Abstract
A healthy diet has long been indicated to be protective against Alzheimer’s diseases (AD). We carried out a systematic review and meta-analysis of published observational studies to explore the relationship between healthy and unhealthy diets and risk of ADs. We screened PubMed, Scopus, Web of Sciences, Google Scholar, Science Direct, and Embase, and screened manually to identify relevant articles published in English and non-English until Jun 2020. We classified the studied dietary patterns into two groups: healthy and unhealthy diets. The pooled weighted mean difference and 95% confidence interval (95% CI) was used to analyze the data using a random-effects model. The data were extracted manually and the preferred reporting items for systematic review and meta-analysis checklist was used to appraise the risk of bias and quality of data. Of the 1,813 articles identified, 21 met the inclusion criteria and were included in the quantitative analysis. A healthy diet was related to a lower risk of AD [odds ratio (OR): 0.45, 95% CI: 0.23 to 0.86, I2=99.7%; n=17 studies]. Moreover, high adherence to an unhealthy diet was not associated with increased risk of AD (OR: 0.99, 95% CI: 0.98 to 0.99, I2=0.0%; n=6 studies). However, the etiology of AD is uncertain and it is difficult draw conclusions about dietary healthy patterns. We concluded that adherence to a healthy diet is associated with a lower risk of AD, but were unable to find evidence that an unhealthy diet increases the risk of AD.
Keywords: Alzheimer’s disease, dietary pattern, healthy diet, meta-analysis, systematic review
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease associated with progressive memory and cognitive impairment (Niu et al., 2017). Although the etiology of AD is unclear, evidence suggests that damage to nerve cells involved in cognitive functions may promote AD development (Alzheimer’s Association, 2015). Extracellular amyloid-β (Aβ) plaques accumulate in the brain, which cause synapse dysfunction and results cell death (Querol-Vilaseca et al., 2019). In 2017, approximately 6.08 million persons in the United States were suffering from clinical or mild cognitive impairment due to AD; this figure is estimated to increase to 15 million by 2060 (Brookmeyer et al., 2018). There is an increasing prevalence of AD worldwide, associated with the rapidly aging population (Niu et al., 2017).
Diet can have a protective role on cognitive function in human aging. In animal models dietary restriction without malnutrition extends life span and decreases the incidence of neurodegenerative disorders by protecting against brain atrophy (Cox et al., 2019; Pifferi and Aujard, 2019). Many studies have evaluated one type of diet and risk of AD (Scarmeas et al., 2006b; Gu et al., 2010a; Eskelinen et al., 2011; Gu et al., 2011; Gardener et al., 2012; Liu et al., 2016). A healthy diet is reflected by adequate consumption of whole grains, nuts, legumes, fruits, vegetables, low-fat dairy, poultry, and fish, and by low consumption of red meat, processed meat, and added sugar foods (Pasdar et al., 2019; Pasdar et al., 2020a). For example, Mediterranean diets (MDs), which emphasizes higher intakes of whole grains, vegetables, nuts, fish, olive, olive oil, and lower intakes of red meat and processed meat, and wine as the main source of alcohol, are considered healthy diets (Scarmeas et al., 2006b; Pasdar et al., 2020b). In contrast, unhealthy diets, such as Western diets, are high in saturated fat, trans-fatty acids, refined grains, red meat, processed meat, and added sugar, promote obesity and many chronic diseases, such as cardiovascular disease, diabetes, depression, and cancer (Pasdar et al., 2019; Moludi et al., 2020).
Dietary components such as vitamin C, vitamin E, flavonoids, and unsaturated fatty acids can delay the degeneration of neurons and nerve tissue (Scarmeas et al., 2006a). Some epidemiological studies have stated protective role of diet on incidence of AD (Scarmeas et al., 2006a; Scarmeas et al., 2006b; Devore et al., 2009; Gu et al., 2010b; Morris et al., 2015). However, no studies have yet categorized or summarized this evidence. In our recent systematic review, we qualitatively summarized the association between diet and risk of AD. These results indicated that high adherence to a healthy diet can decrease the incidence of AD and that an unhealthy diet has neurodegenerative effects (Samadi et al., 2019).
To the best of our knowledge, there no meta-analyses and systematic reviews have quantified the association between diet and risk of AD. Therefore, we carried out a meta-analysis and systematic review of the association between diet and risk of AD, expanding on the results of our previous meta-analysis by exploring the association between a healthy diet and risk of AD.
MATERIALS AND METHODS
Design
This systematic review and meta-analysis was carried out by following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendations (Moher et al., 2015) until Jun 2020. The meta-analysis was registered in PROSPERO (ID: CRD42020171361).
Search strategy
We designed the systematic search terms using related medical subject headings (MeSH) and non-MeSH keywords (Table 1). Two independent researchers searched online electronic databases, including PubMed, Scopus, Web of Sciences, Google Scholar, Science Direct, and Embase. In addition, we manually searched the references of the relevant review articles to identify any additional relevant articles. Also, we did not filter articles by publication time, location of the study or language.
Table 1.
Medical subject headings (MeSH) and non-MeSH keywords used to search relevant publications
| Concept 1 | “diet” OR “food” OR “dietary” OR “dietary pattern” OR “food pattern” |
| Concept 2 | “Alzheimer’s disease” OR “Alzheimer’s ’s disease” |
| Concept 3 | “Cohort studies” OR “Prospective studies” OR “Retrospective studies” OR “Cross sectional” OR “Case control” OR “Cohort” OR “Prospective” OR “Retrospective” |
The combination of keywords was used to search online databases as follows: (“concept 1” AND “concept 2” AND “concept 3”).
