Abstract
Background
Our healthcare institution was one of the first to see SARS CoV-2 cases in the country. We describe the early COVID-19 experience of a private hospital in the Philippines and discuss the healthcare system response in the setting of surge capacity.
Methods
We reviewed the medical records of adult COVID-19 hospitalized patients admitted in March 2020. We reported their demographic and clinical characteristics using descriptive statistics.
Results
Of 40 patients admitted, 23 (57.5%) were male and 19 (47.5%) were aged <60 years. Most (n = 27, 67.5%) had moderate-risk, 9 (22.5%) had high-risk, and 4 (10%) had low-risk COVID-19. SARS-CoV-2 testing took 5.5 (range 1–10) days. Overall mortality rate was 6/40 (15.0%). Clinical cure was documented in all low-risk patients, 25 (92.6%) moderate-risk patients, and only 1 (11.1%) high-risk patient. In response to the surge, the hospital rapidly introduced one-way traffic systems, dedicated screening, triage and Emergency Department areas for COVID-19, a clinical pathway, engineering controls, patient cohorting, and strict infection prevention and control measures.
Conclusion
Majority of patients recovered from COVID-19. Older age and high-risk pneumonia were associated with poor outcomes. Adaptations to hospital structure and staff were quickly made in response to surge capacity, although our response was hampered by prolonged time to COVID-19 confirmation. Our study underscores the urgent need for rapid adaptive response by the healthcare system to address the surge of cases.
Keywords: COVID-19, Surge capacity, Healthcare response, Clinical profile, Outcome
1. Introduction
The novel coronavirus, SARS COV-2, first isolated in Hubei, China in December 2019, has caused a global pandemic.1 As of October 8, 2020 there were 36,002,827 cases worldwide, with 1,049,810 reported deaths by the World Health Organization (WHO) daily tracker. Case series from China have been published, describing the epidemiology and early outcomes of COVID-19.2, 3, 4 The first case of COVID-19 in the Philippines was confirmed on January 27, 20205 and local transmission was reported on March 7, 2020.6
The Medical City (TMC) was one of the first private hospitals in the Philippines to report a confirmed COVID-19 case. To date, published data regarding healthcare system response in the Asia-Pacific region remain limited, with few published case series on COVID-19 in the Philippines, and none in the private health care setting. We aimed to: (1) describe the clinical characteristics, hospital course, and outcomes of the first 40 hospitalized Filipino patients diagnosed with COVID-19; and (2) describe healthcare system measures undertaken to respond rapidly to the COVID-19 surge.
2. Methods
2.1. Patient selection
We reviewed the medical records of all hospitalized, confirmed COVID-19 patients ≥18 years old at TMC, Pasig City, Philippines from March 5, 2020–March 28, 2020. We excluded the following: patients <18 years old, patients with suspected or probable COVID-19 not confirmed by RT-PCR, and those admitted for <24 h. The TMC institutional review board (IRB) approved this retrospective study (IRB # GCS-Med-2020-030) including waiver of patient informed consent. Through chart review, study authors (CLA, EDV, BT, JB, JF) obtained demographic data, information on exposure or travel, clinical symptoms at presentation, relevant physical examination findings, and laboratory and radiologic results on pre-determined days (Day 0, 3, 5 7, 10, 14, 21, and 28). All laboratory tests, radiologic assessments and treatments were performed at the discretion of the healthcare team. For those patients still admitted, patient data were censored at the time of data cutoff, on April 12, 2020. The study authors (CLA, EDV) created a database using the Research Electronic Data Capture software (REDCap, Vanderbilt University) (https://redcapinfo.ucdenver.edu/citing-redcap.html ).
2.2. Definitions
For this study, the following definitions were used: ARI – persons with acute respiratory infection7; Probable COVID-19 – symptomatic individuals suspected to have COVID-198; Confirmed COVID-19 – any individual with a positive RNA RT-PCR test for SARS CoV-2; Low-risk COVID-19 – any individual confirmed to have COVID-19 and fulfilling criteria for community-acquired pneumonia low-risk (CAP-LR) as stated in the interim Philippine Society for Microbiology and Infectious Diseases (PSMID) guidelines9; Moderate-risk COVID-19 – any individual confirmed to have COVID-19 and fulfilling criteria for community-acquired pneumonia moderate-risk (CAP–MR) as stated in the interim PSMID guidelines9; High-risk or severe COVID-19 - any individual confirmed to have COVID-19 and fulfilling criteria for CAP-high risk as stated in the interim PSMID guidelines,9 or admitted in the ICU. We used standard definitions from the United States (US) Centers for Disease Control (CDC) for hospital-acquired infections including hospital acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), and catheter-related bloodstream infections (CRBSI).10 Acute respiratory distress syndrome (ARDS) was defined using the Berlin Definition.11 We defined patient outcomes as follows: Clinical cure – composite of clinical improvement (e.g., no fever for 24 hours, decreasing oxygen requirement, improvement in cough/well being, improvement in imaging, decline in inflammatory parameters); virologic cure – repeat swab for SARS CoV-2 negative at least once at the time of discharge.
