Abstract
Objective
Intravenous fluid administration is a main component of sepsis therapy, but physicians are cautious about giving fluids to end‐stage renal disease (ESRD) patients out of concern for causing volume overload. We compared the outcomes of septic shock patients with and without ESRD and evaluated the association between early intravenous fluid administration and outcomes.
Methods
We analyzed patients enrolled in the Protocolized Care for Early Septic Shock (PROCESS) trial, which studied different resuscitation strategies for early septic shock. Stratifying for ESRD, we compared patient characteristics, course of care, and outcomes between ESRD and non‐ESRD. Using multivariable logistic regression, we determined the association between 6‐hour total fluid volume (> = 30 mL/kg vs < 30 mL/kg) from preenrollment and outcomes.
Results
There were 84 ESRD and 1257 non‐ESRD patients. ESRD patients had a higher median Charlson Comorbidity score (5 vs 2, P < .001), higher median acute physiology and chronic health evaluation (APACHE) II score (26.5 vs 20.0, P < .001), and lower 6‐hour intravenous fluid administration (54.7 vs 68.3 mL/kg, P < .001). Ninety‐day mortality (33.3% vs 29.3%, P = .43) and intubation rate (31.0% vs 33.4%, P = .64) did not differ between groups. Fewer ESRD received > = 30 mL/kg (66.6% vs 86.7% P < .001). For ESRD, receipt of > = 30 mL/kg intravenous fluid did not alter any outcome. For non‐ESRD patients, receiving ≥30 mL/kg of intravenous fluid was associated with increased 90‐day mortality (adjusted odds ratio = 1.64; 95% confidence interval, 1.03‐2.61).
Conclusions
In the PROCESS trial, ESRD patients had similar outcomes to non‐ESRD patients. Although ESRD patients received less intravenous fluid administration, most received over 30 mL/kg in the first 6 hours. In contrast to non‐ESRD patients, receiving ≥30 mL/kg of intravenous fluid was not associated with worse outcomes in ESRD.
Keywords: critical care, ESRD, kidney disease, resuscitation, sepsis, septic shock
1. INTRODUCTION
1.1. Background
End‐stage renal disease (ESRD) is a condition in which kidney dysfunction requires hemodialysis or peritoneal dialysis to maintain electrolyte and fluid homeostasis. 1 , 2 ESRD affects over 700,000 people in the United States, with approximately 125,000 new cases each year. Those who suffer from ESRD have a high mortality, with 164 in 1000 dialysis patients dying per year. 3
Sepsis, defined as infection with a dysregulated physiologic response, also is common, with over 750,000 diagnosed cases in US hospitals each year. Sepsis and other infections account for 12% of ESRD deaths per year. Compared with the general population, annual sepsis mortality is 100‐ to 300‐fold higher for dialysis patients. 3 , 4 ESRD patients have impaired host defenses; with repetitive vascular access or long‐term indwelling catheters, ESRD patients are at increased risk for severe infections. 5 , 6
1.2. Importance
Intravenous fluid resuscitation is a mainstay of septic shock therapy, but many ESRD patients are anuric or oliguric and can tolerate only limited fluid loads. 7 Subsequently, physicians often struggle with the decision to administer intravenous fluids to those with ESRD and sepsis. Although few previous studies have evaluated ESRD patients with sepsis and severe sepsis, 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 there currently are no known studies evaluating fluid administration and outcomes for ESRD patients specifically with septic shock.
1.3. Goals of the investigation
In 2014, the Protocolized Care for Early Septic Shock (PROCESS) trial, a randomized trial of protocol‐based care for early septic shock, evaluated different fluid resuscitation strategies for patients with septic shock. 13 We sought to explore the role of early volume resuscitation for those with septic shock and ESRD. In this study, we used the PROCESS trial data to compare characteristics of care and outcomes between ESRD and non‐ESRD patients with septic shock. We secondarily evaluated the association between fluid administration and outcomes for ESRD and non‐ESRD patients.
The Bottom Line
Although fluids are a mainstay of sepsis therapy, many are cautious about giving fluids to end‐stage renal (ESRD) patients. Reanalyzing PROCESS trial enrollees, researchers discovered that unlike non‐ESRD patients, ESRD ones did not have worse outcomes with 30 mL/kg or greater in the first 6 hours. Resuscitating ESRD patients per sepsis fluid guidelines appears to be safe.
