Abstract
Purpose
Nonalcoholic fatty liver disease (NAFLD) is the most frequent cause of chronic liver diseases in both adults and children with obesity. The aim of this study was to compare the changes in liver enzymes and metabolic profile in adolescents with fatty liver following selected school-based exercise (SBE) and high-intensity interval training (HIIT) interventions.
Methods
In a semi-experimental study, 34 obese male adolescents with clinically defined NAFLD were divided into the HIIT (n=11, age=12.81±1.02 years, body mass index [BMI]=26.68 ±2.32 kg/m2), selected SBE (n=11, age=13.39±0.95 years, BMI=26.47±1.74 kg/m2), and control (n=12, age=13.14±1.49 years, BMI=26.45±2.21 kg/m2) groups. The ultrasonography NAFLD grade, peak oxygen uptake (VO2peak), lipid profile, insulin resistance, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels of the participants were measured before and after the exercise interventions.
Results
The BMI, waist-to-hip ratio, and body fat percentage of the participants decreased, and a significant increase in VO2peak was observed after the intervention; however, the HIIT group showed a significant improvement compared with the SBE group (p<0.01). Significant reductions were observed in the levels of insulin resistance, triglyceride, total cholesterol, ALT, and AST in both groups, although high-density lipoprotein levels decreased only in the HIIT group (p<0.01). Further, a significant reduction in low-density lipoprotein level was observed in the training groups (p<0.01), but this decrease was not significant compared with the control group (p>0.01).
Conclusion
HIIT and SBE are equally effective in improving health parameters in obese children and adolescents.
Keywords: Exercise interventions, Nonalcoholic fatty liver disease, Insulin resistance, Obesity
INTRODUCTION
The prevalence of obesity and obesity-related illnesses in children and adolescents has dramatically increased over the past decades [1]. Nonalcoholic fatty liver disease (NAFLD) is one of the most common causes of liver abnormality in children and adolescents. NAFLD encompasses a spectrum of conditions ranging from simple hepatic steatosis (fat accumulation in hepatocytes) to nonalcoholic steatohepatitis (fat accumulation with inflammation), leading to advanced fibrosis and cirrhosis [2]. The reference standard method for confirming NAFLD is liver biopsy; however, it is an expensive and invasive procedure [3]. At present, the findings of ultrasonography or magnetic resonance imaging and liver function tests are used as surrogate markers for estimating the degree of steatosis and liver fibrosis and the risk of progression to end-stage liver disease [3]. Ultrasonography is the first-line imaging technique used in the clinical setting because it is a safe, low-cost, and repeatable modality [4]. Measurement of serum alanine aminotransferase (ALT) level is an inexpensive test for detecting NAFLD; however, it should be noted that some studies defined the upper limit of normal ALT in healthy-weight, metabolically normal, liver-disease-free children as 25.8 U/L for boys [3]. Moreover, it has been suggested that NAFLD is strongly associated with some cardiovascular risk factors, such as abdominal obesity, dyslipidemia, hypertension, and insulin resistance (IR)/diabetes, in both children and adults [5].
In Asian regions, a similar prevalence of NAFLD has been found, ranging from 15 to 30% in the general population, and >50% in patients with diabetes and metabolic syndrome [6]. An increased prevalence of fatty liver in obese children, ranging from 46.2 to 77.1%, has been reported in various studies [7,8,9].
NAFLD can rapidly progress in children, and some adolescents reach the last stages of liver diseases. Further, it should be noted that having NAFLD in childhood could be a major risk factor for hepatocellular carcinoma in adulthood [10]. Although there are no valid pharmacological therapies for children with NAFLD, exercise is suggested to be beneficial for preventing and treating NAFLD without weight loss and any dietary changes in adults [11,12,13,14]. The recommended treatment interventions for NAFLD usually include nutrition and exercise modifications [10]. Because studies using exercise alone to treat NAFLD in childhood are very limited [10], very little is known to date about the effect of exercise training as an independent treatment strategy to control NAFLD in adolescents. Furthermore, there are sparse data about the optimal exercise regimen (e.g., type, dose, and intensity) that should be prescribed for the treatment of NAFLD in the young population [2]. Studies have revealed that exercise training reduces visceral fat and IR; thus, a strategy addressing abdominal obesity and IR may be an effective treatment for young persons with NAFLD [2].
School-based exercise (SBE) programs consist of physical education routines and different activities at varying intensities performed before or after school hours [15]. Recent studies have shown that SBE programs improve some cardiometabolic risk factors and the cardiorespiratory fitness of children and adolescents [15,16]. Meanwhile, high-intensity interval training (HIIT) consists of short-term high-intensity activities with short rest periods [17]. Despite promising evidence supporting the positive effects of HIIT on metabolic profile in adults, limited research has been conducted on adolescents [17]. Some studies have shown that HIIT training in children and adolescents resulted in improved metabolic health parameters (lipid profile, IR, blood pressure, body composition, and cardiorespiratory fitness) [18]. However, no study has compared the efficacy of HIIT training with that of SBE in children and adolescents with fatty liver. Thus, the aim of this study was to compare the effects of the two modalities of exercise training on some physiological biomarkers and health-related parameters in children and adolescents with NAFLD.
