Supplemental Digital Content is Available in the Text.
Keywords: CHOIR, Body map, Chronic pain, Development, Validation
Abstract
Introduction:
Critical for the diagnosis and treatment of chronic pain is the anatomical distribution of pain. Several body maps allow patients to indicate pain areas on paper; however, each has its limitations.
Objectives:
To provide a comprehensive body map that can be universally applied across pain conditions, we developed the electronic Collaborative Health Outcomes Information Registry (CHOIR) self-report body map by performing an environmental scan and assessing existing body maps.
Methods:
After initial validation using a Delphi technique, we compared (1) pain location questionnaire responses of 530 participants with chronic pain with (2) their pain endorsements on the CHOIR body map (CBM) graphic. A subset of participants (n = 278) repeated the survey 1 week later to assess test–retest reliability. Finally, we interviewed a patient cohort from a tertiary pain management clinic (n = 28) to identify reasons for endorsement discordances.
Results:
The intraclass correlation coefficient between the total number of body areas endorsed on the survey and those from the body map was 0.86 and improved to 0.93 at follow-up. The intraclass correlation coefficient of the 2 body map graphics separated by 1 week was 0.93. Further examination demonstrated high consistency between the questionnaire and CBM graphic (<10% discordance) in most body areas except for the back and shoulders (≈15–19% discordance). Participants attributed inconsistencies to misinterpretation of body regions and laterality, the latter of which was addressed by modifying the instructions.
Conclusions:
Our data suggest that the CBM is a valid and reliable instrument for assessing the distribution of pain.
1. Introduction
Chronic pain affects 100 million Americans and costs over $600 billion annually.8,21 Characterizing chronic pain is challenging because of the heterogeneity of common chronic pain conditions and the high degree of coprevalence26 (ie, chronic overlapping pain conditions [COPCs]). Visual representations of self-reported pain concisely and directly indicate the body parts affected by chronic pain, thereby facilitating precise diagnoses of pain conditions (eg, a standardized visual pain map would assist with quantifying the degree of widespread pain, while delineating COPCs).
Current pain maps, such as the McGill pain questionnaire28 Brief Pain Inventory,7 and Michigan body map (fibromyalgia-specific),4 among others,27,29 are limited by low resolution, condition-specific features, anatomical demarcations not corresponding to clinical pain conditions, or requirements for paper and pencil. Current body maps also omit key characteristics, such as joint pain representation or separate areas on the back side of the body, limiting their use for general-purpose chronic pain applications. Most notably, the rapid development of electronic medical record systems and secure online research platforms such as Research Electronic Data Capture (REDCap)18 has created a strong need for a standardized, digital, general-purpose body map to efficiently collect self-reported pain location data for integrative treatments and large-scale pain characterization research.
As a solution, Stanford University Division of Pain Medicine clinicians and researchers have developed a multipurpose digital body map as part of the Collaborative Health Outcomes Information Registry (CHOIR), an open-source learning healthcare system.34,35 Collaborative Health Outcomes Information Registry uses item-response theory-based measures, including general-specific and condition-specific legacy measures and National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System12 measures. Although the CHOIR body map (CBM) was initially developed for adult chronic pain, it has been applied more broadly for use in pediatric chronic pain1; perioperative medicine, orthopedic, and trauma surgery30; interventional radiology20; primary care16; gastrointestinal medicine; and psychiatry.
Currently, over 100,000 CBM assessments have been collected and analyzed,1,5,6,9,11,13–17,20–23,31–37 including extensive applications within REDCap tools18 and use of a slight variation by the NIH Multidisciplinary Approach to the Study of Pelvic Pain research network.25 In addition, an NIH-supported effort has recently integrated the CBM to develop a COPC screener. In this application, the participant can click on a particular body map area and is then directed to answer condition-specific pain questions. Extensive use of the CBM for clinical and research purposes has motivated us to publish our initial validation of this instrument. Specifically, we present our work evaluating the (1) comprehension and face validity, (2) temporal test–retest reliability, and (3) accuracy (compared with verbal reports) of the CBM.
2. Methods
2.1. Developing the Collaborative Health Outcomes Information Registry body map
We first performed an environmental scan and literature search of existing paper-and-pencil and electronic body maps.7,27–29,38 Using a Delphi process incorporating pain medicine clinicians, researchers, and patients, we identified strengths and weaknesses of these maps. For example, the von Baeyer body map for pediatric pain38 includes a representation for major joints, which was considered important by the group, but codes the anterior and posterior shoulder as one anatomic area. We sought to demarcate the anterior and posterior shoulder regions, as shoulder pain conditions often present differently depending on the pain distribution. We iteratively conducted this process for each existing body map and the subsequent development of the CBM segments.
After expert consensus, we divided the CBM into 36 anterior and 38 posterior segments that best aligned with typical distributions of common chronic pain conditions on the body surface and in joints, without prioritizing particular pain conditions (Fig. 1). Anterior segments located in the head, neck, upper limbs, lower limbs, and hips have a posterior counterpart. On the front, 6 additional anterior segments are divided into the chest, abdomen, and pelvis regions; by contrast, the back is divided into 8 additional segments, including the upper, middle, and lower back and buttocks. The CBM has 2 body silhouettes of identical segmentation to reflect the female and male anatomy. Participants who selected “male” or “female” as their gender were provided the male or female body map, respectively. Participants who selected “other” or “decline to answer” were provided the female body map. We initially validated the CBM through a Delphi process that relied heavily on feedback from expert physicians, pain researchers, and patients with a broad range of complex chronic pain disorders. We also sought input from the Multidisciplinary Approach to the Study of Pelvic Pain, which ultimately adopted the CBM with slight modification. We further assessed CBM validity, reliability, and ease of use through a formal patient-guided process.
Figure 1.
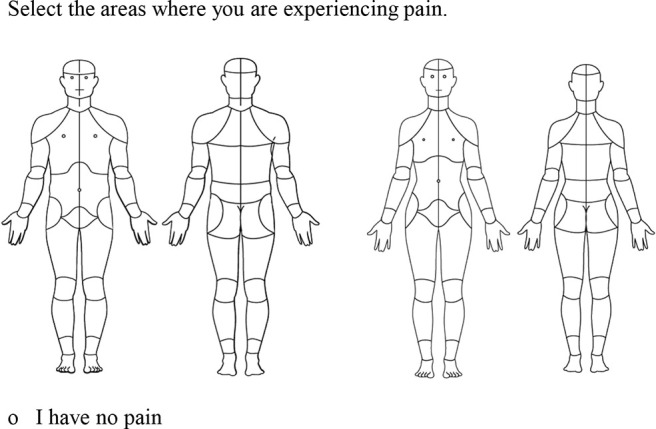
Graphic and instructions. The digital body figure contains 36 anterior segments and 38 posterior segments on which patients can endorse areas of pain. Patients may also endorse that they “have no pain.” The CBM has 2 body silhouettes of identical segmentation to reflect the female and male anatomy. Participants who selected “male” or “female” as their gender were provided the male or female body map, respectively. Participants who selected “other” or “decline to answer” were provided the female body map. CBM, CHOIR body map; CHOIR, Collaborative Health Outcomes Information Registry.
