Abstract
Black radish (Raphanus sativus L. var. niger), which is cultivated worldwide, is used in traditional medicine as it aids liver function, gastric secretion, gallbladder function, and gallstone mitigation. In this study, we examined the anti-inflammatory effects of black radish extract (BRE) on the lipopolysaccharide (LPS)- and interleukin (IL)-6-mediated inflammatory responses in the RAW 264.7 cell lines. Our findings show that BRE significantly ameliorated LPS-induced nitric oxide (NO) release and production of pro-inflammatory cytokines, such as IL-1β, IL-6, tumor necrosis factor (TNF)-α, and prostaglandin E2. The levels of cyclooxygenase (COX)-2 and inducible NO synthase (iNOS) in LPS-stimulated RAW 264.7 cells were found to be suppressed by BRE. Further, BRE significantly suppressed the LPS-induced expression of mRNAs encoding COX-2, iNOS, IL-1β, IL-6, and TNF-α in a concentration-dependent manner. BRE treatment significantly inhibited Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) phosphorylation in IL-6- and LPS-treated RAW 264.7 cells. In addition, BRE decreased the levels of phosphorylated extracellular signal-regulated protein kinases and c-Jun N-terminal kinase under the same conditions. Moreover, BRE induced high nuclear factor erythroid 2-related factor 2 (NRF2) levels and its target gene heme oxygenase 1 (HO-1) in the absence of LPS. These data demonstrate that BRE may be beneficial for treating inflammation through selective immunomodulatory effects, which may be mediated by inhibition of the STAT3/JAK2 and activation of the NRF2/HO-1 signal transduction pathways.
Keywords: anti-inflammatory effects, black radish, JAK2/STAT3, LPS/IL-6, NRF2
INTRODUCTION
Inflammation play an essential role in tissue injury and bacterial and viral infections, and this may induce adverse effects by activating inflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-10], as well as by mediators of inflammation [prostaglandin E2 (PGE2) and nitric oxide (NO)] that are produced by cyclooxygenase (COX)-2 and inducible NO synthase (iNOS), respectively (Adams and Hamilton, 1984; Guzik et al., 2003). However, excessive inflammation can cause severe chronic pathologies, such as arthritis (Berenbaum, 2000; Selmi et al., 2014), cancer (Itzkowitz, 1997; Elinav et al., 2013), obesity, diabetes (Esser et al., 2014; Shay et al., 2015), atherosclerosis (Libby et al., 2002), and non- alcoholic fatty liver disease (NAFLD), while inflammatory cells, such as monocytes and macrophages, can produce a large repertoire of cytokines and induce pathogenic changes in tissues (Duarte et al., 2015). Inflammation is typically initiated through specific cytokine and chemokine production, such as NO, COX-2, and PGE2. Inflammation is also characterized by the recruitment of leukocytes to the injury site (Luster, 1998).
Nuclear factor (NF)-κB and the mitogen-activated protein kinase (MAPK) family proteins extracellular signal- regulated protein kinase (ERK) 1/2, JNK, and P38 MAPK (Kim et al., 2007; Shan et al., 2009; Elinav et al., 2013) in response to lipopolysaccharide (LPS) treatment. Further, the cytokine (IL-6) is seen to bind with glycoprotein-130 and activate Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3). These molecules mediate inflammation-induced cancer or tumor development by activating transcriptional factors of STAT-3, which promotes cell proliferation, cell survival, angiogenesis, oncogene addiction, invasion, and metastasis (Banerjee and Resat, 2016; Zimmers et al., 2016). Activated STAT3 translocates to the nucleus in order to activate inflammatory cytokine expression, such as IL-1β and IL-6, and the receptors of which require STATs for intracellular signal transduction. Thus, STAT3 is critical for activating and signaling factors that mediate inflammatory responses (Ahmed and Ivashkiv, 2000; Ben-Neriah and Karin, 2011).
Radishes are rich in a variety of functional components, such as glucosinolates, flavonoids, organic acids, anthocyanin, polyphenol, and minerals, which helps in the treatment of a number of diseases (Gutiérrez and Perez, 2004; Beevi et al., 2010). Radish has been used as a laxative, stimulant, digestive aid, appetizer, and treatment for stomach disorders (Alqasoumi et al., 2008; Shin et al., 2015; Kapoor, 2017). The main isothiocyanates (ITCs) of radish roots are glucobrassicin (indole-3-carbinol precursor), glucoraphanin, 4-(methylthio)-3-butenyl isothiocyanate (MBITC), allyl isothiocyanate (AITC), benzyl isothiocyanate (BITC), sulforaphene, glucoraphasatin, and phenethyl isothiocyanate (PEITC) (Adachi et al., 1998; Gong et al., 2009; Ediage et al., 2011; Beevi et al., 2012).
Raphanus sativus L. var. niger, a cruciferous vegetable that contains high glucosinolate concentrations, as well as sulfates and cysteine-rich proteins, which are considered glutathione synthesis precursors (Hanlon et al., 2007). BRE has been used in folk medicine in Spain, Iran, China, and Turkey to treat flatulence, gallstones, asthma, bronchitis, and other respiratory disorders (Castro- Torres et al., 2014). BRE is best recognized for its antioxidant and antidiabetic effects, as well as for pulmonary fibrosis treatment (Lugasi et al., 2005; Salah-Abbès et al., 2008; Shukla et al., 2011; Asghari et al., 2015).
There are only a few reports available to confirm the anti-inflammatory capacity and mechanisms of action of BRE using the RAW 264.7 cell model. To the best of our knowledge, this is the first study examining the mechanism of the STAT3 signal transduction pathway in mediating the anti-inflammatory effects of BRE. Specifically, the objectives of the current study are as follows: (1) evaluate the inhibitory effect of BRE on releasing inflammatory cytokines (NO, PGE2, IL-6, IL-1β, and TNF-α); (2) evaluate the levels of mRNAs encoding IL-6, IL-1β, TNF- a, iNOS, and COX-2 in LPS-treated RAW 264.7 cells with or without BRE; (3) determine if BRE inhibited JAK2 and STAT3 phosphorylation (activation); and (4) determine if BRE activated ERK and JNK and induced nuclear factor erythroid 2-related factor 2 (NRF2)-mediated transcription of the gene encoding heme oxygenase-1 (HO-1).
MATERIALS AND METHODS
Chemicals and reagents
The cell culture medium high-glucose Dulbecco’s modified Eagle’s medium (DMEM), trypsinase, and penicillin- streptomycin were all purchased from Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Gibco, while 3-(4,5-dimethy-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) and LPS were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Enzyme-linked immunosorbent assay (ELISA) kits for mouse PGE2, IL-6, IL-1β, and TNF-α were purchased from R&D Systems (Minneapolis, MN, USA). NucleoSpin RNA reagent was obtained from Macherey-Nagel GmbH & Co. KG (Düren, Germany), and the ReverTra Ace-α first strand cDNA synthesis kit was procured from Toyobo (Osaka, Japan). FastStart Universal SYBR Green Master was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA, USA), while AG490 and PEITC were obtained from Invivogen, Inc. (San Diego, CA, USA) and Sigma-Aldrich Co., respectively. Antibodies against COX- 2, β-actin, and iNOS were purchased from Calbiochem (San Diego, CA, USA); antibodies against HO-1 and NRF2 were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); and antibodies against STAT3, p-STAT3 (Tyr 703), JAK2, p-JAK2 (Tyr1007/ 1008), ERK, p-ERK, JNK, and p-JNK were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). All other reagents were of analytical grade.