2.3. Specimen collection and testing
Clinical specimens for COVID-19 diagnostic testing were obtained in accordance with the PSMID guidelines. All clinical specimens were tested using Sansure Biotech diagnostic kits (PCR-Fluorescent Probe). The kit is an RNA-based one-tube technology mobile platform equipped with an automatic nucleic acid extractor, shortening detection time and allowing recognition of suspected cases.12 All RT-PCR tests were run by the Research Institute for Tropical Medicine (RITM), Philippines.
2.4. Data analysis
For this case series, we used descriptive statistics and determined frequency distributions of demographic and clinical characteristics for quantitative variables. We used median as our measure of central tendency in a small patient population with small and large values. For measures of dispersion, we provided the range of the quantitative variables, and added information on the interquartile range (IQR), particularly for skewed distributions and variables with outliers.
3. Results
3.1. Characteristics of the study cohort
Of the first 40 confirmed cases of COVID-19, 23 (57.5%) were male, with a median age of 60.5 (range 22–86) years. Most (n = 27, 67.5%) were diagnosed to have moderate-risk COVID-19; 9 (22.5%) had high-risk; and 4 (10%) had low-risk COVID-19. Among those ≥60 years (n = 21), 15 (71.4%) had moderate-risk COVID-19; 6 (28.6%) had high risk COVID-19; and none were low-risk (Table 1 ). Majority of patients (n = 27, 67.5%) had at least one co-morbid illness, the most common being cardiovascular disease (CVD) (n = 23, 85.2% and diabetes mellitus (DM) (n = 14, 51.9%).
Table 1.
Demographic, clinical, and laboratory profile of the COVID-19 Patients according to Severity of Illness.
| CHARACTERISTIC | ALL CASES (N = 40)< | Low Risk (n = 4) | Moderate Risk (n = 27) | High Risk (n = 9) |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Age in years | ||||
| Median (IQR) min−max |
60.5 (26) 22–86 |
34.5 (19) 22–45 |
62.0 (28) 25–77 |
65.2 (22) 47–86 |
| Age, ≥ 60 years, No. (%) | 21 (52.5) | 0 | 15 (55.6) | 6 (66.7) |
| Sex, Male, No. (%) | 23 (57.5) | 0 | 19 (70.4) | 4 (44.4) |
| History of consult as outpatient prior to admission, No. (%) | 19 (47.5) | 0 | 14 (51.8) | 5 (55.6) |
| Days between onset of symptoms and consult, | ||||
| Median (IQR) min−max |
4 (5) 0–9 |
– | 3 (3) 1–7 |
6 (2) 0–9 |
| Days between onset of symptoms and admission, Median (IQR) min−max |
n = 39 7 (4) 0–42 |
n = 3 8 (12) 4–16 |
n = 27 7 (4) 0–42 |
n = 9 6 (3) 2–14 |
| LABORATORY | ||||
| COVID-19 RT-PCR | ||||
| Turn-around time, days | ||||
| Median (IQR) min−max |
5.5 (4) 1–10 |
3 (4) 2–10 |
6 (5) 1–9 |
5 (4) 2–9 |
| COMPLETE BLOOD COUNT (n = 37) | n = 4 | n = 24 | n = 9 | |
| Hemoglobin (g/L) | ||||
| Median (IQR) min−max |
133 (24) 108–184 |
136 (3) 133–139 |
139 (30.5) 108–184 |
126 (9) 115–140 |
| Hematocrit Median (IQR) min−max |
0.40 (0.08) 0.31–0.56 |
0.41 (0.06) 0.31–0.42 |
0.42 (0.07) 0.31–0.56 |
0.38 (0.03) 0.35–0.41 |
| WBC (x109/L) Median (IQR) min−max |
5.8 (4.6) 3.6–22.4 |
