Abstract
Introduction
Invasive pulmonary aspergillosis is a well-known complication of acute respiratory distress syndrome, the most serious manifestation of COVID-19. Four recent studies have reported its incidence among ICU COVID-19 patients. However, they do not share the same case definition, and have provided conflicting results. In this paper we have aimed at reported the incidence of invasive pulmonary aspergillosis for COVID-19 patients in our ICU, and at comparing the different definitions in order to assess their respective relevance.
Methods
Retrospective cohort study of critically ill patients with severe COVID-19 requiring ICU management between 1st March and 30th April 2020.
Results
Our results showed significantly lower incidence of invasive pulmonary aspergillosis (1.8%;1/53), compared to three out of four previous studies, and wide variation in the numbers of cases with regard to the different definitions.
Conclusion
Large-scale studies are needed for a better definition and a more accurate estimation of invasive pulmonary aspergillosis coinfection during COVID-19.
Keywords: COVID-19, Aspergillosis, Coronavirus Infections/pathology, Pulmonary Aspergillosis/diagnosis
1. Introduction
Invasive pulmonary aspergillosis (IPA) is a well-known complication of acute respiratory distress syndrome (ARDS) in critically ill patients, particularly as reported with severe influenza [1], [2]. In coronavirus disease 2019 (COVID-19) context, ARDS is the main manifestation of COVID-19 managed in intensive care units (ICU) [3], [4]. Consequently, a question arises about the burden of IPA among these patients.
Four recent studies have reported IPA prevalence in COVID-19 patients: in one by Alanio et al. [5], IPA was diagnosed in 9/27 (33%) patients receiving mechanical ventilation. However, their diagnostic criteria differed from those previously applied in ICUs, namely the Asp-ICU algorithm and its variant [1], [2], the EORTC guidelines [6], and the recently published expert consensus case definition [7] for influenza-associated pulmonary aspergillosis (IAPA) in ICU patients. Two other studies used the modified Asp-ICU criteria [2] and found IPA in 9.7% and 26% of COVID-19 patients, respectively [8], [9]. Finally, the most recent study [10] used the expert consensus case definition for IAPA and identified IPA in 27.7% of COVID-19 patients.
The main objectives of our study were to report the prevalence of IPA in COVID-19 patients in our ICU, and to compare the different definitions used during the SARS-Cov-2 pandemics in order to discuss their respective relevance.
2. Methods
This retrospective cohort study was approved by the Ethics Committee of the Société de Réanimation de Langue Française (IRB approval CE SRLF #20-42) and registered with the Institut National des Données de Sante (MR 4109060520). Informed consent was obtained for all individual participants included in the study.
We included all successive patients hospitalized in our ICU for severe COVID-19 (confirmed through RT-PCR assay) between 1st March and 30th April 2020 who had been routinely screened for aspergillosis by both serum galactomannan assay (SGA) and mycological culture of respiratory samples. Patients were excluded if either SGA or mycological culture was missing.
During this study period, all included patients received standard care for ARDS according to international guidelines, including invasive ventilation and prone positioning when needed. It was recommended, but not strictly protocolized, that patients be screened by both SGA and mycological culture of tracheal aspirate (TA) at day 4 after admission and subsequently once a week. BAL was performed when clinically indicated, with mycological culture.
Data were collected prospectively and analysed retrospectively with a focus on mycological data. We initially described the tests used to evaluate the Aspergillus burden in our population, and then evaluated how many cases of IPA were diagnosed using the five different definitions; lastly, we compared our results with those recently published by other teams, (Table 1 ).
Table 1.
Comparison of the number of cases of invasive pulmonary aspergillosis, according to Asp-ICU criteria, modified Asp-ICU criteria, EORTC criteria and Alanio study criteria.
