Abstract
Identifying the individuals and geographical regions witnessing early infections or outbreaks of SARS-CoV-2 and its variants is helpful for studying the early epidemiology or even the origin of the novel coronavirus. Here, we put forward a strategy that can potentially contribute to this goal. Human body fluids and biological materials collected before the COVID-19 pandemic may serve as archives for retrospective testing of early human infections before the recent outbreaks. These have been routinely donated, collected, and archived, creating biorepositories or “biobanks” for clinical or research purposes. SARS-CoV-2 genetic materials and its antibodies have been confirmed in various types of biological samples from COVID-19 patients, including blood, sperm, umbilical cord blood, lung, heart, kidney and so on, making these biological archives as candidates for detecting early COVID-19 infections. Unlike sewage-based epidemiology which only provides information on the geographical aspect, viruses identified in archived human biological samples provide direct links to individuals, from whom a wealth of personal information including their profession, hobbies and activities, travel history, and previous exposure to wildlife can all be retrieved. By analyzing the patterns and links in the behavior of those early infected individuals, it is possible to trace the origin of the virus, for instance, in certain wild animals or local environments.
Keywords: Coronavirus, Origin, Source, Tracking, Donor, Environment
Graphical abstract
1. The quest of tracing early COVID-19 infections
Since the first case reported in Wuhan, China, the origin and earliest outbreak of the coronavirus disease (COVID-19) has been a subject of ongoing investigation and political debate (Lu et al., 2020, Cohen, 2020). Early infections offer the most valuable clues on the possible origin of COVID-19 (CDC, 2020a). Revolving the earliest cases in China, it was previously believed that the first case of COVID-19 was a man in Wuhan, the capital of Hubei province in China, on December 1, 2019 (Huang et al., 2020). On January 24, 2020, the Chinese Center for Disease Control and Prevention published a study, confirming that a cluster of patients with ‘pneumonia of an unknown cause’ had been identified in Wuhan on December 31, 2019 (Zhu et al., 2020). On March 13, 2020, South China Morning Post reported that there had been nine suspected cases of COVID-19 in November 2019, with the earliest one tracing back to November 17, 2019 (Ma, 2020).
Studies showed that archived samples of municipal sewage can be a useful proxy for detecting early COVID-19 outbreaks, given that SARS-CoV-2 is often shed in patients’ stools and urine at infectious loads (Jeong et al., 2020; Sun et al., 2020; Wölfel et al., 2020; Xiao et al., 2020). A recent study in Spain detected SARS-CoV-2 in archived sewage samples collected in Barcelona in March 2019 (Chavarria-Miró et al., 2020), putting the question on the onset of COVID-19 again under the spotlight. In southern Brazil, Fongaro et al. (2020) detected the novel coronavirus in sewage samples collected on November 27, 2019. Meanwhile, researchers found traces of SARS-CoV-2 in municipal wastewater sampled in Milan and Turin on December 18, 2019 (La Rosa et al., 2021), suggesting that COVID-19 had been circulating in northern Italy even before the first clustered infections identified in Wuhan. These findings contribute to a growing body of evidence suggesting that SARS-CoV-2, the causation agent of the COVID-19 pandemic, had been circulating in communities without being noticed, possibly well in advance of the early cases reported in Wuhan.
2. Challenges of using archived sewage for backtracking COVID-19
Sewage-based epidemiology has been advocated as a surveillance tool for monitoring illicit drugs (McCall et al., 2016), antimicrobial resistant genes (Munir et al., 2011) and recently, COVID-19 infections in communities (Lodder and de Roda Husman, 2020; O’Reilly et al., 2020). In the U.S., the Centers for Disease Control and Prevention (CDC) and the Department of Health and Human Services recently initiated the National Wastewater Surveillance System as an augmented tool to measure the extent of COVID-19 infections nationwide (CDC, 2020b). There are, however, several inherent drawbacks and limitations when adopting this approach for tracing early COVID-19 infections. The first and foremost obstacle is that, in most places, there is a complete lack of archived sewage samples available. Prior to recent advocates by the scientific community due to COVID-19, sewage archiving was not conducted as a regular practice, nor was it required by local municipalities or regulatory bodies. There were only about 20 countries which conducted regular sewage archiving, mostly for detecting illicit drug use (Feng et al., 2018). Most countries have not set up protocols or the infrastructure to store sewage samples for epidemiology studies, nor is there an existing global network for sewage archiving. Known as the largest repository of wastewater process flow samples, the Human Health Observatory at Arizona State University collects municipal wastewater and sludge samples from over 220 cities and stored them at −20 °C for further analysis. However, the initiative relies on voluntary submission of samples, with about 3/4 of the sampling sites located within the U.S. and the rest in seven countries, namely, Australia, Brazil, China, France, Ireland, Italy, and Kenya (ASU, 2021).
The detection limit of SARS-CoV-2 in municipal sewage is reported to be within 100 infections per million population (Kumar et al., 2020). Scattered cases of early infections, should they fail to propagate, can be difficult to detect in samples collected at centralized wastewater treatment plants serving large and populated urban catchments. Using data reported in recent studies, Hart and Halden (2020) established a model which, by assuming the absence of rainwater, commercial, and industrial flows in sewer systems, predicted that SARS-CoV-2 could be detected in municipal sewage with at least one person infected in a population of 14,000 to 33,000, at median rates of wastewater generation and virus excretion. It should be noted that such estimates are likely to be optimistic, given that countries are far from completing the work of combined sewer separation in urban communities (Han and He, 2020). Other environmental and human factors can have further impact on virus detection using this approach. For instance, temperature and in-sewer travel time are known to severely impact viral detectability in municipal sewage (Hart and Halden, 2020). Also, some catchments have dynamic population sizes which create additional uncertainties in estimating the magnitude of infections, especially in smaller communities with large numbers of non-residents (e.g., tourist and college towns). There are also technical challenges when applying this method in practice (Belfast, 2020; Sims and Kasprzyk-Hordern, 2020). One of the key challenges is the abundant and diverse range of compounds and microorganisms in municipal wastewater, which presents a rather complex matrix for isolation and analysis of a specific virus strain (Caicedo et al., 2019).