2. METHODS
2.1. Study design and setting
We conducted a secondary analysis of data from the PROCESS trial. The PROCESS trial was a randomized, controlled, multicenter sepsis trial that evaluated different sepsis resuscitation strategies. The trial took place in the United States in multiple academic hospitals. Subjects were eligible for the study if they had sepsis with either refractory hypotension or a lactic acid > 4 mmoL per liter. Upon enrollment, subjects were randomized to 1 of 3 different treatment strategies for septic shock: Early Goal‐Directed Therapy defined by Rivers et al 14 managed by the study team, a non‐invasive protocol based therapy managed by the study team, or usual care, dictated solely by the clinical team. The study team at each site was composed of a local study physician, a study coordinator, and the bedside nurse. The local study team prospectively collected data on patient characteristics, interventions, and hospital course.
We requested and received approval from the PROCESS trial coordinating center. The ‐PROCESS trial coordinating center supplied all data from the PROCESS trial for this analysis. We gained approval as an exempt protocol by the Institutional Committee for the Protection of Human Subjects.
2.2. Selection of participants
Participants in the PROCESS trial met 3 inclusion criteria: suspected infection in the emergency department, 2 or more systemic inflammatory response syndrome criteria, and either hypotension despite 1L of crystalloid resuscitation or a blood lactic acid ≥ 4 mmoL/L. We included all 1341 patients enrolled in the parent trial. We separated the patients according to the presence or absence of ESRD. ESRD history was reported by research personnel. For this secondary analysis, we excluded patients with incomplete resuscitation volume data.
2.3. Outcomes
Patient characteristics included age, sex, race, ethnicity, Charlson Comorbidity score. Course of care included total intravenous fluid given from preenrollment to 6 hours post enrollment. Outcomes include 60‐day mortality, 90‐day mortality, 72‐hour intubation, median days of respiratory support, duration of cardiovascular support, hospital length of stay (LOS), and ICU LOS.
2.4. Analysis
We divided the cohort into patients with and without ESRD. We compared patient characteristics and course of care between ESRD and non‐ESRD using t tests and Wilcoxon rank‐sum tests.
For the secondary analysis evaluating the association between intravenous fluid administration and outcomes, we first developed a list of variables that conceptually served as confounders in the analysis. Given the constraints based on sample size of ESRD patients, we used univarite logistic regression models to evaluate the association of patient characteristics and comorbidities on 90‐day mortality in those with ESRD and those without ESRD. Using this data and a basic understanding of the current literature, we selected 3 confounders to adjust for in the logistic regression model; age, Charlson Comorbidity score, and baseline acute physiology and chronic health evaluation (APACHE) II score.
We then calculated the amount of intravenous fluid given from preenrollment to 6 hours after enrollment and divided this by patient mass in kilograms. Weight data for 4 ESRD patients and 50 non‐ESRD patients were missing so they were excluded from this portion of the analysis. Stratifying for ESRD, we applied a univariate logistic regression to evaluate the association between receiving ≥30 mL/kg of intravenous fluid from preenrollment to 6 hours and outcomes. We evaluated this same association using multivariate logistic regression, adjusting for the selected confounders. Goodness of fit was confirmed using a Hosmer‐Lemeshow test.
3. RESULTS
3.1. Characteristics of study subjects
There were 84 ESRD patients and 1257 non‐ESRD patients. Fewer ESRD were white (43% vs 70%). ESRD had higher median Charlson Comorbidity score (5 vs 2), higher median baseline APACHE II (26.5 vs 20), and lower initial systolic blood pressure (90.9 vs 101.4 mmHg). Other characteristics did not differ between ESRD and non‐ESRD (Table 1).
TABLE 1.