Preparation of BRE
The black radishes used were obtained from Sungsan Ilchubong Nonghyup, whose planting facility is located in Jeju Province, Korea. Its roots (5,000 g) were cut into thin slices and extracted three times at room temperature with 70% ethanol (5,000 mL). The extracts were strained through filter paper, and the combined filtrate was evaporated at 42°C using a Buchi R-210 rotary evaporator (BÜCHI Labortechnik AG, Flawil, Switzerland), producing 152.65 g of BRE. BRE was then sufficiently dissolved in 1,500 mL distilled water and centrifuged at 4,000 rpm for 20 min. The supernatant was loaded onto a Diaion HP-20 column (15 cm×100 cm; Mitsubishi Chemical Corporation, Tokyo, Japan), eluted using an ethanol gradient of 0%∼50% (v/v), and the fraction eluted by 50% ethanol was combined and evaporated to produce BRE (approximately 30 g).
Cell culture
The RAW 264.7 cell line was purchased from the Korean Cell Line Bank (Seoul, Korea). The cells were then cultured in DMEM containing 2 mM glutamine, 4.5 g/L glucose, 100 mg/L sodium pyruvate (Gibco), penicillin (100 units/mL), streptomycin (100 μg/mL), and 10% FBS. The cells were cultured at 37°C in a humidified incubator in an atmosphere containing 5% CO2. Then, the cells (5×105 cells/well) were seeded in a 6-well cell culture plate, incubated for 16 h, and then treated with LPS (1 μg/mL) with or without BRE (200 μg/mL, 100 μg/mL, 50 μg/mL, and 25 μg/mL) for 6∼24 h.
Cell viability assay
Cell viability was measured using the MTT assay. Cells (1×104 cells/well) were seeded in a 96-well cell culture plate, incubated for 24 h, and treated with different concentrations of BRE (125, 250, 500, and 1,000 μg/mL) for 24 h. MTT was dissolved in phosphate-buffered saline to 2 mg/mL. After incubation, the cells were treated with 50 μL of MTT solution for 2 h, then the solution was removed, and dimethyl sulfoxide was added to dissolve the insoluble formazan crystals. The concentration of dissolved formazan was measured at 540 nm using a microplate reader (BioTek Instruments Inc., Winooski, VT, USA).
NO and lactate dehydrogenase (LDH)-release assays
The NO assay was performed as previously described, but with slight modifications (Yoon et al., 2009). After incubating RAW 264.7 cells (1.5×105 cells) with LPS (1 mg/mL) for 24 h, the nitrite (NO2−) level in the culture medium was measured as a surrogate for NO production. The amount of NO2−, a stable metabolite of NO, was measured using Griess reagent [1% sulfanilamide and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride in 2.5% phosphoric acid]. Afterward, 100 μL of cell culture medium was mixed with 100 μL of Griess reagent. This mixture was then incubated at room temperature for 10 min, and the absorbance was measured at 540 nm using a microplate reader. Fresh culture medium was used as a blank. The NO2− level was determined from the standard curve for NO2−. The LDH release assay kit (Promega, Madison, WI, USA) was used to determine LDH activity, as per the manufacturer’s manual.
Measurement of cytokine and PGE2 production
BRE was diluted with DMEM before use. The cells were treated for 24 h with LPS (1 μg/mL) to induce cytokine production. The concentrations of cytokines IL-1, IL-6, TNF-α, and PGE2 in the culture medium supernatants were measured using ELISA (R&D Systems), as per the manufacturer’s instructions.
Real-time quantitative polymerase chain reaction (qPCR) analysis
RAW 264.7 cells were collected from 6-well plates, and the total RNA was obtained using NucleoSpin RNA reagent (Macherey-Nagel GmbH & Co. KG), according to the manufacturer’s instructions. Then, the cDNA was synthesized using a first strand cDNA synthesis kit (Toyobo). Furthermore, qPCR was performed using a premix (5× HOT FIREPol EvaGreen qPCR Supermix; Solis BioDyne OÜ, Tartu, Estonia) through a Hangzhou Bioer Technology Co,. Ltd. (Hangzhou, China) with the primers (Bioneer, Daejeon, Korea) specific for the mRNAs encoding IL-1, IL-6, TNF-α, COX-2, and iNOS (Table 1).
Table 1.
Primer sequences used in real-time quantitative polymerase chain reaction analysis
| Genes | Forward primer (5’→3’) | Reverse primer (5’→3’) |
|---|---|---|
| iNOS | AACATCAGGTCGGCCATCACT | CCAGAGGCAGCACATCAAAGC |
| IL-1β | CGTTCCCATTAGACAACTGCA | GGTATAGATTCTTTCCTTTGAGGC |
| IL-6 | ACGGCCTTCCCTACTTC | TTCCACGATTTCCCAGA |
| COX-2 | GCAAATCCTTGCTGTTCCAAT | GGAGAAGGCTTCCCAGCTTTTG |
| TNF-α | CAAGGGACTAGCCAGGAG | TGCCTCTTCTGCCAGTTC |
| β-Actin | CATCCTGCGTCTGGACCTGG | TAATGTCACGCACGATTTCC |
Western blot analysis
RAW 264.7 cells were lysed in RIPA buffer, and the supernatant samples containing 40 μg of protein were loaded onto the lanes of a 10% sodium dodecyl sulfate- polyacrylamide gel, electrophoresed, and then immunoblotted onto a nitrocellulose membrane (Schleicher & Schuell BioScience, Inc., Keene, NH, USA). The membrane was then incubated with antibodies against iNOS (1:1,000 dilution), COX-2 (1:1,000), JAK2 (1:1,000), STAT3 (1:1,000), p-JNK (1:1,000), p-ERK (1:1,000), JNK (1:1,000), ERK (1:1,000), cytochrome P450 (CYP) family 1 subfamily A member 2 (CYP1A2) (1:1,000), NRF2 (1:1,000), or anti-HO-1 (1:1,000) for 2 h. The immunocomplexes were detected using a chemiluminescent substrate (Miracle-StarTM; iNtRON Biotech, Gyeonggi, Korea), according to the manufacturer’s instructions. After imaging, the membranes were stripped and probed again with an anti-β-actin antibody. The optical density (OD/ mm2) of each band was measured, and the band density relative to that of the β-actin band was compared using ImageJ software. Next, the stripped membranes were incubated with antibodies against COX-2, iNOS, STAT3, p-STAT3, JAK2, and p-JAK2 overnight at 4°C. The membranes were washed three times for 10 min each between each step, incubated with horseradish peroxidase-conjugated secondary antibodies for 30 min at room temperature, and then washed again.
Statistical analysis
The data are presented as the mean±standard deviation (SD). Statistical analysis was performed using the two- tailed Student’s t-test or ANOVA for comparisons between or among two or multiple groups, respectively. Values with P<0.05 were considered as significantly different.
RESULTS
The effects of BRE on RAW 264.7 cell viability
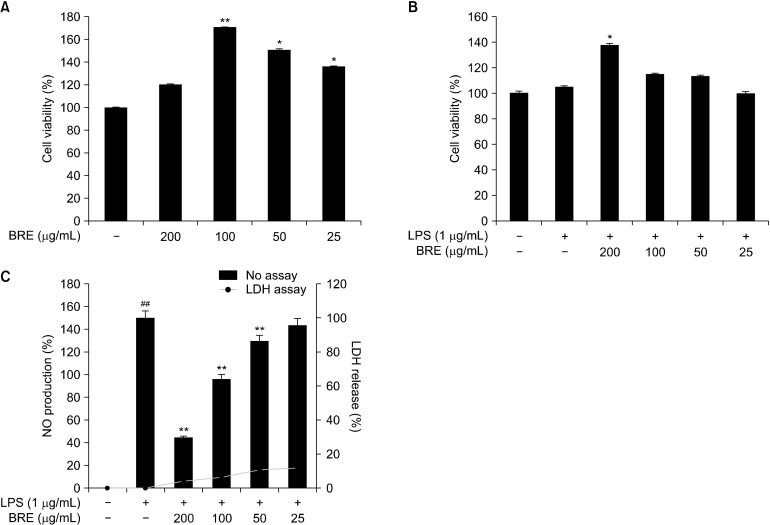
When the cells were treated with BRE, viability has been observed to increase significantly in a concentration-dependent manner (Fig. 1A) when the cells were treated with BRE. Thus, cell viability increased to 20% and 65% after 200 μg/mL and 100 μg/mL of BRE treatment, respectively, compared with the controls. The cytotoxic effects of BRE on LPS-treated RAW 264.7 cells were measured, and the result showed that BRE, at concentrations up to 25 μg/mL, did not significantly affect cell survival, whereas higher concentrations of BRE increased cell viability (Fig. 1B). However, LPS treatment for 18 h slightly reduced cell viability in a concentration-dependent manner. Therefore, NO and cytokine levels were determined. Western blot analyses of the LPS-stimulated RAW 264.7 cells were performed after 25∼200 μg/mL BRE treatment. The LDH release assay showed minor cytotoxic effects (Fig. 1C).
