Abstract
Background
While the landscape of vaccine and treatment candidates against the novel coronavirus disease 2019 (COVID-19) has been reviewed systematically, prophylactic candidates remain unexplored.
Objectives
To map pre- and postexposure prophylactic (PrEP and PEP) candidate for COVID-19.
Data sources
PubMed/Medline, Embase, International Committee of Medical Journal Editors and International Clinical Trials Registry Platform clinical trial registries and medRxiv.
Study eligibility criteria and participants
All studies in humans or animals and randomized controlled trials (RCTs) in humans reporting primary data on prophylactic candidates against COVID-19, excluding studies focused on key populations.
Interventions
PrEP and PEP candidate for COVID-19.
Methods
Systematic review and qualitative synthesis of COVID-19 PrEP and PEP studies and RCTs complemented by search of medRxiv and PubMed and Embase for studies reporting RCT outcomes since systematic review search completion.
Results
We identified 13 studies (from 2119 database records) and 117 RCTs (from 5565 RCTs listed in the registries) that met the inclusion criteria. Non-RCT studies reported on cross-sectional studies using hydroxychloroquine (HCQ) in humans (n = 2) or reported on animal studies (n = 7), most of which used antibodies. All five completed RCTs focused on the use of HCQ as either PrEP or PEP, and these and the cross-sectional studies reported no prophylactic effect. The majority of ongoing RCTs evaluated HCQ or other existing candidates including non–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines, anti(retro)virals or use of vitamins and supplements.
Conclusions
The key message from completed studies and RCTs seems to be that HCQ does not work. There is little evidence regarding other compounds, with all RCTs using candidates other than HCQ still ongoing. It remains to be seen if the portfolio of existing molecules being evaluated in RCTs will identify successful prophylaxis against COVID-19 or if there is a need for the development of new candidates.
Keywords: COVID-19, Prophylaxis, Randomized control trials, SARS-CoV-2, Systematic review
Introduction
The world is facing the biggest global public health emergency of this generation as a result of the novel coronavirus pandemic. Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is the causative agent for coronavirus disease 2019 (COVID-19), characterized by rapid human-to-human transmission and important pathogenicity [1]. At the time of writing, the world has passed a new worrying milestone of one million confirmed deaths due to COVID-19 [2].
Beyond the human suffering, the COVID-19 pandemic has caused unprecedented pressures on healthcare systems and supply chains [3,4], with the ensuing lockdowns resulting in growing frailties in national economies [5,6]. Containing the COVID-19 pandemic will necessitate multipronged strategies, including effective vaccination, prophylaxis and treatment, in addition to existing protective measures such as social distancing, masking and hand hygiene [[7], [8], [9]]. This pandemic has resulted in an unparalleled galvanization of the medical and scientific community to identify pharmacologic candidates for its prevention and treatment. While the landscape of vaccine [10,11] and treatment [12,13] candidates has been reviewed systematically, evidence synthesis of prophylactic candidates remains unexplored.
In this review, we aim to address this gap by performing a systematic review of all published studies and clinical trials registries for prophylactic candidates to map out the landscape of existing and future candidates. As this is a fast-moving field, we aim to provide an updated status of the evidence by performing an updated systematic review in the near future.
Methods
Search strategy and selection criteria
We carried out a systematic review according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines [14] to identify studies reporting on prophylactic candidate for COVID-19 and/or SARS-CoV-2. Prophylactic candidates were defined as any drug, biologic-based molecule, dietary supplement or herbal remedy used to prevent infection of disease, regardless of its administration route. This included both pre- and postexposure prophylaxis (PrEP and PEP) but excluded SARS-CoV-2 vaccines and therapeutic interventions for individuals who are already infected. We excluded studies focused on populations with specific comorbidities, including those undergoing specific surgical procedures or those with specific comorbidities (Table 1 ).
Table 1.