2.2. Study settings, participants, and methods
All 3 validation studies were approved by the university institutional review board. All online surveys were conducted through the HIPAA-compliant REDCap.18
2.2.1. Study 1: comprehension and face validity of the Collaborative Health Outcomes Information Registry body map
To validate the CBM against verbal descriptions of pain location, we assessed the intraclass correlation coefficient (ICC) of the total body areas indicated as having pain and the percentages of discordance between each CBM area and the individual body part questionnaire (IBPQ). We used the Stanford Systems Neuroscience and Pain Lab research recruitment database to distribute email invitations to individuals who had previously self-reported chronic pain and had given permission to be contacted for research purposes. Individuals were prompted to review the study information sheet, give consent, and continue to the initial online survey via REDCap. The 30-minute study 1 online survey included the interactive CBM; the IBPQ; demographics; pain duration, intensity, and diagnoses; a patient experience questionnaire; and the question “may we send you a follow-up survey in 1 week?”
2.2.2. Study 2: test–retest reliability of the Collaborative Health Outcomes Information Registry body map
Participants who consented to be contacted again per their initial survey (278/530) received an electronic invitation to complete a second online survey 1 week later to assess CMB test–retest reliability. This abbreviated version took ∼20 minutes to complete and included name; gender; pain duration, intensity, and diagnoses; the CBM; and the IBPQ.
2.2.3. Study 3: comparing the Collaborative Health Outcomes Information Registry body map and verbal pain location survey
Study 3 used an in-person, treatment-seeking clinical sample to clarify pain location endorsement discordance between the CBM and the IBPQ. After a regularly scheduled clinic visit, providers invited patients from the Stanford Pain Management Center to participate in this study. A research assistant then provided the patient with the study information sheet.
Participants were provided a handheld touchscreen device to obtain informed consent and complete the first part of the online survey in the presence of (but without assistance from) a research assistant. This 10-minute electronic REDCap survey included demographics; pain duration, intensity, and diagnoses; and the CBM. The research assistant took ∼20 minutes to verbally administer the second half of the survey, which included the IBPQ and the patient experience questionnaire. The research assistant clarified and documented laterality, when relevant, and clarified pain location discordance between the CBM and the verbally administered IBPQ with each participant. Pain experts thematically categorized qualitative explanations for discordances and generated frequencies for each theme.
2.3. Measures
2.3.1. Demographics
Demographics included date of birth, gender, race/ethnicity, marital status, and education level.
2.3.2. Self-reported pain characteristics and diagnoses
We assessed pain intensity on a numerical rating scale using a modified Patient-Reported Outcomes Measurement Information System Pain Intensity measure. Patients were asked to rate their current, worst, and average pain intensity over the past 7 days on a scale of 0 to 10.10,19 We assessed pain duration with the following time referents: <3, 3 to 6, 6 to 12 months, 1 to 5 years, and >5 years. In addition, participants reported pain diagnoses received by a physician by selecting one or more items from a list of common chronic pain conditions or by selecting “other” and writing in their condition. Finally, we counted the total number of reported diagnoses for each participant to assess COPCs.
2.3.3. Collaborative Health Outcomes Information Registry body map
The CBM is an electronic, visual representation of the human body that enables participants to indicate the location(s) of their pain (Fig. 1). Participants use a computer mouse or touchscreen device to select each body area in which they experience pain, with the instruction “select the areas where you are experiencing pain” or the option to indicate “I have no pain.”
2.3.4. Individual body part questionnaire
In all 3 studies, the IBPQ assessed concordance between text descriptions of pain location and the CBM representation. Patients responded to yes/no questions, such as “are you experiencing pain in…” followed by one of the 74 CBM segments. If participants indicated pain within a specific body part, additional questions clarified whether the pain occurred in the front or back and left-hand or right-hand side of the body.
2.3.5. Participant experience questionnaire
We assessed CMB utility through a brief list of questions addressing the ease of (1) the CBM instructions and (2) using the CBM graphic on a 5-point Likert scale (very easy, easy, neutral, difficult, and very difficult). Next, a yes/no question assessed the ability to discriminate left vs right sides on the body map. We assessed patient satisfaction with a yes/no question: “On the Body Map, were you able to mark all the areas where you have pain?” Each question was followed by a text-response field (initial survey) or an open-ended question administered by the trained research assistant (in-person interviews) to allow participants to elaborate on their answers, describe any confusion, and suggest potential changes. This questionnaire was not administered as part of the follow-up survey.
2.3.6. Validation measures
Using 2 methods, we assessed face validity by examining the (1) agreement between the CBM and the IBPQ and (2) test–retest reliability of the CBM 1 week later. First, we used intraclass correlations to assess the consistency of the total number of body areas endorsed as having pain. Second, we quantified the percentages of discordance between each specific body area endorsed on the CBM and the IBPQ, as well as between CBM results acquired at 2 time points.
2.3.7. Qualitative evaluation of discordant responses
To evaluate discordant responses in the in-person interviews from study 3, we used a thematic analysis approach3 of data organization and transcription, item coding, theme identification, item analysis, and reporting.
2.4. Statistics
We used descriptive statistics for demographic information. Data were analyzed using t-tests and the R (version 3.5.0) programming language. Demographic and descriptive statistics are presented in Table 1.
Table 1.
