Abstract
Chocolate may affect the glycemic response, which is related the insulin and incretin response. We evaluated the glucose, insulin, and glucagon-like peptide-1 (GLP-1) responses in male adults after consumption of three commonly consumed chocolates. Furthermore, we assessed the glycemic index (GI), insulin index (II), and glycemic load (GL) of the chocolates. The study protocol was adapted from the International Standard Organization recommendations. Test foods were chocolate A (milky chocolate), chocolate B (creamy chocolate), chocolate C (chocolate ball), and reference food (glucose solution). Glucose, insulin, and GLP-1 concentrations were assessed at 0, 15, 30, 45, 60, 90, and 120 min after consumption of the test foods. The glycemic responses of the three chocolates were lower than those of the reference food at 30 and 45 min (P<0.001). However, the insulin and GLP-1 responses did not differ between the three chocolates and the reference food. The GI value of chocolates A, B, and C were 39.2, 47.8, and 33.7, respectively; all GI values were lower than that of the reference food. The II values of all test foods were similar, aside for chocolate B (97.9). All chocolates were classified as low-GL. This study showed that glycemic responses depends on the amount of carbohydrates and the physical properties. Further research is required to examine incretin responses and to determine if the type of chocolate can influence metabolic response beyond glycemia.
Keywords: chocolate, glycemic index, glycemic load, incretin response, insulin
INTRODUCTION
Chocolate is a widely consumed snack throughout the world, with Western countries accounting for the largest proportion of chocolate consumption. In Switzerland, people consume approximately one serving size (25 g) of chocolate per day (Statista, 2018). Recently, the enjoyment of chocolate consumption has extended from Western countries to Asian countries, including South Korea. The frequency of chocolate consumption among adults in South Korea rose steadily from 2012 to 2016 (KCDC, 2016). It has been argued that there is a substantial relationship chocolate consumption and health outcomes in recent years (Grassi et al., 2005; McCullough et al., 2006; Greenberg and Buijsse, 2013). Chocolate has been associated with favorable outcomes, such as protection against cardiovascular disease and improved insulin sensitivity, whereas excessive chocolate consumption leads to weight gain (Grassi et al., 2005; McCullough et al., 2006; Greenberg and Buijsse, 2013).
Chocolate may influence glycemic responses, since it is mostly (approximately 50∼60%) composed of carbohydrates, and is generally consumed as a single snack. One possible mechanism of action is that chocolate elicits low blood glucose response. Indeed, chocolate is classified as having a low glycemic index (GI) and glycemic load (GL), while the insulin index (II) tends to be relatively high compared to the GI (Shively et al., 1986; Holt et al., 1997; Brand-Miller et al., 2003). Since chocolate appears to have specific characteristics in terms of inducing insulin secretion, it is important to examine both the glycemic and insulin response to chocolate and the incretin response, which influences insulin release.
Current evidence on the influence of chocolate on both the glycemic and incretin responses is limited. In human subjects, chocolate induced slower increases in glucose concentrations than reference food, and cocoa powder induced insulin release (Brand-Miller et al., 2003; Zhang et al., 2018). Furthermore, the gut-derived incretin hormones glucagon-like peptide-1 (GLP-1) stimulated insulin secretion in a glucose-dependent manner in response to food intake, and decreased the rate of gastric emptying (Holst, 2007).
Therefore, the aim of the present study was to evaluate glucose, insulin, and GLP-1 responses after intake of chocolate products commonly consumed by Korean adults, according to the protocols of the International Organization for Standardization (ISO) 2010 recommendation (ISO, 2010). In addition, GI, II, and GL values of these chocolates were determined.
MATERIALS AND METHODS
Subjects
Seventeen subjects were recruited through open announcements. Two subjects withdrew consent, therefore, a total of fifteen subjects participated in the study. All subjects were given the full details of the study protocol and provided written informed consent before participating. Subjects who met the following eligibility criteria were enrolled: healthy men 20∼45 years of age; weight maintenance for the previous 3 months; body mass index (BMI) ranging from 18.5 to 25 kg/m2; and normal fasting glucose (<5.5 mmol/L) or normal 2-h postprandial glucose (<7.7 mmol/L). Exclusion criteria were diabetes; history of gastrointestinal surgery; gastric disease; food allergies or intolerances (chocolate and lactose); and use of medications known to affect glucose tolerance, digestion or nutrient absorption. The study was conducted according to the guidelines established in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethical institutional review board of Kyung Hee University Hospital (no. KHUH-080-003). All experiments were performed at Kyung Hee University in Seoul, Korea.