Inclusion and exclusion criteria for identified published studies and RCTs
| Characteristic | Inclusion | Exclusion |
|---|---|---|
| Population | • Humans and animals (for the database search, not clinical trial registry search), including ‘high-risk’ older individuals, healthcare workers and healthy subjects | • In vitro studies; studies focused on key population (e.g. those with specific comorbidities) |
| Intervention | • Drug- or biologic-based prophylaxis (before or after exposure) or those based on dietary supplements or herbal extracts | • Reporting on other prevention approaches (e.g. social distancing, mask wearing or SARS-CoV-2 vaccines); theoretical candidates or reporting on populations on long-term medication for other conditions and their impact on COVID-19 |
| Outcomes | • Studies reporting impact on SARS-CoV-2 or COVID-19 incidence or prevalence | • Safety profiles, pharmacologic outcomes or studies reporting on outcomes related to other prevention approaches or treatment |
| Study | • Primary data of prophylactic candidates for COVID-19 or SARS-CoV-2 (RCTs only for medRxiv and clinical trial registries) | • Studies focusing on previous coronavirus strains (e.g. SARS-CoV, MERS), opinion or narrative pieces, case reports, trial protocols |
COVID-19, coronavirus disease 2019; RCT, randomized controlled trial; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
PubMed/Medline and Embase were searched from database inception up to and including 13 December 2020; searches were not restricted by language or quality of study, and a broad search strategy was used combining the terms ‘SARS-CoV-2’ OR ‘COVID-19’ and ‘prophylaxis’ OR ‘prophylactic’.
In order to provide a complete picture of the current prophylactic landscape, we also searched clinical trials registries (both the International Committee of Medical Journal Editors (ICMJE) and International Clinical Trials Registry Platform (ICTRP)) (Supplementary Table S1) for any randomized controlled trials (RCTs) of prophylaxis against COVID-19 and/or SARS-CoV-2, focusing on RCTs evaluating the impact of prophylactic candidates on SARS-CoV-2 or COVID-19 incidence/new cases in humans as a primary endpoint [15,16]. We included all RCTs irrespective of status, but we excluded RCTs with other primary endpoints such as safety (Table 1). The ICMJE and ICTRP search was conducted up to 13 December 2020 using the same terms as the database search and limiting to interventional studies where possible. Furthermore, medRxiv was searched from inception to 30 December 2020 for any studies reporting the outcomes of prophylaxis RCTs using the search terms ‘COVID-19’ AND ‘prophylaxis’ AND ‘Trial’. Finally, an additional search of PubMed/Medline and Embase was performed to identify peer-reviewed articles reporting on clinical trial outcomes since search completion (13 December 2020) using the search terms ‘SARS-CoV-2’ OR ‘COVID-19’ and ‘prophylaxis’ OR ‘prophylactic’ AND ‘clinical trial’, limited to title and abstract and published between 1 December 2020 to 30 December 2020.
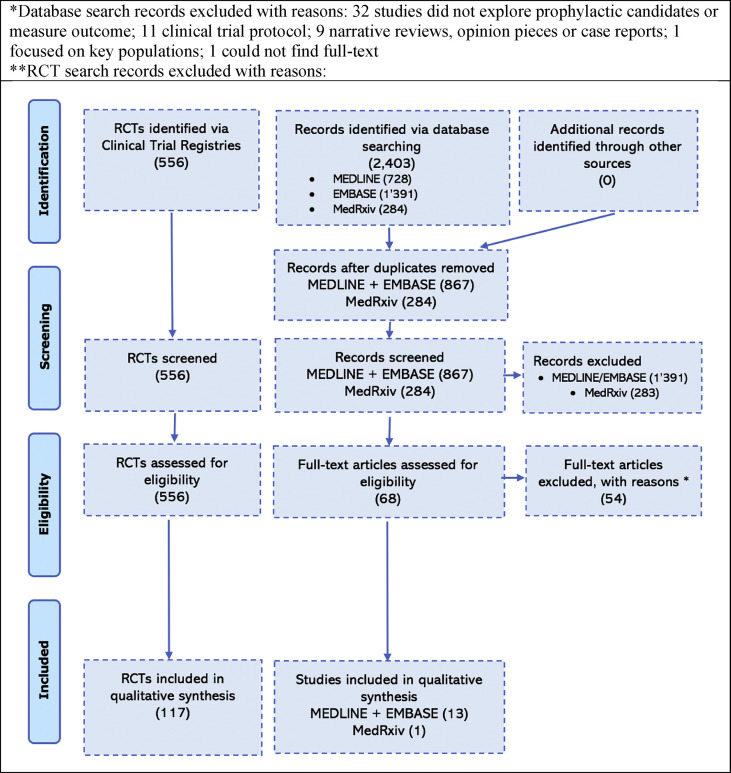
After removal of duplicates, two reviewers (MS and AM) screened abstracts and RCTs independently according to prespecified inclusion and exclusion criteria (Table 1). Where two articles reported on the same study, the most recent one reporting on the impact of the prophylaxis was chosen. Where the same RCT was found in two or more registries or an RCT was also found in a published article, it was only reported once. Conflicts were resolved by the two reviewers on a case-by-case basis, with conflicts resolved with a third reviewer (AC) as needed. Reference lists of included full-text articles were screened to identify additional studies. The screening and selection process is presented in Fig. 1 .