Participant demographics for each study.
| Initial survey (study 1), n = 560 | Follow-up survey (study 2), n = 287 | In-person interviews (study 3), n = 30 | |
|---|---|---|---|
| Age, M (SD) (range) | 53.7 (14.7) (18–89) | 54.8 (14.3) (22–87) | 58.7 (14.7) (32–81) |
| Gender, n (%) | |||
| Male | 159 (28.4%) | 85 (29.6%) | 17 (56.7%) |
| Female | 398 (71.1%) | 200 (69.7%) | 13 (43.3%) |
| Other | 2 (0.4%) | 2 (0.7%) | 0 (0.0%) |
| Decline to answer | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) |
| Race, n (%) | |||
| African American | 14 (2.5%) | 4 (1.4%) | 1 (3.3%) |
| Anglo-American | 446 (79.6%) | 256 (86.8%) | 23 (76.7%) |
| Asian | 45 (8.0%) | 16 (5.4%) | 2 (6.7%) |
| Pacific Islander | 4 (0.7%) | 2 (0.7%) | 0 (0.0%) |
| American Indian or Alaskan Native | 4 (0.7%) | 4 (1.4%) | 0 (0.0%) |
| Others | 47 (8.4%) | 13 (4.4%) | 4 (13.8%) |
| Marital status, n (%) | |||
| Never married | 99 (17.7%) | 44 (15.3%) | 4 (13.3%) |
| Married | 310 (55.4%) | 174 (60.6%) | 16 (53.3%) |
| Separated | 10 (1.8%) | 8 (2.8%) | 0 (0.0%) |
| Divorced | 71 (12.7%) | 34 (11.9%) | 8 (26.7%) |
| Widowed | 22 (3.9%) | 9 (3.1%) | 1 (3.3%) |
| Living Together | 47 (8.4%) | 17 (5.9%) | 1 (3.3%) |
| No response | 1 (0.2%) | 1 (0.4%) | 0 (0.0%) |
| Education, n (%) | |||
| Some high school | 4 (0.7%) | 1 (0.4%) | 0 (0.0%) |
| High school or GED | 16 (2.9%) | 7 (2.4%) | 3 (10.0%) |
| Some college, no degree | 106 (18.9%) | 49 (17.1%) | 5 (16.7%) |
| Associate degree or vocational certificate | 76 (13.6%) | 46 (16.0%) | 6 (20.0%) |
| Bachelor's degree | 170 (30.4%) | 92 (32.1%) | 7 (23.3%) |
| Master's degree | 129 (23.0%) | 58 (20.2%) | 7 (23.3%) |
| Professional degree | 29 (5.2%) | 16 (5.6%) | 2 (6.7%) |
| Doctoral degree | 30 (5.4%) | 18 (6.3%) | 0 (0.0%) |
Age is presented as the mean (SD) and range. All other variables are presented as the number of participants and percent of the study sample size.
Data from the initial and follow-up surveys were individually analyzed with R. We did not include surveys with missing responses or nonsensical input in the calculations. For each demographic variable, missing data percentages ranged from 0.2% to 6.0% and are presented in tables where appropriate.
For age and numerical pain ratings, we calculated mean, standard deviation, and range. To calculate age, date of birth was subtracted from the survey timestamp. For all other demographic, diagnostic, and CBM fields, we calculated frequencies and percentages.
To assess the agreement in the total number of body areas with pain between the CBM and the IBPQ, we calculated the ICC (C, 1) 2-way mixed, single score for the initial survey, follow-up survey, and in-person interviews. To assess the test–retest reliability of the CBM, we calculated the ICC (C,1) for CBM data collected in the initial and follow-up surveys. According to Koo and Li, 2016,24 values of <0.5, 0.5 to 0.75, 0.75 to 0.9, and >0.90 indicate poor, moderate, good, and excellent reliability, respectively.
We calculated the discordance percentage for each of the 74 body parts (1) for the initial survey, follow-up survey, and in-person interviews and (2) between the initial and follow-up survey (to assess test–retest reliability). The discordance percentage represents the ratio of the number of discordant pairs over the total number of pairs. We performed analyses with SAS Enterprise Guide (SAS Institute Inc., Cary, NC).
2.5. Excluded participants
Participants indicating that they did not experience pain were excluded from analyses beyond demographic information (initial survey n = 30; follow-up survey n = 9; and in-person interviews n = 2).
3. Results
3.1. Participant demographics
The mean age across all studies was 55.7 years (Table 1). Approximately 70% of the first 2 cohorts and 43% of the third cohort were women. Most participants were Anglo-American, and ∼50% reported a married status, and had a bachelor's degree, master's degree, or some college education.
3.2. Pain characteristics and participant-reported pain diagnoses
The modal pain duration across studies was >5 years, followed by 1 to 5 years, reflective of those seeking treatment at a tertiary care clinic (Table 2). The mean average pain intensity score for studies 1, 2, and 3 was 4.6, 4.5, and 5.8, respectively. Most patients (75.7% of initial cohort; 83.8% of follow-up cohort; and 75.0% of in-person cohort) reported receiving at least one diagnosis from their physician (Table 3), with the most common being arthritis, back pain, degenerative disc disease, neuropathic pain, and migraine (see Table, supplemental digital content 1, for participant-reported pain conditions within the “other” category, available at http://links.lww.com/PR9/A90). The range of reported diagnoses in the 3 studies was 0 to 16, 0 to 18, and 0 to 6 (Table 4). The most common number of pain conditions for participants was 2 to 3 (32.6%, 38.1%, and 67.9% of participants, respectively), followed by 4 to 5 for studies 1 and 2 (22.6% and 21.6%) and 0 to 1 for study 3 (25.0%).
Table 2.
Participant pain characteristics for each study.
| Initial survey (study 1), n = 530 | Follow-up survey (study 2), n = 278 | In-person interviews (study 3), n = 28 | |
|---|---|---|---|
| Pain duration, n (%) | |||
| <3 months | 5 (0.9%) | 0 (0.0%) | 0 (0.0%) |
| 3–6 months | 6 (1.1%) | 4 (1.4%) | 1 (3.6%) |
| 6–12 months | 21 (4.0%) | 12 (4.3%) | 4 (14.3%) |
| 1–5 years | 151 (28.5%) | 81 (29.1%) | 8 (28.6%) |
| >5 years | 347 (65.5%) | 180 (64.8%) | 15 (53.6%) |
| No response | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) |
| No pain | 30* | 9* | 2* |
| Pain rating over past 7 days, M (SD) | |||
| Worst pain rating | 6.7 (2.0) | 6.5 (2.1) | 8.0 (1.9) |
| Average pain rating | 4.6 (1.9) | 4.5 (1.9) | 5.8 (2.4) |
| Current pain rating | 4.30 (2.21) | 4.25 (2.31) | 5.29 (2.72) |
| Have you received a diagnosis from your doctor?, n (%) | |||
| Yes | 424 (75.7%) | 233 (83.8%) | 21 (75.0%) |
| No | 104 (18.6%) | 40 (14.4%) | 6 (21.4%) |
| Missing | 32 (5.7%) | 5 (1.8%) | 1 (3.6%) |
Pain duration and formal diagnosis are presented as the number of participants and percent of the study sample size, and pain rating is presented as the mean (SD).