MATERIALS AND METHODS
The participants comprised 34 male students aged between 10 and 15 years (body mass index [BMI] >25 kg/m2 or >85%) who were diagnosed with NAFLD using ultrasonography of the liver parenchyma (grade 1 or 2) and enzymatic tests (ALT >25.8 U/L) [3]. The inclusion criteria were as follows: no use of medications, no history of any heart and vascular disease, and a sedentary lifestyle (<30 minutes of physical activity per week). The exclusion criteria were injury during physical activity and absence in more than two sessions during the exercise protocol.
Before performing the exercise test and before involvement in the study, parents and guardians signed an informed consent form in accordance with international ethical standards. The study was approved by the ethics committee of the University of Isfahan, Isfahan Province, Iran (registry no. IR.UI.REC.1396.026).
Pre-exercise testing protocol
The maturity stage of the participants was determined on the basis of the five-stage Tanner classification scale (V-IV-III-II-I) [19]. The fat percentage was measured using a skinfold caliper (SlimGuide; Creative Health Products, Plymouth, MI, USA) at four points and calculated using Peterson's formula [20]. All participants performed the 20-m shuttle run field test to predict peak oxygen uptake (VO2peak) and maximal aerobic velocity under the same environmental conditions. Thereafter, we used the formula of Matsuzaka et al. [21] to estimate VO2peak:
| VO2peak=61.1−2.20×(sex)−0.462×age (years)−0.862×BMI+(0.192×number of laps), where sex=0 (male) or 1 (female). |
Liver fat assessment
An expert radiologist used sonography techniques to measure liver fat with respect to standardized criteria (3.5–5 MHz probe; Voluson Expert 730; GE Healthcare, Seoul, Korea). The degree of fatty liver was reported as follows: grade 1, mildly increased liver echogenicity with normal visualization of the diaphragm and intrahepatic vascular borders; grade 2, moderately increased liver echogenicity with slightly decreased visualization of the diaphragm and intrahepatic vascular margins; and grade 3, severely increased liver echogenicity with poor or no visualization of the diaphragm and vessel borders [22].
Metabolic parameters
Blood samples were collected from the antecubital vein after a 12-hours fast (time interval: 9.00–10.00 am), 48 hours before starting the session, and 48 hours after the last training session. Immediately after blood collection, samples were placed on ice and spun at 3,000 revolutions per minute for 10 minutes. Plasma was stored at −80°C until final analysis. Total cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glucose, insulin, ALT, and aspartate aminotransferase (AST) levels were measured in all participants using enzymatic and electrochemiluminescence methods with an analyzer system (Cobas C-311, Cobas E-411) and Roche kits (Roche Diagnostics, Indianapolis, IN, USA). IR was estimated using the homeostasis assessment model of IR (HOMA-IR) and calculated from fasting insulin and glucose levels according to the following formula: insulin (μIU/mL) glucose (mmoL/L)/22.5 [1].
Training program
Different aerobic physical activities and sports have been shown to have potentially beneficial effects on childhood obesity and cardiorespiratory fitness. In this study, the training groups performed two selected protocols (SBE and HIIT aerobic exercise), 3 days per week for 8 weeks (Table 1). The sessions started with a standardized warm-up, which consisted of 5-minutes jogging followed by 5-minutes dynamic stretching. Thereafter, the participants performed their assigned training program. Selected SBE training included implementation of a 20 m shuttle run test, sports skill training (futsal, handball, and basketball), jump rope, and games. The HIIT protocol was modified from the study of Racil et al. [23]. At the end of the training session, the participants performed 10 minutes of cool-down activities including running at low intensity and static stretching. Two coaches supervised all HIIT and SBE training sessions.
Table 1. Training program protocols.
| Training programs | Weeks 1–2 | Weeks 3–5 | Weeks 6–8 |
|---|---|---|---|
| SBE | Training includes 20-m shuttle run test, futsal basic skills training, futsal games, and jump rope exercise. | Training includes 20-m shuttle run test, basketball basic skills training, basketball games, and jump rope exercise. | Training includes 20-m shuttle run test, handball basic skills training, handball games, and jump rope exercise. |
| Total=50 min | Total=55 min | Total=60 min | |
| ACC≃300–350 | ACC≃350–400 | ACC≃400–450 | |
| HIIT | 2×(6×30 s/30 s) | 2×(7×30 s/30 s) | 2×(8×30 s/30 s) |
| 100%/50% MAS | 105%/50% MAS | 110%/50% MAS | |
| Rest=4 min | Rest=4 min | Rest=4 min | |
| Total=36 min | Total=38 min | Total=40 min | |
| ACC≃250–300 | ACC≃300–350 | ACC≃350–400 |
SBE: school-based exercise, HIIT: high-intensity interval training, ACC: approximate calories consumed in one session, MAS: maximum aerobic speed associated with VO2peak.