5.9 (5.47) 5.19–16.04 |
5.6 (4.48) 3.6–17.79 |
7.1 (7.4) 4.1–22.4 |
| Platelets (x109/L) categories, No. (%) | ||||
| 100–150 | 2/37 (5.4) | 0 | 0 | 2 (22.2) |
| >150 | 35/37 (94.6) | 4 (100) | 24 (100) | 7 (77.8) |
| Cytopenia categories, No. (%) | ||||
| None | 35/37 (94.6) | 4 (100) | 23 (95.8) | 8 (88.9) |
| One-lineage | 2/37 (5.4) | 0 | 1 (4.2) | 1 (11.1) |
| Creatinine (mg/dL) categories, No. (%) | n = 4 | n = 22 | n = 9 | |
| <1 | 25/35 (71.4) | 4 (100.0) | 16 (72.7) | 5 (55.6) |
| 1–2 | 8/35 (22.9) | 0 | 5 (22.7) | 3 (33.3) |
| >2 | 2/35 (5.7) | 0 | 1 (4.5%) | 1 (11.1) |
| ALT (IU/L) categories, No. (%) | ||||
| Normal (0–50) | 12/18 (66.7) | 1 (100) | 10 (83.3) | 1 (20.0) |
| 1-2x elevated (>50 to 100) | 5/18 (27.8) | 0 | 1 (8.3) | 4 (80.0) |
| 2-3x elevated (>100 to <150) | 1/18 (5.6) | 0 | 1 (8.3) | 0 |
| >3x elevated (≥150) | 0 | – | – | – |
| IMAGING | ||||
| Initial chest x-ray findings, No. (%) | ||||
| Bilateral infiltrate | 18 (45.0) | 0 | 12 (44.4) | 6 (66.7) |
| Unilateral | 5 (12.5) | 0 | 3 (11.1) | 2 (22.2) |
| Normal | 10 (25.0) | 3 (75.0) | 6 (22.2) | 1 (11.1) |
| Other Findings | 5 (12.5) | 1 (25.0) | 4 (14.8) | 0 |
| Not Done | 2 (5.0) | 0 | 2 (7.4) | 0 |
| Chest CT Scan, No. (%) | ||||
| Unilateral | 1 (2.5) | 1 (25.0) | 0 | 0 |
| Bilateral infiltrate | 11 (27.5) | 0 | 10 (37.0) | 1 (11.1) |
| Not done | 28 (70.0) | 3 (75.0) | 17 (63.0) | 8 (88.9) |
ALT – alanine aminotransferase, AST – aspartate aminotransferase, IQR – interquartile range, NI – not indicated, TMC – The Medical City.
We also noted a cluster of cases within a family with possible secondary transmission to a health care worker (HCW) (Supplementary Appendix A Fig. A.1). Only five patients (12.5%) had a history of international travel within 14 days of symptom onset – two arrived from the US and one each from Thailand, Saudi Arabia, and the United Kingdom.
3.2. Signs and symptoms
Nineteen patients (47.5%) consulted in the ambulatory setting or at another hospital prior to hospitalization; median time from symptom onset to date of first ambulatory consult was 4 days (IQR = 5).
Majority (n = 39, 97.5%) of patients had symptoms. Median time from onset of first symptom to date of admission was 7 (range 0–42) days. The most common symptoms were: cough (n = 33, 84.6%) and fever (n = 28, 74.3%), followed by dyspnea (n = 15, 38.5%) and generalized weakness (n = 15, 38.5%) (Table 1). Among patients with details on the nature of their cough (n = 29), it was described as dry (n = 14), productive (n = 15), or intermittent (n = 7). Dyspnea was described only in a few patients (n = 7), characterized as occurring at rest (n = 3) or progressive (n = 7), while no further details on the symptom of weakness were available. The temperature was <38°C among 24 (60%) patients, with overall median temperature of 37.8°C (range 36.0–39.7°C). Fever occurred in 19 patients during hospital admission, with a range of 37.8–39.7°C and median duration of 3 (range: 1–29) days. Median time to confirmation of COVID-19 diagnosis by RT-PCR was 5.5 (range 1–10) days.