Study protocol
This randomized, open-label study was conducted over 5 weeks. The protocol was adapted from the ISO 2010 recommendation and reviews described by Brouns et al. (2005) and ISO (2010). The subjects visited once per week (±5 days) to minimize carryover effects. Subjects arrived throughout the morning after fasting overnight 10∼12 h. During the fasting state, a catheter was inserted into an antecubital vein and fasting blood samples were taken. All subjects consumed each test food with 250 mL of water within 12 min after the first bite. Blood samples were taken at 15, 30, 45, 60, 90, and 120 min following intake of the test foods. Plasma glucose, serum insulin, and plasma GLP-1 concentrations were measured in each blood sample at each time point. Blood was conducted by a medical laboratory technologist. Subjects were instructed to maintain their usual diet during the weeks of testing, and were asked to restrict their alcohol consumption and high-intensity physical activity on the day before the test.
Test foods
The nutrient compositions of the test foods are listed in Table 1. The total CHO content of the test foods was set to 25 g for the calculation of the GI in accordance with the ISO 2010 recommendations (ISO, 2010). The levels of the other macronutrients (protein and fat) differed slightly between the three chocolates.
Table 1.
Standard serving sizes and nutrient composition of the chocolates
| Chocolate A | Chocolate B | Chocolate C | |
|---|---|---|---|
| Standard serving size (g) | 25 | 20 | 31 |
| Energy (kcal) | 142.0 | 110.0 | 180.0 |
| Total CHO (g) | 13.6 | 11.6 | 17.0 |
| Sugar (g) | 13.3 | 10.2 | 16.0 |
| Protein (g) | 2.2 | 1.6 | 3.0 |
| Total fat (g) | 8.7 | 6.4 | 11.0 |
| Saturated fat (g) | 5.6 | 3.1 | 7.0 |
No significant differences in the nutrient composition of the chocolates were observed.
Chocolate A, chocolate bar with milky filling; Chocolate B, milk and cocoa cream with crispy wafer balls coated with cocoa cream; Chocolate C, milk chocolate ball.
We prepared four test foods: three chocolates and a reference food. Three chocolates were named chocolate A, chocolate B, and chocolate C. These chocolates included a chocolate bar with a milky filling (chocolate A), a milk and cocoa cream chocolate with crispy wafer balls coated with creamy cocoa (chocolate B), and a milk chocolate ball (chocolate C). Chocolate A contained milk chocolate (40.0%), cocoa mass, cocoa butter, sugar, skimmed milk powder, anhydrous milkfat, vegetable fat, and lecithin. Chocolate B contained semi-chocolate (30.5%), sugar, skimmed milk powder, vegetable fat, fat-reduced cocoa powder, lecithin, and powdered barley malt extract. Chocolate C contained cocoa mass, cocoa butter, cocoa preparation, vegetable oil, lecithin, and almond powder. The reference food was a glucose solution (DiasolⓇ; Taejoon Pharm Co., Ltd., Seoul, Korea) containing 25 g of available carbohydrate. To determine standard values for glucose, the reference food test was conducted twice.
Measurements
On the screening day, height, body weight, and body composition were measured using Inbody 720 (Biospace Co., Ltd., Seoul, Korea). Fingertip capillary blood glucose was also measured by finger pricks (Accu-Chek; Roche Dignostics, Indianapolis, IN, USA) to determine if the subjects were eligible for the study.
On the test day, plasma glucose, serum insulin, and plasma GLP-1 concentrations were measured at all time points by the blood from the antecubital vein. Each blood sample for GLP-1 was injected with a protease inhibitor (aprotinin from bovine lung; Sigma-Aldrich Co., St. Louis, MO, USA) to prevent degradation of intact GLP-1 by dipeptidyl peptidase-4 (DPP-4). All samples were centrifuged and separated after blood collection. The separated glucose samples were analyzed immediately, while the separated insulin and GLP-1 samples were stored at −32 °C and −75°C, respectively, until analysis.
Blood glucose concentrations were measured using the glucose oxidase and hydrogen peroxide electrode method (Amperometry method) on an ADAMS glucose GA- 1171 system (ARKRAY, Inc., Kyoto, Japan). Serum insulin concentrations were measured using an immunoradiometric assay [Insulin (I-125) IRMA kit; Institute of Isotopes Co., Ltd., Budapest, Hungary]. Plasma GLP-1 concentrations were measured using a human GLP-1 EIA enzyme-linked immunosorbent assay kit (RayBiotech, Peachtree Corners, GA, USA).