Indicates participants who were excluded from analyses and gives sample sizes.
Table 3.
Participant-reported formal pain diagnoses given by a physician for each study.
| Diagnoses, n (%) | Initial survey (study 1), n = 530 | Follow-up survey (study 2), n = 278 | In-person interviews (study 3), n = 28 |
|---|---|---|---|
| Arthritis | 168 (31.7%) | 91 (32.7%) | 5 (17.9%) |
| Back pain | 160 (30.2%) | 79 (28.5%) | 0 (0.0%) |
| Burning mouth syndrome | 1 (0.2%) | 1 (0.4%) | 0 (0.0%) |
| Cancer | 7 (1.3%) | 2 (0.7%) | 1 (3.6%) |
| Endometriosis | 12 (2.3%) | 9 (3.2%) | 0 (0.0%) |
| Fibromyalgia | 72 (13.6%) | 38 (13.7%) | 1 (3.6%) |
| Carpal tunnel | 32 (6.0%) | 16 (5.8%) | 0 (0.0%) |
| Complex regional pain syndrome | 41 (7.7%) | 24 (8.6%) | 0 (0.0%) |
| Degenerative disc disease | 150 (28.3%) | 71 (25.5%) | 4 (14.3%) |
| Headache or migraine | 133 (25.1%) | 68 (24.5%) | 1 (3.6%) |
| Irritable bowel syndrome | 31 (5.9%) | 20 (7.2%) | 0 (0.0%) |
| Lupus | 6 (1.1%) | 2 (0.7%) | 0 (0.0%) |
| Mitochondrial disorders | 2 (0.4%) | 0 (0.0%) | 0 (0.0%) |
| Multiple sclerosis | 3 (0.6%) | 1 (0.4%) | 0 (0.0%) |
| Musculoskeletal pain | 76 (14.3%) | 35 (12.6%) | 2 (7.1%) |
| Myofascial pain | 46 (8.9%) | 15 (5.4%) | 0 (0.0%) |
| Neuropathic pain | 143 (27.0%) | 76 (27.3%) | 5 (17.9%) |
| Osteoarthritis | 87 (16.4%) | 50 (18.0%) | 1 (3.6%) |
| Phantom limb pain | 3 (0.6%) | 1 (0.4%) | 0 (0.0%) |
| Post stroke pain | 4 (0.8%) | 2 (0.7%) | 0 (0.0%) |
| Psoriatic arthritis | 4 (0.8%) | 3 (1.1%) | 0 (0.0%) |
| Rheumatoid arthritis | 23 (4.3%) | 10 (3.6%) | 0 (0.0%) |
| Sciatica | 94 (17.7%) | 37 (13.3%) | 0 (0.0%) |
| Scoliosis | 34 (6.4%) | 19 (6.8%) | 1 (3.6%) |
| Spinal cord injury | 25 (4.7%) | 16 (5.8%) | 0 (0.0%) |
| TMJ | 42 (7.9%) | 19 (6.8%) | 0 (0.0%) |
| Trigeminal neuralgia | 18 (3.4%) | 10 (3.6%) | 0 (0.0%) |
| Vascular pain | 13 (2.5%) | 5 (1.8%) | 0 (0.0%) |
| Other | 148 (27.9%) | 82 (29.5%) | 11 (39.3%) |
Participants could select one or more items from a provided list of common chronic pain conditions and/or select and fill in “other” (for diagnoses in the “other” category, see Table, supplemental digital content 1, available at http://links.lww.com/PR9/A90). Data are presented as the number of participants and percent of the study sample size.
Table 4.
Number of formal pain diagnoses self-reported by participants for each study.
| No. of diagnoses, n (%) | Initial survey (study 1), n = 530 | Follow-up survey (study 2), n = 278 | In-person interviews (study 3), n = 28 |
|---|---|---|---|
| 0–1 | 107 (20.2%) | 46 (16.6%) | 7 (25.0%) |
| 2–3 | 173 (32.6%) | 106 (38.1%) | 19 (67.9%) |
| 4–5 | 120 (22.6%) | 60 (21.6%) | 1 (3.4%) |
| 6–7 | 69 (13.0%) | 43 (15.5%) | 1 (3.6%) |
| 8–9 | 33 (6.2%) | 12 (4.3%) | 0 (0.0%) |
| 10–11 | 16 (3.0%) | 5 (1.8%) | 0 (0.0%) |
| 12–13 | 8 (1.5%) | 4 (1.4%) | 0 (0.0%) |
| 14–15 | 2 (0.4%) | 0 (0.0%) | 0 (0.0%) |
| 16–17 | 2 (0.4%) | 1 (0.4%) | 0 (0.0%) |
| 18 | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) |
Data are presented as the number of participants and percent of the study sample size.
3.3. Study 1: assessing participant comprehension of the Collaborative Health Outcomes Information Registry body map
A total of 530 participants completed the CBM and answered questions assessing their comprehension of the CBM and its instructions (Table 5). Half indicated that this was their first time using the CBM, and 93% and 92% indicated that the instructions were easy or very easy to follow, and the CBM was easy or very easy to complete, respectively.
Table 5.
Participant comprehension metrics and reported themes for improvements of the CBM for studies 1 and 3.
| Ease of use survey questions | Initial survey (study 1), n = 530 | In-person interviews (study 3), n = 28 |
|---|---|---|
| First time using the CHOIR body map, n (%) | ||
| Yes | 280 (52.8%) | 7 (25.0%) |
| No | 250 (47.2%) | 21 (75.0%) |
| Ease of CHOIR body map instructions, n (%) | ||
| Very easy | 369 (69.6%) | 21 (75.0%) |
| Easy | 124 (23.4%) | 7 (25.0%) |
| Neutral | 31 (5.9%) | 0 (0.0%) |
| Difficult | 6 (1.1%) | 0 (0.0%) |
| Very difficult | 0 (0.0%) | 0 (0.0%) |
| Ease of filling out the CHOIR body map, n (%) | ||
| Very easy | 367 (69.3%) | 22 (78.6%) |
| Easy | 121 (22.8%) | 4 (14.3%) |
| Neutral | 26 (4.9%) | 2 (7.1%) |
| Difficult | 11 (2.1%) | 0 (0.0%) |
| Very difficult | 3 (0.6%) | 0 (0.0%) |
| No response | 2 (0.4%) | 0 (0.0%) |
| Difficulty identifying the left and right side on the CHOIR body map, n (%) | ||
| Yes | 75 (14.2%) | 24 (85.7%) |
| No | 455 (85.9%) | 3 (10.7%) |
| No response | 0 (0.0%) | 1 (3.6%) |
| Able to mark all areas with pain on the CHOIR body map?, n (%) | ||
| Yes | 440 (83.0%) | 18 (64.3%) |
| No | 88 (16.6%) | 10 (35.7%) |
| No response | 2 (0.4%) | 0 (0.0%) |
| Participant-reported themes for the CHOIR body map needed improvements, n (%) | ||
| Left vs right indicators | 48 (9.2%) | 6 (21.4%) |
| More granularity | 68 (12.8%) | 12 (42.9%) |
| Describe pain type and quality | 34 (6.4%) | 8 (28.6%) |
| Indicate pain frequency | 35 (6.6%) | 1 (3.6%) |
| Map worked well; no suggestions | 92 (17.4%) | 2 (7.1%) |
Data are presented as the number of participants and percent of the study sample size.