3. Using stored human body fluids and biological materials for retrospective investigations
Human biological materials, including body fluids and excretions (saliva, nasal discharge, sputum, sperm, umbilical cord blood), tissues (retinal, skeletal muscle, saphenous vein, rectal), and organs (lung, brain, kidney, heart, liver, pancreas, intestine) showed positive results in viral tests for COVID-19 (Casagrande et al., 2020; Chang et al., 2020; Sekulic et al., 2020; Vivanti et al., 2020; Wichmann et al., 2020; Yang et al., 2020). Sekulic et al. (2020) suggested using tissues from upper and lower human airways, immune organs (e.g., lymph nodes, spleen), and to a lesser degree the intestine, heart, and liver for viral testing of COVID-19 in an autopsy. In an interim guidance issued on June 4, 2020, the CDC recommends postmortem examinations to be performed on patients who died of suspected COVID-19, while any postmortem (upper and lower respiratory tract swab) or remaining specimens (e.g., nasopharyngeal swab, sputum, serum, stool) that may have been collected prior to death should be retained (CDC, 2020c). Postmortem examinations can provide reliable diagnosis of early patients who died of suspected COVID-19 infections, which may offer renewed insights into the early circulation of the virus in the community. For instance, the first COVID-19 death in the United States was believed to have occurred in Washington state on February 29, 2020 (CDC, 2020d). A few weeks later, the CDC confirmed that tissue samples from two individuals who died on 6 February and February 17, 2020 at their homes in Santa Clara County had tested positive for COVID-19 (Santa Clara County, 2020a). Confirmed by postmortem examinations, these cases indicated that while the first community infection of COVID-19 was not identified in the Santa Clara County until February 28, 2020 (Santa Clara County, 2020b), local transmission of the virus had started in this area at least three weeks earlier than previously believed. It should be noted that such examinations do not necessarily require postmortem samples. In a recent report, researchers in Italy detected SARS-CoV-2 RNA in an oropharyngeal swab specimen collected on December 5, 2019 (14 days after symptom onset) from a 4-year-old boy with suspected measles who lived in the surrounding area of Milan and had no travel history (Amendola et al., 2021). Confirmed by retrospective testing, this early case of infection is about 3 months before the first reported COVID-19 case in Italy.
Blood plasma samples from COVID-19 patients were widely tested positive for SARS-CoV-2 antibodies in serological testing (Perera et al., 2020). The CDC recently launched a large-scale seroprevalence survey to test de-identified clinical blood specimens from ten areas and blood donations collected between June 2020 to November 2021 from 25 metropolitan areas for SARS-CoV-2 antibodies (CDC, 2020e). While the survey itself focuses on gaining more accurate estimates on COVID-19 infections in the U.S. and the changes over time, it provides a proof-of-concept for adopting a similar strategy for tracing early COVID-19 infections using archived pre-pandemic blood samples stored in repositories (“blood banks”) as well as those clinical specimens collected at healthcare or research facilities. In fact, researchers have long exploited archived blood samples, with some collected years before the outbreak, to trace early human exposure to novel pathogens (Anderson et al., 2012; Bone et al., 2012). Retrospective investigations on three coronavirus outbreaks in the past 20 years showed that, in all of those events, the viruses had circulated in animals and humans earlier than people realized. Zheng et al. (2004) identified antibodies of SARS-CoV, SARS-CoV-like, or both types in 17 of the serum samples collected from 938 healthy adults in Hong Kong in May 2001. In those serum samples, the study identified a high ratio of antibodies to the SARS-CoV-like virus, isolated from Himalayan palm civets and racoon dogs in a live animal market in Guangdong, China, versus a relatively low ratio of antibodies to SARS-CoV, suggesting that a small portion of adults in Hong Kong had acquired the SARS-CoV-like virus with ineffective propagation at least two years before the SARS outbreak. Using a similar approach, Müller et al. (2014) tested 189 serum samples of dromedary camels originating from Egypt, Sudan, and Somalia, all collected between 1983 and 1997. MERS-CoV antibodies were found in 81.0% of the archived serum samples, showing unequivocal evidence on the long-term and widespread circulation of MERS-CoV or MERS-CoV-like viruses in dromedary camels.