Calculation and statistical analyses
All statistical analyses were performed using SPSS software version 23.0 (SPSS Inc., Chicago, IL, USA). The incremental area under the curve (IAUC), excluding the area below the fasting baseline, was calculated for glucose, insulin, and GLP-1 of each subject and test food in GraphPad Prism version 7 (GraphPad Software, San Diego, CA, USA). The GIs of the three chocolates were calculated from the glucose IAUC using the following equation (Atkinson et al., 2008):
Similarly, the II was calculated from the insulin IAUC (Holt et al., 1997). The GL was calculated as follows (Atkinson et al., 2008):
The serving size of each chocolate was taken from information provided by the manufacturer.
Significant differences in blood glucose, insulin, and GLP-1 concentrations after consumption of the test foods at each time points were determined by repeated measures ANOVA and Student’s t-tests with Bonferroni’s correction. The GI, II, and GL values were analyzed by one- way ANOVA and Duncan’s multiple range tests. All values are presented as mean with standard error. P<0.05 was considered significant.
RESULTS
After excluding outliers as specified in the ISO 2010 and according to the clinician’s decision, there were a total of 10 subjects (ISO, 2010). The inter-individual variation in the glycemic response to the standard test for all ten subjects was 30.7%.
Subjects’ characteristics and anthropometric measurements
Subjects had an average age of 26.7±0.8 years, a mean BMI of 23.1±0.5 kg/m2, a mean waist circumference of 93.7±1.2 cm, and a mean hip circumference of 102.0± 1.0 cm (Table 2). Mean energy intake was 2,082.7±82.7 kcal (data not shown).
Table 2.
Subjects’ characteristics and anthropometric measurements (n=10)
| Characteristics | |
|---|---|
| Age (yrs) | 26.7±0.8 |
| Height (cm) | 178.6±2.1 |
| Weight (kg) | 73.8±2.5 |
| BMI (kg/m2) | 23.1±0.5 |
| Total body fat (%) | 16.1±0.9 |
| Fat mass (kg) | 12.0±0.7 |
| Lean body mass (kg) | 35.2±1.4 |
| Waist circumference (cm) | 93.7±1.2 |
| Hip circumference (cm) | 102.0±1.0 |
| Waist hip ratio | 0.92±0.01 |
| Systolic blood pressure (mmHg) | 121.0±3.5 |
| Diastolic blood pressure (mmHg) | 75.0±2.2 |
All values are mean±SE.
BMI: body mass index.
Postprandial glucose responses after consumption of the chocolates
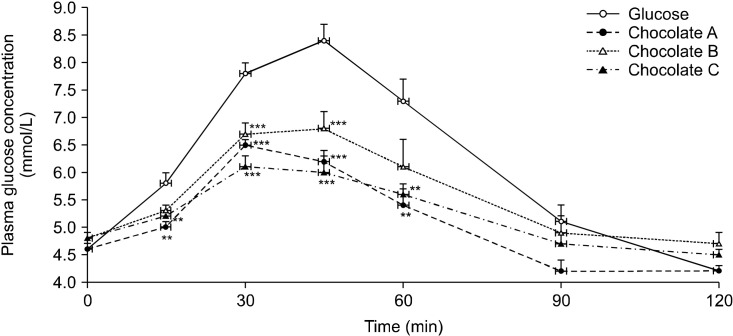
Plasma glucose concentrations peaked 30 min after consumption for chocolates A and C, but peaked 45 min after consumption for chocolate B and the reference food (Fig. 1). At 15 min, the glycemic responses to chocolates A and C were significantly lower than that of the reference food (P<0.005). The postprandial plasma glucose concentrations after consumption of the three chocolates were lower than those of the reference food at 30 and 45 min (all P<0.001). At 60 min, the glycemic responses to chocolates A and C were significantly lower than those to the reference food (all P<0.003; chocolate A: 5.4±0.3 mmol/L, chocolate C: 5.6±0.2 mmol/L, reference food: 7.3±0.4 mmol/L), whereas the glycemic responses to chocolate B were similar to those to the reference food at the same time points. No significant differences were observed between the glucose responses after consumption of three chocolates and reference food at 90 and 120 min (Fig. 1).
Fig. 1.
Postprandial glucose concentration after consumption of three chocolates and reference food (glucose). Values are mean±SE. Significant differences by ANOVA for repeated measures at different time points. Mean values were significantly different from those obtained after reference food consumption (**P<0.01 and ***P<0.001; Student’s t-test with Bonferroni’s correction).