Inclusion and exclusion criteria
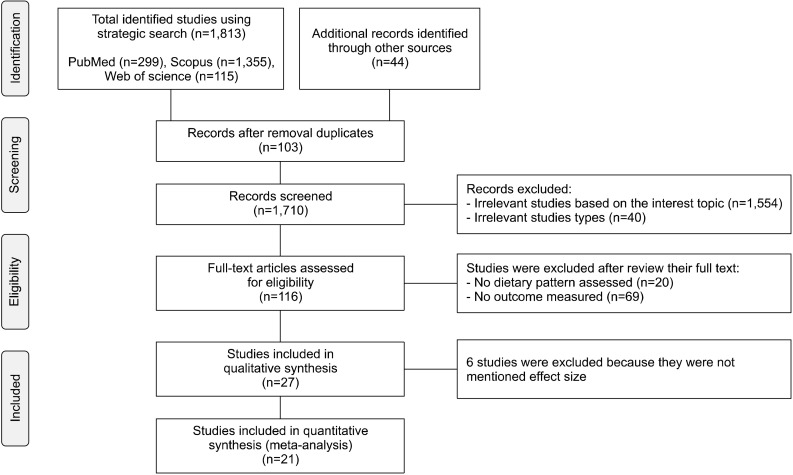
Eligible studies were performed on adults and evaluated the relationship between dietary patterns and risk of AD. We considered all observational studies, including case- control, cross-sectional, prospective, and retrospective studies. Interventional studies were not included to this study because the duration of exposure is generally short. Overall, we identified 1,813 articles in the initial search. We excluded duplicate studies (n=103) and the remaining studies were screened based on topic, following which a further 1,554 studies were excluded. An additional, 40 studies were excluded based on study type. We reviewed the full texts of the remaining 116 studies. Of these, 89 studies were excluded: 20 did not assess dietary patterns and 69 did not evaluate the outcome “Alzheimer’s disease”. In addition, 6 studies were excluded as they did not determine an effect size (Gustaw-Rothenberg, 2009; Mosconi et al., 2014; Berti et al., 2015; Liu et al., 2016; Pase et al., 2017; Calil et al., 2018). In total, 21 eligible studies were identified (Fig. 1).
Fig. 1.
Flow diagram of the literature search.
Quality assessment
The strengthening the reporting of observational studies in epidemiology (STROBE) guidelines was used to evaluate the quality of the studies. STROBE guidelines include a checklist of 22 items that are considered essential for good reporting of observational studies, including for the title, abstract, introduction, methods, results, and discussion. Of these, 18 items were common across all observational studies and the other items were specific for case-control, cohort, and cross-sectional studies (PLoS Medicine at http://www.plosmedicine.org/). This list is liberally available on the PLoS medicine website, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/ (Vandenbroucke et al., 2007). Evidence on the STROBE Initiative is accessible at www.strobe-statement.org. The scores of the methods sections of the included studies are presented in Table 2.
Table 2.
Quality assessment of studies included in the systematic review and meta-analysis based on strengthening the reporting of observational studies in epidemiology statement
| Morris et al. (2003a) | Morris et al. (2003b) | Scarmeas et al. (2006a) | Scarmeas et al. (2006b) | Laitinen et al. (2006) | Luchsinger et al. (2007) | Scarmeas et al. (2009a) | Devore et al. (2009) | Gu et al. (2010a) | Gu et al. (2010b) | Gu et al. (2011) | ||||||||||
| Study design | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Study location and date | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Study sittings: periods of recruitment, exposure, and follow-up | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Sampling method and adequate sample size | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Inclusion and exclusion criteria and demographic characteristics | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Data sources-measurements | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Outcome data | * | * | * | * | * | * | * | * | * | * | * | |||||||||
| Eskelinen et al. (2011) | Gardener et al. (2012) | Ozawa et al. (2013) | Morris et al. (2015) | Olsson et al. (2015) | Morris et al. (2016) | Ylilauri et al. (2017) | Vassilaki et al. (2018) | Fernando et al. (2018) | Hill et al. (2018) | |||||||||||
| Study design | * | * | * | * | * | * | * | * | * | * | ||||||||||
| Study location and date | * | * | * | * | * | * | * | * | * | * | ||||||||||
| Study sittings: periods of recruitment, exposure, and follow-up | * | * | * | * | * | * | * | * | * | * | ||||||||||
| Sampling method and adequate sample size | * | * | * | * | * | * | * | * | * | * | ||||||||||
| Inclusion and exclusion criteria and demographic characteristics | * | * | * | * | * | * | * | * | * | * | ||||||||||
| Data sources- measurements | * | * | * | * | * | * | * | * | * | * | ||||||||||
| Outcome data | * | * | * | * | * | * | * | * | * | * | ||||||||||
Data extraction and quality assessment
Data were extracted by two independent investigators (SM and JM) using a data collection checklist. Any disagreement during quality assessment and data extraction were discussed and resolved accordingly. This checklist included first author name, study population, study year, study design, study country, sample size, age, dietary assessment instruments, and type of dietary pattern.
Definition of adherence to a healthy diet
Based on the reviewed studies, we categorized dietary patterns as healthy and unhealthy. Healthy dietary patterns were related to intake of fiber, vegetables, fruits, sea foods, poultry, low fat dairy, and whole grains, and a high intake of antioxidants and polyunsaturated and monounsaturated fatty acids (PUFA and MUFA, respectively). Unhealthy dietary patterns included a high intake of sugar-sweetened beverages, processed and red meat, refined grains, and fatty foods (Lyros et al., 2014). By these definitions, 16 of the identified articles evaluated healthy dietary patterns and five evaluated unhealthy dietary patterns. All data are presented in the RESULTS.
Statistical analysis
Data analysis was performed using STATA (ver. 15.1) software (StataCorp LLC, College Station, TX, USA). Differences between results were considered significant at P< 0.05. The relationship between dietary patterns and risk of AD was expressed in odds ratios (ORs). To combine the results of the different studies, OR logarithms were employed for each study, and I2 indexes and Cochran Q tests were used to measure study heterogeneities (Higgins and Green, 2019). Furthermore, we used a popular method by Zhang and Yu (1998) for converting risk ratios to OR. The I2 index can be categorized as slight heterogeneity (less than 25%), moderate heterogeneity (25% to 75%), and intense heterogeneity (more than 75%). Since the fixed effects model is used for low heterogeneity and the random effects model is used for high heterogeneity, we used a random effects model (I2=99.7%). In addition, we used Egger’s regression asymmetry test and visual inspection of funnel plots to evaluate potential publication bias.
RESULTS
Literature search
Of the 1,813 articles identified, 21 met the eligibility criteria and were involved in the quantitative analysis. Details of the meta-analysis selection process are shown in Fig. 1.
Study characteristics
Sample sizes ranged from 115 and 5,395, with studies had durations of follow-up ranging from 3.7 to 18 years. The outcome of interest was risk of AD. Only six of the 21 studies were carried out in Mediterranean populations. The other cohorts included populations in the US and Northern Europe, and a cohort of Europeans living in Australia. The overall number of subjects in the included studies was 50,506. All but four prospective studies used a food frequency questionnaire (FFQ) for dietary assessment; these 4 studies used food records and dietary habit questionnaires (Laitinen et al., 2006; Eskelinen et al., 2011; Olsson et al., 2015; Ylilauri et al., 2017). General study characteristics are summarized in Table 3 and 4. Based on the STROBE statement, all studies included in this meta-analysis were of adequate quality (Table 2).