Example: (2×(6×30 s/30 s), 100/50% MAS, rest=4 min) means that the participant has to run two series of six repetitions of 30 s/30 s, composed of 30-s running at 100% of MAS and 30-s active recovery at 50% of MAS. The participant passively recovers for 4 min in between series. Each session is repeated three times a week.
Post-exercise testing protocol
Post-exercise assessments for metabolic parameters, body composition, VO2peak, and blood pressure were performed 24 hours after the final training session.
Statistical analysis
Data are presented as mean±standard deviation. The Shapiro-Wilk test was applied to analyze the normality of the data. The Wilcoxon signed-rank test, analysis of covariance (ANCOVA), and Bonferroni post hoc tests were applied to analyze the collected data. Significance level was set at p<0.05, and statistical analysis was performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Co., Armonk, NY, USA).
RESULTS
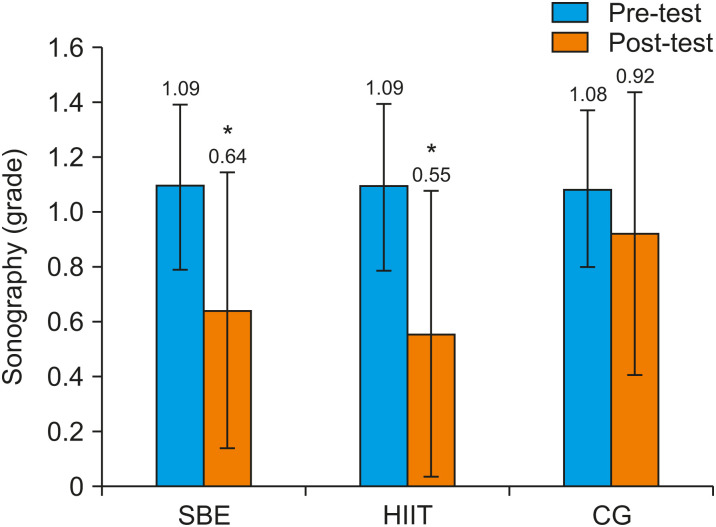
The measured anthropometric and physiological variables are presented in Table 2. Statistical analysis showed that the baseline values of the measured variables, including ultrasonography grade and pubertal stage, were not significantly different (p<0.05). Following the interventions, the training groups had significant decreases in body weight (p<0.001), body fat percentage (%BF) (p<0.001), waist-to-hip ratio (WHR) (p<0.009), BMI (p<0.001), and ultrasonography grade (p<0.05) (Fig. 1), and significant improvement in VO2peak levels (p<0.001) from pre-test to post-test, whereas the variables remained unchanged in the control (CON) group. Additionally, the Bonferroni post hoc test for between-group comparisons revealed significant differences between the training groups and the CON group in mean %BF (HIIT: 29.99% vs. CON: 31.54%, p=0.009; SBE: 29.74% vs. CON: 31.54%, p=0.001; HIIT: 29.99% vs. SBE: 29.74%, p=0.40), WHR (HIIT: 0.952 vs. CON: 0.975, p=0.04; SBE: 0.941 vs. CON: 0.975, p=0.02; HIIT: 0.952 vs. SBE: 0.941, p=0.82), and VO2peak (HIIT: 38.70 mLㆍkg−1ㆍmin−1 vs. CON: 35.23 mLㆍkg−1ㆍmin−1, p=0.001; SBE: 37.33 mLㆍkg−1ㆍmin−1 vs. CON: 35.23 mLㆍkg−1ㆍmin−1, p=0.01; HIIT: 38.70 mLㆍkg−1ㆍmin−1 vs. SBE: 37.33 mLㆍkg−1ㆍmin−1, p=0.001) (Table 2).
Table 2. Selected physical and physiological variables before and after exercise.
