Abstract
Silkworm pupae (Bombyx mori) is an edible insect that has been reported to contain high-quality proteins, lipids, minerals, and vitamins, and to possess high antioxidant activity. However, there have been no studies on the neuroprotective effects of silkworm pupae. Therefore, we investigated a water extract of silkworm pupae with protease (WSP) as a functional and therapeutic candidate for neurodegenerative disorders. First, we evaluated the effect of WSP on oxidative stress-induced mouse hippocampal neuronal cells (HT-22 cells). Cell viability diminished by addition of glutamate but was significantly recovered by WSP treatment. Furthermore, WSP significantly decreased the release of lactate dehydrogenase and generation of intracellular reactive oxygen species in oxidative stress-induced cells. In addition, in scopolamine-treated mice, WSP attenuated memory impairment, as demonstrated in the Morris water maze and passive avoidance tests, indicating protection of neuronal cells against oxidative damage. Moreover, WSP prevented scopolamine-induced increases in acetylcholinesterase activity and decreases in choline-acetyltransferase activity. Finally, treatment with WSP enhanced the antioxidant defense system by regulating the activities of antioxidant enzymes. Overall, this study showed that WSP exerted antioxidant and memory enhancing action against oxidative stress.
Keywords: antioxidant enzymes, cholinergic nervous system, memory enhancing effect, silkworm pupae
INTRODUCTION
The incidence of neurodegenerative diseases in elderly people has gradually increased with the increase in human lifespan. Alzheimer’s disease (AD) is the most common type of neurodegenerative disease, and is caused by progressive loss of neuronal cells (Ross and Poirier, 2004; Lin and Beal, 2006). The characteristic pathological features of the central nervous system (CNS) in AD are senile plaques, neurofibrillary tangles, excessive oxidative stress, and neurotransmitter dysfunction (Kwon et al., 2010). Therefore, recovery or maintenance of cholinergic neurotransmitter levels is a key target in AD treatment and prevention (Kwon et al., 2010) because neurotransmitter disorders in the cholinergic nervous system are consistently associated with memory loss and progression of AD (Giacobini, 1990; Lee et al., 2020). The neurotransmitter acetylcholine (Ach) improves cholinergic function in AD and its release is affected by levels of acetylcholinesterase (AChE) and choline-acetyltransferase (ChAT) (Min et al., 2015; Shin et al., 2019; Baek et al., 2020). Another mechanism treatment and prevention of AD is prevention of oxidative stress in the brain (Min et al., 2015; Shin et al., 2019; Baek et al., 2020; Lee et al., 2020). An association between neuropathological processes and oxidative stress has been reported, whereby oxidative stress leads to neuronal damage and cell death since the brain is more sensitive to oxidative stress than other organs (Calabrese et al., 2004; Baek and Kim, 2020). In neuronal cells of the brain, oxidative stress induces accumulation of polyglutamate aggregates, protein misfolding, changes in intracellular mechanisms, membrane damage, mitochondrial dysfunction, and programmed cell death (Lin and Beal, 2006; Li et al., 2008). Many studies have investigated the benefits of enhancing the activities of antioxidant enzymes against generation of excessive oxidative stress (Min et al., 2015; Shin et al., 2019; Baek et al., 2020; Lee et al., 2020).
Recently, edible insects have been proposed as a new source of food in response to rapid growth of the world’s population, the rising cost of animal protein, growing concerns over environmental issues, and the increasing demand for protein (Mishyna et al., 2020). The intake of insects has become a new trend in food science since around 2013 when the Food and Agriculture Organization of the United Nations published a document entitled “Edible Insects: an Alternative of Nutritional, Functional and Bioactive Compounds” (da Silva Lucas et al., 2020). Edible insects are good sources of proteins and lipids (Mishyna et al., 2020), the protein content varying among and within insect orders from 13% to 77%, with protein digestibility of 76% to 98% (Ramos-Elorduy et al., 1997; Akhtar and Isman, 2018). Moreover, insects can be a source of vitamins, such as riboflavin, pantothenic acid, and biotin and, in some cases, folic acid (Rumpold and Schlüter, 2013; Nowak et al., 2016). Therefore, the number of studies related to the use of insects as a food source has increased. Silkworm pupae (Bombyx mori, a type of edible insect part) are a typical Asian food consumed since ancient times due to the high protein content (Altomare et al., 2020). In addition, many studies have reported that silkworm pupae oil attenuates acute liver injury, the protein extracts exert anticancer activity, and the hydrolysates possess angiotensin converting enzyme inhibitory and antioxidant activities (Yu et al., 2008; Chukiatsiri et al., 2020; Long et al., 2020). Nevertheless, little is known about the neuroprotective effects of silkworm pupae.
In this study, we investigated whether silkworm pupae with protease water extracts (WSP) protect hippocampal neuronal cells against oxidative stress in both in vivo and in vitro models. We report the neuroprotective effects of WSP in cholinergic nervous system regulation and antioxidant enzyme activation. Such effects of WSP may provide basic information for the application of edible insects as preventive supplements or therapeutic agents for use in neurodegenerative diseases.
MATERIALS AND METHODS
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), 10× phosphate-buffered saline, 10× Tris-buffered saline, and Hank’s balanced salt solution (HBSS) were purchased from Welgene, Inc. (Deagu, Korea). Fetal bovine serum (FBS), 2’,7’-dichlorodihydroflorescein diacetate (H2DCFDA), and 2’,7’-dichlorofluorescein were purchased from Invitrogen, Inc. (Carlsbad, CA, USA). Trypsin-ethylenediaminetetraacetic acid (EDTA) and antibiotics (penicillin and streptomycin) were obtained from Gibco BRL (Grand Island, NY, USA). Sodium phosphate-monobasic, sodium phosphate-dibasic, acetic acid, potassium phosphate-monobasic, and potassium phosphate-dibasic were purchased from Daejung, Ltd. (Chungwon, Korea). 4,6-Dihydroxy-2-mercaptopyrimidine was purchased from Alfa Aesar (Seoul, Korea). EDTA was purchased from Junsei Chemical Co, Ltd. (Tokyo, Japan). L-glutamic acid, dimethyl sulfoxide, sodium dodecyl sulfate (SDS), β-nicotinamide adenine dinucleotide 2’-phosphate reduced tetrasodium salt hydrate, 4,6-dihydroxy-2-mercaptopyrimidine (DTNB), glutathione reductase (GR, type Ⅲ from baker’s yeast), and L-glutathione reduced (GSH) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Preparation of WSP
Silkworm pupae was heated, desiccated to remove fat, and the dried silkworm pupae powdered. The powdered silkworm pupae was then hydrolyzed, heated with water nine times, and treated with 0.5% protease P enzyme (Amano Enzyme USA Co., Ltd., Elgin, IL, USA) of the powder. The mixture was reacted at 50°C for 5 h, and then inactivated at 95°C for 30 min. After enzyme decomposition, mixture was centrifuged at 8,000 rpm for 20 min and the supernatant was concentrated in a rotary evaporator (N11, Yamato Co., Tokyo, Japan) under reduced pressure to produce WSP. Samples were stored at −20°C until use. We investigated the proximate composition in WSP by following AOAC (1990). WSP was composed of 12.14% carbohydrate, 0.22% crude fat, 12.15% crude ash, 4.73% moisture, and 70.76% crude protein.