Table 3.
Characteristics of studies that assessed healthy dietary patterns and risk of Alzheimer’s diseases
| Study name | Study design | Country | Sample size | Age (years) | Dietary pattern assessment tools | Kind of diet | Diet components | |
|---|---|---|---|---|---|---|---|---|
| Morris et al. (2003b) | Chicago health and aging project | Prospective study | USA | 815 (101 men and 713 women) | ≥65 | FFQ | Seafood rich diet | Tuna sandwich, fish sticks, cakes; or sandwich, fresh fish as a main dish, and shrimp, lobster, or crab |
| Morris et al. (2003a) | Dietary vegetable oil | Dairy products, removal fat, or poultry skin, specified brand name products for cereals, margarine, oil, and multivitamins | ||||||
| Scarmeas et al. (2006a) | WHICAP 1992 and WHICAP 1999 | Case-control | USA | 1,984 (630 men and 1,354 women) | 76.3 (mean) | FFQ | MD | Fruits, vegetables, legumes, cereals, fish, meat, and dairy products |
| Scarmeas et al. (2006b) | WHICAP 1992 and WHICAP 1999 | Prospective study | USA | 2,226 (720 men and 1,506 women) | 77.2 (mean) | FFQ | MD | Fruits, vegetables, legumes, cereals, fish, meat, and dairy products |
| Scarmeas et al. (2009a) | WHICAP 1992 and WHICAP 1999 | Prospective study | USA | 1,880 (587 men and 1,293 women) | 77.2 (mean) | FFQ | MD | Dairy, meat, fruits, vegetables, legumes, cereals, and fish |
| Devore et al. (2009) | Ommoord cohort study | Prospective study | The Netherlands | 5,395 (2,211 men and 3,184 women) | ≥55 | FFQ | Seafood rich diet | Total fish intake and intake of different fish types (e.g., salmon) |
| Gu et al. (2010a) | WHICAP II | Prospective study | USA | 1,219 (407 men and 812 women) | ≥65 | FFQ | MD | Fruits, vegetables, legumes, cereals, fish, meat, and dairy products |
| Gu et al. (2010b) | WHICAP 1992 and WHICAP 1999 | Prospective study | USA | 2,148 (691 men and 1,457 women) | ≥65 | FFQ | MD | Higher intakes of salad dressing, nuts, fish, tomatoes, poultry, cruciferous vegetables, fruits, and dark and green leafy vegetables, and a lower intake of high fat dairy products, red meat, organ meat, and butter |
| Eskelinen et al. (2011) | The cardiovascular risk factors, aging, and dementia (CAIDE) study | Prospective study | Sweden | 525 (201 men and 324 women) | 65~79 | Dietary habits questionnaire | HEI | Beneficial components (vegetables and roots, berries, and fruits, bread, fish, coffee drinking, MUFAs, and PUFAs from milk products and spreads) and unhealthy (sausage foods, eggs, candies, sweet soft drinks, sugar lumps in coffee, salty fish, and SFAs from milk products, and spreads) |
| Gardener et al. (2012) | The Australian imaging, biomarkers, and lifestyle study of ageing (AIBL) | Prospective study | Australia | 970 (402 men and 568 women) | ≥60 | FFQ | MD | Fruits, vegetables, legumes, cereals, fish, meat, and dairy products |
| Ozawa et al. (2013) | Hisayama study | Prospective study | Japan | 1,006 (433 men and 573 women) | 60~79 | FFQ | Soy based food and diary | High intake of soybeans and soybean products, vegetables, algae, and milk and dairy products, and a low intake of rice |
| Olsson et al. (2015) | The Uppsala longitudinal study of adult men (ULSAM) | Prospective study | Sweden | 1,602 men | 60~70 | Food record | HEI | WHO dietary guidelines |
| MD | PUFA/SFA (ratio), fruits, vegetables, legumes, cereals, fish, meat, dairy products and alcohol | |||||||
| LCHP | Carbohydrate and protein intake | |||||||
| Morris et al. (2015) | Rush memory and aging project | Prospective study | USA | 923 (232 men and 691 women) | 58~98 | FFQ | MD | Fruits, vegetables, legumes, cereals, fish, meat, and dairy products |
| DASH | 7 food groups and 3 dietary components (total fat, saturated fat, and sodium) | |||||||
| MIND | Ten brain healthy food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, fish, poultry, olive oil, and wine) and 5 unhealthy food groups (red meats, butter, and stick margarine, cheese, pastries, and sweets, and fried/fast food) | |||||||
| Morris et al. (2016) | Rush memory and aging project | Cross-sectional | USA | 286 (93 men and 193 women) | 89.9 (mean) | FFQ | Seafood rich diet | Tuna sandwich; fish sticks, cakes, or sandwich; fresh fish as a main dish; and shrimp, lobster, or crab |
| DHA + EPA Food sources | ||||||||
| α-Linolenic 18:3 n-3 | ||||||||
| Vassilaki et al. (2018) | Mayo clinic study of aging (MCSA) | Prospective study | USA | 278 (155 men and 123 women) | 70~89 | FFQ | MD | Fruits, vegetables, legumes, cereals, fish, meat, and dairy products |
| Fernando et al. (2018) | The Australian imaging, biomarkers and lifestyle study of ageing (AIBL) | Cross-sectional | Australia | 541 (222 men and 319 women) | ≥60 | FFQ | High protein and high fiber | Grams per day intake of protein and fiber |
| Hill et al. (2018) | The women’s health aging project | Prospective study | Australia | 115 men | 45~55 | FFQ | MD | Whole grains, vegetables, nuts, fish, and wine as the main source of alcohol |
| low fat | Low-fat dairy products, vegetables, and unsaturated spreads |
FFQ, food frequency questionnaire; MD, Mediterranean diet; HEI, healthy eating index; LCHP, low carbohydrate high protein; DASH, dietary approaches to stop hypertension; MIND, Mediterranean-DASH intervention for neurodegenerative delay; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
Table 4.