| Measured variable | SBE (n=11) | p-value (pre-post) | HIIT (n=11) | p-value (pre-post) | Control (n=12) | p-value (pre-post) | p-value (between groups) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-test | Post-test | Pre-test | Post-test | Pre-test | Post-test | |||||
| PS (II/III/IV) | 4/3/4 | - | 5/2/4 | - | 3/4/5 | - | ||||
| Age (yr) | 13.39±0.95 | - | 12.81±1.02 | - | 13.14±1.49 | - | ||||
| Weight (kg) | 66.46±11.57 | 64.51±10.40*† | 0.001 | 63.20±7.47 | 60.51±7.11*† | 0.001 | 65.30±12.04 | 65.63±11.66 | 0.260 | 0.001† |
| BMI (kg/m2) | 26.47±1.74 | 25.95±2.10*† | 0.001 | 26.68±2.32 | 25.35±1.62*† | 0.001 | 26.45±2.21 | 26.60±1.99 | 0.230 | 0.001† |
| WHR | 0.956±0.04 | 0.941±0.04*† | 0.030 | 0.964±0.06 | 0.952±0.07*† | 0.009 | 0.971±0.03 | 0.975±0.03 | 0.530 | 0.040† |
| Body fat percentage | 30.42±2.08 | 29.74±1.99*† | 0.001 | 30.37±1.95 | 29.99±1.72*† | 0.001 | 31.41±1.19 | 31.54±1.16 | 0.231 | 0.001† |
| VO2peak (mL·kg−1·min−1) | 35.08±3.36 | 37.33±2.58*† | 0.001 | 35.38±2.33 | 38.70±1.68*†‡ | 0.001 | 35.01±2.97 | 35.23±2.68 | 0.215 | 0.001†‡ |
Values are presented as number only or mean±standard deviation.
SBE: school-based exercise, HIIT: high-intensity interval training, PS: parenchymal sonography, BMI: body mass index, WHR: waist-to-hip ratio, VO2peak, peak oxygen uptake.
*Differences between time points within condition (pre-post). †Differences between training groups vs. control group. ‡Differences between experimental groups. p<0.05.
Fig. 1. Ultrasonography grades in the three groups before and after the intervention. Data are mean±standard deviation, p<0.05. *Within group differences.
SBE: school-based exercise, HIIT: high-intensity interval training, CG: control group.
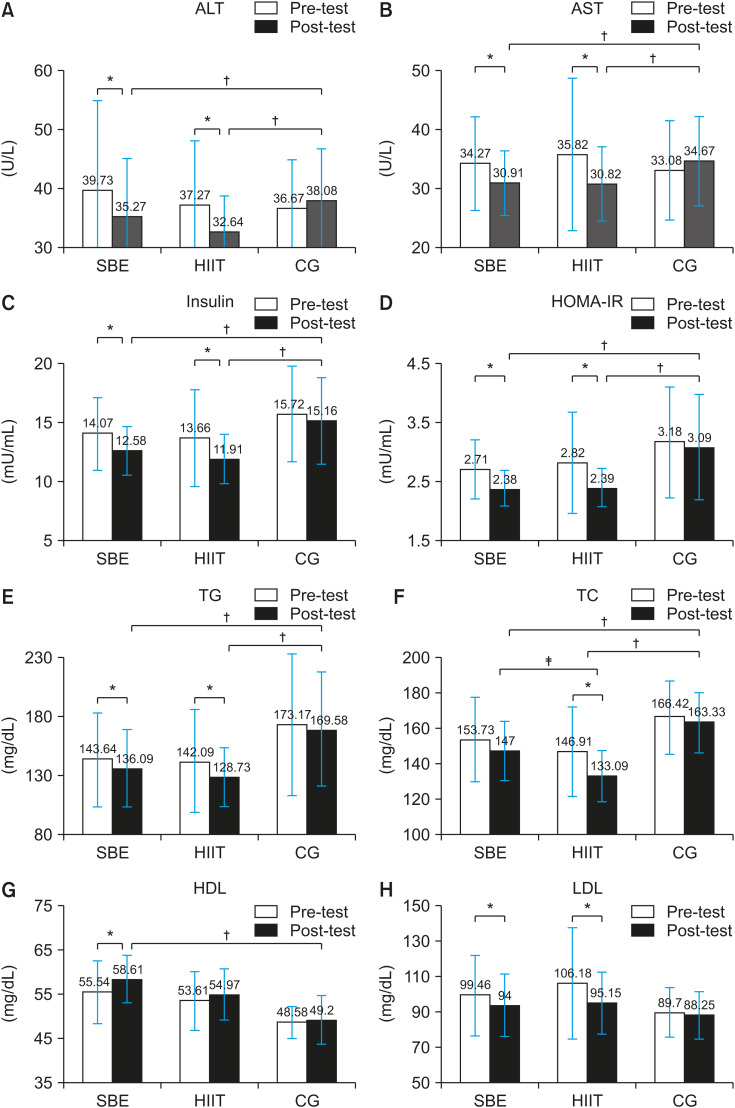
The results of ANCOVA with adjustments for pre-test measurements as covariate variables showed improvements in the mean insulin level (HIIT: 11.91 mU/mL vs. CON: 15.16 mU/mL, p=0.02; SBE: 12.58 mU/mL vs. CON: 15.16 mU/mL, p=0.011; HIIT: 11.91 mU/mL vs. SBE: 12.58 mU/mL, p=0.13), HOMA-IR index (HIIT: 2.39 vs. CON: 3.09, p=0.013; SBE: 2.38 vs. CON: 3.09, p=0.018; HIIT: 2.39 vs. SBE: 2.38, p=0.13), ALT level (HIIT: 32.64 U/L vs. CON: 38.08 U/L, p=0.003; SBE: 35.27 U/L vs. CON: 38.08 U/L, p=0.013; HIIT: 32.64 U/L vs. SBE: 35.27 U/L, p=0.13), and AST level (HIIT: 30.82 U/L vs. CON: 34.67 U/L, p=0.016; SBE: 30.91 U/L vs. CON: 34.67 U/L, p=0.019; HIIT: 30.82 U/L vs. SBE: 30.91 U/L, p=0.94) after the exercise interventions (Fig. 2). At the end of the study, the alterations in LDL-C level in the training groups did not reach statistical significance (p>0.05); however, there were significant differences in HDL-C (HIIT: 54.97 mg/dL vs. CON: 49.2 mg/dL, p=0.507; SBE: 58.61 mg/dL vs. CON: 49.23 mg/dL, p=0.046; SBE: 58.61 mg/dL vs. HIIT: 54.97 mg/dL, p=0.571), total cholesterol (HIIT: 133.09 mg/dL vs. CON: 163.3 mg/dL, p=0.001; SBE: 147 mg/dL vs. CON: 163.3 mg/dL, p=0.049; SBE: 147 mg/dL vs. HIIT: 133.09 mg/dL, p=0.022), and triglyceride (HIIT: 128.73 mg/dL vs. CON: 169.58 mg/dL, p=0.001; SBE: 136.09 mg/dL vs. CON: 169.58 mg/dL, p=0.010; SBE: 136.09 mg/dL vs. HIIT: 128.73 mg/dL, p=0.601) (Fig. 2).
Fig. 2. Selected metabolic variables before and after exercise. (A) ALT, (B) AST, (C) insulin, (D) HOMA-IR, (E) TG, (F) TC, (G) HDL, and (H) LDL. Data are mean±standard error. Significance: p≤error. Significance: lipoprotein cholesterol. Data are mean±-post). *Differences between time points within condition (pre-post). †Differences between training groups vs. control group. ‡Differences between training groups.