Several studies have demonstrated the durability of SARS-CoV-2 antibodies in serum samples of COVID-19 patients (Gudbjartsson et al., 2020; Long et al., 2020), and determined the accuracies of using serological antibody testing for detecting COVID-19 infection (Guo et al., 2020; Long et al., 2020; Xiang et al., 2020). Meanwhile, questions have been raised on the accuracy of antibody-based COVID-19 testing as a rapid method for confirming ongoing COVID-19 infections in the population. Specifically, there were concerns on the sensitivity and specificity of the test, and the prevalence of serological antibodies in infected individuals as antibody levels could wane over time to undetectable levels, resulting in a limited time window for antibody-based COVID-19 testing to be effective. The CDC also pointed out that IgM and IgG antibodies may take 1–3 weeks to develop after infection, and some people may not develop detectable antibodies after COVID-19 infection (CDC, 2020f). While these may diminish its usefulness as an approach for detecting ongoing infections, for which the reliability needs to be improved by adopting smarter testing strategies (CDC, 2020f) or combining with other testing methods (Guo et al., 2020), the method itself serves a useful tool for conducting retrospective investigations on early infections and propagation of COVID-19, especially in the lack of pre-archived samples specifically fulfilling such a purpose. In fact, the usefulness of this approach was demonstrated in two recent serological surveys published in the interim during the review of this article, which showed real-life evidence that antibody testing of archived human blood donations can be a useful tool for identifying early infections of COVID-19 in the population before the first known local case of COVID-19 infection (Apolone et al., 2020; Basavarain et al., 2020). These findings confirmed the earlier observation of SARS-CoV-2 antibodies in a small number of stored plasma samples (n = 5) in Lombardy, Italy and the postulation that a prior unnoticed circulation of the virus had occurred in the area (Percivalle et al., 2020).
4. Benefits of this approach
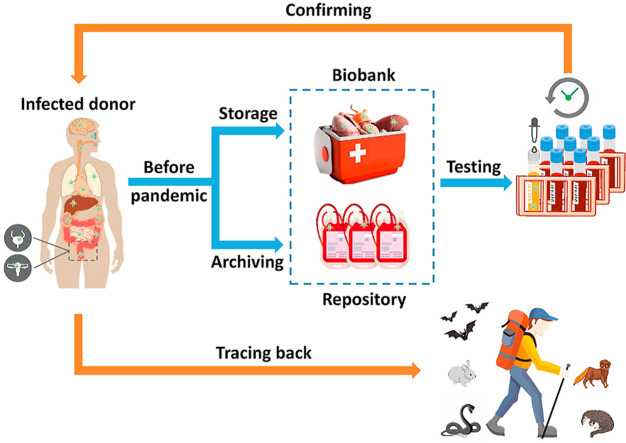
Many human body fluids and biological materials have important clinical and research uses. They have been routinely donated, collected, and stored in large biorepositories or “biobanks”, along with donors’ personal information (GODT, 2020; WHO, 2020a; WMDA, 2021). Given the long and continuous archiving timeline, these provide a vast archive of human biological materials for tracing early infections by the novel coronavirus and the early propagation prior to the eventual outbreaks. Using modern analytical methods and instrumentation, SARS-CoV-2, including its genetic materials, antibodies, and antigens can be detected in an array of human biological matrices with high detection sensitivity and target specificity (Loeffelholz and Tang, 2020; Perera et al., 2020). The wide availability of samples, long archiving timeline, and high detection sensitivity have led us to the proposition of using various biorepositories (“biobanks”) storing human body fluids and bodily materials, as archives for conducting retrospective investigations on COVID-19. Specifically, we propose using the detection of 1) SARS-CoV-2 antibodies in archived human blood samples and 2) SARS-CoV-2 RNA in other human body fluids and bodily materials collected prior to the pandemic, as a viable approach for identifying early infections and possibly the origin of COVID-19.
This approach offers several important advantages over sewage-based epidemiology. First of all, human body fluids and materials constitute an essential part of clinical supplies for blood perfusion, surgeries, implants, fertilization, and so on, which are routinely collected and stored in countries around the world, including in developing countries. The WHO Global Database on Blood Safety reported that, in 2018, over 126 million blood donations were received from voluntary unpaid donors in 108 countries, and donation rates ranged from 5.0 to 31.5 per thousand people worldwide (WHO, 2020a). In the United States, the country with the largest number of blood donors, a total of 17.2 million transfusing blood units were donated by over 13.2 million donors in 2015 (CDC, 2020g). Human body materials are collected and maintained in a similar manner, albeit in smaller quantities. According to the Global Observatory on Donation and Transplantation, there were 38,594 deceased donors and 146,840 organ transplants around the globe in 2018 (GODT, 2020). In the U.S. alone, there were 10,722 deceased donors and 37,386 organ transplants in 2018, followed by China which registered 6302 deceased donors and 20,201 organ transplants in that year. Statistics from the World Marrow Donor Association showed that, as of January 21, 2021, there were 37, 632, 842 cord blood donors and 800, 532 cord blood units in public cord blood banks located in 54 countries (WMDA, 2021). In addition to voluntary unpaid donations, paid donations have been collected as additional resources for clinical uses and research purposes (Katz et al., 2010). Such an enormous amount of human biological materials provides a vast and readily available biological archive for conducting retrospective investigations on the emergence and early prorogation of the novel coronavirus.
Given that archived human blood samples or bodily materials are stored in sterile environments under cryogenic conditions, they usually have long storage lives which allow the detection of the novel coronavirus or its early variants long before the first known outbreak. Many countries have mandates in place requiring years of storage for archived human biological samples along with the information of donors and receptors for look-back investigations in the event of an adverse reaction. Archiving periods of blood donations usually range from one to five years (e.g., Australia, France, the Netherlands) but can be as long as 30 years (e.g., Japan, the UK) or even indefinite in some countries (e.g., Scotland, Ireland) (Bone et al., 2012; Franklin et al., 2007). Organ procurement organizations recommended that blood donations from deceased donors should be kept at −70 °C or lower for at least 10 years (Seem et al., 2013), whereas the UK Guidelines for the Blood Transfusion Services specified that blood samples from tissue donors should be kept for a minimum of 11 years after the expiry date of the tissue with the longest storage life (JPAC, 2013). The NetCord-FACT International Standards for Cord Blood Collection, Banking, and Release for Administration required at least one sample to be retained from each cord blood unit, which should be stored indefinitely at −150 °C or a lower temperature (FACT, 2019). Non-interrupted cryogenic storage of archived human biological samples enables excellent preservation of viruses and their genetic materials. In a retrospective study, Franklin et al. (2007) showed that HCV, HIV RNA, HBV genetic materials and antibodies in human blood samples could survive storage for at least 15 years at −20 °C. An earlier study by Sanlidag et al. (2005) demonstrated that quantities of HBV DNA in human serum samples were stable after being subjected to ten freeze-thaw cycles involving overnight storage at −20 °C and thawing in a 30 °C water bath for 30 min.