Fig. 1.
Systematic search flow diagram and search terms. ∗Database search records excluded, as follows: 32 studies did not explore prophylactic candidates or measure outcome; 11 had a clinical trial protocol; nine were narrative reviews, opinion pieces or case reports; one focused on key populations; and for one study we could not find full text. ∗∗Excluded RCT search records.
Data extraction and synthesis
All data were extracted to Microsoft Excel by MS and AM using a data extraction form which was piloted on five studies and five clinical trials. All data extracted were checked by the other coauthor for quality assurance. Data extracted from full-text articles included first author, publication year, country of study, study type, prophylaxis type, molecule name or combination and class, host and study outcome. For RCTs, data extraction included trial title, country of sponsor, prophylaxis type, name of molecule or combination and class, target population, sample size and status.
A qualitative data synthesis was performed outlining the landscape of prophylactic candidates, geographical distribution of studies, stage of development and trial status. Risk of bias was assessed by a single reviewer (MS) for all published (peer reviewed and preprint) studies using version 2 of the Cochrane risk-of-bias tool for RCTs (RoB 2) and the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [17,18].
Results
The database search identified 2119 records. After removing duplicates, we screened 867 citations and assessed 67 full-text reports (Fig. 1). Of these, 13 met the inclusion and exclusion criteria and were included in the qualitative synthesis. The majority of the studies excluded at the full-text assessment were studies that did not focus on prophylactic candidate, including in vitro studies or studies focused on functional or safety outcomes (n = 20) or because they reported on trial protocols (n = 12). In addition, the search of medRxiv identified one study reporting on the results of prophylactic RCTs. No additional studies were identified through the handpicked search of the database. All studies reporting on RCT results (four from the database search and one from medRxiv) were from RCTs identified in the clinical trial registries and so are only counted once.
The search of the clinical registry identified 556 clinical trials. After removing duplicates and performing a full screening, 117 RCTs were identified that met the inclusion criteria and were thus included in the qualitative synthesis.
The geographical distribution of the included studies and clinical trials was limited in scope (Supplementary Fig. S1). The majority of the studies and RCTs (n = 45) were conducted in the United States, Spain (n = 13) and Canada (n = 8), with the African continent having the fewest studies and RCTs.
Overview of published studies
A total of five RCTs were identified, including one preprint. All the remaining studies reported on cross-sectional studies in humans (n = 2) or animals (n = 7). Here we focus on reporting on non-RCT studies; below we separately report studies reporting on clinical trial results, together with RCTs identified through the clinical trial registries. The majority of the non-RCT studies reported on PrEP (8/9) and one focused on PEP. Five studies focused on hydroxychloroquine (HCQ), four focused on antibodies and one looked at both HCQ and the antiviral favipiravir.
Of the two human studies reporting on HCQ, one found no COVID-19 cases in the intervention arm and 3 cases in the control arm but did not perform statistical analyses on the results [19], and the other found no observed effect [20], although the risk of bias was moderate to high (Supplementary Fig. S2).