SBE: school-based exercise, HIIT: high-intensity interval training, CG: control group, ALT: alanine aminotransferase, AST: aspartate aminotransferase, HOMA-IR: homoeostasis model assessment for insulin resistance, TG: triglyceride, TC: total cholesterol, LDL-C: low-density lipoprotein cholestero, HDL-C: high-density lipoprotein cholesterol.
DISCUSSION
The major focus of this study was to compare the effects of SBE versus HIIT as modes of exercise on some physiological variables in obese adolescents with NAFLD. The results revealed that both types of exercise training were effective in improving parameters such as aerobic fitness, BMI, WHR, %BF, lipid profile, insulin, HOMA-IR, ultrasonography grade, ALT level, and AST level. These results support previous studies and showed improvement in cardiorespiratory fitness, which had the highest inverse correlation with HOMA-IR and ALT [24]. The most practical index of cardiorespiratory fitness is VO2peak, and the improvement in this variable following exercise intervention was significantly greater in the HIIT group than in the SBE group (SBE: 6.41% vs. HIIT: 9.38%). These results support previous studies that showed a higher efficiency of HIIT training in improving VO2peak than moderate-intensity continuous exercise training [25,26]. Although the mechanisms of how HIIT training improves VO2peak is still not well understood, some researchers have stated that the rest periods, or the lower-intensity exercise intervals, make it possible for participants to complete short exercise bouts at higher intensity, which provides a greater exercise stimulus to the heart than what is possible during completing moderate-intensity continuous exercise training [27]. As this selected SBE training is aerobic and low intensity in nature, it was expected that children would participate in long-duration fun activities. Therefore, the results of this study were predictable and in accordance with the findings of most previous investigations in this field [27].
Available evidence suggests that there is a strong relationship between obesity and NAFLD in children, and about 70–90% of children with NAFLD are obese [28]. Hence, weight loss is recommended as a desirable management measure for NAFLD [29]. Studies in children have demonstrated that a moderate reduction in weight and BMI reduces the ALT levels and improves liver function [30]. Additionally, it was indicated that a mixture of dietary constraints and exercises affects the liver function and hepatic steatosis when a body weight loss of 3–10% is achieved [29]. In the present study, a decrease in %BF (SBE: 2.23%; HIIT: 1.25%) was also observed in both training groups. In a study using a similar HIIT protocol, Racil et al. [23] reported improvements in %BF following a 12-week HIIT intervention compared with a moderate-intensity protocol in obese adolescents. Although the mechanism of fat and weight loss after HIIT is uncertain, it could be caused by increased post-exercise metabolism [31]. In addition, HIIT considerably enhances the levels of muscle mitochondrial beta-hydroxy acyl-CoA dehydrogenase, which may increase fat loss [32]. Moreover, glycogen resynthesis occurs during and after HIIT, and the need for removing lactate and H+ increases fat oxidation [32]. In conclusion, HIIT and other training methods represent an important intervention for weight loss, as they have the potential to reduce body mass, increase the fat-free mass, and sustain or enhance the rate of resting metabolism [32].