Fig. 1.
Effects of black radish extract (BRE) on the cell viability and nitric oxide (NO) production of RAW 264.7 macrophages. The cells were incubated for 24 h with different concentrations of BRE (25, 50, 100, and 200 μg/mL) with or without 1 mg/mL lipopolysaccharides (LPS) for 24 h. The cell viability (A and B) and NO (C) production were measured using 3-(4,5-dimethy-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay and enzyme-linked immunosorbent assay kit as described in MATERIALS AND METHODS. Data are expressed as mean± SD (n=3) of the three independent experiments. ##P<0.01 versus control; *P<0.05 and **P<0.01 versus the untreated control group.
The effects of BRE on NO production
NO production was measured in the LPS-treated RAW 264.7 cells with different concentrations of BRE for 24 h (Fig. 1C). NO production was significantly inhibited in a concentration-dependent manner. For example, treatment with 200 mg/mL BRE decreased NO production by 60%, compared with LPS-treated control cells. These results indicate that the NO production was potently inhibited by BRE in a concentration-dependent manner.
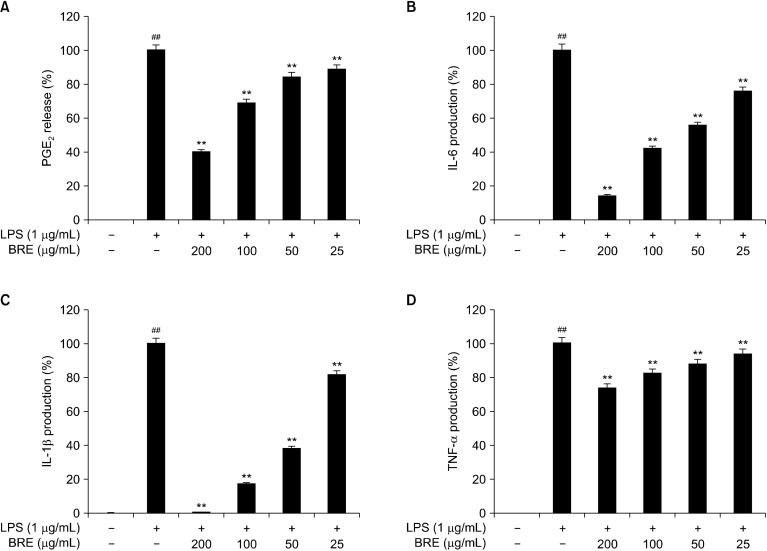
The effects of BRE on PGE2 and cytokine production
The RAW 264.7 cells were treated with LPS with or without BRE to determine its effects on PGE2 production. BRE treatment inhibited PGE2 production in a concentration-dependent manner, compared with LPS-treated control cells. Thus, BRE extracts added to final concentrations of 50, 100, and 200 μg/mL inhibited PGE2 production by 12%, 30%, and 60%, respectively (Fig. 2A).
Fig. 2.
The effects of black radish extract (BRE) on prostaglandin E2 (PGE2) release and pro-inflammatory cytokines interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α production. The RAW 264.7 cells were pre-treated with different BRE concentrations for 1 h and then treated with lipopolysaccharides (LPS, 1 μg/mL) for another 24 h. Release of PGE2 (A), IL-6 (B), IL-1β (C), and TNF-α (D) in culture media were measured using enzyme-linked immunosorbent assay. Data are expressed as mean±SD of the three independent experiments. ##P<0.01 versus control; **P<0.01 versus LPS treatment alone.
Next, we determined the effects of BRE on IL-6 production in the LPS-treated RAW 264.7 cells. BRE treatment inhibited IL-6 production in a concentration-dependent manner, compared with the LPS-treated control cells. Specifically, BRE extracts added to concentrations of 100 and 200 μg/mL were seen to inhibit IL-6 production by 60% and 80%, respectively (Fig. 2B). Moreover, BRE inhibited IL-1β production in a concentration-dependent manner, compared with the LPS-treated control cells. Specifically, 50, 100, or 200 μg/mL BRE treatment inhibited IL-1β production by 60%, 80%, and 95%, respectively (Fig. 2C).
To study the effect of BRE on LPS-induced TNF-α secretion, the RAW 264.7 cells were then treated with different concentrations of BRE to examine its effects on LPS-induced TNF-α secretion. BRE treatment inhibited TNF-α secretion in a concentration-dependent manner, compared with the LPS-treated control cells. BRE (100 and 200 μg/mL) inhibited TNF-α secretion by 12% and 20%, respectively (Fig. 2D). Thus, these results indicate that the BRE extract significantly inhibited the production of PGE2, IL-1β, IL-6, and TNF-α.
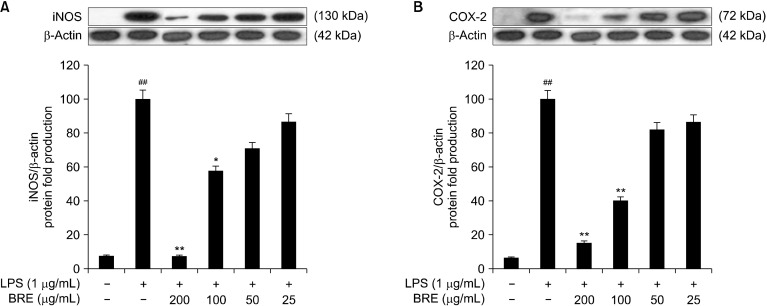
The effects of BRE on iNOS and COX-2 protein expression
We performed Western blot analysis to determine the effects of BRE on iNOS expression. The LPS-treated RAW 264.7 cells with or without BRE were observed to significantly decrease iNOS levels, compared with that of the LPS-treated control cells. Further, treatment with 100 mg/mL of BRE extracts inhibited iNOS expression by 50% relative to that of β-actin (Fig. 3A). COX-2, which is induced during inflammation, produces inflammatory mediators, such as PGE2, which causes pain and fever (Shay et al., 2015). Therefore, we performed Western blot analysis of COX-2 expression using the BRE-treated cells. BRE inhibited COX-2 expression in a concentration-dependent manner. Treatment with 100 μg/mL of BRE inhibited COX-2 expression by 60% relative to that of b- actin (Fig. 3B). These data suggest that BRE reduced NO and PGE2 production by inhibiting iNOS and COX-2 expression, thereby exerting an anti-inflammatory effect.
Fig. 3.
The effects of black radish extract (BRE) on the lipopolysaccharides (LPS)-induced expression of inducible nitric oxide synthase (iNOS) (A) and cyclooxygenase (COX)-2 (B). The cells were pre-treated with BRE (25, 50, 100, or 200 μg/mL) for 1 h and then treated with LPS (1 μg/mL) for 24 h. Representative pictures of Western blot and quantitative analysis of blots. Each immunoreactive band was digitized and expressed as a ratio of β-actin levels. Data are expressed as mean±SD of the three independent experiments. ##P<0.01 versus control; *P<0.05 and **P<0.01 versus LPS treatment alone.