Postprandial insulin responses after consumption of the chocolates
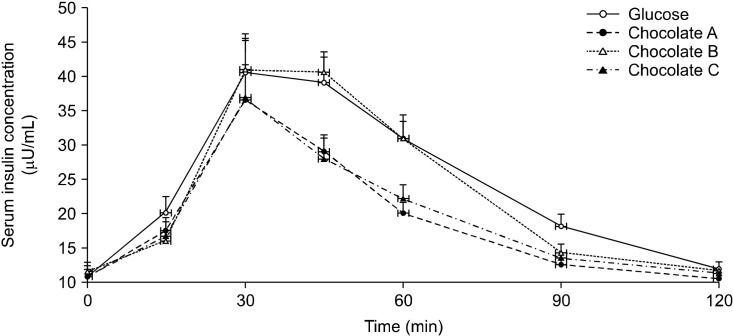
The serum insulin concentrations peaked 30 min after consumption of the three chocolates and reference food (reference food: 40.6±5.7 μU/mL, chocolate A: 36.6± 5.2 μU/mL, chocolate B: 41.1±4.2 μU/mL, and chocolate C: 36.9±8.7 μU/mL). There were no statistically significant differences between the three chocolates and the reference food at all time points (Fig. 2).
Fig. 2.
Postprandial insulin concentration after consumption of three chocolates and reference food (glucose). Values are mean±SE. Significant differences by ANOVA for repeated measures at different time points.
Postprandial GLP-1 responses after consumption of the chocolates
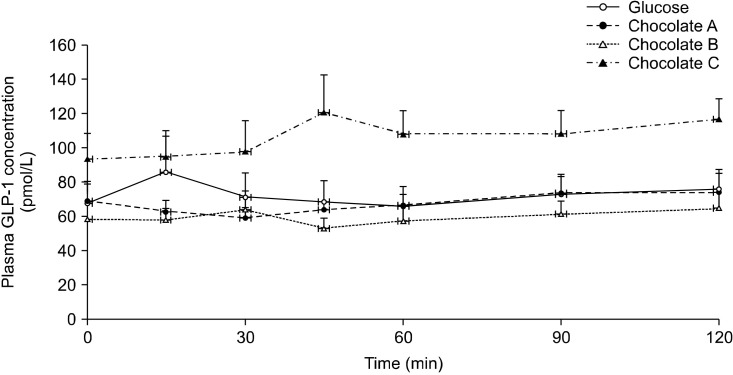
There were no significant differences in the plasma GLP-1 responses following consumption of the chocolates and the reference food at all time points (Fig. 3).
Fig. 3.
Postprandial glucagon-like peptide-1 (GLP-1) concentration after consumption of three chocolates and reference food (glucose). Values are mean±SE. Significant differences by ANOVA for repeated measures at different time points.
IAUCs for glucose, insulin, and GLP-1
The IAUCs for plasma glucose, serum insulin, and plasma GLP-1 following consumption of the test foods are shown in Table 3. The IAUCs for plasma glucose were significantly smaller for the three chocolates than for the reference food at all time points (P<0.01). In contrast, the IAUCs for serum insulin and plasma GLP-1 did not differ significantly between the three chocolates and the reference food at all time points.
Table 3.
Total IAUC for plasma glucose, serum insulin, and plasma GLP-1 following test food consumption
| Reference food | Chocolate A | Chocolate B | Chocolate C | |
|---|---|---|---|---|
| IAUC 0∼120 min (glucose) | 201.5±17.8 | 86.2±15.7*** | 106.7.0±20.5** | 70.2±10.7*** |
| IACU 0∼120 min (insulin) | 1,736.4±171.3 | 1,259.9±187.2 | 1,513.5±143.9 | 1,105.2±182.4 |
| IAUC 0∼120 min (GLP-1) | 1,093.3±429.8 | 704.7±218.0 | 831.1±438.6 | 2,203.7±637.6 |
Values are mean±SE.
Significant differences by ANOVA for repeated measures at total 0∼120 min.
Mean values were significantly different from those obtained after reference food consumption (**P<0.01 and ***P<0.001; Student t-test with Bonferroni’s correction).
Reference food, glucose solution; Chocolate A, chocolate bar with milky filling; Chocolate B, milk and cocoa cream with crispy wafer balls coated with cocoa cream; Chocolate C, milk chocolate ball.
IAUC, incremental area under the curve; GLP-1, glucagon-like peptide-1.