Some studies have reported the association of lipid profile and liver enzymes with the NAFLD process and the ultrasonography grade [33,34]. In the present study, similar to several previous studies [35,36,37], the lipid profile of the participants improved after the exercise interventions. Although the changes in LDL-C in both groups were not significant in comparison with the CON group, the results suggested that LDL and HDL are less affected by exercise [38], and even a 1% decrease in LDL-C can reduce the risk of coronary artery disease by about 2% [39].
AST and ALT are two enzymes that are tested to detect liver damage, and their levels may be high in the blood of persons with NAFLD [40]. As normal amounts of ALT have been also observed in the NAFLD spectra, the interpretation of either the ALT or AST value as a marker of exercised-induced alterations in persons with NAFLD should be performed with caution [41]. Several studies have examined the effects of exercise interventions on NAFLD, and suggested that aerobic training reduces systemic ALT and AST levels [41]. The results of this study showed significant reductions in serum ALT (SBE: 11.22% vs. HIIT: 12.42%) and AST (SBE: 9.8% vs. HIIT: 13.95%); however, there were no differences between the exercise groups. Conversely, the results of ultrasound examinations (changes in almost 50% of the participants in both groups) were consistent with the results of the liver enzymes, demonstrating that the effects of the two training methods were almost identical. Therefore, we concluded that both exercise training protocols may be effective in controlling the hepatic fat level.
Obesity leads to NAFLD development through liver dysfunction caused by hepatic steatosis, and it has been shown that NAFLD is closely associated with IR [14]. Our study revealed that the improvements in the HOMA-IR of the participants after SBE (12.17%) and HIIT (15.25%) training were statistically significant. In this regard, the two training protocols had an almost similar effectiveness in reducing IR. Several studies have also evaluated the effects of aerobic exercise programs on IR in children and adolescents; however, conflicting results were reported with respect to the effect of exercise on insulin sensitivity [42,43]. IR plays an important role in NAFLD by promoting free fatty acid storage [44], and this hyperinsulinemia activates the lipogenic factors of transcriptional regulators including fatty acid synthase and sterol regulatory element binding protein-1c (SREBP1c) [29]. Therefore, changes in the pathways of fatty acid synthesis and fatty acid oxidation play key roles, and exercise training may have a protective effect against fatty liver by activating the AMPK pathway and/or suppressing the SREBP1c pathway [45]. These results support previous studies and showed that there were remarkable improvements in IR, fasting blood glucose, and lipid profile in the training groups, which seemed to be major contributors to the relative improvement of the liver parenchyma in these groups. As the HIIT and SBE training protocols had approximately similar effects on NAFLD and metabolic syndrome markers in this study, we concluded that these exercise modalities can be interchangeably used. Our study is one of the few studies aimed at comparing the effects of selected HIIT and SBE training protocols in children suspected to have NAFLD. Our results suggested that HIIT is preferable in clinical situations involving persons with obesity with higher levels of motivation, whereas SBE is suitable for increasing adherence in schools or communities by providing fun activities. It should also be noted that the SBE protocol in this study is very similar to conventional physical education classes and can be used outside of school. Considering that obese children usually lack the impetus for completing vigorous exercises, SBE or game-oriented interventions may be more suitable for this population. As nutritional limitations may play a key role in decreasing obesity, health-related risk factors, and NAFLD, all participants of this study were housed in a boarding school and provided the same diet.
The control of nutrition and diet was one of the main limitations of this study, as the participants may have eaten snacks between meals. In this regard, it seems that caloric restrictions imposed by family members may provide better results. Moreover, increasing the duration of training in future studies might cause greater improvement in the liver parenchyma. Therefore, new strategies are needed for encouraging adolescents to engage in sufficient physical activity to develop and maintain health-related fitness [18]. One of the most important goals of physical education classes is to provide more enjoyment and increase adherence to exercise training [17]. Although numerous studies have suggested that HIIT exercises in different modes have beneficial effects on the health of children, obese children have some difficulties in participating and show low commitment in adhering to the training programs owing to the monotonous nature and high intensity of this exercise mode. This assumption was proven by the higher tendency shown by the participants of this study in engaging in SBE and in continuing this exercise protocol.
In conclusion, the results of this study demonstrated that SBE could be used in the treatment of obese adolescents with NAFLD, if they are attractively designed with a focus on enjoyability. Finally, although HIIT and SBE have been shown to have approximately equal effects in improving the most important health parameters (e.g., aerobic fitness, lipid profile, insulin sensitivity, BMI, AST, and ALT) in obese children and adolescents with NAFLD, SBE was the favored protocol in recent studies.