CBM, CHOIR body map; CHOIR, Collaborative Health Outcomes Information Registry.
Only 14.2% of participants experienced difficulty in identifying left and right sides on the CBM, whereas 83.0% indicated that they could mark all areas in which they experienced pain. To further explore any challenges encountered by the patients, we administered additional coded, qualitative, text-response questions (see Table 5 for common themes). Among those responding, ∼13% suggested increasing the body map granularity to indicate smaller regions of pain. Approximately 9% suggested adding left-side and right-side indicators to the front and back of the CBM. Finally, 6.6% suggested adding the ability to describe the pain type, quality, and frequency for each segment endorsed.
3.4. Study 1: validating the Collaborative Health Outcomes Information Registry body map
Overall, the ICC of the total number of endorsed painful body areas between the CBM and the IBPQ was 0.86 for the initial survey (n = 530) and 0.93 for the follow-up survey (n = 278), demonstrating good and excellent reliability, respectively. In the granular comparison, the endorsement discordance was 10.3% or lower for the front of the CBM, except for the joints (ie, wrists, knees, ankles, and hips, but not elbows), hands, feet, right side of the face, and left upper arm, which ranged from 10% to 15% (Figs. 2A, B). The highest discordance occurred on the dorsal side, specifically the upper, mid, and low back; posterior shoulder; hip; and neck (15.2%–18.4% discordance). The maximum discordance was 18.4%, observed for the left posterior shoulder.
Figure 2.
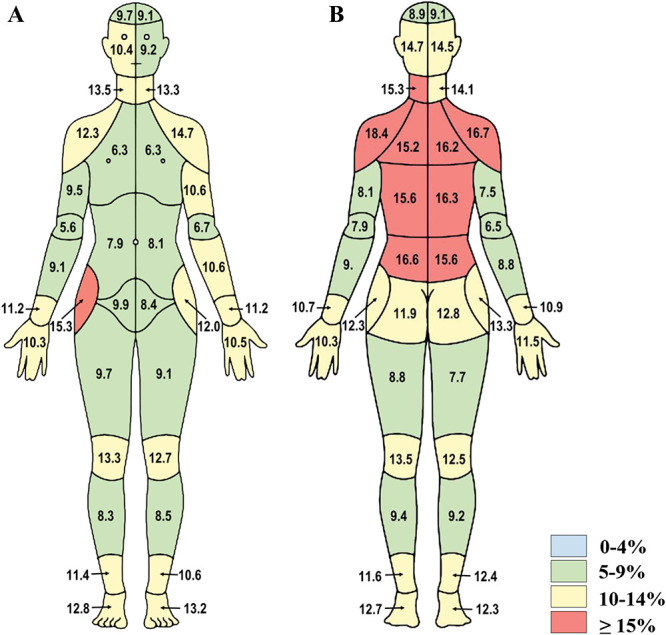
Initial survey discordance data from study 1. Percent discordance between areas of pain endorsed on the CBM and the individual body part questionnaire are presented for (A) front and (B) back segments of the body map (n = 530). CBM, CHOIR body map; CHOIR, Collaborative Health Outcomes Information Registry.
Consistent with the ICC trends, the individual discordance levels were lower at follow-up (Figs. 3A, B). However, these results mirrored those of the initial survey, wherein the highest discordance levels occurred in the upper and lower back and shoulder regions (11.0%–14.9%). For most remaining body regions, the discordance levels were below 10.0%.
Figure 3.
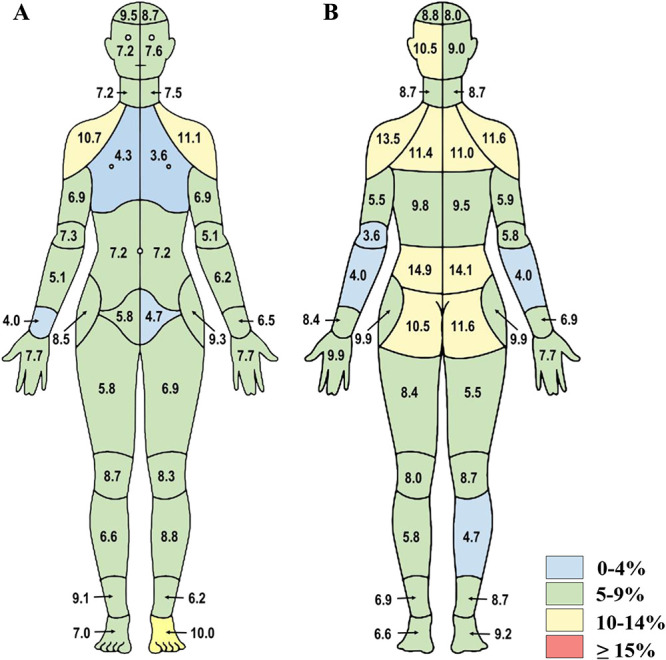
Follow-up survey discordance data from study 1. Percent discordance between areas of pain endorsed on the CBM and the individual body part questionnaire are presented for (A) front and (B) back segments of the body map (n = 278). CBM, CHOIR body map; CHOIR, Collaborative Health Outcomes Information Registry.
3.5. Study 2: evaluating test–retest reliability of the Collaborative Health Outcomes Information Registry body map
Of the 530 participants from the initial survey, 278 completed the CBM and IBPQ again 1 week later. Overall, the ICC (C,1) for the total number of areas marked as painful between the initial and follow-up body map demonstrated excellent reliability, at 0.93. Consistent with the ICC (C,1) results, the discordance percentages were low (<10.0%) for most body areas except the lower back, posterior shoulders, and right hip (13.1%–17.1%; Figs. 4A, B).