Determination of peptide molecular weight distribution (MWD)
WSP (0.1 g to 1 g) was diluted with 100 mL distilled water for 30 min by sonification and the solution was analyzed using a high-performance liquid chromatography (HPLC) autosampler (Agilent Technologies, Inc., Wilmington, DE, USA) with column (Ultrahydrogel 120, 7.8 mm×30 m, Waters Co., Milford, MA, USA). The mobile phase was H2O:CAN:TFA (55:45:0.1). The injection volume was 10 mL and the column temperature was 30 °C. The detector was monitored to a fluorescence of 220 nm and a flow rate of 0.5 mL/min.
Measurement of total amino acid composition
WSP (0.1 g to 1 g) was diluted with 100 mL distilled water, derived with o-phthaldehyde reagent (Agilent Technologies, Inc.), and analyzed using an HPLC autosampler (Agilent Technologies, Inc.) by performing a pre-column and Zorbax Eclipse-AAA column (150×4.6 mm, 3.5 mm, Agilent Technologies, Inc.) at 40°C. NaH2PO4 40 mM (pH 7.8) was used as mobile phase A and ACN:MeOH:H2O (45:45:10) was used as mobile phase B. Analysis of the conditions of the mobile phase gradient is shown in Table 1. Samples (10 mL) were monitored in the detector with a fluorescence of 340 nm to 450 nm and a flow rate of 2.0 mL/min.
Table 1.
Mobile phase gradient conditions used to determine the amino acid composition in the water extract of silkworm pupae
| Time (min) | A (%) | B (%) |
|---|---|---|
| 0.00 | 100 | 0 |
| 1.9 | 100 | 0 |
| 18.1 | 43 | 57 |
| 18.6 | 0 | 100 |
| 22.3 | 0 | 100 |
| 23.2 | 100 | 0 |
| 26 | 100 | 0 |
HT-22 cell culture
HT-22 cells, a mouse hippocampus-derived cell line, were obtained from Medifron (Seoul, Korea) and maintained in DMEM with 10% FBS, 100 units/mL penicillin, and 100 mg/mL streptomycin at 37°C with 5% CO2.
Measurement of cell viability
Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kits, according to the manufacturer’s instructions MTT assay kit, USB Corporation, Cleveland, OH, USA). HT-22 cells were seeded into 48-well plates at a density of 1×104 cells per well for 24 h, with each well containing 500 μL cell culture medium. Cells were treated with glutamate (5 mM) in the presence or absence of WSP (10∼100 μg/mL). After 11 h incubation, MTT solution (0.2 mg/mL) was added to each well, and the plates were incubated at 37°C for 30 min. Then, the absorbance was read using an Emax Precision microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 550 nm. The percentages of surviving cells were determined relative to control cells.
Measurement of lactate dehydrogenase (LDH) leakage
LDH leakage was assessed using a LDH assay kit, according to the manufacturer’s instructions (Roche Diagnostics, Indianapolis, IN, USA). Briefly, HT-22 cells were preincubated with or without WSP (0∼100 μg/mL) for 30 min in 48-well plates before challenge with glutamate. Then, the medium of each well was removed and placed into 96-well plates, and the kit reagents was added and left to react for 10 min. The absorbance was then read using an Emax Precision microplate reader (Molecular Devices) at 490 nm and the percentage of surviving cells determined relative to control cells.
Measurement of intracellular reactive oxygen species (ROS) levels
The level of intracellular ROS was measured using DCFDA as described previously (Baek and Kim, 2020). Twelve hours following glutamate treatment, cells were stained with 10 μM DCFDA in HBSS for 30 min in the dark, and the fluorescence was monitored using a microplate reader (DTX 880 Multimode Detector, Beckman Coulter, Inc., Fullerton, CA, USA) with an excitation wavelength of 485 nm and an emission wavelength of 525 nm.
Animals
Male 6-week-old ICR mice (Shin et al., 2019; Baek et al., 2020; Lee et al., 2020) were purchased from Raonbio, Co. (Daejeon, Korea). Mice were randomly divided into six groups of eight mice and each maintained at a constant temperature and humidity (25.0±2.0°C and 55± 10%) with a 12-h light/dark cycle. The mice were given free access to food and water. Briefly, 2 mg/kg scopolamine was dissolved in saline and injected intraperitoneally daily for two weeks. WSP (dissolved in saline) and tacrine (9-amino-1,2,3,4-tetrahydroacridine hydrochloride hydrate; dissolved in 0.9% NaCl) were administered orally on a daily basis 30 min after the scopolamine injection for two weeks. All mouse care protocols and experiments were approved by the Animal Experimental Care Center of Chungnam National University (Daejeon, Korea) and the experiments were conducted in compliance with the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Registration No. CNU-00859).
Tissue preparation and collection
Mice were euthanized after treatment, and their brains were removed. The brains were homogenized with homogenization buffer (20 mM phosphate buffer containing 0.1 M KCl, 1 mM EDTA, and 0.5% Triton X-100) and centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was used for biochemical assays (AChE, ChAT, and antioxidant enzymes) and the homogenate was used for lipid peroxidation concentration assays. The supernatants were stored as −70°C until use.
Morris water maze tests
The Morris water maze tests were conducted to evaluate cognitive changes arising from WSP treatment. Black circular pools (150 cm×60 cm in depth and diameter) was used, as described in previous studies (Morris, 1984; Baek et al., 2020). The pool was filled with water at 23± 1°C and split four parts. Morris water maze tests were conducted on six days before the end of the experiment. One hour before the behavior test, the mice were orally administered WSP (200∼400 mg/kg, orally) before intraperitoneal injection of scopolamine.