Characteristics of studies that assessed an unhealthy dietary pattern and risk of Alzheimer’s diseases
| Study name | Study design | Country | Sample size | Age (year) | Dietary pattern assessment | Kind of diet | Diet components | |
|---|---|---|---|---|---|---|---|---|
| Morris et al. (2003a) | Chicago health and aging project | Prospective study | USA | 815 (101 men and 713 women) | ≥65 | FFQ | High fat diet and high animal fat diet | Dairy products, removal fat or poultry skin, specified brand name products for cereals, margarine, oil, and multivitamins |
| Morris et al. (2003b) | ||||||||
| Laitinen et al. (2006) | The cardiovascular risk factors, aging and incidence of dementia (CAIDE) study | Prospective study | Finland | 1,449 (549 men and 900 women) | 65∼80 | Dietary habit questionnaire | High fat diet | Milk, sour milk, eggs, coffee, tea, and sugar in tea/coffee |
| Luchsinger et al. (2007) | − | Prospective study | USA | 939 (549 men and 390 women) | ≥65 | FFQ | High glycemic diet | Carbohydrate and sugary food intake |
| Gu et al. (2011) | WHICAP 1992 and WHICAP 1999 | Prospective study | USA | 2,258 (1,526 men and 732 women) | ≥65 | FFQ | DII | Amount and type of fat, essential fatty acids, vitamins, minerals and antioxidants, glycemic index, and anti-inflammatory compounds |
| Ylilauri et al. (2017) | The Kuopio ischemic heart disease risk factor study | Prospective study | Finland | 2,497 men | 42∼60 | Food record | Dietary cholesterol | Cholesterol from all component of diet |
| Dietary cholesterol from egg intake | Cholesterol from egg | |||||||
| Hill et al. (2018) | The women’s health aging project | Prospective study | Australia | 115 women | 45∼55 | FFQ | High fat | High-fat diet loaded heavily on food groups such as processed meats, fried fish, red meats, fried potatoes, and poultry |
| Junk food | High consumption of takeaway foods, added sugar, confectionary and cakes, biscuits, and sweet pastries |
FFQ, food frequency questionnaire; DII, dietary inflammatory index.
Publication bias
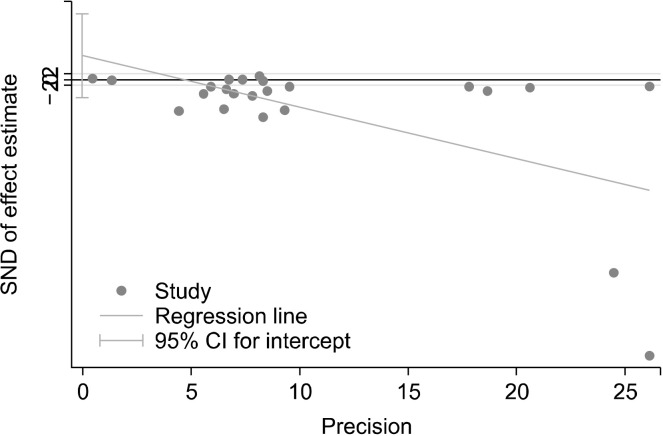
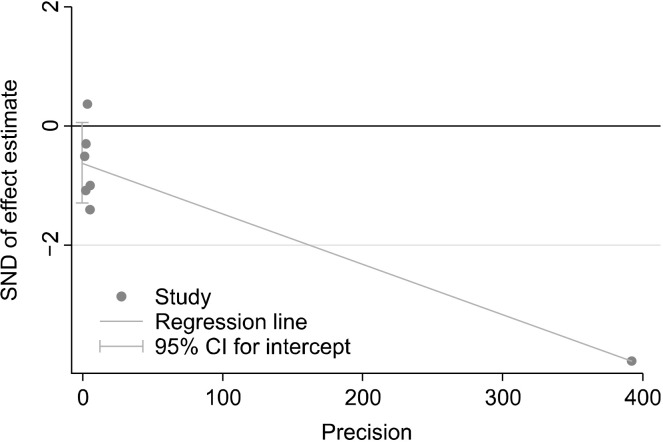
We investigated the heterogeneity of the included studies using chi-square tests. The random-effect model was used due to high heterogeneity (Higgins and Green, 2011). Egger’s linear regression investigations did not identify evidence of publication bias for the healthy (P=0.24, Fig. 2) or unhealthy (P=0.066, Fig. 3) groups.
Fig. 2.
Publication bias for articles on healthy dietary pattern and risk of Alzheimer’s diseases. SND, standard normal distribution; CI, confidence interval.
Fig. 3.
Publication bias for articles on unhealthy dietary pattern and risk of Alzheimer’s diseases. SND, standard normal distribution; CI, confidence interval.
Diet and risk of AD
In this updated systematic review and meta-analyses, data of new studies were combined with data included in former reports. Overall risk of AD was reported in 18 prospective studies, 2 cross-sectional studies, and 1 case-control study.
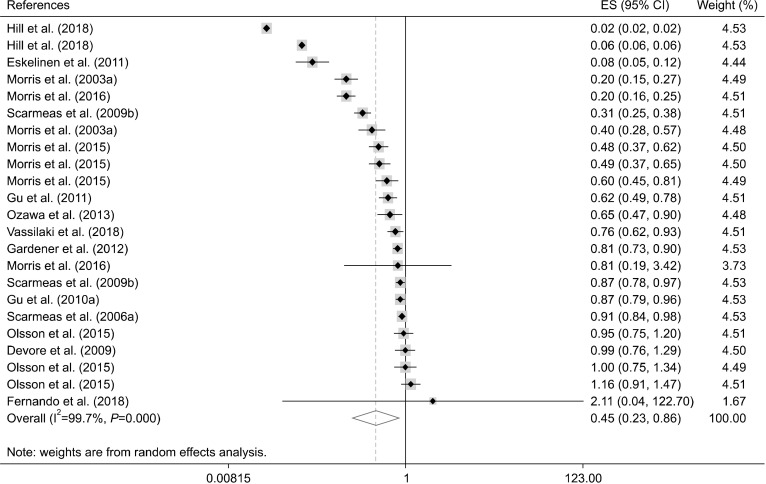
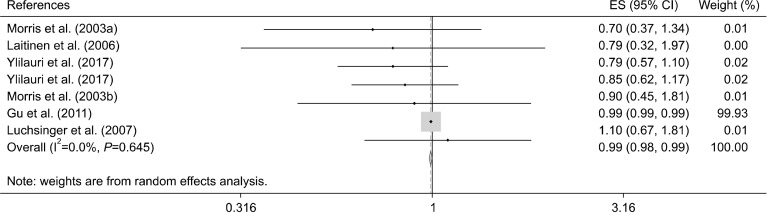
Using the random effects model, we observed that maximum adherence to a healthy diet was inversely associated with lower risk of AD [OR: 0.45, 95% confidence interval (CI): 0.23 to 0.86, I2=99.7%; n=17 studies] (Fig. 4, Table 5). Moreover, high adherence to an unhealthy dietary pattern was not associated with risk of AD (OR: 0.99, 95% CI: 0.98 to 0.99, I2=0.0%; n=6 studies) (Fig. 5, Table 6).