Figure 4.
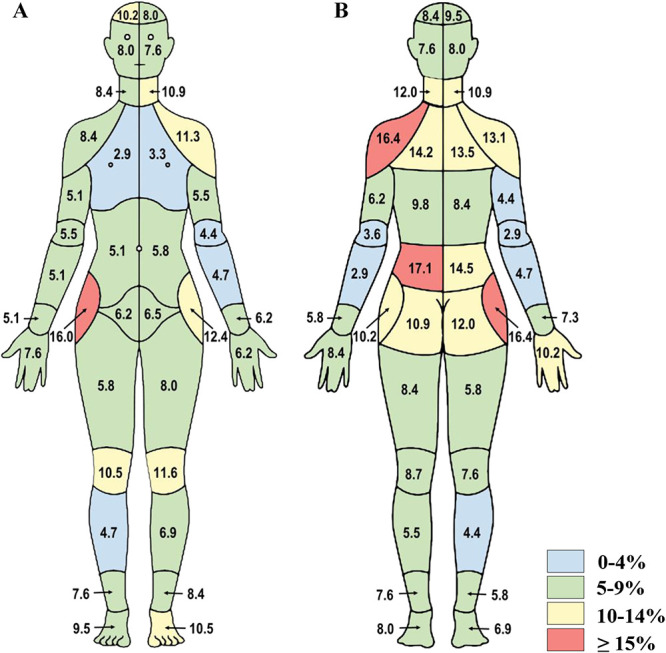
Test–retest assessment of the CBM from study 2. Percent discordance between areas of pain endorsed on the CBM graphic from the initial survey to the follow-up survey are presented for (A) front and (B) back segments of the body map (n = 278). CBM, CHOIR body map; CHOIR, Collaborative Health Outcomes Information Registry.
3.6. Study 3: evaluating discordance between Collaborative Health Outcomes Information Registry body map and verbal body region endorsement
For the in-person interviews (n = 28), the ICC (C,1) for the total number of areas endorsed as painful between the CBM and the verbally administered IBPQ was good, at 0.84. The research assistant identified at least one discordant region in ∼90% of the sample (Figs. 5A, B). The discordance levels were 0.0% to 26.7%, with the highest discordance observed for the mid-back (23.3% and 26.7%), followed by the wrists, dorsal hand, abdomen, shoulders, posterior neck, and right anterior knee (16.7%–20.0%).
Figure 5.
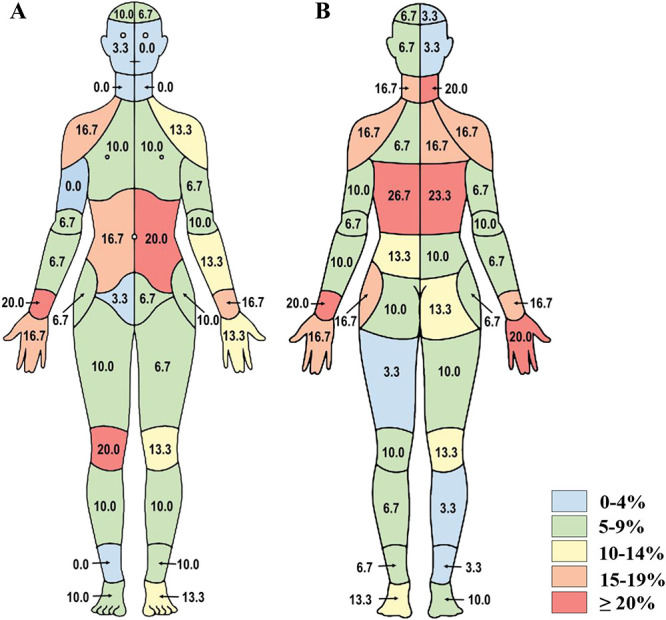
In-person interview discordance data from study 3. Percent discordance between areas of pain endorsed on the CBM and the individual body part questionnaire are presented for (A) front and (B) back segments of the body map (n = 28). CBM, CHOIR body map; CHOIR, Collaborative Health Outcomes Information Registry.
Similar to study 1, participants from study 3 were asked to assess the usability of the CBM and to suggest improvements (Table 5). In this cohort, 25.0% indicated having not used this body map before. All participants reported that the instructions were easy or very easy to understand, and ∼93% indicated that the CBM was easy or very easy to complete. Notably, 85.7% experienced difficulty in identifying the left or right side of the body on the CBM, whereas 64.3% indicated that they could mark all areas in which they experienced pain. Consistent with study 1, the most common participant-reported themes for improvements included more granularity; left-side and right-side labels; and the ability to indicate pain type, quality, and frequency.
3.7. Study 3: qualitatively evaluating reasons for discordance
In study 3, a trained research assistant clarified discordances in CBM endorsement during in-person interviews. The primary reason for discordance, as reported by most patients (75.0%), was that patients only endorsed regions on the CBM for which they were seeking care at the pain clinic (Table 6). When prompted by the individual items on the body part questionnaire, patients tended to report all regions of acute and chronic pain, even if managed at a different clinic. A subset (25.0%) indicated that they forgot about certain areas of pain when completing the CBM or did not interpret the regions accurately.
Table 6.
Participant-reported explanations for CBM endorsement discordance in study 3.
| Themes for discordance explanations, n (%) | In-person interviews (study 3), n = 28 |
|---|---|
| Only endorsed regions on body map for which patient is presenting to the clinic | 21 (75.0%) |
| Forgot about pain in a certain region until prompted | 7 (25.0%) |
| Reported only chronic (vs acute) pain on the body map | 5 (17.9%) |
| Interpreted the region indicated by the body map inaccurately | 7 (25.0%) |
| Confused between front/back and left/right on the body map | 4 (14.3%) |
Data are presented as the number of participants and percent of the study sample size.
CBM, CHOIR body map; CHOIR, Collaborative Health Outcomes Information Registry.
4. Discussion
This work provides an initial validation of the CBM, a standardized, digital, general-purpose body map for the collection of self-reported visual body pain locations. We used a Delphi technique, expert panel consensus, and quantitative and qualitative methods across 3 studies and 2 distinct participant samples, non–treatment-seeking and treatment-seeking, which both included COPCs. Our findings demonstrate that the CBM and its instructions (1) are intuitive and easy to use by research participants and patients, (2) have a high degree of concordance with textual/verbal measures of pain location, and (3) have excellent test–retest reliability. Moreover, by capturing larger musculoskeletal and joint-specific regions, the CBM presents higher granularity than other body maps. This feature may be particularly important for patients with multiple cooccurring conditions resulting in pain, where a multifactorial etiology is likely.
