q., Wang C., Shi Q., Lu S., Ma Y. Clinical characteristics of children with COVID-19: a rapid review and meta-analysis. Ann Transl Med. 2020;8(10) doi: 10.21037/atm-20-3302. 620–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Coronavirus (COVID-19)Oman. https://doi.org/https://covid19.who.int/region/emro/country/om. [Google Scholar]
- WHO . 2020. Multisystem inflammatory syndrome in children and adolescents with COVID-19. Available from: https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. May, 1–3. [Google Scholar]
- Zachariah P., Zachariah P., Johnson C.L., Johnson C.L., Halabi K.C., Ahn D. Epidemiology, Clinical Features, and Disease Severity in Patients with Coronavirus Disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr. 2020;2019 doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]