Fig. 4.
Forest plot of healthy dietary pattern and risk of Alzheimer’s diseases. ES, effect size; CI, confidence interval.
Table 5.
Studies that investigated the association between a healthy dietary pattern and Alzheimer’s diseases
| Kind of diet | Comparison | Effect size | Confidence interval | Outcome1) | |
|---|---|---|---|---|---|
| Morris et al. (2003b) | Seafood-rich diet | Q4 vs. Q1 | RR: 0.4 | 0.2∼0.9 | 1, 2, 3, 4, 7, 28 |
| Morris et al. (2003a) | Dietary vegetable oil | RR: 0.6 | 0.3∼1.3 | 1, 2, 3, 4, 7, 27, 28 | |
| Scarmeas et al. (2006a) | MD | Continuous | HR: 0.91 | 0.83∼0.98 | 1, 2, 3, 4, 8, 10, 12, 13, 28 |
| Scarmeas et al. (2006b) | MD | T3 vs. T1 | OR: 0.31 | 0.16∼0.58 | 1, 2, 3, 4, 8, 10, 11, 12, 13, 24, 25, 28 |
| Devore et al. (2009) | Seafood-rich diet | T3 vs. T1 | HR: 0.99 | 0.76∼1.29 | 1, 2, 3, 4, 21, 22, 23 |
| Scarmeas et al. (2009a) | MD | Continuous | HR: 0.87 | 0.77∼0.99 | 1, 2, 3, 4, 5, 8, 10, 12, 14, 16, 17, 18, 28 |
| Gu et al. (2010a) | MD | Continuous | HR: 0.87 | 0.78∼0.97 | − |
| Gu et al. (2010b) | MD | T3 vs. T1 | HR: 0.62 | 0.43∼0.89 | 1,2, 4, 11, 12, 17, 28 |
| Eskelinen et al. (2011) | HEI | High adherence vs. low | OR: 0.08 | 0.01∼0.89 | 1, 2, 4, 12 |
| Gardener et al. (2012) | MD | Continuous | OR: 0.806 | 0.71∼0.92 | − |
| Ozawa et al. (2013) | Soy-based food and dairy | Q4 vs. Q1 | HR: 0.65 | 0.40∼1.06 | 1, 2, 3, 4, 8, 10, 12, 13, 14, 16, 17, 18, 19, 28 |
| Olsson et al. (2015) | HEI | Continuous | HR: 0.95 | 0.75∼−1.22 | − |
| MD | HR: 1 | 0.75∼1.33 | |||
| LCHP | HR: 1.16 | 0.95∼1.43 | |||
| Morris et al. (2015) | MD | T3 vs. T1 | HR: 0.48 | 0.29∼0.79 | 1, 2, 4, 8, 9, 10, 12, 14, 15, 16, 30 |
| DASH | HR: 0.6 | 0.37∼0.96 | |||
| MIND | HR: 0.49 | 0.29∼0.85 | |||
| Morris et al. (2016) | Seafood-rich diet | Continuous | Beta: 0.2 | −0.04∼0.43 | 1, 2, 4, 5, 10, 12, 28 |
| DHA+EPA food sources | Beta: 0.81 | −0.63∼2.25 | |||
| α-Linolenic 18:3 n-3 | Beta: −0.37 | −0.93∼0.18 | |||
| Vassilaki et al. (2018) | MD | Continuous | OR: 0.76 | 0.58∼0.99 | 1, 2, 4, 8, 10, 11, 12, 14, 16, 18, 26 |
| Fernando et al. (2018) | High protein | T1 vs. T3 | OR: 12.594 | 1.70∼93.01 | 1, 2, 3, 4, 8, 10, 12, 13, 28 |
| High fiber | OR: 2.106 | 0.51∼8.64 | |||
| Hill et al. (2018) | MD | Coefficient: 0.06 | −0.02∼0.14 | 1, 4, 17, 18 | |
| Low fat | Liner | Coefficient: 0.023 | −0.05∼0.1 |
MD, Mediterranean diet; HEI, healthy eating index; LCHP, low carbohydrate high protein; DASH, dietary approaches to stop hypertension; MIND, Mediterranean-DASH intervention for neurodegenerative delay; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; RR, relative risk; HR, hazard ratio; OR, odd ratio.
1)Age (1), gender (2), race/ ethnic (3), education (4), country (5), enrollment time (6), follow-up time (7), smoking status (8), alcohol drinking (9), body mass index (10), physical activity (11), caloric intake (12), medical comorbidity index (13), diabetes (14), history of myocardial infarction (15), stroke (16), coronary heart diseases (17), hypertension (18), dyslipidemia (19), serum total cholesterol (20), high sensitivity C-reavtive protein (21), fasting insulin (22), adiponectin level (23), depression (24), dementia (25), cholesterol intake (26), other fat (27), apolipoprotein E e4 allele (28), cognition (29), supplement use (30).
Fig. 5.
Forest plot of unhealthy dietary pattern and risk of Alzheimer’s diseases. ES, effect size; CI, confidence interval.
Table 6.