| Alanio study n = 27 | Koehler study n = 19 | Van Arkel study n = 31 | Bartolleti study n = 108 | Present study n = 53 | ||
|---|---|---|---|---|---|---|
| Alanio criteria |
Aspergillus positive culture from BAL Or 2 of the followings: Aspergillus positive TA Positive Aspergillus qPCR in BAL, TA, or serum Galactomannan index > 0.8 on BAL or > 0.5 on serum β-D-glucan > 80 pg/ml in serum |
9 | 5 | 3 | 20 | 1 |
| Asp-ICU criteria | All 4 criteria (if one missing, colonisation): Aspergillus-positive lower respiratory tract sample (entry criterion) Compatible clinical signs and symptoms Abnormal medical imaging Either host risk factor or Aspergillus-positive culture from BAL |
6 and colonisation in one case | 2 and colonisation in one case | 3 and colonisation in 3 cases | 19 and colonisation in one case | 1 and colonisation in one case |
| Modified Asp-ICU criteria (used by Koehler and van Arkel) | Clinical criteria, radiological criteria and one or more of the followings: Aspergillus positive culture from BAL Galactomannan index ≥ 1 on BAL or ≥ 0.5 on serum |
7 | 4 | 3 | 30 | 2 |
| EORTC criteria | At least one host factora, one clinical feature and mycologic evidence in the followings: Aspergillus positive culture from sputum, BAL or TA Positive Aspergillus qPCR on BAL or serum Galactomannan index > 1 on BAL or serum |
7 | 5 | 6 | 36 | 2 |
| Expert consensus case criteria for IAPA (used by Bartolleti) | A: Pulmonary infiltrate and at least one of the following: Aspergillus positive culture from BAL Galactomannan index ≥ 1 on BAL or ≥ 0.5 on serum OR B: Cavitating infiltrate (not attributed to another cause) and at least one of the following: Aspergillus positive culture from sputum or TA |
7 | 4 | 3 | 30 | 2 |
| Antifungal Treatment | 1 | 4 | 3 | 16 | 1 |
For the COVID-19 population, COVID-19 with ARDS was considered as a host factor by itself.
The five definitions we used for diagnosis of to diagnose IPA during COVID-19 were as follows: the Asp-ICU algorithm and its variant [1], [2], the EORTC criteria [6], the expert consensus case criteria for IAPA [7], and Alanio's definition [5]. Patients receiving antifungal treatment for aspergillosis were also recorded.
For descriptive analysis, categorical variables are presented as counts and percentages.
Relationships between categorical variables were tested using the Fisher exact test for group comparison; statistical significance was considered for P-values < 0.05. All statistical analyses were performed using the R 3.5 software package (http://cran.r-project.org).
3. Results
Among the 76 patients admitted to our ICU with confirmed COVID-19, 53 met our inclusion criteria, and 23 were excluded (Fig. 1 ). The main patient characteristics are described in Table 2 . One patient presented with a positive culture from TA and BAL, which was associated with pulmonary cavitating infiltrate. One patient had positive culture from TA, with both negative BAL and SGA, and another had positive SGA without positive culture. According to the criteria used by Alanio, IPA incidence in our study was then 1/53 (1.9%), significantly lower than in three of the four earlier studies (Alanio [5], P = 0.0001; Koehler [9], P = 0.004; van Arkel [8], P = 0.14; Bartolleti [10], P = 0.00003, Fisher's exact tests). The same incidence was found with the Asp-ICU criteria (plus one colonisation), while the three other definitions showed an incidence of 2/53 (3.8%), which was still significantly lower than in three out of four studies (Alanio [5], P = 0.0006; Koehler [9], P = 0.01; van Arkel [8], P = 0.35; Bartolleti [10], P = 0.0002, Fisher's exact tests).
Figure 1.
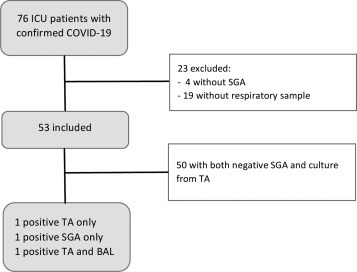
Flow-chart. SGA: serum galactomannan assay; TA: tracheal aspirate; BAL: broncho-alveolar lavage.
Table 2.