Lastly and perhaps most importantly, archived human biological samples contain the IDs of donors, from which links can be established between infections and donors’ personal information. Unlike sewage-based epidemiology which only provides information on the geographical aspect, viruses identified in human biological fluids or bodily materials give direct links to host individuals, from whom a wealth of information including their profession, hobbies and activities, travel history, and prior exposure to wild animals could be retrieved. By analyzing the patterns and links in the behavior of early infected individuals, it is possible to trace the origin of the virus, for instance, in certain wild animals or local natural environments. It is also feasible to isolate early virus strains from archived human biological samples. Full genome sequencing can be performed on SARS-CoV-2 strains isolated from frozen or formalin-fixed-paraffin-embedded tissues for phylogenetic analysis, which may help identify the closest coronavirus strains in wild species (Chan et al., 2020; Forster et al., 2020; Maitra et al., 2020; Sekulic et al., 2020). These benefits have been demonstrated in a real-life event where researchers inadvertently used this approach in tracing the transmission of rabies, a zoonotic virus, in an early infected individual that was previously unidentified (Wallace et al., 2014). An organ recipient who had not been exposed to infected animals was confirmed of rabies infection in February 2013, after receiving a kidney transplant 18 months earlier. Researchers explored the possibility of transplant-acquired infection by testing tissues of the organ donor stored since 2011. Rabies antigen was found in the archived brain tissues of the donor, and genetic sequence of the virus was found to be identical to the strain isolated from the recipient, confirming kidney transplantation as the route of transmission. The genetic sequence was closely associated with a raccoon variant circulating in North Carolina, USA, which was the state of residence by the donor. Exposure history of the donor was later obtained from his family and friends, confirming that the individual was an avid hunter and trapper with extensive exposures to wildlife, who was bitten by raccoons twice in February 2010 and January 2011, without seeking medical attention. A conclusion was drawn from the investigation that transmission of the zoonotic virus most probably occurred when the donor was bitten by an infected raccoon in his home state.
5. A proposed roadmap
The enormous amount of archived human body fluids and biological materials makes it a daunting task for researchers to conduct sample testing on any significant portion of the existing collection. With this in mind, sample screening and prioritization is essential for this approach to be adopted. As a first step, countries and regions reporting early cases of COVID-19 infections (e.g., Wuhan, Barcelona, California, Lombardy) should be given priority for conducting look-back investigations. Also, given that people infected with COVID-19 often develop flu-like symptoms or respiratory illness (e.g., persistent fever, coughing, difficulty in breathing) (Singhal, 2020), priority could also be given to communities where flu infections or mortality rates from respiratory diseases increased abnormally prior to the COVID-19 outbreak, regardless of earlier diagnoses on these individuals. All samples should be tested in a reverse chronological order, starting with the date of the first confirmed local case then working backwards to earlier samples. Also, despite the existing challenges, exploration of human blood-based COVID-19 detection methods is strategically important given the fact that blood donations constitute the most abundant and widely available form of regularly archived human biological samples, compared with other body fluids, organs, tissues, and excreta.
Where a significant number of positive samples are identified in archived human biological materials originated from a particular area within a certain period of time, scientists can then use these as unequivocal clues to identify signs of COVID-19 infection in the wider population in the target area by, for instance, reviewing the medical records of patients admitted to local hospitals and clinics in that particular area within a defined set of dates. In many places, records such as transcripts of verbal communications revolving patients’ symptoms, medical diagnoses, and lab testing results of the patients’ biological samples are routinely archived and stored, for possible follow-up visits or conducting medical reviews. These are readily accessible by medical professionals and patients, and consents can be obtained by public health authorities for research purposes. Given the general small percentages of donors in the entire population, medical records may be exploited as a supplementary source of information, preferably in conjunction with analyses of donors’ biological materials, to identify early infections of COVID-19 in the wider population in the target geographical region.
Our proposed strategy may help identify the places where the novel coronavirus first appeared or during its early stage of propagation, possibly well before the first reported outbreak, by narrowing down the communities, timeframes, and identifying the early infected individuals and even the genome sequence of early virus strains circulating in these communities. Ultimately, such information will help pin-point the source of SARS-CoV-2 and the initial transmission route to humans, which will also be valuable for preventing the spread of zoonotic pathogens in the future. Since it has been over ten months since the onset of COVID-19, we call for urgent actions on preserving human body fluids and biological materials collected pre-pandemic, including those submitted to various biorepositories and others inadvertently kept in intermittent storage facilities, as useful archives for future look-back investigations on COVID-19. This is particularly relevant for countries requiring relatively short periods of storage (e.g., one year) for archived human biological materials. Meanwhile, hospitals, clinics, and laboratories should preserve their clinical samples with intact records of patients’ information for epidemiology studies on COVID-19. It is important for developing countries to have access to the necessary facilities and infrastructures for storing their archived human biological materials collected both before and through the current pandemic. Lastly, we wish to point out that given the month-to-year long stability of coronaviruses in both refrigerated and frozen states (Aboubakr et al., 2020; WHO, 2020b), it may be necessary to sample blood donations and human biological materials collected shortly before and during the current pandemic and test them for COVID-19 before clinical use, to rule out possible donor-to-receptor transmission of the virus.