Characteristics of patients enrolled in the PROCESS trial, stratified by ESRD
| Characteristic | ESRD (n = 84) | Non‐ESRD (n = 1257) | P value |
|---|---|---|---|
| Age, 1 y | 60 (49.5‐67) | 62 (50‐74) | 0.1 5 |
| Sex, Male 2 | 52 (62%) | 696 (55%) | 0.2 6 |
| Race 2 | <0.01 6 | ||
| White | 36 (43%) | 880 (70%) | |
| Black or African American | 40 (48%) | 293 (23%) | |
| Asian | 3 (3%) | 23 (2%) | |
| Other | 5 (6%) | 61 (5%) | |
| Ethnicity 2 | 0.7 6 | ||
| Non‐Hispanic | 74 (88%) | 1,122 (89%) | |
| Hispanic | 10 (12%) | 133 (11%) | |
| Charlson Comorbidity score 1 | 5 (3‐7) | 2 (1‐4) | <0.01 7 |
| Baseline APACHE II 1 | 26.5 (23.5‐30.5) | 20 (15‐25) | <0.01 7 |
| Entry criterion 3 | |||
| Refractory hypotension | 55.9% (45.1‐66.8%) | 55.9% (51.3‐56.9%) | 0.7 6 |
| Hyperlactatemia | 58.3% (47.6‐69.1%) | 59.8% (57.0‐62.5%) | 0.8 6 |
| Physiological variables 4 | |||
| Systolic blood pressure (mmHg) | 90.9 (84.0‐97.9) | 101.4 (99.8‐103.0 | <0.01 5 |
| Lactic acid | 4.7 (4.0‐5.4) | 4.9 (4.7‐5.1) | 0.6 5 |
| Vasopressors 3 | 17.9% (9.5‐26.2%) | 16.9% (14.9‐19.0%) | 0.8 6 |
| Study arm 2 | 0.7 6 | ||
| EGDT 5 | 27 (32%) | 412 (33%) | |
| Protocol | 25 (30%) | 421 (33%) | |
| Usual care | 32 (38%) | 424 (4%) |
Reported as median (interquartile range).
Reported as N (%).
Reported as % (95% confidence interval).
Reported as mean (95% confidence interval).
Compared using a t test.
Compared using a chi‐square test.
Compared using a Wilcoxon rank‐sum test.
APACHE, acute physiology and chronic health evaluation; EGDT, Early Goal Directed Therapy; PROCESS, Protocolized Care for Early Septic Shock.
3.2. Main results
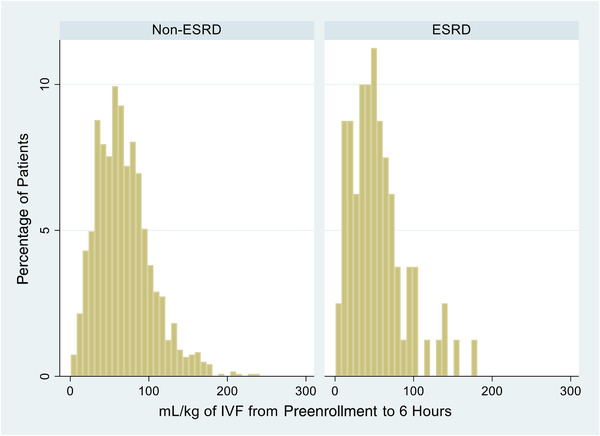
ESRD patients received less fluid from preenrollment to 6 hours after enrollment (47.2 vs 64.3 mL/kg, P < .001) (Appendix 1); 90‐day mortality (33.3% vs 29.3%, P = .4) and 60‐day mortality (22.6% vs 19.1%, P = .4) did not differ significantly between ESRD and non‐ESRD patients. The 72‐hour intubation rate (31.0% vs 35.5%, P = .6), median days of respiratory support (1.5 vs 1, P = .1), median days in the ICU (4 vs 3, P = .3), and median days in the hospital (9 vs 8, P = .1) did not differ between the groups (Figure 1 and Appendix 2).
FIGURE 1.
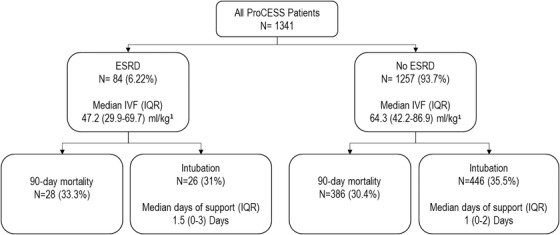
Outcomes for all patients from the PROCESS trial, stratified by ESRD. 1Intravenous fluids administered from preenrollment to 6 hours. ESRD, end‐stage renal disease; IQR, interquartile range; IVF, intravenous fluid; PROCESS, Protocolized Care for Early Septic Shock
In the first 6 hours of resuscitation, fewer ESRD patients received > = 30 mL/kg IVF (20/80, 66.6% vs 1067/1207, 86.7%, P > .001). For ESRD, receiving ≥30 mL/kg was not associated with increased odds of 60‐day mortality, 90‐day mortality, 72‐hour intubation rate, ICU LOS, or hospital LOS. For non‐ESRD, receiving > = 30 mL/kg was not associated with worse outcomes with the exception of adjusted 90‐day mortality (adjusted odds ratio = 1.6; 95% confidence interval, 1.03‐2.6) (Table 2).