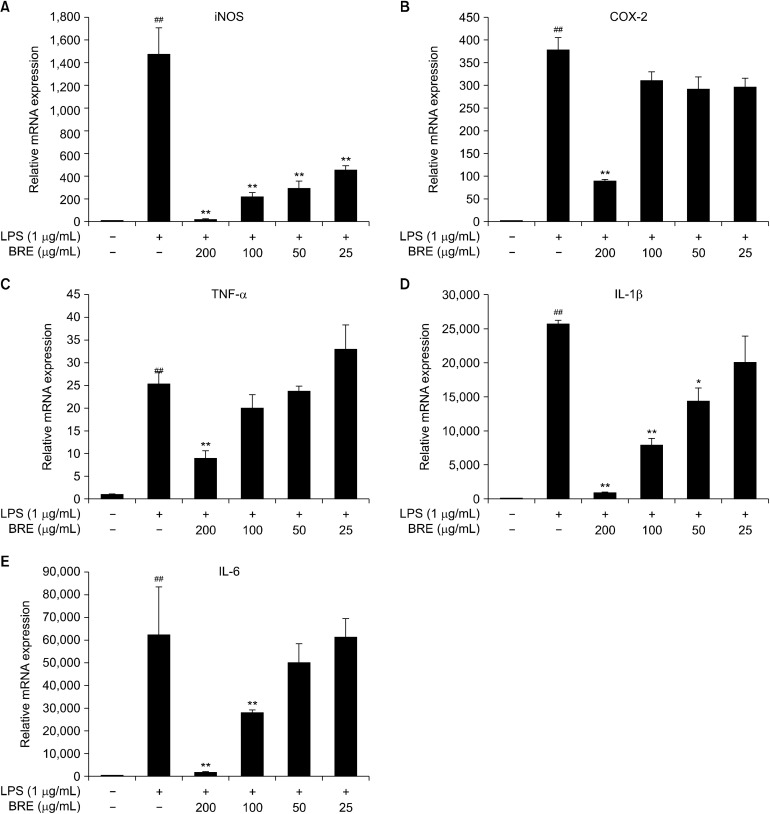
BRE inhibits the expression of mRNAs encoding COX-2, iNOS, TNF-α, IL-1β, and IL-6
Next investigated whether BRE affected the levels of mRNAs encoding COX-2, iNOS, TNF-α, IL-1β, and IL-6. The LPS-treated control has significantly stimulated the transcription of each of these mRNAs encoding COX-2, iNOS, TNF-α, IL-1β, and IL-6 (Fig. 4). After 200 μg/mL concentration of the BRE extract were incubated with LPS, we observed that the levels of iNOS, COX-2, TNF-α, IL-1β, and IL-6 mRNA were reduced to 92.4±40, 4.1± 0.5, 2.8±0.3, 26.0±0.2, and 34.8±0.5 folds, respectively. Further, BRE pre-treatment was seen to significantly reduce the levels of these mRNAs (Fig. 4). Although COX- 2 levels in LPS+BRE-treated cells were consistently reduced compared with that in the LPS-treated control, the differences were insignificant, except for 200 μg/mL BRE- treated cells (Fig. 4B). Further, BRE treatment alone minimally affected the basal levels of mRNAs and the production of cognate cytokines (Fig. 2). Furthermore, qPCR analysis appears that mRNA expression levels of iNOS and COX-2 are correlated with their protein levels (Fig. 3, 4A, and 4B). These results indicate that BRE has a significant anti-inflammatory potential by suppressing LPS- stimulated pro-inflammatory cytokine expression.
Fig. 4.
The effects of black radish extract (BRE) on inducible nitric oxide synthase (iNOS) (A), cyclooxygenase (COX)-2 (B), tumor necrosis factor (TNF)-α (C), interleukin (IL)-1β (D), and IL-6 (E) mRNA expression in lipopolysaccharides (LPS)-stimulated RAW 264.7 macrophages. The cells were pre-treated with BRE (200, 100, 50, or 25 μg/mL) for 1 h and then treated with LPS (1 μg/mL) for 4 h. Cellular mRNA expression analysis was determined using quantitative polymerase chain reaction. iNOS, COX-2, TNF-α, IL-1β, and IL-6 expression levels were calculated after normalizing the signal against the β-actin gene. Data are expressed as mean±SD of the three independent experiments. ##P<0.01 versus control; *P<0.05 and **P<0.01 versus LPS treatment alone.
BRE inhibits IL6-induced JAK2/STAT3 phosphorylation in RAW 264.7 cells
BRE has been observed to significantly inhibit IL-6 protein and mRNA expression (Fig. 2B and 4E). Next, we examined whether the anti-inflammatory activity of BRE can be explained by the inhibition of the activity of the IL-6/STAT3 (JAK2/STAT3) signal transduction pathway to investigate the underlying mechanism. JAK2 and STAT3 phosphorylation levels were significantly increased 30 min after the RAW 264.7 cells were treated with IL-6 (Fig. 5A and 5C).
Fig. 5.
The effects of black radish extract (BRE), AG490, and phenethyl isothiocyanate (PEITC) on interleukin (IL)-6-induced Janus kinase 2 (JAK2) (A and B) and signal transducer and activator of transcription 3 (STAT3) (C, D, and E) phosphorylation. The RAW 264.7 cells were pre-treated with different BRE concentrations for 30 min and then treated with IL-6 (10 ng/ mL) for 30 min. Representative pictures of Western blot and quantitative analyses. Each immunoreactive band was digitized and expressed as a ratio of STAT3 protein levels. Data are expressed as mean±SD of the three independent experiments. ##P<0.01 versus control; *P<0.05 and **P<0.01 versus IL-6 treatment alone (B, D, and E).
The IL-6 (10 ng/mL)-induced phosphorylation of JAK2 and STAT3 tyrosine residues was significantly inhibited after the cells were treated for 30 min. These results indicate that IL-6 induces JAK2 and STAT3 activation. Moreover, BRE dramatically inhibited IL-6-induced JAK2 and STAT3 phosphorylation at the indicated concentration (Fig. 5B and 5D).
AG490 is widely used as a therapeutic JAK inhibitor because it specifically inhibits JAK2/STAT3 signaling (Huang et al., 2010). PEITC, which is one of the most extensively studied ITCs, is a product of gluconasturtiin hydrolysis, a glucosinolate typically found in watercress from cruciferous vegetables, such as radishes and turnips (Wang and Chiao, 2010). Therefore, we tested whether AG490 and PEITC affected IL-6-induced STAT3 phosphorylation. For this purpose, IL-6-treated RAW 264.7 cells were treated with 30 μM or 10 μM of each compound. As shown in Fig. 5E, compared with the IL-6- treated control cells, AG490 and PEITC inhibited the phosphorylation of STAT3 in a concentration-dependent manner. These results demonstrate that AG490, PEITC, and BRE inhibited STAT3 activity and may therefore serve as potential anti-inflammatory agents.
BRE inhibits LPS-induced STAT3 phosphorylation
Recently, the STAT cascade has been reported to be essential in mediating cytokine signaling and inflammatory response. The JAK2/STAT3 cascade in LPS-stimulated RAW 264.7 cells is an essential signaling pathway in mediating immune responses and pro-inflammatory cytokine production (Guo et al., 2014; Yang et al., 2014). To investigate the activation of STAT3 in the LPS-induced inflammatory response, we assessed the levels of the total and phosphorylated STAT3 in RAW 264.7 cells treated with LPS for 4 h to examine the activation of STAT3 in the LPS-induced inflammatory response. Our findings show that there was a significant increase in phosphorylated STAT3 levels, compared with the untreated control cells (Fig. 6). The significant decrease in the levels of LPS-induced phosphorylated STAT3 in BRE-treated cells, as compared with cells treated with LPS-treated alone, suggests that BRE may function as a STAT3 activity modulator. Thus, these results are consistent with the inhibition of the production of pro-inflammatory factors by BRE.
Fig. 6.