The GI, II, and GL
The GI and II values of the three chocolates are shown in Table 4. All the chocolates were classified as low GI (low≤55). The GI values of chocolates A, B, and C were 39.2, 47.8, and 33.7, all of which were significantly lower than that of the reference food (P<0.001). The II value of chocolate B (97.9±12.5) was similar to that of the reference food, while the II values of chocolate A and C (68.9±9.0 and 63.8±9.3, respectively) were significantly lower than the II of the reference food (P=0.009). The GL values of the three chocolates are shown in Table 5. The GL values of chocolates A, B, and C were 5.5, 5.3, and 5.7, repectively; thus, all three chocolates were categorized as low GL (low ≤10).
Table 4.
Glycemic indexes (GI) and insulin indexes (II) of the three chocolates
| Food | n | GI | Min∼Max | P-value | GI classification2) | II | Min∼Max | P-value |
|---|---|---|---|---|---|---|---|---|
| Reference food1) | 10 | 100a | 100a | |||||
| Chocolate A | 10 | 39.2±6.0bc | 26∼53 | <0.001 | Low | 68.9±9.0b | 29∼89 | 0.009 |
| Chocolate B | 10 | 47.8±5.6b | 35∼61 | Low | 97.9±12.5a | 70∼126 | ||
| Chocolate C | 10 | 33.7±3.9c | 25∼43 | Low | 63.8±9.3b | 43∼85 |
Values are mean±SE.
Mean values were found to differ significantly from those after reference food consumption by ANOVA and Duncan’s multiple range test.
Different letters (a-c) in the same column indicate significant differences compared to the reference food.
Reference food, glucose solution; Chocolate A, chocolate bar with milky filling; Chocolate B, milk and cocoa cream with crispy wafer balls coated with cocoa cream; Chocolate C, milk chocolate ball.
1)Glucose solution.
2)Low ≤55; medium 56∼69; high ≥70.
Table 5.
Glycemic loads (GL) of the three chocolates
| Food | n | Standard serving size (g) | Carbohydrate (g/portion) | GL (per serving) | GL classification1) |
|---|---|---|---|---|---|
| Chocolate A | 10 | 25 | 14 | 5.5 | Low |
| Chocolate B | 10 | 20 | 11 | 5.3 | Low |
| Chocolate C | 10 | 31 | 17 | 5.7 | Low |
Chocolate A, chocolate bar with milky filling; Chocolate B, milk and cocoa cream with crispy wafer balls coated with cocoa cream; Chocolate C, milk chocolate ball.
1)Low, ≤10; medium, 11∼19; high, ≥20.
DISCUSSION
The glycemic responses induced by the chocolates were lower than those induced by the reference food. However, the insulin responses were similar for the chocolates and the reference food at all time points, and the GLP-1 responses to the chocolates were inconsistent. Furthermore, all the chocolates were classified as low GI and low GL. In addition, most of the GI and II values of the chocolates were significantly lower than those of the reference food, apart from the II value for chocolate B (creamy chocolate), which did not differ from that of the reference food.
In the present study, glycemic responses after chocolate consumption were lower and less fluctuating than those induced by the reference food, which is consistent with results from a previous study (Zhang et al., 2018). These findings may be due to nutritional factors in chocolate-products that elicit lower glycemic responses than reference foods due to factors such as fat and protein. Several studies have revealed that co-ingestion of fat or protein reduces glycemic response by delaying gastric emptying and promoting insulin release compared with carbohydrates alone (Collier et al., 1984; Nuttall et al., 1984; Gannon et al., 1993; Karamanlis et al., 2007; Hätönen et al., 2011).
Our results also revealed that a creamy chocolate (chocolate B) elicited more similar glycemic responses to the reference food than chocolate balls (chocolates A and C) at 15 and 60 min. It is possible that the semi-solid and solid consistencies of the two chocolates contributed to this difference. The physical form of food has been recognized to influence the rate of digestion and absorption. Liquid foods are digested and absorbed more rapidly than solid foods, so the glycemic response is more rapid following consumption (Haber et al., 1977; O’Dea et al., 1980). Thus, in the present study, creamy chocolate with a semi-solid consistency could have caused a higher postprandial glycemic response because it was digested and absorbed more rapidly than the chocolate ball with a solid consistency.