TABLE 2.
Association between receiving over 30 mL IVF/kg of body weight and outcomes for patient enrolled in the PROCESS trial, stratified by ESRD
| Models adjusted for sex, race, Charlson Comorbidity score, and APACHE II score | ||||
|---|---|---|---|---|
| Outcome | ESRD a N = 80 Unadjusted | ESRD a N = 80 Adjusted | Non‐ESRD a N = 1207 Unadjusted | Non‐ESRD a N = 1207 Adjusted |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reported as odds ratio (95% confidence interval); compared using univariate logistic regressions for the unadjusted analyses and multivariate logistic regressions for the multivariate analyses.
APACHE, acute physiology and chronic health evaluation; ESRD, end‐stage renal disease; IVF, intravenous fluid; LOS, length of stay; PROCESS, Protocolized Care for Early Septic Shock.
4. LIMITATIONS
Although using a sepsis trial data set with goal‐directed resuscitation might bias the analysis, one would expect more fluid administration to be associated with sicker patients and worse outcomes. However, ESRD patients receiving greater than 30 mL/kg did not experience worse outcomes in our study. The retrospective nature of this analysis limits our ability to draw conclusions about the effect of resuscitating with higher amounts of intravenous fluid.
The small sample size limited the complexity of the analysis to a stratified descriptive study, and the results could be underpowered and biased toward the null hypothesis. ESRD patients did have a trend toward worse mortality and longer ICU LOS, but we could have failed to identify a significant relationship because of the small sample size. The small sample size also limited the number of variables that could be included in our secondary analysis, but we attempted to select for the most evidence‐based and sensible variables to include in the analysis.
Although the APACHE II score and Charlson Comorbidity scores both contain kidney disease as a component, they encompass many more risk factors for poor outcomes and represent high yield variables in an analysis that is limited owing to sample size. Additionally, although age is part of APACHE II and Charlson Comorbidity scores, early inspection of covariates revealed that age had a significant association with mortality, so we felt it was an important independent variable to include in our analysis. A larger sample size would allow for a more robust analysis, as well as a direct, propensity matched comparison between ESRD and non‐ESRD patients. Future prospective trials are necessary to evaluate the effect of fluid administration on outcomes for ESRD patients with septic shock.
5. DISCUSSION
We found that ESRD and non‐ESRD patients with septic shock had similar outcomes when resuscitated as part of a septic shock trial. Although ESRD patients received less fluid than non‐ESRD patients, two thirds of ESRD still received over 30 mL/kg in the first 6 hours, and the median fluid received in the ESRD group was over 45 mL/kg. Additionally, although receiving ≥30 mL/kg was linked to increased mortality for non‐ESRD patients in our adjusted analysis, receiving > = 30 mL/kg was not associated with worse outcomes for ESRD patients. This provides reassurance that ESRD patients can likely receive substantial amounts of early fluid resuscitation when indicated without negatively affecting mortality or need for mechanical ventilation.
Prior data has suggested that ESRD patients have higher mortality from sepsis, with epidemiologic studies finding a 100‐ to 300‐fold increased yearly mortality rate of sepsis mortality for ESRD patients. 4 , 15 Although more recent studies have not found this difference, 8 , 16 no studies have specifically evaluated patients with septic shock. Our study suggests that ESRD is not associated with worse outcomes in septic shock.