Passive avoidance tests
Passive avoidance was evaluated in a light and dark chamber (Jung Bio & Plant Co., Ltd., Seoul, Korea) to investigate the cognitive ability of mice, as previously reported (Lorenzini et al., 1997; Baek et al., 2020). WSP and tacrine were injected orally, followed by scopolamine 1 h later. Passive avoidance tests were conducted 30 min later after scopolamine administration.
Protein determination
Protein concentration was measured with Bio-Rad protein assay dye reagent (Bradford, 1976). Supernatants from mouse brains were mixed with 1:5 diluted dye reagent and incubated at room temperature for 10 min. The absorbances was measured by a spectrophotometer at 595 nm. Bovine serum albumin was used to generate a standard curve in the range of 0.2 to 1.5 mg/mL.
Measurement of AChE and ChAT activities
AChE activity was measured by a colorimetric reaction with an acetylthiocholine iodide substrate, as previously reported (Min et al., 2015). Brain supernatants were mixed with 0.1 M phosphate buffer, 10 mM Ellman’s reagent, and 75 mM acetylthiochloride iodide. The absorbances of the mixtures were measured by a spectrophotometer at 410 nm for 5 min, at 1 min intervals. ChAT activity was analyzed using a kit (Elabscience, Houston, TX, USA), following the manufacturer’s instructions.
Measurement of lipid peroxide concentration in brain
Brain tissues were maintained in an ice box and homogenized with 50 mM sodium phosphate buffer using a tissue homogenizer with a Teflon pestle (DuPont, Wilmington, DE, USA), as previously reported (Min et al., 2015; Baek et al., 2020). Briefly, homogenates (1 mL) were mixed with 1 mL of 8.1% SDS, 2 mL of 20% acetic acid, and 1 mL of 0.75% thiobarbituric acid (TBA) and boiled for 30 min. The absorbances of the malondialdehyde (MDA)-TBA adduct formed in the supernatants were measured at 532 nm, as previously described. The MDA values were compared to a standard curve that was prepared with tetramethoxypropane, and expressed in TBA values.
Measurement of antioxidant enzyme activities in brain
GSH, GR, and glutathione peroxidase (GPx) concentrations in the brain homogenates were measured as previously reported (Min et al., 2015; Baek et al., 2020). GSH, GR, and GPx activities were measured at absorbances of 412 nm, 340 nm, and 340 nm, respectively, using a spectrophotometer.
Statistical analyses
Results are expressed as mean±standard error of the mean (SEM). All statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) software. One-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test and Duncan’s test were used to analyze differences between the groups. P<0.05 and P<0.01 were considered statistically significant.
RESULTS
Peptide MWD of WSP
In previous studies, it has been reported that some peptides and amino acid possess antioxidant effects (Shevyakova et al., 2009; Zhao et al., 2011; Kim et al., 2014). Therefore, we analyzed the peptide MWD of WSP because WSP is mostly composed of crude protein (70.76 %). The MWD of WSP was mainly in the range of 200∼ 500 kDa (about 37.4%) (Table 2). Peptides in range of 500∼1,000 kDa and <200 kDa accounted for approximately 21.7% and 18.6%, respectively (Table 2).
Table 2.
The peptide molecular weight distribution (MWD) of water extract from silkworm pupae
| MWD of peptide (kDa) | Percentage |
|---|---|
| <200 | 18.63±0.61 |
| 200∼500 | 37.43±0.46 |
| 500∼1,000 | 21.72±0.29 |
| 1,000∼1,500 | 10.15±0.27 |
| 1,500∼2,000 | 5.44±0.12 |
| 2,000∼2,500 | 1.97±0.02 |
| 2,500∼3,000 | 1.35±0.02 |
| >3,000 | 3.30±0.02 |
All data are mean±SEM.
In addition, WSP contained 136.06 mg/g amino acids (Table 3). The highest amino acid content in WSP was proline (40.09 mg/g), followed by arginine (14.9 mg/g), methionine (13.9 mg/g), tyrosine (12.9 mg/g), and histidine (12.0 mg/g).
Table 3.
The amino acid composition of water extract from silkworm pupae
| Amino acid | (mg/g) |
|---|---|
| Aspartate | 1.09±0.04 |
| Glutamate | 8.57±0.22 |
| Serine | 2.67±0.06 |
| Histidine | 12.00±0.04 |
| Tyrosine | 12.88±0.15 |
| Methionine | 13.86±0.15 |
| Phenylalanine | 3.26±0.06 |
| Isoleucine | 5.08±0.06 |
| Glycine | 1.91±0.06 |
| Threonine | 2.11±0.04 |
| Arginine | 14.90±0.18 |
| Alanine | 8.38±0.14 |
| Leucine | 4.94±0.06 |
| Lysine | 4.32±0.06 |
| Proline | 40.09±0.66 |
| Total | 136.06±1.08 |
All data are mean±SEM.
Neuroprotective effect of WSP on glutamate-induced oxidative stress in HT-22 cells
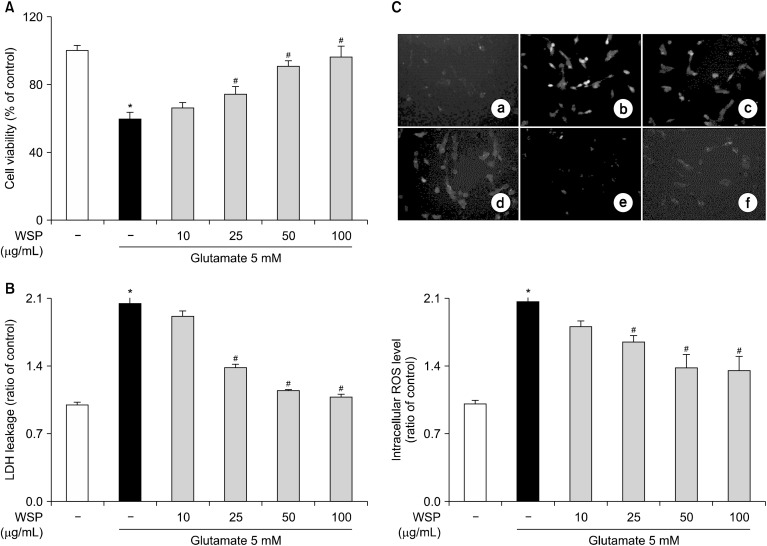
We treated HT-22 cells with glutamate to induce cytotoxicity (Fukui et al., 2009) and investigated the effect of WSP on glutamate-induced cytotoxicity in hippocampal neuronal cells. Compared with the vehicle control, treatment with glutamate reduced cell viability by about 60% (Fig. 1A). However, when HT-22 cells were incubated with WSP before glutamate treatment, WSP increased cell viability in a dose dependent manner. Furthermore, treatment with 100 μg/mL WSP recovered cell viability to the level of the vehicle control. Next, we investigated LDH leakage in glutamate-induced HT-22 cells. In glutamate-treated cells, LDH leakage was twice that of the vehicle control. In contrast, pretreatment with WSP recovered the release of LDH in glutamate-induced HT-22 cells in a dose-dependent manner (Fig. 1B). In addition, treatment with glutamate increased generation of intracellular ROS in HT-22 cells (Fig. 1C). However, pretreatment with WSP reduced the ROS levels in glutamate-treated cells in a dose dependent manner. WSP at 100 μg/mL was sufficient to restore LDH and ROS levels to those of the control group. These results suggest that WSP exerts neuroprotective action by suppressing oxidative stress in a hippocampal neuronal cell line.