The effects of AG490, phenethyl isothiocyanate (PEITC), and black radish extract (BRE) on lipopolysaccharides (LPS)-induced signal transducer and activator of transcription 3 (STAT3) phosphorylation. The RAW 264.7 cells were pre-treated with different concentrations of AG490, PEITC, and BRE for 30 min and then treated with LPS (1 μg/mL) for 4 h. Representative pictures of Western blot and quantitative analyses. Each immunoreactive band was digitized and expressed as a ratio of STAT3 protein levels. Data are expressed as mean±SD of the three independent experiments. ##P<0.01 versus control; *P<0.05 and **P<0.01 versus LPS treatment alone.
We also investigated whether the STAT3 activity inhibition by BRE was sufficient to cause the downregulation of inflammation induced by LPS to determine the target of BRE that caused the inhibition of the inflammatory effects of LPS signaling. We found that the total STAT3 levels were not significantly different with or without BRE treatment of LPS-treated RAW 264.7 cells. In contrast, we found that phospho-STAT3 levels were significantly increased in LPS-treated RAW 264.7 cells and were significantly decreased when the cells were treated with different concentrations of BRE. Thus, BRE attenuated LPS-induced inflammation by inhibiting STAT3 phosphorylation. As shown in Fig. 5B and 5D, BRE has significantly inhibited the IL-6-induced JAK2 and STAT3 tyrosine phosphorylation. Therefore, we performed an investigation on the effects of BRE on the LPS- mediated phosphorylation of STAT3, compared with that of AG490 and PEITC. As expected, PEITC and BRE inhibited the LPS-mediated STAT3 phosphorylation in a concentration-dependent manner (Fig. 6).
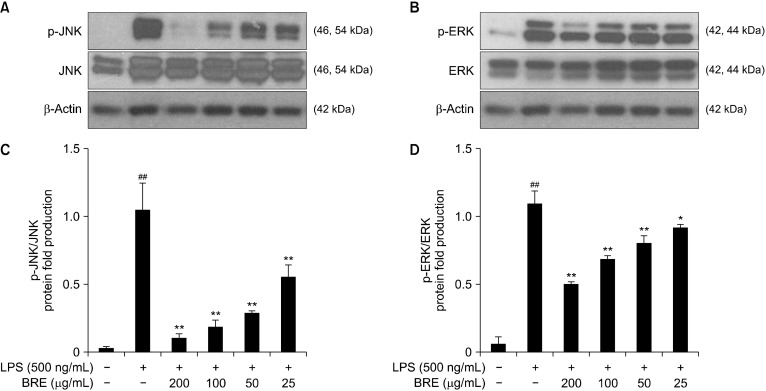
The effects of BRE on MAPK phosphorylation and HO-1 expression
The MAPK family comprises stress-activated protein kinase/JNK, ERK1/2, and p38 MAPK. These signal transducers have been seen to play significant roles in proliferation regulation, inflammatory mediator expression, and macrophage apoptosis (Chen and Wang, 1999). IL-6 and LPS activate the JAK2/STAT3 and toll-like receptor 4-mediated signal transduction pathways, which contribute to MAPK activation that regulates the generation of pro-inflammatory mediators. We performed Western blot analysis of the phosphorylation of ERK1/2 and JNK to determine whether BRE’s inhibitory effect on the inflammatory response was mediated through the MAPK signal transduction pathway. We found that LPS significantly decreased ERK1/2 and JNK phosphorylation, compared with the control cells. Furthermore, BRE pre-treatment of the cells for 1 h significantly decreased ERK and JNK activation that was induced by LPS (Fig. 7). Therefore, BRE exerted a significant effect on the downregulation of ERK1/2 and JNK phosphorylation in LPS-treated cells, suggesting that these pathways are involved in the anti-inflammatory activity of BRE.
Fig. 7.
The inhibitory effects of black radish extract (BRE) on extracellular signal-regulated protein kinase (ERK) and c-Jun N-terminal kinase (JNK) phosphorylation in RAW 264.7 macrophage cells. The cells were pre-treated with BRE for 1 h prior to lipopolysaccharides (LPS) treatment. After treatment with 1 μg/mL LPS, the phosphorylated JNK (A and C) and ERK (B and D) levels were analyzed by immunoblotting. ERK and JNK levels were estimated by the loaded protein as each control. Data are expressed as mean±SD of the three independent experiments. ##P<0.01 versus control; *P<0.05 and **P<0.01 versus LPS treatment alone.
However, the MAPK signal transduction pathway activation may explain, in part, the ability of BRE to rescue cells from the cytotoxic effects of the inflammatory response. Moreover, we detected a decrease of p-ERK and p-JNK but not in the total ERK or JNK levels.
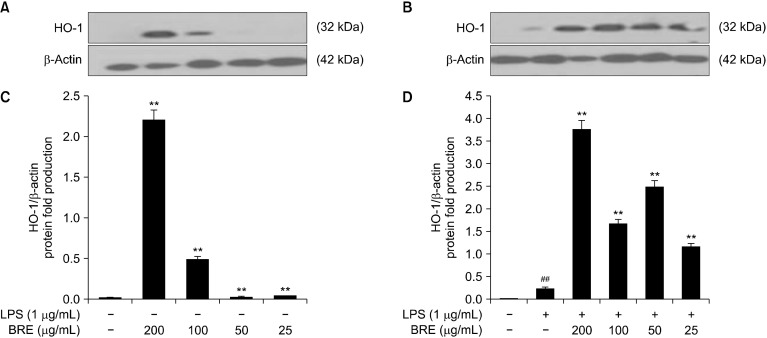
The HO-1 induction is principally regulated by the NRF2/Kelch-like epichlorohydrin-associated protein 1 system, which is considered as the main sensor system of the antioxidative response that regulates the expression of a series of antioxidant enzymes. Furthermore, the NRF2/HO-1 pathway protects cells against oxidative stressors or organic compounds, such as sulforaphane, curcumin, resveratrol, and epigallocatechin gallate (EGCG). HO-1 also confers protection against cell death through its anti-apoptotic, anti-inflammatory, and anti-proliferative effects (Huang et al., 2015).
To investigate the cells were then treated with or without LPS to examine the potential of BRE in modulating the NRF2 signal transduction pathway in RAW 264.7 cells, and the cell extracts were analyzed using Western blotting to determine the levels of HO-1 (Fig. 8A and 8C). The cells were treated with BRE and LPS for 4 h. LPS treatment induced slightly higher HO-1 levels, and BRE was found to increase HO-1 levels in a concentration-dependent manner (Fig. 8B and 8D). These results indicate that the anti-inflammatory effects of LPS on RAW 264.7 cells may be mediated by the NRF2/HO-1-pathway. However, BRE may contribute to the anti-inflammatory effects of signaling through the STAT3/NF-κB/ERK/JNK pathway. These results further indicate that the NRF2 pathway regulation may contribute to BRE’s anti-inflammatory effects.
Fig. 8.
The effects of black radish extract (BRE) on heme oxygenase-1 (HO-1) expression without (A and C) or with (B and D) lipopolysaccharide (LPS). The RAW 264.7 cells were pre-treated with different BRE concentrations. Representative pictures of Western blot and quantitative analyses. Each immunoreactive band was digitized and expressed as a ratio of β-actin levels. Data are expressed as mean±SD of the three independent experiments. ##P<0.01 versus control; **P<0.01 versus LPS treatment alone.