3.3. Diagnostic findings
On admission, 37 (92.5%) had a baseline complete blood count. Median (min-max) white blood cell count and absolute lymphocyte count were 5.8 × 109 cells/L (3.6–22.4), and >1 × 109 cells/L, respectively. Viral film array (6/40) and rapid influenza tests (3/40) were negative. Admission chest radiographs were obtained in 38 patients (95%), showing bilateral interstitial infiltrates in 18 (47.3%), normal radiographic findings in 10 (26.3%) and unilateral infiltrates in 5 (13.1%) patients. Only 12 patients had high-resolution computed tomographic (CT) scan of the chest with majority (11/12, 91.67%) showing bilateral findings (Table 1).
3.4. Drugs used for treatment
3.4.1. Medications for COVID-19
After routine consent was obtained, at least one investigational drug for COVID-19 was administered (n = 29, 72.5%). Chloroquine (CQ) (n = 18) or hydroxychloroquine (HCQ) (n = 9) was given most frequently, followed by lopinavir/ritonavir (LPV/r) (7). Only 5 patients (18.5%) received tocilizumab.
Most patients (35/40, 87.5%) were given at least one antibiotic for bacterial pneumonia. Out of 14 patients given azithromycin, 6 were given concomitant CQ and another 6 received HCQ. Only 6 patients were given corticosteroid therapy (Supplementary Appendix B Table B.1).
3.5. Intensive care unit admission
Nine (22.5%) patients needed admission to the intensive care unit (ICU), with 1 unable to transfer from the Emergency Department (ED) for 3 days due to bed unavailability. Of those admitted to the ICU, 5 had at least one co-morbid disease (2 CVD, 2 CVD/DM, and 1 CVD/DM/chronic lung disease). Five patients were given corticosteroids. All ICU admissions except 1 required at least 1 critical care intervention, including initiation of pressor support (n = 8), invasive mechanical ventilation (n = 8), renal replacement therapy (n = 6), and proning (n = 6). Median length of ICU stay was 14 (range 3–24) days. Initial sequential organ failure assessment (SOFA) score was calculable for 8 patients, with median SOFA scores increasing with length of stay: on Day 0 – median score of 3 (range 1–4); on day 3 –median score of 5.5 (range 0–11); and on day 7 – median score of 8 (range 0–14).
3.6. Outcomes
At the time of reporting, 30 (75%) patients were discharged improved; 1 (2.5%) was discharged against medical advice; 3 (7.5%) remain hospitalized; and 6 (15%) died, all of whom had high-risk COVID-19. Mortality rate was 12.5% (6/40), with five deaths attributable to COVID-19. Median hospital length of stay among survivors (n = 31, 3 still admitted) and non-survivors (n = 6) was 12 (range: 3–32 days) and 14 (range: 8–24) days, respectively (Table 2 ).
Table 2.
Complications and outcomes according to severity of COVID-19.
| OUTCOMES | ALL CASES (N = 40) | Low-Risk (n = 4) | Moderate-Risk (n = 27) | High-Risk (n = 9) |
|---|---|---|---|---|
| OUTCOMES, No. (%) | ||||
| Discharged recovered | 30 (75.0) | 4 (100) | 25 (92.6) | 1 (11.1) |
| Discharged against medical advice | 1 (2.5) | 0 | 1 (3.7) | 0 |
| Hospitalized/Still admitted | 3 (7.5) | 0 | 1 (3.7) | 2 (22.2) |
| Mortality | 6 (15.0) | 0 | 0 | 6 (66.7) |
| COMPLICATIONS, No. (%) | ||||
| Number of patients who experienced complications | 12 (30.0) | 0 | 4 (14.8) | 8 (88.9) |
| ARDS | 8 (20.0) | 0 | 0 | 8 (88.9) |
| Nosocomial infectiona | 5 (12.5) | 0 | 3 (11.1) | 2 (22.2) |
| Septic shock | 5 (12.5) | 0 | 0 | 5 (55.6) |
| AKI requiring RRT | 6 (15.0) | 0 | 0 | 6 (66.7) |
| Otherb: | 3 (7.5) | 0 | 1 (3.7) | 2 (22.2) |
| LENGTH OF STAY | ||||
| Hospital LOS, survivors, days Median (IQR) min-max |