6. An imperative task requiring cooperative efforts
The COVID-19 pandemic is becoming the greatest public health crisis since the influenza pandemic in 1918. At the time of writing, there have been nearly 95 million confirmed cases around the globe, including over 2 million deaths (WHO, 2021). The United Nations (UN) estimated that the global economy would lose nearly $8.5 trillion in output over the next two years due to the pandemic, wiping out nearly all gains of the previous four years and pushing more than 34 million people into extreme poverty in 2020 (UN, 2020). Tracing the origin of SARS-CoV-2 is of great importance for prevention and preparedness for similar diseases in the future, a goal that is of common interests to all mankind. The proposed strategy, using human body fluids and biological materials to trace early COVID-19 infections, had to be met with collaborations from scientists, medical practitioners, and regulatory authorities in different counties. This is particularly important given the fact that, since biobanks were established, the lack of transparency in their usage has been a major hurdle in their continuing development as an essential public health infrastructure for medical and research purposes, for which recommendations have been made to promote the transparency in the use and governance of biobanks (European Commission, 2012; Harris et al., 2012). For biobanks to be used as sources of archived human biological samples, openness and transparency will be the key for conducting productive retrospective investigations on the early infections of COVID-19 and toward the goal of tracing the geographical and natural origin of COVID-19.
There are a number of reasons for conducting such retrospective investigations, apart from solving the political disputes on the geographical origin of the novel coronavirus – an elusive goal that is also beyond the scope of our discussion. Essentially, there are three reasons that make such retrospective investigations meaningful and potentially important (CDC, 2020h; Hu et al., 2017; Reingold, 1998). First, findings from these investigations will provide useful data for studying the epidemiology of COVID-19, particularly with respect to its early propagation in the population. Secondly, any early variants of the SARS-CoV-2 virus detected in pre-pandemic samples provide important insights into the evolution of the virus. Lastly but perhaps most importantly, linking virus detection to human behavior is one of the key benefits of using human biological samples – as opposed to sewage samples – for detecting COVID-19 infections. Provided that animal-to-human transmission can be identified from such investigations, as in the North Carolina case in the United States (Wallace et al., 2014), one may be able to identify the natural host of the novel coronavirus as well as the specific transmission path and environment causing early human infections of the virus or its early variants. These will help public health authorities determine high-risk activities and environments and implement policies or monitoring programs for long-term prevention and control of zoonotic pathogens spilling over to humans via similar hosts or transmission paths. Ultimately, findings from these investigations will help prevent additional cases and avoid future emergence of disease outbreaks caused by zoonotic pathogens.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was funded by the Young Talent Support Plan of Xi’an Jiaotong University.
Footnotes
This paper has been recommended for acceptance by Dr. Da Chen.
References
- Aboubakr H.A., Sharafeldin T.A., Goyal S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: a review. Transbound. Emerg. Dis. 2020:1–17. doi: 10.1111/tbed.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola A., Bianchi S., Gori M., Colzani D., Canuti M., Borghi E., Raviglione M.C., Zuccotti G.V., Tanzi E. Evidence of SARS-CoV-2 RNA in an oropharyngeal swab specimen, milan, Italy, early December 2019. Emerg. Infect. Dis. 2021 doi: 10.3201/eid2702.204632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T.C., Bromfield C.R., Crawford P.C., Dodds W.J., Gibbs E.P.J., Hernandez J.A. Serological evidence of H3N8 canine influenza-like virus circulation in USA dogs prior to 2004. Vet. J. 2012;191(3):312–316. doi: 10.1016/j.tvjl.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Apolone G., Montomoli E., Manenti A., Boeri M., Sabia F., Hyseni I., Mazzini L., Martinuzzi D., Cantone L., Milanese G., Sestini S., Suatoni P., Marchianò A., Bollati V., Sozzi G., Pastorino U. Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy. Tumori J. 2020 doi: 10.1177/0300891620974755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASU (Arizona State University) 2021. Human Health Observatory.https://biodesign.asu.edu/environmental-health-engineering/human-health-observatory [Google Scholar]
- Basavaraju S.V., Patton M.E., Grimm K., Rasheed M.A.U., Lester S., Mills L., Stumpf M., Freeman B., Tamin A., Harcourt J., Schiffer J., Semenova V., Li H., Alston B., Ategbole M., Bolcen S., Boulay D., Browning P., Cronin Li, David E., Desai R., Epperson M., Gorantla Y., Jia T., Maniatis P., Moss K., Ortiz K., Park S.H., Patel P., Qin Y., Steward-Clark E., Tatum H., Vogan A., Zellner B., Drobeniuc J., Sapiano M.R.P., Havers F., Reed C., Gerber S., Thornburg N.J., Stramer S.L. Serologic testing of U.S. blood donations to identify SARS-CoV-2-reactive antibodies: December 2019-January 2020. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfast C. Sewage monitoring is the UK’s next defense against covid-19. BMJ. 2020;370:m2599. doi: 10.1136/bmj.m2599. [DOI] [PubMed] [Google Scholar]