DISCUSSION
Radish is a vegetable that is mainly cultivated in Europe, Spain, Myanmar, Nepal, China, Japan, and Korea. Radish roots are primarily used as food and food supplement. The beneficial effects of its roots, sprouts, seeds, and leaves include its detoxification in HepG2 cells (Hanlon et al., 2007; Scholl et al., 2011) and its hepatoprotective (Lee et al., 2012), antioxidant (Lugasi et al., 1998; Lugasi et al., 2005; Beevi et al., 2012), anti-inflammatory (Park and Song, 2017), gastroprotective (Alqasoumi et al., 2008; Ahn et al., 2013), antimicrobial (Nehrash, 1961; Gutiérrez and Perez, 2004), anti-fibrotic (Asghari et al., 2015), anti-obesity (Kim et al., 2014), anti-lipogenic (Kim et al., 2015b), and anti-diabetic (Taniguchi et al., 2007; Shukla et al., 2011; Banihani, 2017) properties. These beneficial effects are often attributed to its sulfur compounds, phenolic compounds, glucosinolates, and flavonoids (Gutiérrez and Perez, 2004; Ediage et al., 2011; Shin et al., 2015).
For example, radish juice and radish sprouts (Japanese radishes) were observed to attenuate oxidative stress-induced inflammatory responses and pancreatic β-cell damage in streptozotocin-induced diabetic rats (Taniguchi et al., 2007; Shukla et al., 2011). Furthermore, dietary supplementation with radish roots has been reported to lower blood glucose levels and improve lipid metabolism in type 2 diabetic rats and normal mice. In vitro and in vivo models demonstrated that black radish induced phase I and II enzymes (Evans et al., 2014). For example, the phase I detoxification enzymes (CYP isoenzymes CYP1A1 and CYP1A2) and phase II detoxification enzymes (glutathione S-transferase, quinone reductase, and microsomal epoxide hydrolase) were significantly stimulated by a diet containing 20% freeze-dried radish (Hanlon et al., 2007; Hanlon et al., 2009). Furthermore, a 20% radish diet protected mice following exposure to the model carcinogen, 7,12-dimethylbenz(a)anthracene (DMBA), through phase I and II enzymes involved in DMBA clearance (N’jai et al., 2012). Moreover, ingesting radish was seen to induce detoxification enzymes in mice, and although there were numerous human studies investigating the effects of fresh crucifers (Hanlon et al., 2007; Scholl et al., 2011), there are only a few studies examining the effects of BRE on inflammation (Sipos et al., 2002; Jin et al., 2016; Park and Song, 2017).
An indole glucoside isolated from the seeds of R. sativus inhibited IL-6 production by TNF-α-stimulated MG- 63 cells (Jin et al., 2016), and the phenylpropanoid sitosterol sucrose, which was isolated from the seeds of R. sativus, inhibited NO production by LPS-activated murine microglial BV-2 cells (Kim et al., 2014; Kim et al., 2015a). Therefore, BRE can be potentially used to reduce inflammation by inhibiting NO production. Moreover, the chloroform fraction of R. sativus leaves inhibited iNOS and COX-2 expression, thereby reducing inflammation (Park and Song, 2017). These results are consistent with those of the present study.
Overexpressing inflammation-related mediators and cytokines generated from macrophage activation can result in inflammatory disorders (Itzkowitz, 1997; Libby et al., 2002; Elinav et al., 2013; Esser et al., 2014). In this study, we found that BRE significantly inhibited the increased NO, TNF-α, IL-6, and IL-1β production by RAW 264.7 cells. Furthermore, BRE significantly inhibited iNOS and COX-2 expression. Together, these results indicate that BRE may serve as a potential anti-inflammatory agent worthy of further study. For example, we must determine whether BRE inhibits STAT3 activity to interfere with the inflammatory response of activated macrophages. We focused on the IL-6-mediated JAK2/STAT3 pathway to identify the mechanism of the anti-inflammatory effect of BRE, which functions in the immune response and is normally active in the liver in NAFLD.
For example, STAT3 activation was reported to induce the increased TNF-α, IL-10, and IL-1β release (Kim et al., 2007; Duarte et al., 2015) and was ultimately responsible for increased iNOS and COX-2 expression. In this part of the study, we showed that the levels of IL- 1b, TNF-α, and IL-6 were decreased in LPS-treated RAW 264.7 cells via the inhibiting effect of BRE on STAT3 activation. These data were further supported by our detection of the significant inhibitory effects of BRE on STAT3 activity in IL-6- or LPS-treated RAW 264.7 cells. Consistent with these findings, silibinin and tricin 4’-O-(threo-β-guaiacylglyceryl) inhibited iNOS and COX-2 expression, as well as NO production via inhibition of STAT3 activation (Tyagi et al., 2012; Jung et al., 2014). Furthermore, silibinin synergized with a STAT3 inhibitor via inhibition of the activation of STAT3 transcription, and this inhibitory effect reduced iNOS and COX-2 expression, as well as NO generation in LPS-treated RAW 264.7 cells (Guo et al., 2014; Yang et al., 2014). Moreover, STAT3 may also act as an oncogene by activating the expression of several anti-apoptotic genes, such as those that encode B-cell lymphoma-2, cyclin D1, and myeloid cell leukemia-1 (Banerjee and Resat, 2016).
Whether BRE affects these processes requires further research. Notably, BRE affects the NRF2, NF-κB, and STAT3 signal transduction pathways similar to electrophilic compounds and health foods, as well as nutraceuticals, such as sulforaphane, curcumin, resveratrol, EGCG, indole-3-carbinol, PEITC, and quercetin (Huang et al., 2015). Whether the positive influence of BRE, including that of glucosinolate, can be extended to NAFLD, inflammation, cancer, lipogenesis, and other diseases remains to be determined. For example, a recent study found that oxidative disease is mitigated in vivo and in vitro by glucosinolate (Fuentes et al., 2015; Ahn et al., 2016; Ferramosca et al., 2017).
BRE was seen to inhibite the LPS-induced ERK and JNK activation in RAW 264.7 cells. MAPKs are activated by diverse stimuli to regulate cell proliferation, differentiation, and apoptosis (Chun et al., 2012). ERK is generally activated by growth factors, cytokines, and phorbol esters, which regulate cell proliferation or differentiation. Meanwhile, JNK is activated by pro-inflammatory cytokines, ultraviolet irradiation, heat, and osmotic shock, which inhibit proliferation and induce apoptosis. However, this present study shows that BRE treatment significantly inhibited p-ERK, p-JNK were involved in LPS- induced inflammation.
HO-1 is considered as an important cytoprotective enzyme, which is as widely accepted source of oxidative stress. In this study, we showed that HO-1 expression significantly increased in LPS-treated RAW 264.7 cells and that BRE treatment increased the LPS-induced HO-1 expression (Fig. 8). Similar to the LPS-inducible proteins iNOS and COX-2, BRE inhibited the protein expression in LPS-stimulated RAW 264.7 cells (Fig. 3).
To conclude, we found that microgram quantities of BRE inhibited the inflammatory response of RAW 264.7 cells that was associated with iNOS and STAT3 inhibition to enhance signaling through the MAPK/NRF2 signal transduction pathway (Fig. 9). Thus, this study complements our knowledge of BRE’s bioactivity profile, confirms that STAT3 is a promising drug target for treating vascular diseases, and stresses the potential of thiol-reactive compounds, such as glucosinolate, as pharmacological agents with an overall favorable bioactivity profile. Together, our study’s results provide a compelling evidence indicating that the anti-inflammatory effect of BRE on LPS-stimulated RAW 264.7 cells is explained by JAK2 /STAT3 signaling inhibition, leading to the activation of pro-inflammatory regulators and the inhibition of anti- inflammatory regulators, rather than through a general cytotoxicity mechanism. To the best of our knowledge, this is the first study that demonstrated the use of BRE as a JAK2 or STAT3 inhibitor, which induces anti-inflammatory effects.
Fig. 9.
Schematic illustration of the inhibitory effect of black radish extract (BRE) in lipopolysaccharides (LPS)-stimulated RAW 264.7 cells. BRE may be beneficial in treating inflammation through its selective immunomodulatory effects, which may be mediated by Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) inhibition and nuclear factor erythroid-2-related factor 2 (NRF2)/heme oxygenase-1 (HO-1) signal transduction pathway activation, respectively. The arrows represent the activation, whiles the T-shaped arrows indicate inhibition. ATF4, activating transcription factor 4; IL, interleukin; NO, nitric oxide; iNOS, inducible NO synthase; KEAP1, Kelch-like epichlorohydrin-associated protein 1; MAPK, mitogen-activated protein kinase; P, phosphorylation; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor alpha.