Studies that investigated the association between an unhealthy dietary pattern and Alzheimer’s diseases
| Kind of diet | Comparison | Effect size | Confidence interval | Adjustments1) | |
|---|---|---|---|---|---|
| Morris et al. (2003a) | High fat diet | Q5 vs. Q1 | RR: 0.9 | 0.4∼1.8 | 1, 2, 3, 4, 6, 17, 18 |
| Morris et al. (2003b) | High animal fat diet | Q5 vs. Q1 | RR: 0.7 | 0.3∼1.6 | |
| Laitinen et al. (2006) | High fat diet | Q4 vs. Q1 | OR: 0.79 | 0.29∼2.12 | 1, 2, 5, 7, 9, 12, 13, 14, 15, 16, 17, 18 |
| Luchsinger et al. (2007) | High-glycemic diet | Q4 vs. Q1 | HR: 1.1 | 0.7∼1.7 | 1, 2, 3, 4, 12, 18 |
| Gu et al. (2011) | DII | Continuous | HR: 0.99 | 0.99∼1 | − |
| Ylilauri et al. (2017) | High cholesterol diet | Q4 vs. Q1 | HR: 0.79 | 0.53∼1.19 | 1, 2, 3, 4, 5, 7, 8, 9, 10, 11, 18 |
| High dietary egg | Q4 vs. Q1 | HR: 0.85 | 0.59∼1.23 | ||
| Hill et al. (2018) | High fat | Liner | Coefficient: −0.007 | −0.09∼0.07 | 1, 4, 18, 19 |
| Junk food | Liner | Coefficient: −0.09 | −0.18∼−0.008 |
DII, dietary inflammatory index; ES, effect size; CI, confidence interval; RR, relative risk; OR, odd ratio; HR, hazard ratio.
1)Age (1), gender (2), race/ethnic (3), education (4), enrollment time (5), follow-up time (6), smoking status (7), alcohol drinking (8), body mass index (9), caloric intake (10), medical comorbidity index (11), diabetes (12), history of myocardial infarction (13), stroke (14), midlife systolic blood pressure (15), cholesterol (16), other fat (17), apolipoprotein E e4 allele (18), cognition (19).
DISSCUSION
The current meta-analysis evaluating >50,506 subjects showed a significant relationship between adherence to a healthy diet and decreased risk of AD. Indeed, adhering to a healthy diet was associated with a significantly lower overall risk of AD (by approximately 55%). However, we showed an unhealthy diet had a minimal effect on risk of AD.
A healthy diet can improve overall health by providing fluids, macronutrients, micronutrients and adequate calories (Swain et al., 2008), and helps protect against many chronic diseases, including heart disease, cancer, and diabetes (de Ridder et al., 2017). Our results suggest the combination of food groups considered a healthy diet exerts benefits for the brain (Olsson et al., 2015). However, it ultimately remains challenging to define a healthy diet (Tangney et al., 2017). A healthy diet may contain fruits, vegetables, whole grains and white meat (fish and poultry), and little or no sweetened beverages and processed food (Swain et al., 2008). Whereas unhealthy diets, constituting refined grains, sweetened desserts, high fat dairy products, and processed/red meat, have been associated with a higher risk of AD (Olsson et al., 2015; Samadi et al., 2020). The main healthy dietary patterns identified in respect to risk of AD, include the MD (Scarmeas et al., 2006a; Scarmeas et al., 2006b; Scarmeas et al., 2009b; Gu et al., 2010a; Gu et al., 2010b; Gardener et al., 2012; Morris et al., 2015; Olsson et al., 2015; Hill et al., 2018; Vassilaki et al., 2018), dietary approaches to stop hypertension (DASH) diet (Morris et al., 2015), Mediterranean-DASH intervention for neurodegenerative delay (MIND) diet (Morris et al., 2015), healthy eating index (HEI) diet (Eskelinen et al., 2011; Olsson et al., 2015), seafood-rich diet (Morris et al., 2003b; Devore et al., 2009; Morris et al., 2016), soy-based diet (Ozawa et al., 2013), and low-fat diet and high-protein diet (Olsson et al., 2015; Fernando et al., 2018; Hill et al., 2018).
Our findings indicate that healthy dietary patterns decrease the incidence of AD. This is not unpredicted because all components of a healthy diet (e.g., vegetables, fruits, plants proteins, and polyunsaturated-to-saturated fat ratio) have protective roles against AD (Devore et al., 2009; Holt et al., 2009; Farooqui, 2012; Berti et al., 2015; Calil et al., 2018). The health-related benefits of foods consumed together are additive or even synergistic. The current study showed that the inverse link between adherence to a healthy diet and AD risk is not as a result of a lone constituent of this diet but rather the whole dietary pattern. Similarly, to our results, beneficial trends have been identified between AD and fruits and vegetables, fish, dairy, and nuts (Morris et al., 2003a; Swain et al., 2008; Olsson et al., 2015; Pifferi and Aujard, 2019). A healthy diet is recognized to have beneficial anti-inflammatory, antioxidant, and metabolic effects, which may in turn induce anti-neurodegenerative benefits (Seeram, 2006; Berti et al., 2015). Therefore, healthy diets (including DASH, MD, MIND, seafood-rich, HEI, soy-based, capsaicin-rich, and low-fat and high-protein diets) may reduce oxidative stress, chronic inflammation and accumulation of amyloid-β (Morris et al., 2003a; Morris et al., 2003b; Scarmeas et al., 2006a; Scarmeas et al., 2006b; Devore et al., 2009; Scarmeas et al., 2009b; Gu et al., 2010a; Gardener et al., 2012; Ozawa et al., 2013; Olsson et al., 2015; Morris et al., 2015; Morris et al., 2016; Fernando et al., 2018; Hill et al., 2018; Vassilaki et al., 2018). Moreover, few studies have focused on the ability of unhealthy diets, particularly diets high in fat and sugar-sweetened beverages, to promote oxidative stress, inflammation, and the developing of amyloid-β and, consequentially, AD (Morris et al., 2003b; Laitinen et al., 2006; Luchsinger et al., 2007; Gu et al., 2011; Ylilauri et al., 2017; Hill et al., 2018).
The present study did not show a relationship between an unhealthy diet (i.e. high in refined grains, sweetened puddings, full-fat dairy products, and processed/red meat) and risk of AD. However, our results highlights that little is known about the relationship between an unhealthy diet and risk of AD. To our knowledge, few previous studies has investigated the relationship between Western diets, risk of AD, and the possible mechanism of action (Graham et al., 2016). Although we did not find a significant relationship between an unhealthy diet and risk of AD, we are unable to draw conclusions. For example, if the sample size increases, the statistical power is increased and may reach a false level of significance. Although the relationship between an unhealthy diet and risk of AD was not statically significant (approximately 1%), it may be clinically important (Filho et al., 2013; Gholizadeh et al., 2018). However, the constituents of an unhealthy diet, in contrast to a healthy diet, do not have a clear definition. The constituents of Western and unhealthy diets regularly include a high intake of high-fat dairy products, butter, processed meat, saturated- and trans-fat, and refined sugar, which may lead to inflammation and oxidative stress and play an important role in the etiology of AD (Gu et al., 2010a; Graham et al., 2016; Ylilauri et al., 2017).