- Bone A., Guthmann J., Assal A., Rousset D., Degeorges A., Morel P., Valette M., Enouf V., Jacquot E., Pelletier B., Strat Y.L., Pillonel J., Fonteneau L., van der Werf S., Lina B., Tiberghien P., Lévy-Bruhl D. Incidence of H1N1 2009 virus infection through the analysis of paired plasma specimens among blood donors, France. PloS One. 2012;7(3) doi: 10.1371/journal.pone.0033056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo C., Rosenwinkel K., Exner M., Verstraete W., Suchenwirth R., Hartemann P., Nogueira R. Legionella occurrence in municipal and industrial wastewater treatment plants and risks of reclaimed wastewater reuse: Review. Water Res. 2019;149:21–34. doi: 10.1016/j.watres.2018.10.080. [DOI] [PubMed] [Google Scholar]
- Casagrande M., Fitzek A., Püschel K., Aleshcheva G., Schultheiss H., Berneking L., Spitzer M.S., Schultheiss M. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul. Immunol. Inflamm. 2020;28(5):721–725. doi: 10.1080/09273948.2020.1770301. [DOI] [PubMed] [Google Scholar]
- CDC . 2020. About COVID-19 Epidemiology: Investigating COVID-19: The Science Behind CDC’s Response.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/about-epidemiology/index.html [Google Scholar]
- CDC . 2020. National Wastewater surveillance system (NWSS): a New Public Health Tool to Understand COVID-19 Spread in a Community.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/wastewater-surveillance.html [Google Scholar]
- CDC . 2020. Collection and Submission of Postmortem Specimens from Deceased Persons with Known or Suspected COVID-19: Interim Guidance.https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html [Google Scholar]
- CDC . 2020. CDC, Washington State Report First COVID-19 Death.https://www.cdc.gov/media/releases/2020/s0229-COVID-19-first-death.html [Google Scholar]
- CDC . 2020. Large-Scale Geographic Seroprevalence Surveys.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/geographic-seroprevalence-surveys.html [Google Scholar]
- CDC . 2020. Interim Guidelines for COVID-19 Antibody Testing.https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html [Google Scholar]
- CDC . 2020. Blood Safety Basics.https://www.cdc.gov/bloodsafety/basics.html [Google Scholar]
- CDC . 2020. About COVID-19 Epidemiology, Investigating COVID-19: The Science Behind CDC’s Response.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/about-epidemiology/index.html [Google Scholar]
- Chan J.F., Yuan S., Kok K., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H., Lo S.K., Chan K., Poon V.K., Chan W., Ip J.D., Cai J., Cheng V.C., Chen H., Hui C.K., Yuen K. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Zhao L., Gong H., Wang L., Wang L. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg. Infect. Dis. 2020;26:1631–1633. doi: 10.3201/eid2607.200839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria-Miró G., Anfruns-Estrada E., Guix S., Paraira M., Galofré B., Sáanchez G., Pintó R., Bosch A. Sentinel surveillance of SARS-CoV-2 in wastewater anticipates the occurrence of COVID-19 cases. medRxiv. 2020 doi: 10.1101/2020.06.13.20129627. [DOI] [Google Scholar]
- Cohen J. 2020. Trump ‘owes us an apology.’ Chinese scientist at the center of COVID-19 origin theories speaks out. [DOI] [Google Scholar]
- European Commission . 2012. Biobanks for Europe: A challenge for governance, report of the expert group with ethical and regulatory challenges of international biobank research. [DOI] [Google Scholar]
- FACT (Foundation for the Accreditation of Cellular Therapy) 2019. NetCord-FACT International Standards for Cord Blood Collection, Banking, and Release for Administration Seventh Edition.http://www.factwebsite.org/cbstandards/ [Google Scholar]
- Feng L., Zhang W., Li X. Monitoring of regional drug abuse through wastewater-based epidemiology - a critical review. Sci. China Earth Sci. 2018;61:239–255. doi: 10.1007/s11430-017-9129-x. [DOI] [Google Scholar]
- Fongaro G., Stoco P.H., Souza D.S.M., Grisard E.C., Magri M.E., Rogovski P., Schorner M.A., Barazzetti F.H., Christoff A.P., de Oliveira L.F.V., Bazzo M.L., Wagner G., Hernandez M., Rodriguez-Lazaro D. 2020. SARS-CoV-2 in Human Sewage in Santa Catalina, Brazil, November 2019. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. U.S.A. 2020;117(17):9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin I.M., Dow B.C., Jordan A.D. Benefits of a blood donation archive repository: international survey of donor repository procedures and Scottish experiences. Transfusion. 2007;47(7):1172–1179. doi: 10.1111/j.1537-2995.2007.01251.x. [DOI] [PubMed] [Google Scholar]
- GODT (Global Observatory on Donation and Transplantation) 2020. Data (Charts and tables)http://www.transplant-observatory.org/ [DOI] [PubMed] [Google Scholar]
- Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., Arnthorsson A.O., Helgason D., Bjarnadottir K., Ingvarsson R.F., Thorsteinsdottir B., Kristjansdottir S., Birgisdottir K., Kristinsdottir A.M., Sigurdsson M.I., Arnadottir G.A., Ivarsdottir E.V., Andresdottir M., Jonsson F., Agustsdottir A.B., Berglund J., Eiriksdottir B., Fridriksdottir R., Gardarsdottir E.E., Gottfredsson M., Gretarsdottir O.S., Gudmundsdottir S., Gudmundsson K.R., Gunnarsdottir T.R., Gylfason A., Helgason A., Jensson B.O., Jonasdottir A., Jonsson H., Kristjansson T., Kristinsson K.G., Magnusdottir D.N., Magnusson O.T., Olafsdottir L.B., Rognvaldsson S., le Roux L., Sigmundsdottir G., Sigurdsson A., Sveinbjornsson G., Sveinsdottir K.E., Sveinsdottir M., Thorarensen E.A., Thorbjornsson B., Thordardottir M., Saemundsdottir J., Kristjansson S.H., Josefsdottir K.S., Masson G., Georgsson G., Kristjansson M., Moller A., Palsson R., Gudnason T., Thorsteinsdottir U., Jonsdottir I., Sulem P., Stefansson K. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020;383(18):1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Ren L., Yang S., Xiao M., Chang D., Yang F., Cruz C.D.C., Wang Y., Wu C., Xiao Y., Zhang L., Han L., Dang S., Xu Y., Yang Q., Xu S., Zhu H., Xu Y., Jin Q., Sharma L., Wang L., Wang J. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin. Infect. Dis. 2020;71(15):778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., He S. Urban flooding events pose risks of virus spread during the novel coronavirus (COVID-19) pandemic. Sci. Total Environ. 2020;142491 doi: 10.1016/j.scitotenv.2020.142491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J.R., Burton P., Knoppers B.M., Lindpaintner K., Bledsoe M., Brookes A.J., Budin-Ljøsne I., Chisholm R., Cox D., Deschênes M., Fortier I., Hainaut P., Hewitt R., Kaye J., Litton J., Metspalu A., Ollier B., Palmer L.J., Palotie A., Pasterk M., Perola M., Riegman P.H.J., van Ommen G., Yuille M., Zatloukal K. Toward a roadmap in global biobanking for health. Eur. J. Hum. Genet. 2012;20:1105–1111. doi: 10.1038/ejhg.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Zeng L., Yang X., Ge X., Zhang W., Li B., Xie J., Shen X., Zhang Y., Wang N., Luo D., Zheng X., Wang M., Daszak P., Wang L., Cui J., Shi Z. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus on providing new insight into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H.M., Kim S., Kim H., Kim Y., Kim J.H., Cho J.Y., Kim S., Kang H., KimS, Park S., Kim E., Choi Y.K. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020;26(11):1520–1524. doi: 10.1016/j.cmi.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JPAC (Joint United Kingdom Blood Transfusion and Tissue Transplantation Services Professional Advisory Committee) 2013. Archiving of Donor Samples.https://www.transfusionguidelines.org/red-book/chapter-20-tissue-banking-selection-of-donors/20-10-archiving-of-donor-samples [Google Scholar]
- Katz G., Mills A., Garcia J., Hooper K., McGuckin C., Platz A., Rebulla P., Salvaterra E., Schmidt A.H., Torrabadella M. Banking cord blood stem cells: attitude and knowledge of pregnant women in five European countries. Transfusion. 2010;51(3):578–586. doi: 10.1111/j.1537-2995.2010.02954.x. [DOI] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Ferraro G.B., Veneri C., Iaconelli M., Bonadonna L., Lucentini L., Suffredini E. SARS-CoV-2 has been circulating in northern Italy since December 2019: evidence from environmental monitoring. Sci. Total Environ. 2021;750:141711. doi: 10.1016/j.scitotenv.2020.141711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffelholz M.J., Tang Y. Laboratory diagnosis of emerging human coronavirus infections – the state of the art. Emerg. Microb. Infect. 2020;9(1):747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q., Liu B., Deng H., Wu G., Deng K., Chen Y., Liao P., Qiu J., Lin Y., Cai X., Wang D., Hu Y., Ren J., Tang N., Xu Y., Yu L., Mo Z., Gong F., Zhang X., Tian W., Hu L., Zhang X., Xiang J., Du H., Liu H., Lang C., Luo X., Wu S., Cui X., Zhou Z., Zhu M., Wang J., Xue C., Li X., Wang L., Li Z., Wang K., Niu C., Yang Q., Tang X., Zhang Y., Liu X., Li J., Zhang D., Zhang F., Liu P., Yuan J., Li Q., Hu J., Chen J., Huang A. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. 10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. 2020. Coronavirus: China’s First Confirmed Covid-19 Case Traced Back to November 17. South China Morning Post.https://www.scmp.com/news/china/society/article/3074991/coronavirus-chinas-first-confirmed-covid-19-case-traced-back [Google Scholar]
- Maitra A., Sarkar M.C., Raheja H., Biswas N.K., Chakraborti S., Singh A.K., Ghosh S., Sarkar S., Patra S., Mondal R.K., Ghosh T., Chatterjee A., Banu H., Majumdar A., Chinnaswamy S., Srinivasan N., Dutta S., Das S. Mutations in SARS-CoV-2 viral RNA identified in Eastern India: possible implications for the ongoing outbreak in India and impact on viral structure and host susceptibility. J. Biosci. 2020;45:76. doi: 10.1007/s12038-020-00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall A., Bade R., Kinyua J., Lai F.Y., Thai P.K., Covaci A., Bijlsma L., van Nuijs A.L.N., Orta C. Critical review on the stability of illicit drugs in sewers and wastewater samples. Water Res. 2016;88:933–947. doi: 10.1016/j.watres.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Munir M., Wong K., Xagoraraki I. Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res. 2011;45(2):681–693. doi: 10.1016/j.watres.2010.08.033. [DOI] [PubMed] [Google Scholar]