Amongst the seven animal studies, four looked at the use of antibodies [[21], [22], [23], [24]]. All found an effect, although they did not report comparable outcomes. Jones et al. [21] reported reduced virus replication, Li et al. [22] and Tortorici et al. [24] reported high prophylactic efficacy and Rogers et al. [23] reported a 50% reduction in disease as measured by weight loss. The three remaining animal studies looked at the effect of HCQ alone [25,26] or compared to favipiravir [25]. All three demonstrated no observed effect of HCQ, but Kaptein et al. [25] did show that favipiravir significantly reduced infectious titre. Again, risk of bias amongst studies evaluating candidates other than HCQ has a moderate to high risk of bias (Supplementary Fig. S2). Table 2 details the full-text studies.
Table 2.
Summary of peer-reviewed non-RCT studies on prophylactic candidates against COVID-19 and/or SARS-CoV-2
| Study |
P(r)EP details |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Country | Study type | P(r)EP type | Molecule or combination | Molecule type | Host | Host details | Sample size | Study conclusions |
| Revollo [20] | 2020 | Spain | Cross-sectional | PrEP | HCQ | Antimalarial | Human | HCQ | 69 intervention arm; 418 control arm | No observed effect |
| Simova [19] | 2020 | Bulgaria | Cross-sectional | PEP | HCQ | Antimalarial | Human | HCW | 156 intervention arm; 48 control arm | No COVID-19 in intervention; 3 cases in control arm |
| Jones [21] | 2020 | USA | Animal | PrEP | Neutralizing antibody | Antibody | Animal | Macaque | Not specified | Reduced virus replication |
| Kaptein [25] | 2020 | Belgium | Animal | PrEP | HCQ; favipiravir | Antimalarial; antiviral | Animal | Syrian hamster | Not specified | HCQ showed No observed effect; high doses of favipiravir significantly reduced infectious titre |
| Li [22] | 2020 | USA | Animal | PrEP | Monoclonal antibodies | Antibody | Animal | Mice and hamster | Not specified | High efficacy |
| Maisonnasse [31] | 2020 | France | Animal | PrEP | HCQ | Antimalarial | Animal | Macaque | 13 | No observed effect |
| Rogers [23] | 2020 | USA | Animal | PrEP | Neutralizing antibodies | Antibody | Animal | Syrian hamster | Not specified | 50% reduction in disease (measured by weight loss) |
| Rosenke [26] | 2020 | USA and UK | Animal | PrEP | HCQ | Antimalarial | Animal | Hamster; rhesus macaque | 30 Hamsters; 10 macaques | No observed effect |
| Tortorici [24] | 2020 | USA | Animal | PrEP | Antibodies | Antibody | Animal | Syrian hamster | Not specified | Notable protective efficacy |
COVID-19, coronavirus disease 19; HCQ, hydroxychloroquine; HCW, healthcare workers; HR, hazard ratio; IgG1, immunoglobulin G1; PEP, postexposure prophylaxis; PrEP, preexposure prophylaxis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VH-Fc, heavy variable domain fragment crystallization region.
Overview of planned or ongoing RCTs
The search of the databases and medRxiv identified five published studies reporting on the results of RCTs. The search of clinical trial registries identified 117 RCTs that met the inclusion criteria. Of these, 85 focused on PrEP, 29 on PEP and three on both PrEP and PEP (Supplementary Table S2). The RCTs mainly targeted healthcare workers alone (n = 72) or in combination with close contacts, patients, first responders or nursing residents (n = 11), with 15 RCTs targeting close contacts of index cases alone. Nine studies focused on at-risk populations such as geriatric patients, nursing home residents and frontline workers, and two studies focused on military staff. Only seven clinical trials were completed, with 57 either recruiting or ongoing, 38 not yet recruiting and five either suspended of prematurely ended.
With regards to the molecules being tested, the majority focused on antimalarials including HCQ and chloroquine (n = 63) either alone (n = 57) or together with antivirals (n = 3), antibiotics (n = 2) or antiseptic and anthelmintic (n = 1). Eighteen RCT investigated the use of non–SARS-CoV-2 vaccines, especially bacillus Calmette-Guérin vaccine (n = 12). Ten RCT evaluated the impact of antivirals or antiretrovirals. Seven RCTs investigated the use of vitamin D or supplements such as lactoferrin, probiotics and quercetin on COVID-19, and seven others investigated the impact of anthelmintic or antiprotozoal. RCTs focused on HCQ mainly tested HCQ alone against either placebo or surveillance.