In contrast, the insulin responses to the chocolates were comparable to those to the reference food at all time points, and the IAUC for insulin did not differ significantly for the chocolates and reference food, in contrast to the glucose response. This finding is in line with a report by Brand-Miller et al. (2003) that showed cocoa powder may increase insulin release. Indeed, cocoa powder contains a variety of amino acids and biogenic amines, such as theobromine, arginine, and phenylethylamine, which may influence insulin secretion (Bruinsma and Taren, 1999). In earlier studies, a mixture of amino acids were shown to affect insulin release and milk was shown to be a potent insulin secretagogue, suggesting that lactose and insulinogenic amino acids from milk are responsible for high insulin responses (Floyd et al., 1970; van Loon et al., 2000; Liljeberg Elmståhl and Björck, 2001; Hoyt et al., 2005). Furthermore, Brand-Miller et al. (2003) suggested that cephalic-phase insulin release (CPIR) enhanced insulin secretion due to the high preference for chocolate, but it cannot be used to draw conclusions about the effect of chocolate consumption on CPIR in this study, since the very early phase of insulin release (within 10 min of food intake) were not measured.
Unlike the effects on insulin release, the effects of the chocolates on GLP-1 were inconsistent. GLP-1 is an incretin hormone secreted from endocrine L-cells in the distal small intestine and colon in response to food ingestion. GLP-1 regulates glucose and insulin responses by stimulating glucose-dependent insulin secretion, inhibiting glucagon release and delaying gastric emptying (Holst, 2007; Marathe et al., 2013).
There were no significant differences in GLP-1 responses between chocolates and reference food at all time points measured. GLP-1 response to chocolate consumption have not been previously studied, however, several studies investigated the effect of polyphenol-containing foods on GLP-1 responses. Ryan et al. (2017) determined that in vitro cocoa extracts (cocoa beans, unfermented cocoa beans, and fermented cocoa beans) containing flavanols exert mild DPP-4 inhibitor activity compared to controls (Diprotin A, peptide of DPP-4 inhibitor); however, the effects of cocoa extracts on GLP-1 were not analyzed. In human trials, Törrönen et al. (2012) showed that polyphenols in berry with sucrose may induce greater increases in GLP-1 concentrations than sucrose alone, although these results were not statistically significant. In contrast, Castro-Acosta et al. (2016) found that blackcurrants, which are rich in anthocyanin, inversely inhibit secretion of incretin hormones. Most commercial chocolate product contain small amounts of polyphenol due to the conventional chocolate making process (Di Mattia et al., 2017). However, up to 85% of the polyphenols of cocoa are lost during the manufacturing process of unfermented beans into the final chocolate product (Visioli et al., 2009). Therefore, we were unable to conclude that our findings were influenced by the polyphenol content. Also, considering the large variations in individual GLP- 1s, GLP-1 may be sensitive, and there is a lack of prior research to conclude and our results are limited. Therefore, further study is required to examine incretin responses after consumption of chocolate.
In the present study, all the chocolates were categorized as low GI and low GL. According to the international tables of GI and GL values (Foster-Powell et al., 2002), plain milk chocolates from 4 different studies were classed as having a low GI (mean GI value: 34) and medium GL values (mean GL value: 12). Scazzina et al. (2016) reported that GI values of commercial chocolate products from Italy varied from 42 (wafer balls coated with chocolate) to 55 (milk chocolate bar); these chocolates had GL values of 9 and 11, respectively. On the other hand, the present results suggest disparities between GI and II values of chocolate products. Indeed, the II of chocolate B was comparable to that of the reference food, unlike its GI. In a previous study, Holt et al. (1997) confirmed that the II of chocolate bar was 50% higher than its GI (Brand-Miller et al., 2003). The present results obtained using commonly consumed chocolate with higher insulin responses and II are consistent with those of the prior study (Shively et al., 1986; Holt et al., 1997). In addition, since differences exist between the physical properties of each chocolate, the II of creamy chocolate B was not significantly different to the reference food compared with the other chocolates. The foods with semi-solid properties may have been digested and absorbed more rapidly, thus resulting in higher insulin indexes than the chocolate balls (solid property) (Haber et al., 1977; O’Dea et al., 1980).
Strengths of the present study include that, to the best of our knowledge, this was the first to investigate postprandial glucose, insulin, and GLP-1 responses after consumption of chocolate products. Furthermore, it was a well-designed study based on the ISO 2010 standardized protocol (ISO, 2010) and reviews of Brouns et al. (2005). However, the present study has several limitations. Although all subjects met the eligibility criteria, their average age was 26 years, with most belonging to a young age group; this was because the study was conducted at an university. In addition, for economic reasons, the GLP-1 experiment was not repeated. Further studies are required to examine the effects of chocolate intake on other hormones, such as glucose-dependent insulinotropic polypeptide and ghrelin.