Rajdev evaluated ESRD patients with severe sepsis and septic shock in their case control retrospective review. They found that ESRD patients were less likely to receive 30 mL/kg and received less fluids then non‐ESRD patients. On subanalysis, they found no association between receiving > 30 mL/kg and worse outcomes. 8 These results coincide with findings from this analysis and prior studies. 9 , 10 , 11 , 12 , 17
Recent studies have evaluated fluid restrictive strategies in septic shock. Pilot studies suggest that fluid restrictive strategies decrease the amount of fluid resuscitation without impacting outcomes, 18 , 19 suggesting that fluid restriction may be a safe alternative in fluid‐sensitive populations. The Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) is an ongoing multicenter, randomized control trial evaluating different fluid resuscitative strategies, the results of which will provide valuable insight into the best approach to fluid resuscitation of septic shock patients. 20 Studies have also evaluated ultrasound as an adjunct for guiding fluid resuscitation. Inferior vena cava measurements, lung ultrasound, and echocardiograms have been found to be a useful for predicting fluid responsiveness and risk of developing volume overload. 21 , 22 , 23 , 24 Ultrasound might provide useful clinical information to help guide volume resuscitation for ESRD patients with septic shock.
Our study provides further evidence suggesting that early fluid administration is not linked with worse outcomes for ESRD patients. Although this and prior studies have shown that ESRD patients might receive less fluids then non‐ESRD patients, ESRD patients with septic shock likely still benefit from volume resuscitation. Physicians should exercise judgment and use clinical signs of volume overload to guide their resuscitation of these patients.
In conclusion, as part of a protocol‐based sepsis trial, ESRD patients had similar outcomes to non‐ESRD patients. Although ESRD patients received less intravenous fluid than non‐ESRD patients, most received over 30 mL/kg in the first 6 hours. In contrast to non‐ESRD patients, receiving over 30 mL/kg intravenous fluid was not associated with worse outcomes for ESRD patients.
Biography
Ryan Huebinger, MD, is an Assistant Professor in the Department of Emergency Medicine at McGaw Medical Center of UTHealth at Houston.

APPENDIX 1.
Total fluids administered from pre‐enrollment to 6‐hour after randomization, Stratified by end‐stage renal disease (ESRD)
APPENDIX 2.
Outcomes of patient from the PROCESS trial, stratified by ESRD
| Outcome | ESRD(n = 84) | Non‐ESRD(n = 1257) | P value |
|---|---|---|---|
| 90‐day mortality1 | 28, 33.3% (23.0‐43.6%) | 368, 29.3% (26.8‐31.8%) | 0.43 |
| 60‐day in hospital mortality1 | 19, 22.6% (4.6‐42.1%) | 240, 19.1% (16.9‐21.3%) | 0.43 |
| 72‐hour intubation1 | 26, 31.0% (5.1‐46.5%) | 420, 33.4% (1.3‐47.2%) | 0.63 |
| Duration of support | |||
| Cardiovascular2 | 0 (0‐2) | 0 (0‐2) | 0.14 |
| Respiratory2 | 1.5 (0‐3) | 1 (0‐2) | 0.34 |
| LOS ICU2 | 4 (2‐7) | 3 (2‐6) | 0.34 |
| LOS hospital2 | 9 (6‐14.5) | 8 (5‐13) | 0.14 |
1Reported as N, % (95% confidence interval).
2Reported as median days (interquartile range).
3Compared using a chi square test.
4Compared using a Wilcoxon rank‐sum test.
ESRD, end‐stage renal disease; LOS, length of stay; PROCESS, Protocolized Care for Early Septic Shock.
Huebinger RM, Walia S, Yealy DM, Kellum JA, Huang DT, Wang HE. Outcomes of end‐stage renal disease patients in the PROCESS trial. JACEP Open. 2021;2:e12358 10.1002/emp2.12358
Funding and support: Supported by a grant (P50 GM076659) from the National Institute of General Medical Sciences, National Institutes of Health.
Supervising Editor: Nicholas Caputo, MD, MSc.