Fig. 1.
Neuroprotective action of water extract from silkworm pupae (WSP) on glutamate-induced cytotoxicity in HT-22 cells. (A) Cell viability, (B) lactate dehydrogenase (LDH) leakage, and (C) intracellular reactive oxygen species (ROS) level in ⓐ vehicle control, ⓑ cells treated with glutamate 5 mM, ⓒ glutamate 5 mM+WSP 10 μg/mL, ⓓ glutamate 5 mM+WSP 25 μg/mL, ⓔ glutamate 5 mM+WSP 50 μg/mL, and ⓕ glutamate 5 mM+WSP 100 μg/mL. HT-22 cells were seeded at 1×104 cells/well for 24 h. Then, the cells were treated with glutamate in the presence or absence of varying concentrations (0∼100 μg/mL) of WSP for 12 h. Cell viability, LDH leakage and intracellular ROS levels were estimated as described in the Materials and Methods. All data are presented as mean±SEM. Results were calculated as a percentage of the values obtained for control cells. *P<0.05 vs vehicle control and #P<0.05 vs glutamate-treated group.
Effect of WSP on spatial learning ability in the Morris water maze test
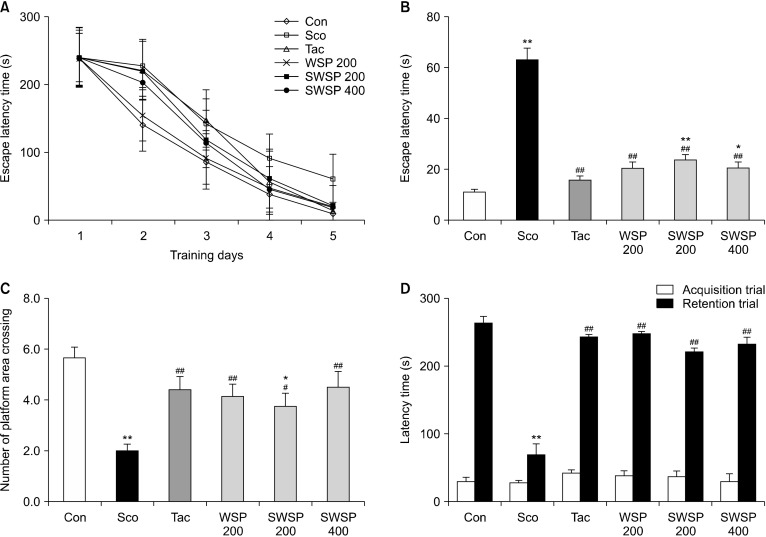
Considering the in vitro results, we investigated the neuroprotective action of WSP in scopolamine-treated mice. To confirm the neuroprotective action of WSP in an in vivo system, mice were orally administered WSP (200 or 400 mg/kg) before the scopolamine challenge. Scopolamine has been reported to induce oxidative stress in brain tissue (Marcus et al., 1998). Tacrine, a scopolamine and Ach inhibitor, was one of the first medicinal materials widely used to alleviate symptoms of Alzheimer’s disease (Saxena et al., 2008). To investigate the effect of WSP on spatial learning ability of scopolamine-treated mice, the Morris water maze test was performed sequentially for five days. The effects of WSP (200∼400 mg/kg) on the escape latency time (ELT) and the number of platform area crossings (NPAC) were confirmed (Fig. 2A∼2C). The ELT of scopolamine-alone-treated mice was significantly longer than that of the non-treated control mice for five days (P<0.05) (Fig. 2A and 2B), showing successful induction of memory impairment in the dementia animal model. Oral administration of WSP significantly recovered the ELT extended by scopolamine in a dose-dependent manner. On the final day of the experiment, the vehicle control mice took an average of 11.8 s to find the platform, whereas the scopolamine-treated mice took an average of 62.8 s. Oral administration of WSP reversed the ELT extended by scopolamine. The ELTs in scopolamine-induced mice administered with 200 and 400 mg/kg WSP were 23.5 and 20.4 s, respectively. In addition, the NPAC was examined by removing the platform to investigate spatial learning memory. The NPAC in the scopolamine-treated mice was two, which was significantly decreased compared to that in the vehicle control (Fig. 2C). However, the NPAC in scopolamine-treated mice administered 200 or 400 mg/kg WSP was 3.8 and 4.5 times, respectively, demonstrating dose-dependent improvements. Moreover, the NPAC following administration of 400 mg/kg WSP was similar to that following the administration of tacrine to scopolamine-induced memory impaired mice. Thus, WPS may improve scopolamine-induced memory impairment in mice.
Fig. 2.