In conclusion, our results revealed that chocolates elicit low glycemic responses, resulting in low GI values. Also, creamy chocolate with semi-solid consistencies showed higher glycemic responses than chocolates with solid consistencies. While the insulin responses induced by the chocolates were similar to those induced by the reference food, the GLP-1 responses to the chocolates were inconsistent. The macronutrients, and physical and biochemical properties of the chocolates impact on glucose, insulin, and GLP-1 responses after ingestion. In particular, it is considered that the insulin-stimulating effect of chocolate may increase insulin responses after consumption of chocolate compared to glycemic responses. Since there are limited data on the glycemic, insulinemic, and GLP-1 responses following chocolate consumption, our finding may help consumers and health practitioners make informed dietary choices. In the present study, the GIs of the chocolates were low; this may prevent glycemic responses from rising rapidly after consumption of chocolate. However, it is necessary to consider that chocolate may cause health problems such as weight gain due to its relatively high fat and sugar content. Therefore, consumers should make more informed choices based on the results of this study.
To conclude, it may be potentially helpful to consider the GI and II when developing chocolate products. However, further research is required to clarify the mechanisms of the incretin response following chocolate consumption, and to determine if chocolate affects metabolic responses.
ACKNOWLEDGEMENTS
The research was carried out thanks to the financial support of Ferrero Asia Limited and Soremartec Italia Srl, Ferrero Group.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest. The findings and conclusions in this report are those of the authors and do not represent the official views or positions of the supporting company.
References
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Miller J, Holt SH, de Jong V, Petocz P. Cocoa powder increases postprandial insulinemia in lean young adults. J Nutr. 2003;133:3149–3152. doi: 10.1093/jn/133.10.3149. [DOI] [PubMed] [Google Scholar]
- Brouns F, Bjorck I, Frayn KN, Gibbs AL, Lang V, Slama G, et al. Glycaemic index methodology. Nutr Res Rev. 2005;18:145–171. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- Bruinsma K, Taren DL. Chocolate: food or drug? J Am Diet Assoc. 1999;99:1249–1256. doi: 10.1016/S0002-8223(99)00307-7. [DOI] [PubMed] [Google Scholar]
- Castro-Acosta ML, Smith L, Miller RJ, McCarthy DI, Farrimond JA, Hall WL. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J Nutr Biochem. 2016;38:154–161. doi: 10.1016/j.jnutbio.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier G, McLean A, O'Dea K. Effect of co-ingestion of fat on the metabolic responses to slowly and rapidly absorbed carbohydrates. Diabetologia. 1984;26:50–54. doi: 10.1007/BF00252263. [DOI] [PubMed] [Google Scholar]
- Di Mattia CD, Sacchetti G, Mastrocola D, Serafini M. From cocoa to chocolate: the impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Front Immunol. 2017;8:1207. doi: 10.3389/fimmu.2017.01207. https://doi.org/10.3389/ fimmu.2017.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd JC, Jr, Fajans SS, Pek S, Thiffault CA, Knopf RF, Conn JW. Synergistic effect of certain amino acid pairs upon insulin secretion in man. Diabetes. 1970;19:102–108. doi: 10.2337/diab.19.2.102. [DOI] [PubMed] [Google Scholar]
- Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- Gannon MC, Nuttall FQ, Westphal SA, Seaquist ER. The effect of fat and carbohydrate on plasma glucose, insulin, C-peptide, and triglycerides in normal male subjects. J Am Coll Nutr. 1993;12:36–41. doi: 10.1080/07315724.1993.10718280. [DOI] [PubMed] [Google Scholar]
- Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- Greenberg JA, Buijsse B. Habitual chocolate consumption may increase body weight in a dose-response manner. PLoS One. 2013;8:e70271. doi: 10.1371/journal.pone.0070271. https://doi.org/10.1371/journal.pone.0070271 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber GB, Heaton KW, Murphy D, Burroughs LF. Depletion and disruption of dietary fibre. Effects on satiety, plasma-glucose, and serum-insulin. Lancet. 1977;2:679–682. doi: 10.1016/S0140-6736(77)90494-9. [DOI] [PubMed] [Google Scholar]