REFERENCES
- 1. Chapter 1: definition and classification of CKD. Kidney Int Suppl. 2013;3(1):19‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arulkumaran N, Montero RM, Singer M. Management of the dialysis patient in general intensive care. Br J Anaesth. 2012;108(2):183‐192. [DOI] [PubMed] [Google Scholar]
- 3. Saran R, Robinson B, Abbott KC, et al. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3):A7‐A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end‐stage renal disease compared with the general population. Kidney Int. 2000;58(4):1758‐1764. [DOI] [PubMed] [Google Scholar]
- 5. Hoen B, Paul‐Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9(5):869‐876. [DOI] [PubMed] [Google Scholar]
- 6. Hoen B, le Kessler M, Hestin D, Mayeux D. Risk factors for bacterial infections in chronic haemodialysis adult patients: a multicentre prospective survey. Nephrol Dial Transplant. 1995;10(3):377‐381. [PubMed] [Google Scholar]
- 7. Halle MP, Hertig A, Kengne AP, Ashuntantang G, Rondeau E, Ridel C. Acute pulmonary oedema in chronic dialysis patients admitted into an intensive care unit. Nephrol Dial Transplant. 2012;27(2):603‐607. [DOI] [PubMed] [Google Scholar]
- 8. Rajdev K, Leifer L, Sandhu G, et al. Fluid resuscitation in patients with end‐stage renal disease on hemodialysis presenting with severe sepsis or septic shock: a case control study. J Crit Care. 2020;55:157‐162. [DOI] [PubMed] [Google Scholar]
- 9. Ozuzun P, Fried J, Grotts J. Early fluid resuscitation of end stage renal disease patients with severe sepsis and septic shock. Crit. Care Med. 2014;42:A1582. [Google Scholar]
- 10. Lee JJ, Taylor SL, Adams JY. Fluid resuscitation and mortality in sepsis with end‐stage renal disease. Am J Respir Crit Care Med. 2017;195:A5020. [Google Scholar]
- 11. Akhter M, Hallare M, Roontiva A, Stowell J. 154 Fluid resuscitation of septic patients at risk for fluid overload. Ann Emerg Med. 2017;70(4):S61‐S62. [Google Scholar]
- 12. Khan RA, Khan NA, Bauer SR, et al. Association between volume of fluid resuscitation and intubation in high‐risk patients with sepsis, heart failure, end‐stage renal disease, and cirrhosis. Chest. 2020;157:286‐292. [DOI] [PubMed] [Google Scholar]
- 13. Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370(18):1683‐1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rivers E, Nguyen B, Havstad S, et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368‐1377. [DOI] [PubMed] [Google Scholar]
- 15. Abou Dagher G, Harmouche E, Jabbour E, Bachir R, Zebian D. Bou Chebl R. Sepsis in hemodialysis patients. BMC Emerg Med. 2015;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Omar Y, Kaczmarczyk K, El Solh AA, Jaoude PE. Fluid resuscitation in patients with end‐stage renal disease presenting with severe sepsis or septic shock. Am J Respir Crit Care Med. 2017; 201:A7140. [Google Scholar]
- 17. Imam AM, Shaikh R, Ivanovic S, et al. Impact of intravenous volume resuscitation in dialysis‐dependent End Stage Renal Disease (ESRD) patients with severe sepsis or septic shock. In: d51. Critical Care: the fountainhead ‐ fluids, monitoring and managment of shock. American Thoracic Society. 2019:A6706. https://doi.org/10.1164/ajrccm‐conference.2019.199.1_meetingabstracts.a6706. [Google Scholar]
- 18. Corl KA, Prodromou M, Merchant RC, et al. The Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock (RIFTS): a randomized pilot study. Crit Care Med. 2019;47(7):951‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel‐group, multicentre feasibility trial. Intensive Care Med. 2016;42:1695‐1705. [DOI] [PubMed] [Google Scholar]
- 20. Self WH, Semler MW, Bellomo R, et al. Liberal versus restrictive intravenous fluid therapy for early septic shock: rationale for a randomized trial. Ann Emerg Med. 2018;72:457‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbier C, Loubières Y, Schmit C, et al. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30(9):1740‐1746. [DOI] [PubMed] [Google Scholar]
- 22. Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30(9):1834‐1837. [DOI] [PubMed] [Google Scholar]
- 23. Lichtenstein D, Karakitsos D. Integrating lung ultrasound in the hemodynamic evaluation of acute circulatory failure (the fluid administration limited by lung sonography protocol). J Crit Care. 2012;27(5):533.e11‐533.e19. [DOI] [PubMed] [Google Scholar]
- 24. Charron C, Caille V, Jardin F, Vieillard‐Baron A. Echocardiographic measurement of fluid responsiveness. Curr Opin Crit Care. 2006;12(3):249‐254. [DOI] [PubMed] [Google Scholar]