ACKNOWLEDGEMENTS
This study was supported by the vice dean for research and technology of the University of Isfahan for doctoral students. Therefore, we express our gratitude for his unconditional support.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.van der Heijden GJ, Wang ZJ, Chu ZD, Sauer PJ, Haymond MW, Rodriguez LM, et al. A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obesity (Silver Spring) 2010;18:384–390. doi: 10.1038/oby.2009.274. [DOI] [PubMed] [Google Scholar]
- 2.Deldin AR, Lee S. Role of physical activity in the treatment of nonalcoholic fatty liver disease in children and adolescents. Appl Physiol Nutr Metab. 2013;38:805–812. doi: 10.1139/apnm-2012-0503. [DOI] [PubMed] [Google Scholar]
- 3.Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700–713. doi: 10.1097/MPG.0b013e318252a13f. [DOI] [PubMed] [Google Scholar]
- 4.Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010;42:272–282. doi: 10.1016/j.dld.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Koot BG, de Groot E, van der Baan-Slootweg OH, Bohte AE, Nederveen AJ, Jansen PL, et al. Nonalcoholic fatty liver disease and cardiovascular risk in children with obesity. Obesity (Silver Spring) 2015;23:1239–1243. doi: 10.1002/oby.21076. [DOI] [PubMed] [Google Scholar]
- 6.Wong VW. Nonalcoholic fatty liver disease in Asia: a story of growth. J Gastroenterol Hepatol. 2013;28:18–23. doi: 10.1111/jgh.12011. [DOI] [PubMed] [Google Scholar]
- 7.Chan DF, Li AM, Chu WC, Chan MH, Wong EM, Liu EK, et al. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004;28:1257–1263. doi: 10.1038/sj.ijo.0802734. [DOI] [PubMed] [Google Scholar]
- 8.Papandreou D, Karabouta Z, Pantoleon A, Rousso I. Investigation of anthropometric, biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite. 2012;59:939–944. doi: 10.1016/j.appet.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Sartorio A, Del Col A, Agosti F, Mazzilli G, Bellentani S, Tiribelli C, et al. Predictors of non-alcoholic fatty liver disease in obese children. Eur J Clin Nutr. 2007;61:877–883. doi: 10.1038/sj.ejcn.1602588. [DOI] [PubMed] [Google Scholar]
- 10.Africa JA, Newton KP, Schwimmer JB. Lifestyle interventions including nutrition, exercise, and supplements for nonalcoholic fatty liver disease in children. Dig Dis Sci. 2016;61:1375–1386. doi: 10.1007/s10620-016-4126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial) Hepatology. 2013;58:1287–1295. doi: 10.1002/hep.26393. [DOI] [PubMed] [Google Scholar]
- 12.Bacchi E, Negri C, Zanolin ME, Milanese C, Faccioli N, Trombetta M, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study) Diabetes Care. 2012;35:676–682. doi: 10.2337/dc11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278–1283. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 15.Stenevi-Lundgren S, Daly RM, Karlsson MK. A school-based exercise intervention program increases muscle strength in prepubertal boys. Int J Pediatr. 2010;2010:307063. doi: 10.1155/2010/307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pozuelo-Carrascosa DP, Cavero-Redondo I, Herráiz-Adillo Á, Díez-Fernández A, Sánchez-López M, Martínez-Vizcaíno V. School-based exercise programs and cardiometabolic risk factors: a meta-analysis. Pediatrics. 2018;142:e20181033. doi: 10.1542/peds.2018-1033. [DOI] [PubMed] [Google Scholar]
- 17.Logan GR, Harris N, Duncan S, Schofield G. A review of adolescent high-intensity interval training. Sports Med. 2014;44:1071–1085. doi: 10.1007/s40279-014-0187-5. [DOI] [PubMed] [Google Scholar]
- 18.Costigan SA, Eather N, Plotnikoff RC, Taaffe DR, Lubans DR. High-intensity interval training for improving health-related fitness in adolescents: a systematic review and meta-analysis. Br J Sports Med. 2015;49:1253–1261. doi: 10.1136/bjsports-2014-094490. [DOI] [PubMed] [Google Scholar]
- 19.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson MJ, Czerwinski SA, Siervogel RM. Development and validation of skinfold-thickness prediction equations with a 4-compartment model. Am J Clin Nutr. 2003;77:1186–1191. doi: 10.1093/ajcn/77.5.1186. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzaka A, Takahashi Y, Yamazoe M, Kumakura N, Ikeda A, Wilk B, et al. Validity of the multistage 20-m shuttle-run test for Japanese children, adolescents, and adults. Pediatr Exerc Sci. 2004;16:113–125. [Google Scholar]
- 22.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 23.Racil G, Ben Ounis O, Hammouda O, Kallel A, Zouhal H, Chamari K, et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol. 2013;113:2531–2540. doi: 10.1007/s00421-013-2689-5. [DOI] [PubMed] [Google Scholar]