Main patient characteristics.
| n = 53 | n (%) or Median [IQR] |
|---|---|
| Age (years) | 64.0 [55.0–74.0] |
| Sex, Male | 36 (67.9) |
| Active smokers | 2 (3.8) |
| Comorbidities | |
| Ischemic heart disease | 4 (7.5) |
| Hypertension | 23 (43.4) |
| Diabetes | 13 (24.5) |
| Immunodeficiencya | 1 (1.9) |
| Body Mass Index > 30 kg/m2 | 18 (34.0) |
| COPD | 7 (13.2) |
| ICU management | |
| Time between first symptoms to ICU admission | 9.0 [7.0–13.0] |
| SAPS2 | 41 [34–50] |
| High-flow oxygen before intubation | 7 (13.2) |
| Invasive mechanical ventilation | 53 (100) |
| PaO2/FiO2 after intubation | 153 [117–192] |
| Prone position | 36 (67.5) |
| ECMO | 4 (7.5) |
| Tracheotomy | 9 (17.0) |
| Ventilator-associated pneumonia | 29 (54.7) |
| Duration of invasive ventilation (days) | 20 [13.0–36.0] |
| Catecholamine | 50 (94.3) |
| Renal replacement therapy | 11 (20.8) |
| Antibiotherapy at admission | 100% |
| Steroids | 12 (22.6) |
| Antifungal therapy during ICU stay | 2 |
| Biology at ICU admission | |
| LDH (UI/L) | 803 [660–1149] |
| Lymphocytes (G/L) | 0.8 [0.5–1.0] |
| Outcome | |
| ICU length of stay (days) | 24 [14–45] |
| ICU mortality | 19 (35.8) |
| Hospital mortality | 19 (35.8) |
| Day-90 mortality | 20 (37.7) |
defined as haematological malignancies, allogenic stem cell transplant, solid organ transplant, prolonged use of steroids at a therapeutic dose of ≥ 0.3 mg/kg/day for more than 3 weeks in the past 60 days, treatment with recognized T-cell or B-cell immunotherapies during the past 90 days, inherited severe immunodeficiency, or acute graft versus host disease grade III or IV.
In our study, the patient with a diagnosis of COVID-19-associated IPA received antifungal treatment. Comparatively, 1/9 (Alanio), 4/5 (Koehler), 3/3 (van Arkel) and 16/30 (Bartoletti) patients in the previous studies received antifungal treatments, respectively (Table 1).
4. Discussion
We identified COVID-19-associated IPA in 1.9% of our population. According to the various definitions used in previous reports, prevalence of COVID-19-associated IPA ranged from 1.9% to 33% (Table 1).
Prevalence mismatch might be explained not only by varied diagnostic criteria, but also by differences in baseline populations. For example, we included only one patient with underlying immunodepression, whereas Bartoletti and Alanio included 10 and 20% of immunocompromised patients, respectively. Our results might be more extendable to the whole COVID-19 ICU patient population, given that immunosuppression is not known to be an underlying factor of COVID-19. It should be noted that similar results were mentioned in a recent letter [11] reporting a prevalence of 3.8% in a non-immunocompromised population, as was the case in a preliminary French study [12].
It should also be acknowledged that incidence may be influenced by the design of mycological testing; too much testing results in a higher risk of false positive, whereas not enough testing reduces sensitivity. As we have suggested, when SGA and mycological TA culture are carried out at day 4 after admission and subsequently once a week, this bias can be reduced.
However, the highest reported incidences might be overestimated, as fewer patients receive antifungal treatment for IPA compared to the diagnosed cases. Indeed, we believe each IPA confirmed case must receive antifungal therapy; if a patient does not, we could assume that his practitioner did not believe in IPA diagnosis.
Furthermore, multiple criteria for IPA diagnostic are available, and the most reliable for COVID-19 patient are presently being determined, as was recently the case for IAPA [7]. Whereas, as recently noted by experts [13], there exists no perfect definition, we strongly believe the most realistic one should focus on specificity, as Asp-ICU, focuses on specificity. Depending on the criteria used, the number of cases might support major variations, thereby underlining the importance of carefully choosing which definition to use. That is why we carefully examined the number of IPA cases in the four studies, using the five previously mentioned definitions for IPA, and indeed found variation, with different numbers of cases for each definition of IPA (Table 1). In our study, incidence variation was reduced from 3.7% to 1.9%, but the difference was much more pronounced in other studies, ranging from 33% to 22% (Alanio), from 26% to 10% (Koehler), from 19% to 10% (van Arkel), and from 33% to 18% (Bartolleti).