- Hätönen KA, Virtamo J, Eriksson JG, Sinkko HK, Sundvall JE, Valsta LM. Protein and fat modify the glycaemic and insulinaemic responses to a mashed potato-based meal. Br J Nutr. 2011;106:248–253. doi: 10.1017/S0007114511000080. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Holt SH, Miller JC, Petocz P. An insulin index of foods: the insulin demand generated by 1000-kJ portions of common foods. Am J Clin Nutr. 1997;66:1264–1276. doi: 10.1093/ajcn/66.5.1264. [DOI] [PubMed] [Google Scholar]
- Hoyt G, Hickey MS, Cordain L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. Br J Nutr. 2005;93:175–177. doi: 10.1079/BJN20041304. [DOI] [PubMed] [Google Scholar]
- ISO. ISO 26642:2010. International Organization for Standardization; Geneva, Switzerland: 2010. Food products-determination of the glycaemic index (GI) and recommendation for food classification; pp. 1–18. [Google Scholar]
- Karamanlis A, Chaikomin R, Doran S, Bellon M, Bartholomeusz FD, Wishart JM, et al. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr. 2007;86:1364–1368. doi: 10.1093/ajcn/86.5.1364. [DOI] [PubMed] [Google Scholar]
- KCDC. Korea National Health and Nutrition Examination Survey VII. Korea Centers for Disease Control and Prevention; Cheongju, Korea: 2016. [Google Scholar]
- Liljeberg Elmståhl H, Björck I. Milk as a supplement to mixed meals may elevate postprandial insulinaemia. Eur J Clin Nutr. 2001;55:994–999. doi: 10.1038/sj.ejcn.1601259. [DOI] [PubMed] [Google Scholar]
- Marathe CS, Rayner CK, Jones KL, Horowitz M. Glucagon-like peptides 1 and 2 in health and disease: a review. Peptides. 2013;44:75–86. doi: 10.1016/j.peptides.2013.01.014. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Chevaux K, Jackson L, Preston M, Martinez G, Schmitz HH, et al. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol. 2006;47:S103–S121. doi: 10.1097/00005344-200606001-00003. [DOI] [PubMed] [Google Scholar]
- Nuttall FQ, Mooradian AD, Gannon MC, Billington C, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care. 1984;7:465–470. doi: 10.2337/diacare.7.5.465. [DOI] [PubMed] [Google Scholar]
- O'Dea K, Nestel PJ, Antonoff L. Physical factors influencing postprandial glucose and insulin responses to starch. Am J Clin Nutr. 1980;33:760–765. doi: 10.1093/ajcn/33.4.760. [DOI] [PubMed] [Google Scholar]
- Ryan CM, Khoo W, Stewart AC, O'Keefe SF, Lambert JD, Neilson AP. Flavanol concentrations do not predict dipeptidyl peptidase-IV inhibitory activities of four cocoas with different processing histories. Food Funct. 2017;8:746–756. doi: 10.1039/C6FO01730D. [DOI] [PubMed] [Google Scholar]
- Scazzina F, Dall'Asta M, Casiraghi MC, Sieri S, Del Rio D, Pellegrini N, et al. Glycemic index and glycemic load of commercial Italian foods. Nutr Metab Cardiovasc Dis. 2016;26:419–429. doi: 10.1016/j.numecd.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Shively CA, Apgar JL, Tarka SM., Jr Postprandial glucose and insulin responses to various snacks of equivalent carbohydrate content in normal subjects. Am J Clin Nutr. 1986;43:335–342. doi: 10.1093/ajcn/43.3.335. [DOI] [PubMed] [Google Scholar]
- Statista. [cited 2018 May 17];Per capita chocolate consumption worldwide in 2017, by country (in kilograms) 2018 Available from: https://www.statista.com/statistics/819288/worldwide-chocolate-consumption-by-country/
- Törrönen R, Sarkkinen E, Niskanen T, Tapola N, Kilpi K, Niskanen L. Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects. Br J Nutr. 2012;107:1445–1451. doi: 10.1017/S0007114511004557. [DOI] [PubMed] [Google Scholar]
- van Loon LJ, Saris WH, Verhagen H, Wagenmakers AJ. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr. 2000;72:96–105. doi: 10.1093/ajcn/72.1.96. [DOI] [PubMed] [Google Scholar]
- Visioli F, Bernaert H, Corti R, Ferri C, Heptinstall S, Molinari E, et al. Chocolate, lifestyle, and health. Crit Rev Food Sci Nutr. 2009;49:299–312. doi: 10.1080/10408390802066805. [DOI] [PubMed] [Google Scholar]
- Zhang CX, Long WQ, Ye YB, Lu MS, Zhang NQ, Xu M, et al. Effects of chocolate-based products intake on blood glucose, insulin and ghrelin levels and on satiety in young people: a cross- over experimental study. Int J Food Sci Nutr. 2018;69:882–891. doi: 10.1080/09637486.2018.1426737. [DOI] [PubMed] [Google Scholar]