The MD diet was the most frequently investigated healthy diet in this study (Scarmeas et al., 2006a; Scarmeas et al., 2006b; Scarmeas et al., 2009b; Gu et al., 2010a; Gardener et al., 2012; Vassilaki et al., 2018) regarding the decrease in risk of AD. Morris et al. (2015) demonstrated that high adherence to a DASH diet or MD, and moderate adherence to a MIND diet could reduce the incidence of AD. Other diets do not necessarily show positive results in all studies; for example, some studies have demonstrated that intake of seafood may decrease risk of AD, whereas Devore et al. (2009) did not detect any association between moderate consumption of fish and omega-3 PUFAs and AD. We speculate that this may be due to the MD containing a higher or more diverse nutrient content than seafood and/or soy- based foods, and high protein diets. With regard to the possible mechanisms of action, there appears to be a close relationship between inflammation, endothelial dysfunction, oxidative stress, and AD (Lyros et al., 2014). Healthy diet constituents, including green leafy vegetables, nuts, berries, and fish as well as unhealthy food such as butter and red meats are contain a high content of flavonoids, beta carotene, n-3 fatty acids, folate, and carotenoids (Solfrizzi et al., 2017). These components of healthy diets, may contribute synergistically to reducing oxidative stress and inflammation, leading to reduced risk of AD (Holt et al., 2009).
What constitutes a healthy diet is complex problem and understanding requires development of exact dietary pattern. Morris (Morris et al., 2015) developed a new diet highlighting intake of natural and plant-based foods and restriction of saturated fat and animal foods. The MIND diet, has 15 components, including 10 food groups beneficial for the brain [green leafy vegetables, other vegetables, berries, nuts, whole grains, beans, white meat (fish and poultry), olive oil, and limited intake of wine] and recommends decreasing intake of 5 unhealthy food groups, including stick margarine and butter, pastries and sweets, red meats, cheese, and fried/fast food. The MIND diet it thought to help prevent dementia and loss of brain function (Morris et al., 2015).
Possible mechanisms for the effect of a healthy diet on AD are related to high intake of n-3 fatty acids from fish consumption, leading to a decrease in oxidative stress, inflammation and Ab development (Farooqui, 2012). Furthermore, complications related to AD are improved with a high intake of beta carotene, flavonoids, folate and carotenoids, such as consumption of green leafy vegetables (Holt et al., 2009).
The limitations of this meta-analysis is inclusion of studies that use a FFQ to assess diet, which may might not unavoidably signify a dietary pattern. Furthermore, in some included studies confounders were not taken into account. Furthermore, the meta-analysis combined results from studies that had different definitions of healthy and unhealthy diets. In addition, since there was an inadequate number of studies investigation the relationship between AD and diet, characterization of healthy and unhealthy diet is too detailed. Further well-designed clinical studies are required to identify healthy diets in adults with AD.
Our systematic review and meta-analysis provides evidence that high adherence to a healthy diet is related to a lower risk of AD. In addition, we show that greatest adherence to a healthy diet is associated with a decrease in risk of AD of 55%. These findings may be clinically applicable for public health, in order to decrease the risk of disability in the general population. However, it is difficult to draw conclusions about regional healthy diets. The main healthy diets investigated in relation to the risk of AD included DASH, MD, MIND, seafood-rich, HEI, soy- based diet, high-protein and low-fat diets. Future studies should consider the potential effects of total diet scores more carefully, for example, to assess the extent of a healthy diet by HEI scores.
ACKNOWLEDGEMENTS
We appreciate department of nutrition, Kermanshah University of Medical Sciences.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Alzheimer’s Association. 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Berti V, Murray J, Davies M, Spector N, Tsui WH, Li Y, et al. Nutrient patterns and brain biomarkers of Alzheimer's disease in cognitively normal individuals. J Nutr Health Aging. 2015;19:413–423. doi: 10.1007/s12603-014-0534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer's disease in the United States. Alzheimers Dement. 2018;14:121–129. doi: 10.1016/j.jalz.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calil SRB, Brucki SMD, Nitrini R, Yassuda MS. Adherence to the Mediterranean and MIND diets is associated with better cognition in healthy seniors but not in MCI or AD. Clin Nutr ESPEN. 2018;28:201–207. doi: 10.1016/j.clnesp.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Cox LM, Schafer MJ, Sohn J, Vincentini J, Weiner HL, Ginsberg SD, et al. Calorie restriction slows age-related microbiota changes in an Alzheimer's disease model in female mice. Sci Rep. 2019;9:17904. doi: 10.1038/s41598-019-54187-x. https://doi.org/10.1038/s41598-019-54187-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore EE, Grodstein F, van Rooij FJ, Hofman A, Rosner B, Stampfer MJ, et al. Dietary intake of fish and omega-3 fatty acids in relation to long-term dementia risk. Am J Clin Nutr. 2009;90:170–176. doi: 10.3945/ajcn.2008.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ridder D, Kroese F, Evers C, Adriaanse M, Gillebaart M. Healthy diet: health impact, prevalence, correlates, and interventions. Psychol Health. 2017;32:907–941. doi: 10.1080/08870446.2017.1316849. [DOI] [PubMed] [Google Scholar]
- Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife healthy-diet index and late-life dementia and Alzheimer's disease. Dement Geriatr Cogn Dis Extra. 2011;1:103–112. doi: 10.1159/000327518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA. n-3 fatty acid-derived lipid mediators in the brain: new weapons against oxidative stress and inflammation. Curr Med Chem. 2012;19:532–543. doi: 10.2174/092986712798918851. [DOI] [PubMed] [Google Scholar]
- Fernando WMADB, Rainey-Smith SR, Gardener SL, Villemagne VL, Burnham SC, Macaulay SL, et al. Associations of dietary protein and fiber intake with brain and blood amyloid-β. J Alzheimers Dis. 2018;61:1589–1598. doi: 10.3233/JAD-170742. [DOI] [PubMed] [Google Scholar]