Effect of water extract from silkworm pupae (WSP) on scopolamine-treated mice in behavior tests. Scopolamine (2 mg/kg) dissolved in saline was injected intraperitoneally daily for two weeks. WSP (0∼400 mg/kg) or tacrine was dissolved in saline or 0.9% NaCl, respectively. WSP and tacrine were administered orally daily 30 min after the scopolamine injection for two weeks. After 1 h, mice were tested in the Morris water maze or the passive avoidance test (acquisition trial). (A) Escape latency time during experimental days, (B) the latency time on the last experimental day, and (C) the number of platform area crossings in the Morris water maze. (D) Latency time in the passive avoidance test. Con, non-treated group; Sco, scopolamine 2 mg/kg-treated group; Tac, scopolamine 2 mg/kg+tacrine 10 mg/kg-treated group; WSP 200, WSP 200 mg/kg-treated group; SWSP 200, scopolamine 2 mg/kg+WSP 200 mg/kg-treated group; SWSP 400, scopolamine 2 mg/kg+WSP 400 mg/kg-treated group. All data are mean±SEM. *P<0.05 and **P<0.01 vs vehicle control group. #P<0.05 and ##P<0.01 vs scopolamine-treated group.
Effect of WSP on memory ability in the passive avoidance test
Passive avoidance tests were carried out two days prior to the end of the experiment. To investigate the effect of WSP on memory and learning ability, acquisition trial times (AT), and retention trial times (RT) were evaluated (Fig. 2D). AT values were similar among all groups. However, RT values in scopolamine-treated mice were an average of 69.9 s, which was significantly lower than the vehicle control mice. Administration of WSP (200∼400 mg/kg) improved memory impairment in scopolamine-injected mice. The findings suggest that WSP could alleviate memory impairment induced by scopolamine-induced amnesia in mice.
Effect of WSP on activities of ChAT and AChE
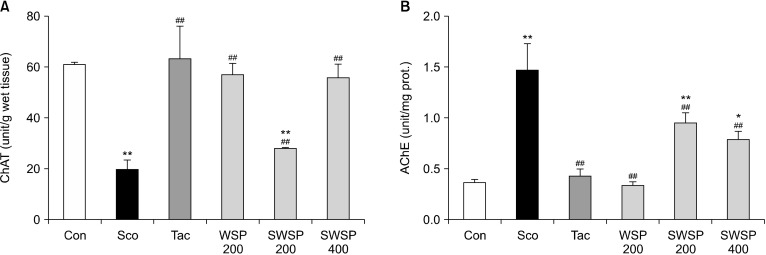
Ach, an important neurotransmitter, plays a role in learning and memory formation (Min et al., 2015). ChAT and AChE, which synthesize and hydrolyze Ach, respectively, are related to neurotransmitters in the brain. Changes in the activities of these enzymes affect behavior tests in memory-impaired animals (Min et al., 2015; Baek et al., 2020). Therefore, we measured the activities of these enzymes to elucidate the underlying mechanisms of WSP in ameliorating scopolamine-induced amnesia. The activity of ChAT in memory impairment-induced mice decreased below those in the vehicle control mice (P<0.05) (Fig. 3A). Oral administration of WSP proved ChAT activity in scopolamine-treated mice in a dose-dependent manner. In addition, AChE activity in the brains of scopolamine-treated mice was highest compared with the other groups (Fig. 3B). Treatment with WSP partially but significantly restored AChE activity, which was increased by scopolamine injection. Hence, treatment with WSP partially prevented memory impairment due to scopolamine-induced decreases in ChAT and increases in AChE. These results indicate that the neuroprotective action of WSP on scopolamine-induced cholinergic dysfunction may be regulated via the cholinergic nervous system.
Fig. 3.
Effect of water extract from silkworm pupae (WSP) on activities of (A) choline-acetyltransferase (ChAT) and (B) acetylcholinesterase (AChE) in whole brains of scopolamine-induced memory impaired mice. Scopolamine (2 mg/kg) dissolved in saline was injected intraperitoneally daily for two weeks. WSP (0∼400 mg/kg) or tacrine was dissolved in saline or 0.9% NaCl, respectively. WSP and tacrine were administered orally daily 30 min after the scopolamine injection for two weeks. The mice were euthanized after the experimental trial, and their brains were removed and homogenized. Con, non-treated group; Sco, scopolamine 2 mg/kg-treated group; Tac, scopolamine 2 mg/kg+tacrine 10 mg/kg-treated group; WSP 200, WSP 200 mg/kg-treated group; SWSP 200, scopolamine 2 mg/kg+WSP 200 mg/kg-treated group; SWSP 400, scopolamine 2 mg/kg+WSP 400 mg/kg-treated group. All data are mean±SEM. *P<0.05 and **P<0.01 vs vehicle control group. ##P<0.01 vs scopolamine-treated group.
Effect of WSP on the antioxidant defense system
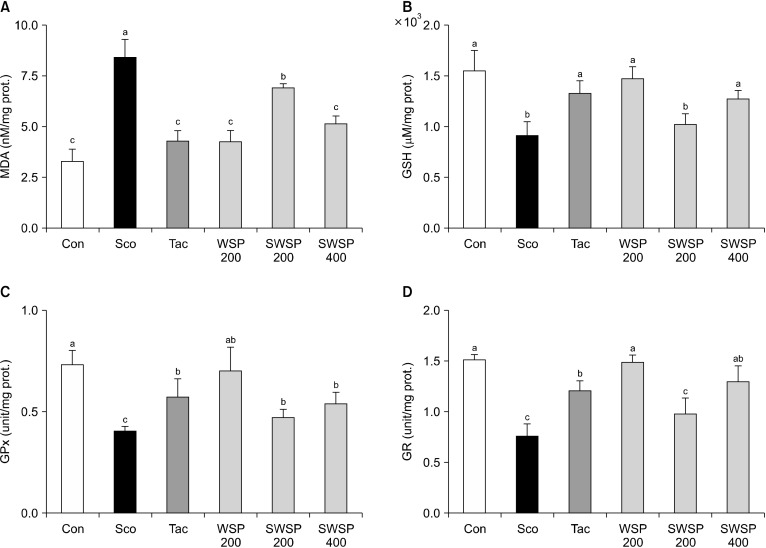
To further investigate the mechanism involved in preventing memory impairment, the effects of WSP on lipid peroxide content and antioxidant enzyme (e.g., GSH, GPx, and GR) activities were assessed in the brains of mice (Min et al., 2015; Lee et al., 2020). First, lipid peroxidation, which is produced by ROS, was evaluated. Levels of MDA, a product of lipid peroxidation, was significantly elevated in scopolamine-treated mice compared to vehicle control mice (P<0.05) (Fig. 4A). In contrast, oral administration of WSP prevented the formation of MDA in scopolamine-induced mice in a dose-dependent manner. In addition, injection of scopolamine reduced the levels of the antioxidative enzymes GSH, GPX, and GR compared with vehicle control mice, whereas pretreatment with WSP reversed the reduction in total GSH, GPX, and GR levels in a dose-dependent manner (Fig. 4B∼4D). Moreover, the neuroprotective effect of WSP (400 mg/kg) was similar to that of tacrine. Therefore, these results suggest that WSP could protect the brain against scopolamine-induced oxidative stress by regulating the activities of antioxidant enzymes.