n = 31 12.0 (7) 3–32 |
n = 3 9.0 (12) 4–16 |
n = 25 12.0 (7) 3–32 |
n = 1 14 days |
| Hospital LOS, non-survivors, days Median (IQR) min-max |
n = 6 14.0 (11) 8–24 |
n = 0 – |
n = 0 – |
n = 6 14.0 (11) 8–24 |
| ICU stay, No. (%) | 9 (22.5) | 0 | 1 (3.7) | 8 (88.9) |
| Duration of ICU stay, days Median (IQR) min-max |
n = 9 14.0 (11) 3–24 |
n = 0 – |
n = 1 1 day |
n = 8 15.0 (10.5) 8–24 |
| Clinical cure, No. (%) | ||||
| Yes | 30 (75.0) | 4 (100) | 25 (92.6) | 1 (11.1) |
| No | 10 (25.0) | 0 | 2 (7.7) | 8 (88.9) |
| Virologic cure, No. (%) | ||||
| Yes | 25 (62.5) | 4 (100) | 19 (70.4) | 2 (22.2) |
| No | 6 (15.0) | 0 | 5 (18.5) | 1 (11.1) |
| Unknown | 9 (22.5) | 0 | 3 (11.1) | 6 (66.7) |
| Time to virologic cure, days Median (IQR) min-max |
n = 25 13.0 (4) 8–27 |
n = 4 16.0 (5) 14–24 |
n = 19 13.0 (6) 8–27 |
n = 2 19.5 (13) 13–26 |
| Mortality, No. (%) | 6 (15.0) | – | – | 6 (66.7) |
| COVID-related | 5 (12.5) | – | – | 5 (55.6) |
| Not COVID-related | 1 (2.5) | – | – | 1 (11.1) |
AKI – acute kidney injury, ARDS – Acute Respiratory Distress Syndrome, CRBSI – Catheter Related Bloodstream Infection HAP –Hospital Acquired Pneumonia, IQR −interquartile range, LOS – length of stay, RRT – renal replacement therapy VAP – Ventilator Associated Pneumonia.
VAP (3), HAP (2), CRBSI (2).
Encephalopathy (1), pneumomediastinum (1), pneumothorax (1).
3.7. Complications
Twelve (30%) patients developed at least one complication during the hospital course (Table 2). Of those with complications, 8 developed ARDS; 6 developed acute kidney injury needing renal replacement therapy; and 5 were diagnosed with septic shock and secondary bacteremia. Hospital-acquired infections including HAP/VAP developed in 5, and CRBSI occurred in 2. Eight of the 12 patients (66.7%) who developed complications had CAP–HR while the remaining 4 had CAP–MR, of whom 3 acquired HAP, and 1 developed encephalopathy. More patients in the >60 age group developed complications (8/21 [38.1%] vs. 4/19 [21.05%]) and needed ICU level care (7/21 [34.09%] vs. 2/19 [10.53%]).
3.8. Clinical and virologic cure
Clinical cure was documented in all low-risk COVID-19 patients; 24 out of 27 moderate-risk COVID-19 patients (88.9%); and only 1 of 9 high-risk patients (11.1%). Time to clinical cure for low to moderate risk COVID-19 took a median of 18 (5–54) days. Time to virologic cure was also shorter in the low and moderate-risk categories, taking a median of 16 (range 14–24) and 13 (range 8–27) days, respectively, compared to a median of 19.5 (range 13–26) days in the high-risk group (Table 2).
Among survivors, median length of hospital stay between the two different age groups was similar [12 (range 4–30) days vs. 11 (range 3–32) days]. The younger age group had a higher frequency of clinical (17/19 [89.5%] vs. 13/21 [61.9%]) and virologic cures (15/19 [78.9%] vs. 10/21 [47.5%]), and a lower mortality rate (2/19 [10.5%] vs. 4/21 [19%]) compared to the older age group (Supplementary Appendix B Table B.2).
4. The TMC healthcare systems response
As a Joint Commission International-accredited hospital, TMC has established systems in place for health disasters and emerging and re-emerging diseases such as SARS in 2003, leptospirosis in 2009, and dengue in 2018. The surge of COVID-19 cases in the TMC ED and the critical care units posed a unique healthcare challenge like no other. In response, TMC made initial preparations by convening its Epidemic Rapid Response Team on January 20, 2020, subsequently meeting weekly to prepare the hospital for the surge of COVID-19 patients.