- 24.Kelishadi R, Cook SR, Amra B, Adibi A. Factors associated with insulin resistance and non-alcoholic fatty liver disease among youths. Atherosclerosis. 2009;204:538–543. doi: 10.1016/j.atherosclerosis.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Cornish AK, Broadbent S, Cheema BS. Interval training for patients with coronary artery disease: a systematic review. Eur J Appl Physiol. 2011;111:579–589. doi: 10.1007/s00421-010-1682-5. [DOI] [PubMed] [Google Scholar]
- 26.Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med. 2012;42:587–605. doi: 10.2165/11631910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Ross LM, Porter RR, Durstine JL. High-intensity interval training (HIIT) for patients with chronic diseases. J Sport Health Sci. 2016;5:139–144. doi: 10.1016/j.jshs.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alisi A, Manco M, Vania A, Nobili V. Pediatric nonalcoholic fatty liver disease in 2009. J Pediatr. 2009;155:469–474. doi: 10.1016/j.jpeds.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Oh S, Han G, Kim B, Shoda J. Regular exercise as a secondary practical treatment for nonalcoholic fatty liver disease. Exerc Med. 2018;2:4. [Google Scholar]
- 30.Nobili V, Alisi A, Raponi M. Pediatric non-alcoholic fatty liver disease: preventive and therapeutic value of lifestyle intervention. World J Gastroenterol. 2009;15:6017–6022. doi: 10.3748/wjg.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trapp EG, Chisholm DJ, Boutcher SH. Metabolic response of trained and untrained women during high-intensity intermittent cycle exercise. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2370–5. doi: 10.1152/ajpregu.00780.2006. [DOI] [PubMed] [Google Scholar]
- 32.Alahmadi MA. High-intensity interval training and obesity. J Nov Physiother. 2014;4:211. [Google Scholar]
- 33.Lin YC, Chou SC, Huang PT, Chiou HY. Risk factors and predictors of non-alcoholic fatty liver disease in Taiwan. Ann Hepatol. 2011;10:125–132. [PubMed] [Google Scholar]
- 34.Sogabe M, Okahisa T, Tsujigami K, Fukuno H, Hibino S, Yamanoi A. Visceral fat predominance is associated with non-alcoholic fatty liver disease in Japanese women with metabolic syndrome. Hepatol Res. 2014;44:515–522. doi: 10.1111/hepr.12146. [DOI] [PubMed] [Google Scholar]
- 35.Leite N, Milano GE, Cieslak F, Lopes WA, Rodacki A, Radominski RB. Effects of physical exercise and nutritional guidance on metabolic syndrome in obese adolescents. Braz J Phys Ther. 2009;13:73–81. [Google Scholar]
- 36.Silva DAS, Petroski EL, Pelegrini A. Effects of aerobic exercise on the body composition and lipid profile of overweight adolescents. Rev Bras Ciênc Esporte. 2014;36:295–309. [Google Scholar]
- 37.Thomas NE, Cooper SM, Williams SP, Baker JS, Davies B. Relationship of fitness, fatness, and coronary-heart-disease risk factors in 12- to 13-year-olds. Pediatr Exerc Sci. 2007;19:93–101. doi: 10.1123/pes.19.1.93. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017;16:132. doi: 10.1186/s12944-017-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martins RA, Veríssimo MT, Coelho e Silva MJ, Cumming SP, Teixeira AM. Effects of aerobic and strength-based training on metabolic health indicators in older adults. Lipids Health Dis. 2010;9:76. doi: 10.1186/1476-511X-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. BMJ. 2014;349:g4596. doi: 10.1136/bmj.g4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glass OK, Radia A, Kraus WE, Abdelmalek MF. Exercise training as treatment of nonalcoholic fatty liver disease. J Funct Morphol Kinesiol. 2017;2:35. [Google Scholar]
- 42.Nassis GP, Papantakou K, Skenderi K, Triandafillopoulou M, Kavouras SA, Yannakoulia M, et al. Aerobic exercise training improves insulin sensitivity without changes in body weight, body fat, adiponectin, and inflammatory markers in overweight and obese girls. Metabolism. 2005;54:1472–1479. doi: 10.1016/j.metabol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Devries MC, Samjoo IA, Hamadeh MJ, Tarnopolsky MA. Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity (Silver Spring) 2008;16:2281–2288. doi: 10.1038/oby.2008.358. [DOI] [PubMed] [Google Scholar]
- 44.Vajro P, Lenta S, Pignata C, Salerno M, D'Aniello R, De Micco I, et al. Therapeutic options in pediatric non alcoholic fatty liver disease: current status and future directions. Ital J Pediatr. 2012;38:55. doi: 10.1186/1824-7288-38-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osaka T, Hashimoto Y, Hamaguchi M, Kojima T, Obora A, Fukui M. Nonalcoholic fatty liver disease remission in men through regular exercise. J Clin Biochem Nutr. 2018;62:242–246. doi: 10.3164/jcbn.17-115. [DOI] [PMC free article] [PubMed] [Google Scholar]