We applied 2 methods to assess the validity and test–retest reliability of the CBM: an ICC approach to demonstrate the stability of the total number of areas endorsed between instruments and across time points and a detailed analysis of percent discordance for each body area (Figs. 2–5) to reveal specific patterns of inconsistency.
Two explanations may clarify the discordances observed between the CBM and textual surveys. First, a subset of participants encountered difficulty in delineating symptoms on the left and right sides. We addressed this issue by clarifying the instructional set to include “left” and “right” labels on the CBM. Moreover, the largest discordances were observed in the extremities, where it may be more difficult for patients to delineate pain symptoms on the anterior–posterior axis. Second, we noted some discordance among endorsement patterns on body map regions and textual/verbal items assessing the same regions, particularly in the lower back, wrists, neck, shoulders, and abdomen, which could simply indicate a difference between how individuals verbally describe pain vs highlight it on an interactive body map. It is also possible that the body map may represent both bodily and referred pain. For instance, during the qualitative study, patients complaining of “lower back pain” pointed to their hips or buttocks, common areas of referred pain for patients with low back pain.2 Consequently, a graphical body map may more effectively assess pain location than a text-based description.
The moderate variability noted in specific regions endorsed on the test–retest assay (between weeks 1 and 2) may result from fluctuating symptoms or practice effects. As noted qualitatively, some participants indicated that they may differentially endorse pain symptoms in certain areas during any given medical visit, possibly because of an idiosyncratic understanding of the body map's intention or vagaries of memory. Indeed, interviewed patients showed the greatest variability in endorsements between the CBM and the verbal inquiry of individual body parts; these patients sought care for a specific issue and may have had their memories rekindled when the research assistant read the body regions aloud. We subsequently modified the instructions to include (1) the specific timeframe of pain and (2) all body regions experiencing pain, not just those for which they are seeking medical attention.
Finally, the presence of COPCs in our sample may have introduced additional challenges for consistently endorsing the CBM. For example, some participants reported difficulty determining whether to endorse areas of acute pain or pain arising from other chronic conditions. Although these factors might seem self-evident, responses from study participants highlight the complexity of symptom endorsement. This finding has implications for existing chronic pain studies, as patients may differentially endorse painful areas based on factors not readily apparent or easily separable, such as patient motivation, misunderstanding of the assessment's purpose, cognitive factors, or fluctuations in disease activity. Thus, instructions should explicitly define inclusion or exclusion parameters.
We note 2 limitations of the current studies. First, studies 1 and 2 included online convenience samples of previous study participants who primarily reside in or near the Bay Area, with ∼65% having at least a bachelor's degree, potentially limiting the generalizability of our findings. Future studies will examine subgroup variability and generalizability among differing education levels. Second, despite the overall reliability among participant endorsement of pain symptoms, discordances reached 20% to 25% for some regions. Such differences may arise from participant factors such as COPCs, difficulty understanding the map's purpose (ie, clinical inquiry vs general pain representation), or confusion regarding the precise meaning of a textual body part description. For the latter possibility, the graphical body map would more accurately represent the patient's pain.
Finally, participants reported difficulties in endorsing certain regions. Further investigation should explore the value of continued refinement of the CBM and assessments of somatic distribution. For example, increasing the granularity of certain regions, implementing a more flexible data capture method, assessing subsets that capture anatomically derived dermatomes, or characterizing the pain intensity, quality, and duration of each region may further improve accuracy in assessing pain distributions. However, each potential modification that adds granularity must be balanced by the increased patient time burden. As an external factor, the type of electronic device used (eg, computer or touchscreen device) may influence the patients' ability to accurately endorse the CBM. We believe this is unlikely, as both CHOIR and REDCap use a responsive web design, scaling the CBM with the device display. Although no participants reported technical difficulty, further examination should assess whether the electronic format impacts self-reports or endorsement discordance between formats.
Although this study validated the CBM using a diverse and complex sample, cross-sectional and longitudinal examinations of specific pain conditions may further establish the validity and reliability of this instrument as a clinical tool and support its use in studies with narrower inclusion criteria. Future studies should also corroborate CBM data with objective markers of disease activity, including pain intensity scales, interference, physical and psychosocial function, and structural or exaination-based findings. Such studies are currently underway, with the goal that CBM-generated data may provide an additional clinical marker of chronic pain.
5. Conclusions
The current study validated the CBM in community-based, treatment-seeking individuals with diverse chronic pain diagnoses. Our findings indicated ease of use, high consistency with conventional textual pain reports, and excellent test–retest reliability. This validation was bolstered by an iterative approach based on quantitative and qualitative patient feedback, which highlighted potential shortcomings and ultimately yielded a clearer and more precise instructional assessment set. This iterative process also revealed patient-specific and situational factors inherent to the process of self-reported pain location assessment, with potential implications for the clinical and empirical utility of patient-reported outcome assessments. This study establishes the CBM as a valid and reliable tool for self-reported assessments of pain location and distribution for clinical and research purposes.
Disclosures
The authors have no conflicts of interest to declare.
Redlich Pain Endowment, MRTG-HSR-02-15-2018-Salmasi, NIH K24DA029262, T32 DA035165, HHSN 2712011200728P, K23AT008477, K23DA047473, R01AT008561, and P01-AT006651.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A90.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
K H. Scherrer and M.S. Ziadni contributed equally to this work. S. Mackey and M.C. Kao contributed equally to this work.
Contributor Information
Kristen Hymel Scherrer, Email: kristen_scherrer@med.unc.edu.
Maisa S. Ziadni, Email: mziadni@stanford.edu.
Jiang-Ti Kong, Email: jtkong@stanford.edu.
John A. Sturgeon, Email: jasturge@asu.edu.
Vafi Salmasi, Email: vsalmasi@stanford.edu.
Juliette Hong, Email: jhong5@stanford.edu.
Eric Cramer, Email: emcramer@stanford.edu.
Abby L. Chen, Email: achen95@stanford.edu.
Teresa Pacht, Email: tpacht@stanford.edu.
Garrick Olson, Email: garricko@stanford.edu.
Beth D. Darnall, Email: bdarnall@stanford.edu.
Ming-Chih Kao, Email: mckao@stanford.edu.