Amongst the five studies reporting on the results of completed RCTs, all focused on HCQ [[27], [28], [29], [30],32]. Two studies focused on PrEP [27,30] and three on PEP [28,29,32]. None of the studies established a prophylactic effect of HCQ against COVID-19 (Table 3 ).
Table 3.
Summary of RCT results of prophylactic candidates against COVID-19 and/or SARS-CoV-2
| Study | P(r)EP type | Intervention | Control | Target population | Intention to treat | Sample size | Study conclusions |
|---|---|---|---|---|---|---|---|
| Abella [27] | PrEP | HCQ (600 mg daily, 8 weeks) | Placebo | HCW | HCQ 4/64, 6.3% vs control 4/61, 6.6% (p >0.99) | Total: 132 HCQ: 66 Control: 66 |
No observed effect |
| Barnabas [28] | PEP | HCQ (400 mg daily; 3 days and 200 mg daily; 11 days) | Ascorbic acid (500 mg daily; 250 mg daily) | Contacts | HR = 1.1 (95% CI 0.73–1.66, p > 0.20) | Total: 671 HCQ: 337 Control: 334 |
No observed effect |
| Boulware [29] | PEP | HCQ (800 mg once; 600 mg in 6-8 hours, 600 mg for 4 days) | Placebo | Household contacts; HCW | HCQ 49/414, 11.8% vs control 58/407, 14.3% (p 0.35) | Total: 821 HCQ: 414; Control: 407 |
No observed effect |
| Mitja [32]a | PEP | HCQ (800 mg once; 400 mg daily for 6 days) | No intervention | Contacts | Risk ratio = 0.89 (95% CI 0.54–1.46) | Total; 2314 HCQ: 1116 Control: 1198 |
No observed effect |
| Rajasingham [30] | PrEP | HCQ (400 mg twice in 6-8 hours; 400 mg once weekly for 12 weeks or 400 mg twice weekly for 12 weeks) | Placebo | HCW | Once weekly: HR = 0.72 (95% CI 0.44–1.16; p 0.18) Twice weekly: HR = 0.74 (95% CI 0.46–1.19; p 0.22) |
Total: 1483 HCQ once weekly: 494 HCQ twice weekly: 495 Control: 494 |
No observed effect |
Abbreviation: CI, confidence intervals; COVID-19, coronavirus disease 19; HCQ, hydroxychloroquine; HCW, healthcare workers; PEP, postexposure prophylaxis; PrEP, preexposure prophylaxis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VH-Fc, heavy variable domain fragment crystallization region.
∗∗ Studies identified post database search.
Studies identified through medRxiv.
Discussion
A range of prophylactic candidates against COVID-19 are being evaluated in RCTs across the world. While the key message from completed studies and RCTs seems to be that HCQ does not work, there is little evidence regarding other compounds, with all RCTs using candidates other than HCQ still ongoing. It remains to be seen if the portfolio of candidates being evaluated in RCTs will identify successful prophylaxis against COVID-19 or if there is a need for the development of new candidates.
The large number of studies and ongoing RCTs into prophylactic candidates for COVID-19 highlights the global efforts to rapidly identify effective strategies to mitigate the pandemic. Despite these efforts, around half of the registered RCTs are either not yet recruiting or suspended; only a handful have been completed, none of them demonstrating an impact. This highlights two important points. Firstly, despite the high level of commitment, the conduct of RCTs faces a number of constraints and challenges [33,34]. Secondly, the only preventative measures the world currently possesses are social distancing, mask wearing and hand hygiene [7], until effective prophylactic and vaccine candidates can be identified.