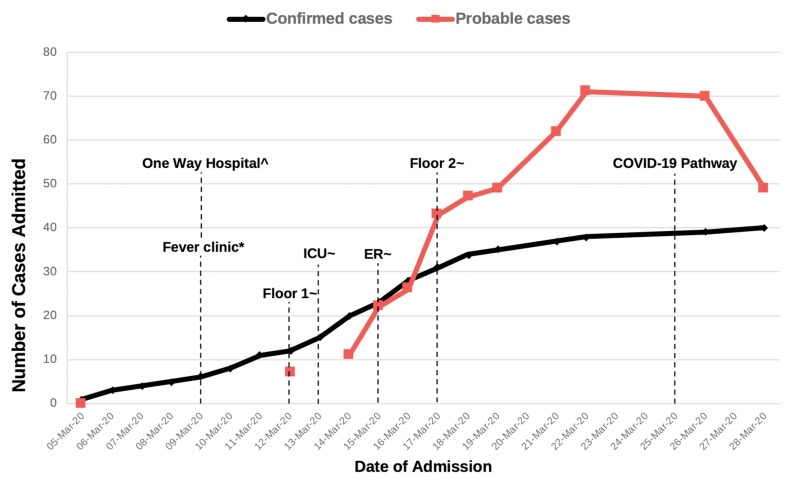
After confirmation of the first case in TMC and a rapidly growing number of cases, several changes were made to the hospital system. A one-way hospital traffic system was created by sealing several entry ways and designating a single point of entry and exit. A screening area in the ED was set up, and separated into COVID-19 and non-COVID areas, with the appropriate engineering controls and uni-directional flow of traffic. A supplemental triage area was built near the ambulatory area in order to effectively direct febrile patients to the ED, instead of the outpatient clinics. Several units, including the acute stroke unit, and two general medical floors were designated as dedicated units for probable and confirmed COVID-19 cases, with each unit having assigned donning and doffing areas, and one-way traffic. Finally, a clinical pathway with clear case definitions was created in order to easily capture COVID-19 patients and integrate rapidly evolving evidence on clinical management (Fig. 1 ). Enhanced infection prevention and control measures, including intensive training of HCWs on donning and doffing of full personal protective equipment (PPE), and patient and staff cohorting were also implemented. HCWs with advanced age and co-morbid disease were also assigned to non-COVID-19 areas.
Fig. 1.
Legend: Graph showing probable and confirmed COVID-19 cases and hospital response.
Footnote:
Number of probable COVID-19 cases from March 6–11 and March 13 not captured.
^ Only one entrance-exit and uni-directional flow allowed.
* Triage clinic for patients entering the ambulatory area.
~ Units w/negative pressure rooms or rooms with hepa filters assigned for probable or confirmed COVID-19 patients only.
5. Discussion
We describe the first 40 confirmed COVID-19 cases admitted in our institution and highlight several clinical findings and observations regarding our health systems response to surge capacity. TMC admitted patients during the early course of the COVID-19 epidemic. Our first few cases were from a family cluster and portrayed person-to-person transmission among close contacts. The study by Chan et al.13 documented familial transmission by showing that genome sequences from two different family members showed almost identical nucleotides with each other. In our cluster, although we were unable to perform genomic analysis, the index patient and his wife stayed with a family of five – and only the three who spent more time with the index patient were confirmed to have COVID-19 infection. (Supplementary Appendix A Fig. A.1). This validates existing data that transmission risk is more likely with recurrent, close contact.13 That the index case and the rest of his family sought consult late also suggests that the index of suspicion for COVID-19 was low despite informational campaigns, the presence of travel history, and compatible symptoms. At this time, many were likely unaware of community transmission, and there remained a need to significantly raise the awareness of people about the signs and symptoms of COVID-19.
The median time from symptom onset to admission was 7 days (range 0–42 days) in our cohort, mirroring the findings in other studies.3 , 4 However, 19 patients first presented a median of 4 days after symptom onset but were not hospitalized. This suggests that persistence or progression of symptoms was necessary before patients were considered for hospital admission. This was consistent with the Philippines' Department of Health directives, which mandated prioritization of older and sicker patients. However, 4 of 19 patients who were seen in the ambulatory setting died from COVID-19; this potential delay in admission may have contributed to poor outcomes in these patients.
From a clinical perspective, we confirm the findings of a recent meta-analysis14 that fever, cough and weakness are the most frequent symptoms, and that severe disease is more common in the older age group and those with co-morbidities.3 , 4 Our rates of complication, severe illness, and ICU admission are also comparable to other case series.3 , 4 , 15
Majority of patients, including all patients in the high-risk group, were given at least one drug repurposed for the treatment of COVID-19 infection, despite the lack of robust evidence to support their use. Several clinical guidelines9 , 16 cautioned against the routine use of these drugs outside of clinical trials, since the benefits from these drugs were unproven. Recent evidence from the Solidarity and Recovery randomized clinical trials17 , 18 show that the use of HCQ, CQ and LPV/r were indeed unwarranted, and these are no longer recommended. Despite the poor quality of evidence,16 , 19 however, physicians are often compelled to start these drugs because of many reasons-- the severity of illness, external pressure from other physicians or patients’ family and relatives, and social media. Moving forward, the urge to use unproven treatments based on anecdotal success and outside of well-designed randomized clinical trials needs to be resisted, especially in light of doing possible harm.