Even though SGA was seldom positive, it was included in all studies as a diagnostic criteria, suggesting, as previously observed [14], poor sensitivity of this test in COVID-19 patients. On the other hand, while BAL galactomannan was not always available, when tested it was more relevant to IPA diagnosis, being found positive in all 30 cases in the Bartolleti study. As it was not routinely available for our study population, it could help to explain the incidence mismatch. While mycological TA cultures may be more sensitive and are widely available, they are insufficiently specific to distinguish colonisation from IPA. At this point, the Asp-ICU algorithm seems to be the most restrictive but also the most accurate and realistic way to identify patients who need treatment. However, this algorithm requires BAL, which has not been widely performed in COVID-19 patients due to the risk of disseminating the virus by aerosolization and thereby infecting the operator. The expert consensus case definition for CAPA seems quite similar to the modified Asp-ICU criteria, with the addition of positive culture from sputum or TA in case of pulmonary cavitating infiltrates. Nevertheless, they share the same flaw as the Asp-ICU algorithm, namely the need for BAL in order to make a diagnosis. Compared to the Asp-ICU algorithm, they seem to be more sensitive and less specific, which could lead to overtreatment. The fact that a single patient in the study by Alanio et al. received treatment suggests overdiagnosis of IPA using their criteria, and the same can be said for the EORTC criteria with COVID-19 as a host factor, which rule out these two definitions as the best candidates for COVID-19-associated IPA.
5. Conclusion
Although less common than previously reported, COVID-19-associated IPA should not be neglected. Standardized investigations are needed to distinguish Aspergillus colonisation from IPA, and there is also a need to develop a consensus case definition for COVID-19-associated IPA. Asp-ICU algorithm and the expert consensus case definition for IAPA applied to COVID-19 seem to be the best candidates. Large-scale cohort studies in COVID-19 ICU patients are needed, in view of achieving better understanding, diagnosis and estimation of the IPA burden in these patients.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments.
Funding
This work has been supported by: Centre Hospitalier de Versailles André Mignot.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship.
The corresponding author had full access to all the study data and takes responsibility for the integrity of the data and accuracy of the data analysis.
Conceptualization: Gouzien, Bruneel
Data curation: Gouzien, Ferré
Formal analysis: Gouzien, Ferré
Funding acquisition: Bruneel
Investigation: Gouzien, Cocherie, Eloy, Ferré, Bedos, Simon, Marque-Juillet, Legriel, Bruneel
Methodology: Gouzien, Eloy, Ferré, Marque-Juillet, Legriel, Bruneel
Project administration: Gouzien, Legriel, Bruneel
Resources: Gouzien, Legriel, Bruneel
Software: Gouzien, Legriel, Bruneel
Supervision: Bruneel
Validation: Gouzien, Eloy, Marque-Juillet, Legriel, Bruneel
Visualization: Gouzien, Legriel, Bruneel
Roles/ Writing – Original draft: Gouzien
Writing – review and editing: Gouzien, Eloy, Marque-Juillet, Legriel, Ferré, Bruneel.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgment
We thank the Centre Hospitalier de Versailles for editorial assistance. We are indebted to Antoinette Wolfe for assistance in English language editing.
References
- 1.Blot S.I., Taccone F.S., Van den Abeele A.-M., et al. A clinical algorithm to diagnose invasive pulmonary Aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 2.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respirat Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 5.Alanio A., Dellière S., Fodil S., et al. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. The Lancet Respiratory Medicine. 2020 doi: 10.1016/S2213-2600(20)30237-X. [S221326002030237X] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed]
- 7.Verweij P.E., Rijnders B.J.A., Brüggemann R.J.M., et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., et al. COVID-19 associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehler P, Cornely OA, Böttiger BW, et al. COVID-19 Associated Pulmonary Aspergillosis. Mycoses n/a: https://doi.org/10.1111/myc.13096.
- 10.Bartoletti M., Pascale R., Cricca M., et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamoth F., Glampedakis E., Boillat-Blanco N., et al. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saint Leger P., Zarrougui W., Sedrati F., et al. Aspergillose pulmonaire invasive chez les patients avec forme sévère de COVID-19: résultats d’une cohorte monocentrique française. Med Malad Infect. 2020;50:S86. doi: 10.1016/j.medmal.2020.06.173. [DOI] [Google Scholar]
- 13.Bassetti M., Kollef M.H., Timsit J.-F. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. 2020;46:2071–2074. doi: 10.1007/s00134-020-06219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verweij P.E., Gangneux J.-P., Bassetti M., et al. Diagnosing COVID-19-associated pulmonary aspergillosis. The Lancet Microbe 0. 2020 doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