- Müller M.A., Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Bosch B., Lattwein E., Hilali M., Musa B.E., Bornstein S., Drosten C. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983-1997. Emerg. Infect. Dis. 2014;20(12):2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reill K.M., Allen D.J., Fine P., Asghar H. The challenges of informative wastewater sampling for SARS-CoV-2 must be met: lessons from polio eradication. The Lancet Microbe. 2020;1(5):e189–e190. doi: 10.1016/S2666-5247(20)30100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percivalle E., Cambiè G., Cassaniti I., Nepita E.V., Maserati R., Ferrari A., Martino R.D., Isernia P., Mojoli F., Bruno R., Tirani M., Cereda D., Nicora C., Lombardo M., Baldanti F. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill. 2020;25(28):2001285. doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R.A., Mok C.P., Tsang O.T., Lv H., Ko R.L., Wu N.C., Yuan M., Leung W.S., Chan J.M., Chik T.S., Choi C.Y., Leung K., Chan K.H., Chan K.C., Li K., Wu J.T., Wilson I.A., Monto A.S., Poon L.L., Peiris M. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill. 2020;25(16):2000421. doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold A.L. Outbreak investigations—a perspective. Emerg. Infect. Dis. 1998;4(1):21–27. doi: 10.3201/eid0401.980104. https://wwwnc.cdc.gov/eid/article/4/1/98-0104_article [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlidag T., Akcali S., Ozbakkaloglu B. Serum hepatitis B DNA: stability in relation to multiple freeze-thaw procedures. J. Virol. Methods. 2005;123(1):49–52. doi: 10.1016/j.jviromet.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Santa Clara County . 2020. County of Santa Clara Identifies Three Additional Early COVID-19 Deaths.https://www.sccgov.org/sites/covid19/Pages/press-release-04-21-20-early.aspx [Google Scholar]
- Santa Clara County . 2020. County of Santa Clara Public Health Department Announces First Death from COVID-19 in Santa Clara County.https://www.sccgov.org/sites/phd/news/Pages/first-covid-19-death.aspx [Google Scholar]
- Seem D.L., Lee I., Umscheid C.A., Kuehnert M.J. Excerpt from PHS guideline for reducing HIV, HBV and HCV transmission through organ transplantation. Am. J. Transplant. 2013;13(8):1953–1962. doi: 10.1111/ajt.12386. [DOI] [PubMed] [Google Scholar]
- Sekulic M., Harper H., Nezami B.G., Shen D.L., Sekulic S.P., Koeth A.T., Harding C.V., Gilmore H., Sadri N. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am. J. Clin. Pathol. 2020;154(2):190–200. doi: 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N., Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020;139:105689. doi: 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J. Pediatr. 2020;87(4):281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., Shi Y., Zhang Z., Chen S., Liu X., Dai J., Li X., Huang S., Huang X., Luo L., Wen L., Zhuo J., Li Y., W ang Y., Zhang L., Zhang Y., Li F., Feng L., Chen X., Zhong N., Yang Z., Huang J., Zhao J., Li Y. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microb. Infect. 2020;9(1):991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN (United Nations) 2020. COVID-19 to Slash Global Economic Output by $8.5 Trillion over Next Two Years.https://www.un.org/development/desa/en/news/policy/wesp-mid-2020-report.html [Google Scholar]
- Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Cao J.D., Benachi A., Luca D.D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R.M., Stanek D., Griese S., Krulak D., Vora N.M., Pacha L., Kan V., Said M., Williams C., Burgess T.J., Clausen S.S., Austin C., Gabel J., Lehman M., Finelli L.N., Selvaggi G., Joyce P., Gordin F., Benator D., Bettano A., Cersovsky S., Blackmore C., Jones S.V., Buchanan B.D., Fernandez A.I., Dinelli D., Agnes K., Clark A., Gill J., Irmler M., Blythe D., Mitchell K., Whitman T.J., Zapor M.J., Zorich S., Witkop C., Jenkins P., Mora P., Droller D., Turner S., Dunn L., Williams P., Richards C., Ewing G., Chapman K., Corbitt C., Girimont T., Franka R., Recuenco S., Blanton J.D., Feldman K.A. A large-scale, rapid public health response to rabies in an organ recipient and the previously undiagnosed organ donor. Zoonoses Public Health. 2014;61(8):560–570. doi: 10.1111/zph.12105. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) 2020. Blood Safety and Availability.https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability [Google Scholar]
- WHO (World Health Organization) 2020. Coronavirus Disease 2019 (COVID-19) Situation Report 32.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200221-sitrep-32-covid-19.pdf [Google Scholar]
- WHO (World Health Organization) 2021. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ [Google Scholar]
- Wichmann D., Sperhake J., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer C., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel M., Kluge S. Autospy findings and venous thromboem bolism in patients with COVID-19. Ann. Intern. Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WMDA (World Marrow Donor Association) 2021. Total Number of Donors and Cord Blood Units.https://statistics.wmda.info/ [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J., Zhou Q., Ye H., Ma Y., Li H., Wei X., Cai P., Ma W. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin. Infect. Dis. 2020;71(8):1930–1934. doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833. doi: 10.1053/j.gastro.2020.02.055. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Chen S., Huang B., Zhong J., Su H., Chen Y., Cao Q., Ma J., He J., Li X., Li X., Zhou J., Fan J., Luo D., Chang X., Arkun K., Zhou M., Nie X. Pathological findings in the Testes of COVID-19 patients: clinical implications. Eur. Urol. Focus. 2020;6(5):1124–1129. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B.J., Wong K.H., Zhou J., Wong K.L., Young B.W.Y., Lu L.W., Lee S.S. SARS-related virus predating SARS outbreak, Hong Kong. Emerg. Infect. Dis. 2004;10:176–178. doi: 10.3201/eid1002.030533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Xi Zhao, Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]