Time to virologic cure took several days, and was longer with greater disease severity and older age. Current guidelines9 , 20 report the need to document 2 negative RT-PCR results before patients are declared “virologically cured.” In our study, we chose at least 1 negative test result since the subsequent test is usually performed elsewhere and we are unable to capture that data. Studies21 , 22 have shown that although viral shedding can last several weeks, this virus could be non-viable by day 822. The test-based strategy – having to repeat RT-PCR until twice negative has been revised and a “non-testing-based strategy” considered instead. Except for high-risk and severely ill patients, discontinuing isolation along with universal source control and standard precautions may be more cost-effective than recurrent testing, especially in resource-poor settings.
The abrupt increase in both probable and confirmed COVID-19 cases during the first month highlighted the need for the hospital to adapt quickly to the surge. Surge capacity, often defined as the ability of a healthcare system to respond to a sudden increase in patient care demands, conceptually contains the following components: supplies, personnel, physical space, and management infrastructure, sometimes referenced as “stuff, staff, and structure”.23 , 24
In our study, “stuff” in the form of testing capacity took too long, with confirmation of SARS-CoV-2 infection by RT-PCR taking a median of 5 days. In the US and China, turn-around time is usually within a few hours of testing.3 , 15 The long delay was primarily because the test was sent out and performed at a reference laboratory. This prolonged turn-around time (TAT) had several healthcare and infection control implications, including the inability to effectively triage non-COVID-19 patients outside of airborne isolation precautions and ration the use of PPE. The long wait also caused increased anxiety for HCWs, the patients, and their family members. At the time of this report, testing is now done at TMC, and TAT is much shorter at 2 days. Nationally, from one reference laboratory performing RT-PCR at the start of the COVID-19 outbreak in the country, the number of licensed RT-PCR laboratories has grown to 108, supplemented by 34 licensed cartridge-based PCR or GeneXpert laboratories (as of October 9, 2020, https://www.doh.gov.ph/covid19tracker).
Our staff were also reshuffled and assigned to patients in COVID-19 units to limit cross-contamination. This method of patient and staff cohorting is often used to curtail outbreaks of multi-drug resistant organisms25 , 26 and emerging infections.26 However, isolation and cohorting are difficult to sustain because of higher cost and increased workload for the healthcare team.27 , 28 In addition, adverse events such as increased patient anxiety, anger or feeling of isolation, increased falls, and less time spent with the healthcare team, have been reported29-- events that we also observed for some of our patients.
Adequate physical space and appropriate structures are often underestimated needs in surge capacity.23 However, our hospital space was rapidly re-organized to accommodate the rise in COVID-19 cases -- several units were dedicated for probable and confirmed COVID-19 cases, with each unit having assigned donning and doffing areas and one-way traffic. These adaptations made it easier for the staff to safely and adequately manage these patients.
Our study has some limitations inherent to a retrospective study reporting on the first 40 patients in our institution. We used categories of COVID-19 severity based on national guidelines, but which are comparable to international definitions. Despite these limitations, our study is the first to describe COVID-19 patients hospitalized in a private tertiary-level hospital in the Philippines. Our experience may not necessarily represent the patient profile and health care system in the Philippines’ public sector, but illustrates the challenges that even well-resourced health facilities in developing countries face.
We validate findings from studies in Wuhan, China during the early days of the pandemic that both older age and presence of a co-morbid disease are associated with more severe disease and poor outcome.2, 3, 4 We identified specific issues that affected initial response to surge capacity, including the prolonged TAT for disease confirmation, the need to re-organize the hospital space and staff, and the need to increase level of awareness of ongoing COVID-19 community transmission. Finally, our report highlights the need for rapid adaptive actions by the healthcare system to respond to the surge of COVID-19 cases and for long-term, innovative strategies for continuing essential hospital services in the new health context created by the COVID-19 pandemic.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare that there is no conflict of interest.