ACKNOWLEDGEMENTS
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of agriculture, food and Rural affairs (MAFRA) (Grant number: 316006-05).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Adachi M, Uchida Y, Shimokawa K. Effect of various inhibitors on ethylene-enhanced degreening of radish (Raphanus sativus L.) cotyledons. J Jpn Soc Hortic Sci. 1998;67:849–855. doi: 10.2503/jjshs.67.849. [DOI] [Google Scholar]
- Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Ahmed ST, Ivashkiv LB. Inhibition of IL-6 and IL-10 signaling and Stat activation by inflammatory and stress pathways. J Immunol. 2000;165:5227–5237. doi: 10.4049/jimmunol.165.9.5227. [DOI] [PubMed] [Google Scholar]
- Ahn M, Kim J, Bang H, Moon J, Kim GO, Shin T. Hepatoprotective effects of allyl isothiocyanate against carbon tetrachloride-induced hepatotoxicity in rat. Chem Biol Interact. 2016;254:102–108. doi: 10.1016/j.cbi.2016.05.037. [DOI] [PubMed] [Google Scholar]
- Ahn M, Koh RK, Kim GO, Shin T. Aqueous extract of purple Bordeaux radish, Raphanus sativus L. ameliorates ethanol-induced gastric injury in rats. Orient Pharm Exp Med. 2013;13:247–252. doi: 10.1007/s13596-013-0131-5. [DOI] [Google Scholar]
- Alqasoumi S, Al-Yahya M, Al-Howiriny T, Rafatullah S. Gastroprotective effect of radish "Raphanus sativus" L. On experimental gastric ulcer models in rats. FARMACIA. 2008;2:204–214. [Google Scholar]
- Asghari MH, Hobbenaghi R, Nazarizadeh A, Mikaili P. Hydro-alcoholic extract of Raphanus sativus L. var niger attenuates bleomycin-induced pulmonary fibrosis via decreasing transforming growth factor b1 level. Res Pharm Sci. 2015;10:429–435. [PMC free article] [PubMed] [Google Scholar]
- Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: A review. Int J Cancer. 2016;138:2570–2578. doi: 10.1002/ijc.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihani SA. Radish (Raphanus sativus) and diabetes. Nutrients. 2017;9:1014. doi: 10.3390/nu9091014. https://doi.org/10.3390/nu9091014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevi SS, Mangamoori LN, Gowda BB. Polyphenolics profile and antioxidant properties of Raphanus sativus L. Nat Prod Res. 2012;26:557–563. doi: 10.1080/14786419.2010.521884. [DOI] [PubMed] [Google Scholar]
- Beevi SS, Narasu ML, Gowda BB. Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Foods Hum Nutr. 2010;65:8–17. doi: 10.1007/s11130-009-0148-6. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- Berenbaum F. Proinflammatory cytokines, prostaglandins, and the chondrocyte: mechanisms of intracellular activation. Joint Bone Spine. 2000;67:561–564. doi: 10.1016/S1297-319X(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Castro-Torres IG, De la O-Arciniega M, Gallegos-Estudillo J, Naranjo-Rodríguez EB, Domínguez-Ortíz MÁ. Raphanus sativus L. var niger as a source of phytochemicals for the prevention of cholesterol gallstones. Phytother Res. 2014;28:167–171. doi: 10.1002/ptr.4964. [DOI] [PubMed] [Google Scholar]
- Chen CC, Wang JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol Pharmacol. 1999;55:481–488. [PubMed] [Google Scholar]
- Chun J, Choi RJ, Khan S, Lee DS, Kim YC, Nam YJ, et al. Alantolactone suppresses inducible nitric oxide synthase and cyclooxygenase-2 expression by down-regulating NF-κB, MAPK and AP-1 via the MyD88 signaling pathway in LPS-activated RAW 264.7 cells. Int Immunopharmacol. 2012;14:375–383. doi: 10.1016/j.intimp.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Duarte N, Coelho IC, Patarrão RS, Almeida JI, Penha-Gonçalves C, Macedo MP. How inflammation impinges on NAFLD: a role for Kupffer cells. BioMed Res Int. 2015;2015:984578. doi: 10.1155/2015/984578. https://doi.org/10.1155/2015/984578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ediage EN, Di Mavungu JD, Scippo ML, Schneider YJ, Larondelle Y, Callebaut A, et al. Screening, identification and quantification of glucosinolates in black radish (Raphanus sativus L. niger) based dietary supplements using liquid chromatography coupled with a photodiode array and liquid chromatography-mass spectrometry. J Chromatogr A. 2011;1218:4395–4405. doi: 10.1016/j.chroma.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Elinav E, Nowarski R, Thaiss C, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Evans M, Paterson E, Barnes DM. An open label pilot study to evaluate the efficacy of Spanish black radish on the induction of phase I and phase II enzymes in healthy male subjects. BMC Complement Altern Med. 2014;14:475. doi: 10.1186/1472-6882-14-475. https://doi.org/10. 1186/1472-6882-14-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferramosca A, Di Giacomo M, Zara V. Antioxidant dietary approach in treatment of fatty liver: new insights and updates. World J Gastroenterol. 2017;23:4146–4157. doi: 10.3748/wjg.v23.i23.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes F, Paredes-Gonzalez X, Kong AN. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3, 3'-diindolylmethane: anti-oxidative stress/inflammation, nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr Pharmacol Rep. 2015;1:179–196. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong A, He M, Krishna Vanaja D, Yin P, Karnes RJ, Young CY. Phenethyl isothiocyanate inhibits STAT3 activation in prostate cancer cells. Mol Nutr Food Res. 2009;53:878–886. doi: 10.1002/mnfr.200800253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Li JR, Wang Y, Lei LS, Yu CL, Chen NN. Cyclovirobuxinum D suppresses lipopolysaccharide-induced inflammatory responses in murine macrophages in vitro by blocking JAK- STAT signaling pathway. Acta Pharmacol Sin. 2014;35:770–778. doi: 10.1038/aps.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez RMP, Perez RL. Raphanus sativus (radish): their chemistry and biology. Sci World J. 2004;4:873172. doi: 10.1100/tsw.2004.131. https://doi.org/10.1100/tsw.2004.131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- Hanlon PR, Robbins MG, Hammon LD, Barnes DM. Aqueous extract from the vegetative portion of Spanish black radish (Raphanus sativus L. var. niger) induces detoxification enzyme expression in HepG2 cells. J Funct Foods. 2009;1:356–365. doi: 10.1016/j.jff.2009.08.001. [DOI] [Google Scholar]
- Hanlon PR, Webber DM, Barnes DM. Aqueous extract from Spanish black radish (Raphanus sativus L. var. niger) induces detoxification enzymes in the HepG2 human hepatoma cell line. J Agric Food Chem. 2007;55:6439–6446. doi: 10.1021/jf070530f. [DOI] [PubMed] [Google Scholar]
- Huang C, Yang G, Jiang T, Huang K, Cao J, Qiu Z. Effects of IL-6 and AG490 on regulation of Stat3 signaling pathway and invasion of human pancreatic cancer cells in vitro. J Exp Clin Cancer Res. 2010;29:51. doi: 10.1186/1756-9966-29-51. https://doi.org/10.1186/1756-9966-29-51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li W, Su ZY, Kong AN. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkowitz SH. Inflammatory bowel disease and cancer. Gastroenterol Clin North Am. 1997;26:129–139. doi: 10.1016/S0889-8553(05)70287-9. [DOI] [PubMed] [Google Scholar]