Most ongoing efforts focus on evaluating the use of existing molecules on COVID-19, with few studies and registered RCTs evaluating new molecules. The repurposing of existing drugs is in part driven by observational studies which seemed to suggest that individuals receiving long-term treatment – for example, HCQ [35], arbidol [36] and thymosin [37] – may benefit from a protective effect against COVID-19 compared to individuals not receiving those treatments. Numerous pharmaceuticals and biotech firms have joined the race to find vaccines as well as prophylactic and therapeutic candidates against COVID-19 [38]. This includes a focus on new candidates. Apart from the candidates identified in this review, other molecules cited in the published literature and in the media as possible prophylactic candidates include existing herbal extracts such as Echinacea [39,40], nicotine [41] and new molecules including PAC-MAN (prophylactic antiviral CRISPR in human) for viral inhibition [42] and DARPin as well as multitarget binding neutralizing proteins [43]. The underrepresentation of new molecules in ongoing RCTs is likely in part due to the relative novelty of this virus and the necessary time lag to develop new molecules before testing them in clinical trials.
The world is certainly not unaccustomed to infectious diseases. From the discovery of penicillin at the start of the 20th century [44] and the fight against HIV, which started in the 1980s [45,46], the scientific and medical community has demonstrated its ability to galvanize rapidly in order to collate knowledge on transmission, prevention and treatment of infectious diseases. COVID-19 has undisputedly brought the urgency to understand an infectious disease to a new level. However, it has also highlighted the importance of coordinated and aligned efforts, in order to identify effective strategies to fight the pandemic. The large number of prophylactic trials testing the same prophylactic candidate, for example, highlights two points. While the world stood still to halt the spread of the disease, the medical and scientific community has worked under never-before seen pressure, resulting in often fragmented efforts. It has made it hard for communities to coordinate their efforts and join forces. Yet every trial faces limitations, including selection bias and sample size issues. Pooling the wealth of clinical trial data that are being produced will allow us to construct a clearer of the true effect of these candidates.
Strengths and limitations
To our knowledge, this is one of the first global systematic reviews to map the landscape of existing and future prophylactic candidates against COVID-19, providing a detailed summary of published studies and both completed and ongoing clinical trials. This review comes at an important time in the pandemic, succeeding the first pandemic wave and preceding a potential second wave.
This study has a number of limitations. Firstly, it is limited to published data and registered trials and thus may have missed ongoing unregistered trials. Given the rapid pace of knowledge generation in relation to this pandemic, data are being published across preprint platforms which are not peer reviewed. This review thus provides the best understanding of this large field according to available high-quality data. In fact, since the database search on 17 September 2020, a number of additional studies have emerged, including an additional study reporting that HCQ was not effective as prophylaxis [27]. Secondly, given the pace of knowledge generation, it provides information on candidates being tested in the prevention of COVID-19, with limited data available on their clinical and epidemiologic effects. Thirdly, the large heterogeneity in the existing data on prophylactic impact and the range of candidates being evaluated means that statistical comparison could not be made to provide an indication of their relative effect.
Conclusions
A range of prophylactic candidates against COVID-19 are currently being evaluated in clinical trials across a number of countries and settings. Data from completed studies and RCTs seem to suggest that HCQ does not work, but the evidence regarding other compounds remains scare, with RCTs using candidates other than HCQ still ongoing. It remains to be seen if the portfolio of existing candidates will identify successful prophylaxis against COVID-19 or if there is a need for the development of new candidates.
Transparency declaration
All authors report no conflicts of interest relevant to this article.