References
- [1].Bhandari RP, Feinstein AB, Huestis SE, Krane EJ, Dunn AL, Cohen LL, Kao MC, Darnall BD, Mackey SC. Pediatric-Collaborative Health Outcomes Information Registry (Peds-CHOIR): a learning health system to guide pediatric pain research and treatment. PAIN 2016;157:2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. PAIN 2009;147:17–19. [DOI] [PubMed] [Google Scholar]
- [3].Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psych 2006;3:77–101. [Google Scholar]
- [4].Brummett CM, Bakshi RR, Goesling J, Leung D, Moser SE, Zollars JW, Williams DA, Clauw DJ, Hassett AL. Preliminary validation of the Michigan body map. PAIN 2016;157:1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carriere JS, Martel MO, Kao MC, Sullivan MJ, Darnall BD. Pain behavior mediates the relationship between perceived injustice and opioid prescription for chronic pain: a Collaborative Health Outcomes Information Registry study. J Pain Res 2017;10:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carriere JS, Sturgeon JA, Yakobov E, Kao MC, Mackey SC, Darnall BD. The impact of perceived injustice on pain-related outcomes: a combined model examining the mediating roles of pain acceptance and anger in a chronic pain sample. Clin J Pain 2018;34:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- [8].Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dressler AM, Gillman AG, Wasan AD. A narrative review of data collection and analysis guidelines for comparative effectiveness research in chronic pain using patient-reported outcomes and electronic health records. J Pain Res 2019;12:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. PAIN 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [11].Feinstein AB, Sturgeon JA, Darnall BD, Dunn AL, Rico T, Kao MC, Bhandari RP. The effect of pain catastrophizing on outcomes: a developmental perspective across children, adolescents, and young adults with chronic pain. J Pain 2017;18:144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas 2010;11:304–14. [PMC free article] [PubMed] [Google Scholar]
- [13].Gilam G, Sturgeon JA, You DS, Wasan AD, Darnall BD, Mackey SC. Negative affect-related factors have the strongest association with prescription opioid misuse in a cross-sectional cohort of patients with chronic pain. Pain Med 2019;21:e127–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hah JM, Sturgeon JA, Zocca J, Sharifzadeh Y, Mackey SC. Factors associated with prescription opioid misuse in a cross-sectional cohort of patients with chronic non-cancer pain. J Pain Res 2017;10:979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Harle CA, Lipori G, Hurley RW. Collecting, integrating, and disseminating patient-reported outcomes for research in a learning healthcare system. EGEMS 2016;4:1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harle CA, Listhaus A, Covarrubias CM, Schmidt SO, Mackey S, Carek PJ, Fillingim RB, Hurley RW. Overcoming barriers to implementing patient-reported outcomes in an electronic health record: a case report. J Am Med Inform Assoc 2016;23:74–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harle CA, Marlow NM, Schmidt SO, Shuster JJ, Listhaus A, Fillingim RB, Hurley RW. The effect of EHR-integrated patient-reported outcomes on satisfaction with chronic pain care. Am J Manag Care 2016;22:e403–8. [PMC free article] [PubMed] [Google Scholar]
- [18].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S, Collaborative EPCR. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manag 2011;41:1073–93. [DOI] [PubMed] [Google Scholar]
- [20].Hoang NS, Hwang W, Katz DA, Mackey SC, Hofmann LV. Electronic patient-reported outcomes: semi-automated data collection in the interventional radiology clinic. J Am Coll Radiol 2019;16:472–7. [DOI] [PubMed] [Google Scholar]
- [21].Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press (US), 2011. PMID: 22553896. [PubMed] [Google Scholar]
- [22].Karayannis NV, Sturgeon JA, Chih-Kao M, Cooley C, Mackey SC. Pain interference and physical function demonstrate poor longitudinal association in people living with pain: a PROMIS investigation. PAIN 2017;158:1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Khan JS, Hah JM, Mackey SC. Effects of smoking on patients with chronic pain: a propensity-weighted analysis on the Collaborative Health Outcomes Information Registry. PAIN 2019;160:2374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV, Mackey S, Kusek JW, Mullins C, Clemens JQ. The MAPP research network: design, patient characterization and operations. BMC Urol 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain 2016;17(9 suppl):T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. PAIN 1986;24:57–65. [DOI] [PubMed] [Google Scholar]
- [28].Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. PAIN 1975;1:277–99. [DOI] [PubMed] [Google Scholar]
- [29].Moseley GL. I can't find it! Distorted body image and tactile dysfunction in patients with chronic back pain. PAIN 2008;140:239–43. [DOI] [PubMed] [Google Scholar]
- [30].Rosenberg GM, Shearer EJ, Zion SR, Mackey SC, Morris AM, Spain DA, Weiser TG. Implementation challenges using a novel method for collecting patient-reported outcomes after injury. J Surg Res 2019;241:277–84. [DOI] [PubMed] [Google Scholar]
- [31].Ross AC, Simons LE, Feinstein AB, Yoon IA, Bhandari RP. Social risk and resilience factors in adolescent chronic pain: examining the role of parents and peers. J Pediatr Psychol 2018;43:303–13. [DOI] [PubMed] [Google Scholar]
- [32].Sharifzadeh Y, Kao MC, Sturgeon JA, Rico TJ, Mackey S, Darnall BD. Pain catastrophizing moderates relationships between pain intensity and opioid prescription: nonlinear sex differences revealed using a learning health system. Anesthesiology 2017;127:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sturgeon JA, Carriere JS, Kao MJ, Rico T, Darnall BD, Mackey SC. Social disruption mediates the relationship between perceived injustice and anger in chronic pain: a collaborative health outcomes information Registry study. Ann Behav Med 2016;50:802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sturgeon JA, Darnall BD, Kao MC, Mackey SC. Physical and psychological correlates of fatigue and physical function: a Collaborative Health Outcomes Information Registry (CHOIR) study. J Pain 2015;16:291–8.e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sturgeon JA, Dixon EA, Darnall BD, Mackey SC. Contributions of physical function and satisfaction with social roles to emotional distress in chronic pain: a Collaborative Health Outcomes Information Registry (CHOIR) study. PAIN 2015;156:2627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sturgeon JA, Ziadni MS, Trost Z, Darnall BD, Mackey SC. Pain catastrophizing, perceived injustice, and pain intensity impair life satisfaction through differential patterns of physical and psychological disruption. Scand J Pain 2017;17:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tran P, Sturgeon JA, Nilakantan A, Foote A, Mackey S, Johnson K. Pain catastrophizing mediates the relationship between trait happiness and depressive symptoms in individuals with current pain. J Appl Biobehav Res 2017;22:e12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].von Baeyer CL, Lin V, Seidman LC, Tsao JC, Zeltzer LK. Pain charts (body maps or manikins) in assessment of the location of pediatric pain. Pain Manag 2011;1:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A90.