- Jin HG, Ko HJ, Chowdhury MA, Lee DS, Woo ER. A new indole glycoside from the seeds of Raphanus sativus. Arch Pharm Res. 2016;39:755–761. doi: 10.1007/s12272-016-0758-0. [DOI] [PubMed] [Google Scholar]
- Jung YS, Kim DH, Hwang JY, Yun NY, Lee YH, Han SB, et al. Anti-inflammatory effect of tricin 4'-O-(threo-β-guaiacylglyceryl) ether, a novel flavonolignan compound isolated from Njavara on in RAW264.7 cells and in ear mice edema. Toxicol Appl Pharmacol. 2014;277:67–76. doi: 10.1016/j.taap.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Kapoor LD. Handbook of ayurvedic medicinal plants: herbal reference library. CRC Press; Boca Raton, FL, USA: 2017. p. 283. [DOI] [Google Scholar]
- Kim HG, Yoon DH, Lee WH, Han SK, Shrestha B, Kim CH, et al. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J Ethnopharmacol. 2007;114:307–315. doi: 10.1016/j.jep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Kim KH, Kim CS, Park YJ, Moon E, Choi SU, Lee JH, et al. Anti-inflammatory and antitumor phenylpropanoid sucrosides from the seeds of Raphanus sativus. Bioorg Med Chem Lett. 2015a;25:96–99. doi: 10.1016/j.bmcl.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Kim KH, Moon E, Kim SY, Choi SU, Lee JH, Lee KR. 4-Methylthio-butanyl derivatives from the seeds of Raphanus sativus and their biological evaluation on anti-inflammatory and antitumor activities. J Ethnopharmacol. 2014;151:503–508. doi: 10.1016/j.jep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Lee DH, Ahn J, Chung WJ, Jang YJ, Seong KS, et al. Pharmacokinetics, tissue distribution, and anti-lipogenic/adipogenic effects of allyl-isothiocyanate metabolites. PLoS One. 2015b;10:e0132151. doi: 10.1371/journal.pone.0132151. https://doi.org/10.1371/journal.pone.0132151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Yang KM, Kim JK, Nam BH, Lee CM, Jeong MH, et al. Effects of white radish (Raphanus sativus) enzyme extract on hepatotoxicity. Toxicol Res. 2012;28:165–172. doi: 10.5487/TR.2012.28.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lugasi A, Blázovics A, Hagymási K, Kocsis I, Kéry A. Antioxidant effect of squeezed juice from black radish (Raphanus sativus L. var niger) in alimentary hyperlipidaemia in rats. Phytother Res. 2005;19:587–591. doi: 10.1002/ptr.1655. [DOI] [PubMed] [Google Scholar]
- Lugasi A, Dworschák E, Blázovics A, Kéry Á. Antioxidant and free radical scavenging properties of squeezed juice from black radish (Raphanus sativus L. var niger) root. Phytother Res. 1998;12:502–506. doi: 10.1002/(SICI)1099-1573(199811)12:7<502::AID-PTR336>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- N'jai AU, Kemp MQ, Metzger BT, Hanlon PR, Robbins M, Czuyprynski C, et al. Spanish black radish (Raphanus sativus L. Var. niger) diet enhances clearance of DMBA and diminishes toxic effects on bone marrow progenitor cells. Nutr Cancer. 2012;64:1038–1048. doi: 10.1080/01635581.2012.714831. [DOI] [PubMed] [Google Scholar]
- Nehrash AK. Report I. The antimicrobial activity of extracts and essential oil from cultivated and wild radish. J Microbiol Kiev. 1961;23:32–37. [Google Scholar]
- Park HJ, Song M. Leaves of Raphanus sativus L. shows anti-inflammatory activity in LPS-stimulated macrophages via suppression of COX-2 and iNOS expression. Prev Nutr Food Sci. 2017;22:50–55. doi: 10.3746/pnf.2017.22.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah-Abbès JB, Abbès S, Ouanes Z, Houas Z, Abdel-Wahhab MA, Bacha H, et al. Tunisian radish extract (Raphanus sativus) enhances the antioxidant status and protects against oxidative stress induced by zearalenone in Balb/c mice. J Appl Toxicol. 2008;28:6–14. doi: 10.1002/jat.1240. [DOI] [PubMed] [Google Scholar]
- Scholl C, Eshelman BD, Barnes DM, Hanlon PR. Raphasatin is a more potent inducer of the detoxification enzymes than its degradation products. J Food Sci. 2011;76:C504–C511. doi: 10.1111/j.1750-3841.2011.02078.x. [DOI] [PubMed] [Google Scholar]
- Selmi C, Generali E, Massarotti M, Bianchi G, Sciré CA. New treatments for inflammatory rheumatic disease. Immunol Res. 2014;60:277–288. doi: 10.1007/s12026-014-8565-5. [DOI] [PubMed] [Google Scholar]
- Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo L, et al. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-κB and JNK/AP-1 activation. Int Immunopharmacol. 2009;9:1042–1048. doi: 10.1016/j.intimp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Shay J, Elbaz HA, Lee I, Zielske SP, Malek MH, Hüttemann M. Molecular mechanisms and therapeutic effects of (—)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid Med Cell Longev. 2015;2015:181260. doi: 10.1155/2015/181260. https://doi.org/10.1155/2015/181260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Ahn M, Kim GO, Park SU. Biological activity of various radish species. Orient Pharm Exp Med. 2015;15:105–111. doi: 10.1007/s13596-015-0183-9. [DOI] [Google Scholar]
- Shukla S, Chatterji S, Mehta S, Rai PK, Singh RK, Yadav DK, et al. Antidiabetic effect of Raphanus sativus root juice. Pharm Biol. 2011;49:32–37. doi: 10.3109/13880209.2010.493178. [DOI] [PubMed] [Google Scholar]
- Sipos P, Hagymási K, Lugasi A, Fehér E, Blázovics A. Effects of black radish root (Raphanus sativus L. var niger) on the colon mucosa in rats fed a fat rich diet. Phytother Res. 2002;16:677–679. doi: 10.1002/ptr.950. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Muroi R, Kobayashi-Hattori K, Uda Y, Oishi Y, Takita T. Differing effects of water-soluble and fat-soluble extracts from Japanese radish (Raphanus sativus) sprouts on carbohydrate and lipid metabolism in normal and streptozotocin- induced diabetic rats. J Nutr Sci Vitaminol. 2007;53:261–266. doi: 10.3177/jnsv.53.261. [DOI] [PubMed] [Google Scholar]
- Tyagi A, Agarwal C, Dwyer-Nield LD, Singh RP, Malkinson AM, Agarwal R. Silibinin modulates TNF-α and IFN-γ mediated signaling to regulate COX2 and iNOS expression in tumorigenic mouse lung epithelial LM2 cells. Mol Carcinog. 2012;51:832–842. doi: 10.1002/mc.20851. [DOI] [PubMed] [Google Scholar]
- Wang LG, Chiao JW. Prostate cancer chemopreventive activity of phenethyl isothiocyanate through epigenetic regulation (review) Int J Oncol. 2010;37:533–539. doi: 10.3892/ijo_00000702. [DOI] [PubMed] [Google Scholar]
- Yang DJ, Chang YY, Lin HW, Chen YC, Hsu SH, Lin JT. Inhibitory effect of litchi (Litchi chinensis Sonn.) flower on lipopolysaccharide-induced expression of proinflammatory mediators in RAW264.7 cells through NF-κB, ERK, and JAK2/STAT3 inactivation. J Agric Food Chem. 2014;62:3458–3465. doi: 10.1021/jf5003705. [DOI] [PubMed] [Google Scholar]
- Yoon WJ, Ham YM, Kim KN, Park SY, Lee NH, Hyun CG, et al. Anti-inflammatory activity of brown alga Dictyota dichotoma in murine macrophage RAW 264.7 cells. J Med Plants Res. 2009;3:1–8. [Google Scholar]
- Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol. 2016;54:28–41. doi: 10.1016/j.semcdb.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]