Fig. 4.
Effect of water extract from silkworm pupae (WSP) on lipid peroxide contents and antioxidant enzyme activities in whole brains of scopolamine-induced memory impaired mice. Scopolamine (2 mg/kg) dissolved in saline was injected intraperitoneally dailys for two weeks. WSP (0∼400 mg/kg) or tacrine was dissolved in saline or 0.9% NaCl, respectively. WSP and tacrine were administered orally daily 30 min after the scopolamine injection for two weeks. The mice were euthanized after treatment, and their brains were removed and homogenized. The levels of (A) malondialdehyde (MDA), (B) glutathione (GSH), (C) glutathione peroxidase (GPx), and (D) glutamate reductase (GR) activities in mice. Con, non-treated group; Sco, scopolamine 2 mg/kg-treated group; Tac, scopolamine 2 mg/kg+tacrine 10 mg/kg-treated group; WSP 200, WSP 200 mg/kg-treated group; SWSP 200, scopolamine 2 mg/kg+WSP 200 mg/kg-treated group; SWSP 400, scopolamine 2 mg/kg+WSP 400 mg/kg-treated group. All data are mean±SEM. Different letters (a-c) above the bars are significantly different by Duncan’s multiple range test at P<0.05.
DISCUSSION
Edible insects have received much attention as sustainable and alternative food sources to meet future demands since they contain high-quality protein and long-chain polyunsaturated fatty acids (Yang et al., 2006; Mishyna et al., 2020; Pyo et al., 2020). In addition, edible insects are a source of vitamins, including riboflavin, pantothenic acid, and biotin and, in some cases, folic acid (Rumpold and Schlüter, 2013; Nowak et al., 2016). Silkworm, a type of edible insect, is very efficient at producing silk. After collection of silk from its cocoon, the silkworm pupae are discarded (Felix et al., 2020). However, in Korea, silkworm pupae are used as food and in traditional medicine because they are rich in essential amino acids, protein, lipids, and minerals (Pemberton, 1999; Yang et al., 2006). Moreover, a protein-rich fraction of the larvae of silkworm pupae and aqueous protein extracts from silkworm pupae have been shown to possess high antioxidant activities (Takechi et al., 2014; Chatsuwan et al., 2018). Nevertheless, whether edible insects exert neuroprotective effects has not previously been investigated. In previous reports, we showed a connection between in vitro and in vivo studies, which evaluated the ability to prevent oxidative stress in glutamate-treated hippocampal neuronal cells (HT-22 cells) and a scopolamine-treated ICR mouse model (Shin et al., 2019; Baek and Kim, 2020; Baek et al., 2020; Lee et al., 2020). Therefore, in the present study we investigated whether a WSP can protect neuronal cells against oxidative stress in both in vivo and in vitro models.
As the incidence of neurodegenerative diseases has increased in elderly people, there have been many studies on the abilities of neuroprotective compounds in preventing neurodegenerative diseases (Min et al., 2015; Shin et al., 2019; Baek et al., 2020; Lee et al., 2020). Most of those studies have aimed to repress the effects of oxidative stress in the brain because AD is caused by excessive oxidative stress (Kwon et al., 2010). Indeed, excessive accumulation of oxidative stress leads to cell damage and death, such as through apoptosis and necrosis (Nunomura et al., 2006; Du et al., 2015). Scopolamine is a muscarinic cholinergic antagonist because it blocks muscarinic Ach receptors (Broks et al., 1988). In addition, scopolamine has been reported to induce oxidative stress in brain tissues, further inducing lipid peroxidation and decreasing levels of antioxidant enzymes (Marcus et al., 1998). Furthermore, the deficit in cognition caused by repeated administration of scopolamine was found to be accompanied by a reduction in hippocampal volume as well as increased lipid peroxidation associated with AD (Yamada et al., 2008). Herein, scopolamine was used to induce transient memory impairment and cognitive deficits in the normal brains of mice (Marcus et al., 1988).
One mechanism for the neuroprotective action of WSP is related to the cholinergic nervous system, a crucial factor in neuronal cells. Cognitive deficits and memory impairment in neuropsychiatric disorders, such as AD, have been closely related to cholinergic deficits (Marcus et al., 1998). These memory disorders have previously assessed by evaluating the activities of markers such as Ach, AChE, and ChAT (Gil-Bea et al., 2005; Min et al., 2015; Baek et al., 2020). In scopolamine-treated mice, the cholinergic neurotransmitter is disabled, resulting in increased levels of AChE, an enzyme that breaks down Ach, and decreased levels of ChAT, an ACh-synthesizing enzyme (Min et al., 2015). WSP at concentrations of 200 ∼400 mg/mL improved spatial learning and memory ability of scopolamine-induced memory deficit-mice in two behavior tests. Cholinergic disorders cause dysregulation of cholinergic signaling in attention, spatial memory, and cognitive processes, which play important roles in encoding and memory processing (Maurer and Williams, 2017). Since the end of cholinergic transmission is affected by levels of ACh, AChE-inhibiting drugs including tacrine, donepezil hydrochloride, and rivastigmine have been used to improve symptoms in dementia patients (Holden and Kelly, 2002). For this reason, we used tacrine as both an inhibitor of AChE and a positive control because tacrine has been used to improve cognitive dysfunction and memory impairment by reversing the activity of cholinergic enzymes in the brain (Min et al., 2015; Lee et al., 2019; Shin et al., 2019; Baek et al., 2020). In our present study, administration of WSP reversed the increases in AChE activity and decreases in ChAT activity in the brains of scopolamine-treated mice. Therefore, the cholinergic-regulating effect of WSP on scopolamine-induced cholinergic dysfunction may be associated with memory and learning ability.