Acknowledgements
We thank the Swiss National Science Fund for supporting this work.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.01.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Han Y., Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): a Chinese perspective. J Med Virol. 2020;92:639–644. doi: 10.1002/jmv.25749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughan A. 16 September 2020. Coronavirus death toll reaches 1 million – how did we get here? New Scientist.https://www.newscientist.com/article/mg24733003-600-coronavirus-death-toll-reaches-1-million-how-did-we-get-here/ Available at: [Google Scholar]
- 3.European Data Portal . 30 June 2020. Pressure on healthcare systems: coping with demand for ICU and hospital beds.https://www.europeandataportal.eu/en/impact-studies/covid-19/pressure-healthcare-systems-coping-demand-icu-and-hospital-beds Available at: [Google Scholar]
- 4.Tanne J.H., Hayasaki E., Zastrow M., Pulla P., Smith P., Rada A.G. COVID-19: how doctors and healthcare systems are tackling coronavirus worldwide. BMJ. 2020;368 doi: 10.1136/bmj.m1090. [DOI] [PubMed] [Google Scholar]
- 5.World Bank . 8 June 2020. The global economic outlook during the COVID-19 pandemic: a changed world.https://www.worldbank.org/en/news/feature/2020/06/08/the-global-economic-outlook-during-the-covid-19-pandemic-a-changed-world Available at: [Google Scholar]
- 6.Organisation for Economic Co-operation and Development (OECD) 24 June 2020. The impact of the coronavirus (COVID-19) crisis on development finance.http://www.oecd.org/coronavirus/policy-responses/the-impact-of-the-coronavirus-covid-19-crisis-on-development-finance-9de00b3b/ Available at: [Google Scholar]
- 7.Flaxman S., Mishra S., Gandy A., Unwin H.J.T., Mellan T.A., Coupland H. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 8.Walker P.G.T., Whittaker C., Watson O.J., Baguelin M., Winskill P., Hamlet A. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science. 2020;369:413–422. doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartsch S.M., O’Shea K.J., Ferguson M.C., Bottazzi M.E., Wedlock P.T., Strych U. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med. 2020;59:493–503. doi: 10.1016/j.amepre.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Checcucci E., Piramide F., Pecoraro A., Amparore D., Campi R., Fiori C. The vaccine journey for COVID-19: a comprehensive systematic review of current clinical trials in humans. Panminerva Med. 2020 doi: 10.23736/S0031-0808.20.03958-0. [DOI] [PubMed] [Google Scholar]
- 11.Bhagavathula A.S., Aldhaleei W.A., Rovetta A., Rahmani J. Vaccines and drug therapeutics to lock down novel coronavirus disease 2019 (COVID-19): a systematic review of clinical trials. Cureus. 2020;12 doi: 10.7759/cureus.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siemieniuk R.A., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Kum E. Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsen A.P.H., Wiberg S., Laigaard J., Pedersen C., Rokamp K.Z., Mathiesen O. A systematic review of trial registry entries for randomized clinical trials investigating COVID-19 medical prevention and treatment. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Committee of Medical Journal Editors (ICMJE) Clinical trials registration. http://www.icmje.org/about-icmje/faqs/clinical-trials-registration/ Available at:
- 16.World Health Organization (WHO) Primary registries in the WHO registry network. https://www.who.int/clinical-trials-registry-platform/network/primary-registries Available at:
- 17.Cochrane Training Chapter 25: assessing risk of bias in a non-randomized study. https://training.cochrane.org/handbook/current/chapter-25 Available at:
- 18.Cochrane Methods Bias RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials Available at:
- 19.Simova I., Vekov T., Krasnaliev J., Kornovski V., Bozhinov P. Hydroxychloroquine for prophylaxis and treatment of COVID-19 in health-care workers. New Microbes New Infect. 2020;38:100813. doi: 10.1016/j.nmni.2020.100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revollo B., Tebe C., Penafiel J., Blanco I., Perez-Alvarez N., Lopez R. Hydroxychloroquine pre-exposure prophylaxis for COVID-19 in healthcare workers. J Antimicrob Chemother. 2020 doi: 10.1093/jac/dkaa477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones B.E., Brown-Augsburger P.L., Corbett K.S., Westendorf K., Davies J., Cujec T.P. LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection. BioRxiv. 2020 doi: 10.1101/2020.09.30.318972. [DOI] [Google Scholar]