Author contributions
All authors contributed to study design; CLA, CPC, MAL – contributed to data analysis, manuscript writing; CLA/JB/JF/EV/MFT/BT/MBB – data collection and management; MCS/NPB/EPP/BT/KH – protocol feedback, manuscript writing.
Acknowledgments
We would like to acknowledge Kathy Rayos, clinical pharmacist, for her help with the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cegh.2020.100695.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China. The New England journal of medicine. 2020. 2019;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edrada E.M., Lopez E.B., Villarama J.B., et al. First COVID-19 infections in the Philippines: a case report. Trop Med Health. 2020;48:21. doi: 10.1186/s41182-020-00203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health Philippines COVID-19 tracker: Philippines. https://ncovtracker.doh.gov.ph/
- 7.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (COVID) infection is suspected. Interim guidance. 28 January 2020 [Google Scholar]
- 8.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 9.Philippine Society for Microbiology and Infectious Diseases . 28 March 2020. Interim guideline on the clinical management of adult patients with suspected or confirmed COVID-19 infection. Version 2.0. [Google Scholar]
- 10.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Contr. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Ranieri V.M., Rubenfeld G.D., Thompson B.T., et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 12.Asia Pacific Biotech news http://www.worldscientific.com p. 26.
- 13.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (London, England) 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J Med Virol. 2020;92(6):612–617. doi: 10.1002/jmv.25735. Epub 2020 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, Comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. Jama. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhimraj A., Morgan R.L., Shumaker A.H., et al. an official publication of the Infectious Diseases Society of America; 2020. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horby P., Mafham M., Linsell L., et al. Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. https://www.medrxiv.org/content/10.1101/2020.07.15.20151852v1. 2020
- 18.Pan H., Peto R., Karim Q., et al. Repurposed antiviral drugs for COVID-19–interim WHO SOLIDARITY trial results. https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1.full.pdf. 2020 [DOI] [PMC free article] [PubMed]
- 19.Siedner M.J., Gandhi R.T., Kim A.Y. Desperate times call for temperate measures: practicing infectious diseases during a novel pandemic. J Infect Dis. 2020;222(7):1084–1085. doi: 10.1093/infdis/jiaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criteria for return to Work for healthcare personnel with suspected or confirmed COVID-19 (interim guidance) 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html
- 21.Wolfel R., Corman V.M., Guggemos W., et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 22.Xiao A.T., Tong Y.X., Zhang S. an official publication of the Infectious Diseases Society of America; 2020. Profile of RT-PCR for SARS-CoV-2: A Preliminary Study from 56 COVID-19 Patients. Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hick J.L., Koenig K.L., Barbisch D., Bey T.A. Surge capacity concepts for health care facilities: the CO-S-TR model for initial incident assessment. Disaster medicine and public health preparedness. 2008;2(Suppl 1):S51–S57. doi: 10.1097/DMP.0b013e31817fffe8. [DOI] [PubMed] [Google Scholar]
- 24.Kaji A., Koenig K.L., Bey T. Surge capacity for healthcare systems: a conceptual framework. Acad Emerg Med : official journal of the Society for Academic Emergency Medicine. 2006;13:1157–1159. doi: 10.1197/j.aem.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 25.Abad C.L., Barker A.K., Safdar N. A systematic review of the effectiveness of cohorting to reduce transmission of healthcare-associated C. difficile and multidrug-resistant organisms. Infect Contr Hosp Epidemiol. 2020:1–19. doi: 10.1017/ice.2020.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan Y.M., Chow P.K., Tan B.H., et al. Management of inpatients exposed to an outbreak of severe acute respiratory syndrome (SARS) J Hosp Infect. 2004;58:210–215. doi: 10.1016/j.jhin.2004.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucet J.C., Armand-Lefevre L., Laurichesse J.J., et al. Rapid control of an outbreak of vancomycin-resistant enterococci in a French university hospital. J Hosp Infect. 2007;67:42–48. doi: 10.1016/j.jhin.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Reeme A.E., Bowler S.L., Buchan B.W., et al. Use of a cohorting-unit and systematic surveillance cultures to control a Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae outbreak. Infect Contr Hosp Epidemiol. 2019;40:767–773. doi: 10.1017/ice.2019.99. [DOI] [PubMed] [Google Scholar]
- 29.Abad C., Fearday A., Safdar N. Adverse effects of isolation in hospitalised patients: a systematic review. J Hosp Infect. 2010;76:97–102. doi: 10.1016/j.jhin.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.