The other mechanism for the neuroprotective effect of WSP was associated with production of antioxidant enzymes such as GSH, GPx, and GR. GSH is the brain’s major antioxidant system and plays a critical role against oxidative stress (Dringen, 2000). GSH exists in either a reduced GSH form or an oxidized GSH state (GSSG); it is the reduced state that detoxifies ROS (Gawryluk et al., 2011). Accumulation of oxidative stress represents an imbalance between the oxidant and antioxidant defense systems, whereby production of free radicals outweighs the system’s ability to detoxify reactive intermediates (Halliwell, 2001). Hydrogen peroxide is detoxified by GSH through GPx, leading to formation of GSSG, which is recycled back to GSH by GR (Meister, 1988). Maintaining appropriate levels of GSH, GPx, and GR is essential for preventing oxidative damage in the brain, which causes AD. MDA, a marker of lipid peroxidation, is generated by excessive oxidative stress (Meister, 1988; Gawryluk et al., 2011). Thus, deficiencies in antioxidant enzymes also result in oxidative stress, lipid peroxidation, and neuronal cell death associated with memory impairment. The present study showed that scopolamine elevated lipid peroxidation in mouse brains, whereas it reduced the activities of antioxidant enzymes, including GSH, GPx, and GR. In contrast, administration of WSP significantly improved the antioxidant defense system by suppressing lipid peroxidation and activating antioxidant enzymes against scopolamine-induced oxidative stress. Consistent with our results, in a previous study, increases in oxidative stress were related to the activities of ChAT and GPx (Min et al., 2015).
We also investigated the peptide MWD of WSP, as previous studies have reported that some peptides or amino acids contain antioxidant amino acid residues and play roles as antioxidants (Shevyakova et al., 2009; Zhao et al., 2011; Kim et al., 2014). We therefore predicted that the effect of active compounds in WSP on neuroprotective and antioxidant action may be related to the contained peptide. In support of this suggestion, our unpublished data indicates that WSP at 0.1 % in distilled water increases DPPH radical scavenging activity (about 66.0%).
In conclusion, the results of the present study suggest that WSP exert neuroprotective effects by activating both the cholinergic system and antioxidant enzymes. Therefore, WSP may possess therapeutic and preventive potential against neurodegenerative diseases. Nevertheless, further study is required to confirm the identify of and analyze the main biochemical peptide fragment in WSP and the mechanisms of action, using protein markers related to memory. Moreover, studies on other animal models should be conducted to assess the effects WSP on memory improvement for preventive and clinical purposes.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Akhtar Y, Isman MB. Insects as an alternative protein source. In: Yada RY, editor. Proteins in Food Processing. 2nd ed. Woodhead Publishing; Duxford, UK: 2018. pp. 263–288. [DOI] [Google Scholar]
- Altomare AA, Baron G, Aldini G, Carini M, D'Amato A. Silkworm pupae as source of high-value edible proteins and of bioactive peptides. Food Sci Nutr. 2020;8:2652–2661. doi: 10.1002/fsn3.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC. Association of Official Analytical Chemists. 15th ed. Washington, DC, USA: 1990. Official methods of analysis; pp. 8–35. [Google Scholar]
- Baek SY, Kim MR. Neuroprotective effect of carotenoid-rich Enteromorpha prolifera extract via TrkB/Akt pathway against oxidative stress in hippocampal neuronal cells. Mar Drugs. 2020;18:372. doi: 10.3390/md18070372. https://doi.org/10.3390/md18070372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SY, Li FY, Kim DH, Kim SJ, Kim MR. Enteromorpha prolifera extract improves memory in scopolamine-treated mice via downregulating amyloid-β expression and upregulating BDNF/TrkB pathway. Antioxidants. 2020;9:620. doi: 10.3390/antiox9070620. https://doi.org/10. 3390/antiox9070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broks P, Preston GC, Traub M, Poppleton P, Ward C, Stahl SM. Modelling dementia: effects of scopolamine on memory and attention. Neuropsychologia. 1988;26:685–700. doi: 10.1016/0028-3932(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Boyd-Kimball D, Scapagnini G, Butterfield DA. Nitric oxide and cellular stress response in brain aging and neurodegenerative disorders: the role of vitagenes. In Vivo. 2004;18:245–267. [PubMed] [Google Scholar]
- Chatsuwan N, Puechkamut Y, Pinsirodom P. Characterization, functionality and antioxidant activity of water-soluble proteins extracted from Bombyx mori Linn. Curr Appl Sci Technol. 2018;18:83–96. doi: 10.1155/2018/6528312. [DOI] [Google Scholar]
- Chukiatsiri S, Siriwong S, Thumanu K. Pupae protein extracts exert anticancer effects by downregulating the expression of IL-6, IL-1β and TNF-α through biomolecular changes in human breast cancer cells. Biomed Pharmacother. 2020;128:110278. doi: 10.1016/j.biopha.2020.110278. https://doi.org/10.1016/j.biopha.2020.110278 . [DOI] [PubMed] [Google Scholar]
- de Oliveira LM, da Rocha M, Prenticea C , da Silva Lucas AJ. Edible insects: an alternative of nutritional, functional and bioactive compounds. Food Chem. 2020;311:126022. doi: 10.1016/j.foodchem.2019.126022. https://doi.org/10.1016/j.foodchem.2019.126022 . [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/S0301-0082(99)00060-X. [DOI] [PubMed] [Google Scholar]
- Du CN, Min AY, Kim HJ, Shin SK, Yu HN, Sohn EJ, et al. Deer bone extract prevents against scopolamine-induced memory impairment in mice. J Med Food. 2015;18:157–165. doi: 10.1089/jmf.2014.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix M, Bascon C, Cermeño M, FitzGerald RJ, de la Fuente J, Carrera-Sánchez C. Interfacial/foaming properties and antioxidant activity of a silkworm (Bombyx mori) pupae protein concentrate. Food Hydrocoll. 2020;103:105645. doi: 10.1016/j.foodhyd.2020.105645. https://doi.org/10.1016/j.foodhyd.2020.105645 . [DOI] [Google Scholar]
- Fukui M, Song JH, Choi J, Choi HJ, Zhu BT. Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol. 2009;617:1–11. doi: 10.1016/j.ejphar.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- Giacobini E. The cholinergic system in Alzheimer disease. In: Aquilonius SM, Gillberg PG, editors. Progress in Brain Research. Vol 84. Elsevier B.V.; Amsterdam, Netherlands: 1990. pp. 321–332. [DOI] [PubMed] [Google Scholar]