- 22.Li W., Chen C., Drelich A., Martinez D.R., Gralinski L.E., Sun Z. Rapid identification of a human antibody with high prophylactic and therapeutic efficacy in three animal models of SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2020;117:29832–29838. doi: 10.1073/pnas.2010197117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020 doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tortorici M.A., Beltramello M., Lempp F.A., Pinto D., Dang H.V., Rosen L.E. Ultrapotent human antibodies protect against SARS-CoV-2 challenge via multiple mechanisms. Science. 2020;370:950–957. doi: 10.1126/science.abe3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaptein S.J.F., Jacobs S., Langendries L., Seldeslachts L., Ter Horst S., Liesenborghs L. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2–infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci U S A. 2020;117:26955–26965. doi: 10.1073/pnas.2014441117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenke K., Jarvis M.A., Feldmann F., Schwarz B., Okumura A., Lovaglio J. Hydroxychloroquine proves ineffective in hamsters and macaques infected with SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.06.10.145144. [DOI] [Google Scholar]
- 27.Abella B.S., Jolkovsky E.L., Biney B.T., Uspal J.E., Hyman M.C., Frank I. Efficacy and safety of hydroxychloroquine vs. placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnabas R.V., Brown E.R., Bershteyn A., Stankiewicz Karita H.C., Johnston C., Thorpe L.E. Hydroxychloroquine as postexposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 infection: a randomized trial. Ann Intern Med. 2020 doi: 10.7326/M20-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajasingham R., Bangdiwala A.S., Nicol M.R., Skipper C.P., Pastick K.A., Axelrod M.L. Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 32.Mitja O., Ubals M., Corbacho M., Alemany A., Suner C., Tebe C. A cluster-randomized trial of hydroxychloroquine as prevention of COVID-19 transmission and disease. medRxiv. 2020 doi: 10.1101/2020.07.20.20157651. [DOI] [Google Scholar]
- 33.Treweek S., Jüni P., Li T., Collin J., Briel M., Chan A.W. COVID-19 randomised trial protocols: rapid publication without barriers. Trials. 2020;21:327. doi: 10.1186/s13063-020-04304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briel M., Speich B., von Elm E., Gloy V. Comparison of randomized controlled trials discontinued or revised for poor recruitment and completed trials with the same research question: a matched qualitative study. Trials. 2019;20:800. doi: 10.1186/s13063-019-3957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen S., Werner A., Shekhar A. Within a large healthcare system, the incidence of positive COVID-19 results and mortality are lower in patients on chronic hydroxychloroquine therapy. Drugs Ther Perspect. 2020 doi: 10.1007/s40267-020-00741-x. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J., Wang W., Peng B., Peng W., Zhang Y., Wang Y. Potential of arbidol for post-exposure prophylaxis of COVID-19 transmission—a preliminary report of a retrospective cohort study. Curr Med Sci. 2020 doi: 10.1007/s11596-020-2203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X., Liu Y., Wang L., Hu L., Liu D., Li J. Analysis of the prophylactic effect of thymosin drugs on COVID-19 for 435 medical staff: a hospital-based retrospective study. J Med Virol. 2020 doi: 10.1002/jmv.26492. [DOI] [PubMed] [Google Scholar]
- 38.Association of the British Pharmaceutical Industry (ABPI) What are pharmaceutical companies doing to tackle COVID-19? https://www.abpi.org.uk/medicine-discovery/covid-19/what-are-pharmaceutical-companies-doing-to-tackle-the-disease/ Available at:
- 39.Signer J., Jonsdottir H.R., Albrich W.C., Strasser M., Züst R., Ryter S. In vitro virucidal activity of Echinaforce, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol J. 2020;17:136. doi: 10.1186/s12985-020-01401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aucoin M., Cooley K., Saunders P.R., Carè J., Anheyer D., Medina D.N. The effect of Echinacea spp. on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: a rapid review. Adv Integr Med. 2020 doi: 10.1016/j.aimed.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nau J.Y. COVID-19: la troublante découverte des possibles vertus de la nicotine. Rev Med Suisse. 2020;16:966–967. [PubMed] [Google Scholar]
- 42.Abbott T.R., Dhamdhere G., Liu Y., Lin X., Goudy L., Zeng L. Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell. 2020;181:865–876. doi: 10.1016/j.cell.2020.04.020. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lever A.M.L. Wiley; Hoboken, NJ: 1996. The molecular biology of HIV/AIDS. [Google Scholar]
- 44.Tan S.Y., Tatsumura Y. Alexander Fleming (1881–1955): discoverer of penicillin. Singapore Med J. 2015;56:366–367. doi: 10.11622/smedj.2015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control (CDC) Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men—New York City and California. MMWR Morb Mortal Wkly Rep. 1981;30:305–308. [PubMed] [Google Scholar]
- 46.Barre-Sinoussi F., Chermann J.C., Rey F., Nugeyre M.T., Chamaret S., Gruest J. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.