- Gil-Bea FJ, García-Alloza M, Domínguez J, Marcos B, Ramírez MJ. Evaluation of cholinergic markers in Alzheimer's disease and in a model of cholinergic deficit. Neurosci Lett. 2005;375:37–41. doi: 10.1016/j.neulet.2004.10.062. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- Holden M, Kelly C. Use of cholinesterase inhibitors in dementia. Adv Psychiatr Treat. 2002;8:89–96. doi: 10.1192/apt.8.2.89. [DOI] [Google Scholar]
- Kim CR, Jeon HL, Shin SK, Kim HJ, Ahn CW, Jung SU, et al. Neuroprotective action of deer bone extract against glutamate or Aβ1-42-induced oxidative stress in mouse hippocampal cells. J Med Food. 2014;17:226–235. doi: 10.1089/jmf.2013.2951. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Lee HK, Kim JA, Hong SI, Kim HC, Jo TH, et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur J Pharmacol. 2010;649:210–217. doi: 10.1016/j.ejphar.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Lee BD, Yoo JM, Baek SY, Li FY, Sok DE, Kim MR. 3,3'-Diindolylmethane promotes BDNF and antioxidant enzyme formation via TrkB/Akt pathway activation for neuroprotection against oxidative stress-induced apoptosis in hippocampal neuronal cells. Antioxidants. 2020;9:3. doi: 10.3390/antiox9010003. https://doi.org/10.3390/antiox9010003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li H, Li XJ. Intracellular degradation of misfolded proteins in polyglutamine neurodegenerative diseases. Brain Res Rev. 2008;59:245–252. doi: 10.1016/j.brainresrev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Long X, Song J, Zhao X, Zhang Y, Wang H, Liu X, et al. Silkworm pupa oil attenuates acetaminophen-induced acute liver injury by inhibiting oxidative stress-mediated NF-κB signaling. Food Sci Nutr. 2020;8:237–245. doi: 10.1002/fsn3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzini CGA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of ventral hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response memory trace. Brain Res. 1997;768:242–248. doi: 10.1016/S0006-8993(97)00651-3. [DOI] [PubMed] [Google Scholar]
- Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Exp Neurol. 1998;150:40–44. doi: 10.1006/exnr.1997.6750. [DOI] [PubMed] [Google Scholar]
- Maurer SV, Williams CL. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front Immunol. 2017;8:1489. doi: 10.3389/fimmu.2017.01489. https://doi.org/10.3389/fimmu.2017.01489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. doi: 10.1016/S0021-9258(19)77815-6. [DOI] [PubMed] [Google Scholar]
- Min AY, Doo CN, Son EJ, Sung NY, Lee KJ, Sok DE, et al. N-palmitoyl serotonin alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of BDNF and p-CREB in mice. Chem Biol Interact. 2015;242:153–162. doi: 10.1016/j.cbi.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Mishyna M, Chen J, Benjamin O. Sensory attributes of edible insects and insect-based foods-future outlooks for enhancing consumer appeal. Trends Food Sci Technol. 2020;95:141–148. doi: 10.1016/j.tifs.2019.11.016. [DOI] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nowak V, Persijn D, Rittenschober D, Charrondiere UR. Review of food composition data for edible insects. Food Chem. 2016;193:39–46. doi: 10.1016/j.foodchem.2014.10.114. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- Pemberton RW. Insects and other arthropods used as drugs in Korean traditional medicine. J Ethnopharmacol. 1999;65:207–216. doi: 10.1016/S0378-8741(98)00209-8. [DOI] [PubMed] [Google Scholar]
- Pyo SJ, Kang DG, Jung C, Sohn HY. Anti-thrombotic, anti-oxidant and haemolysis activities of six edible insect species. Foods. 2020;9:401. doi: 10.3390/foods9040401. https://doi.org/10.3390/foods9040401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Elorduy J, Moreno JMP, Prado EE, Perez MA, Otero JL, Guevara OL. Nutritional value of edible insects from the state of Oaxaca, Mexico. J Food Compos Anal. 1997;10:142–157. doi: 10.1006/jfca.1997.0530. [DOI] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10:S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Rumpold BA, Schlüter OK. Nutritional composition and safety aspects of edible insects. Mol Nutr Food Res. 2013;57:802–823. doi: 10.1002/mnfr.201200735. [DOI] [PubMed] [Google Scholar]
- Saxena G, Singh SP, Agrawal R, Nath C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur J Pharmacol. 2008;581:283–289. doi: 10.1016/j.ejphar.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Shevyakova NI, Bakulina EA, Kuznetsov VV. Proline antioxidant role in the common ice plant subjected to salinity and paraquat treatment inducing oxidative stress. Russ J Plant Physiol. 2009;56:663–669. doi: 10.1134/S1021443709050124. [DOI] [Google Scholar]
- Shin SK, Yoo JM, Li FY, Baek SY, Kim MR. Mulberry fruit improves memory in scopolamine-treated mice: role of cholinergic function, antioxidant system, and TrkB/Akt signaling. Nutr Neurosci. 2019 doi: 10.1080/1028415X.2019.1696613. https://doi.org/10.1080/1028415X.2019.1696613 . Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Takechi T, Wada R, Fukuda T, Harada K, Takamura H. Antioxidant activities of two sericin proteins extracted from cocoon of silkworm (Bombyx mori) measured by DPPH, chemiluminescence, ORAC and ESR methods. Biomed Rep. 2014;2:364–369. doi: 10.3892/br.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Chiba T, Sasabe J, Terashita K, Aiso S, Matsuoka M. Nasal colivelin treatment ameliorates memory impairment related to Alzheimer's disease. Neuropsychopharmacology. 2008;33:2020–2032. doi: 10.1038/sj.npp.1301591. [DOI] [PubMed] [Google Scholar]
- Yang LF, Siriamornpun S, Li D. Polyunsaturated fatty acid content of edible insects in Thailand. J Food Lipids. 2006;13:277–285. doi: 10.1111/j.1745-4522.2006.00051.x. [DOI] [Google Scholar]
- Yu JS, Woo KS, Hwang IG, Lee YR, Kang TS, Jeong HS. ACE inhibitory and antioxidative activities of silkworm larvae (Bombyx mori) hydrolysate. J Korean Soc Food Sci Nutr. 2008;37:136–140. doi: 10.3746/jkfn.2008.37.2.136. [DOI] [Google Scholar]
- Zhao L, Luo YC, Wang CT, Ji BP. Antioxidant activity of protein hydrolysates from aqueous extract of velvet antler (Cervus elaphus) as influenced by molecular weight and enzymes. Nat Prod Commun. 2011;6:1683–1688. doi: 10.1177/1934578X1100601130. [DOI] [PubMed] [Google Scholar]