- Filho DBF, Paranhos R, da Rocha EC, Batista M, da Silva JA, Jr, Santos MLWD, et al. When is statistical significance not significant? Bras Political Sci Rev. 2013;7:31–55. doi: 10.1590/S1981-38212013000100002. [DOI] [Google Scholar]
- Gardener S, Gu Y, Rainey-Smith SR, Keogh JB, Clifton PM, Mathieson SL, et al. Adherence to a Mediterranean diet and Alzheimer's disease risk in an Australian population. Transl Psychiatry. 2012;2:e164. doi: 10.1038/tp.2012.91. https://doi.org/10.1038/tp.2012.91 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholizadeh F, Moludi J, Lotfi Yagin N, Alizadeh M, Mostafa Nachvak S, Abdollahzad H, et al. The relation of dietary diversity score and food insecurity to metabolic syndrome features and glucose level among pre-diabetes subjects. Prim Care Diabetes. 2018;12:338–344. doi: 10.1016/j.pcd.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Graham LC, Harder JM, Soto I, de Vries WN, John SWM, Howell GR. Chronic consumption of a western diet induces robust glial activation in aging mice and in a mouse model of Alzheimer's disease. Sci Rep. 2016;6:21568. doi: 10.1038/srep21568. https://doi.org/10.1038/srep21568 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer's disease. J Alzheimers Dis. 2010a;22:483–492. doi: 10.3233/JAD-2010-100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Nieves JW, Luchsinger JA, Scarmeas N. Dietary inflammation factor rating system and risk of Alzheimer disease in elders. Alzheimer Dis Assoc Disord. 2011;25:149–154. doi: 10.1097/WAD.0b013e3181ff3c6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch Neurol. 2010b;67:699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustaw-Rothenberg K. Dietary patterns associated with Alzheimer's disease: population based study. Int J Environ Res Public Health. 2009;6:1335–1340. doi: 10.3390/ijerph6041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; London, UK: 2011. [updated March, cited 2020 May 06]. Part 1: Cochrane reviews. Chapter 3: Maintaining reviews: updates, amendments and feedback. Available from: https://handbook-5-1.cochrane.org/ [Google Scholar]
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. 2nd ed. Wiley Blackwell; Hoboken, NJ, USA: 2019. p. 203. [Google Scholar]
- Hill E, Clifton P, Goodwill AM, Dennerstein L, Campbell S, Szoeke C. Dietary patterns and β-amyloid deposition in aging Australian women. Alzheimers Dement. 2018;4:535–541. doi: 10.1016/j.trci.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, et al. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009;109:414–421. doi: 10.1016/j.jada.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen MH, Ngandu T, Rovio S, Helkala EL, Uusitalo U, Viitanen M, et al. Fat intake at midlife and risk of dementia and Alzheimer's disease: a population-based study. Dement Geriatr Cogn Disord. 2006;22:99–107. doi: 10.1159/000093478. [DOI] [PubMed] [Google Scholar]
- Liu CH, Bu XL, Wang J, Zhang T, Xiang Y, Shen LL, et al. The associations between a capsaicin-rich diet and blood amyloid-β levels and cognitive function. J Alzheimers Dis. 2016;52:1081–1088. doi: 10.3233/JAD-151079. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Mayeux R. Glycemic load and risk of Alzheimer's disease. J Nutr Health Aging. 2007;11:238–241. [PubMed] [Google Scholar]
- Lyros E, Bakogiannis C, Liu Y, Fassbender K. Molecular links between endothelial dysfunction and neurodegeneration in Alzheimer's disease. Curr Alzheimer Res. 2014;11:18–26. doi: 10.2174/1567205010666131119235254. [DOI] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. https://doi.org/10.1186/2046-4053-4-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moludi J, Maleki V, Jafari-Vayghyan H, Vaghef-Mehrabany E, Alizadeh M. Metabolic endotoxemia and cardiovascular disease: A systematic review about potential roles of prebiotics and probiotics. Clin Exp Pharmacol Physiol. 2020;47:927–939. doi: 10.1111/1440-1681.13250. [DOI] [PubMed] [Google Scholar]
- Morris MC, Brockman J, Schneider JA, Wang Y, Bennett DA, Tangney CC, et al. Association of seafood consumption, brain mercury level, and APOE e4 status with brain neuropathology in older adults. JAMA. 2016;315:489–497. doi: 10.1001/jama.2015.19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, et al. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003a;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003b;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11:1015–1022. doi: 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Murray J, Davies M, Williams S, Pirraglia E, Spector N, et al. Nutrient intake and brain biomarkers of Alzheimer's disease in at-risk cognitively normal individuals: a cross-sectional neuroimaging pilot study. BMJ Open. 2014;4:e004850. doi: 10.1136/bmjopen-2014-004850. https://doi.org/10.1136/bmjopen-2014-004850 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Álvarez-Álvarez I, Guillén-Grima F, Aguinaga-Ontoso I. Prevalence and incidence of Alzheimer's disease in Europe: a meta-analysis. Neurologia. 2017;32:523–532. doi: 10.1016/j.nrleng.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Olsson E, Karlström B, Kilander L, Byberg L, Cederholm T, Sjögren P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis. 2015;43:109–119. doi: 10.3233/JAD-140867. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Ninomiya T, Ohara T, Doi Y, Uchida K, Shirota T, et al. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama Study. Am J Clin Nutr. 2013;97:1076–1082. doi: 10.3945/ajcn.112.045575. [DOI] [PubMed] [Google Scholar]
- Pasdar Y, Hamzeh B, Moludi J, Mehaki B, Darbandi M, Moradi S. Dietary intake and risk of depression among male and female with HIV/AIDS. Eat Weight Disord. 2020a;25:1029–1038. doi: 10.1007/s40519-019-00726-4. [DOI] [PubMed] [Google Scholar]
- Pasdar Y, Moradi S, Esfahani NH, Darbandi M, Niazi P. Intake of animal source foods in relation to risk of metabolic syndrome. Prev Nutr Food Sci. 2020b;25:133–139. doi: 10.3746/pnf.2020.25.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdar Y, Moradi S, Moludi J, Darbandi M, Niazi P, Nachvak SM, et al. Risk of metabolic syndromein non-alcoholic fatty liver disease patients. Med J Nutr Metab. 2019;12:353–363. doi: 10.3233/MNM-190290. [DOI] [Google Scholar]
- Pase MP, Himali JJ, Jacques PF, DeCarli C, Satizabal CL, Aparicio H, et al. Sugary beverage intake and preclinical Alzheimer's disease in the community. Alzheimers Dement. 2017;13:955–964. doi: 10.1016/j.jalz.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifferi F, Aujard F. Caloric restriction, longevity and aging: recent contributions from human and non-human primate studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;95:109702. doi: 10.1016/j.pnpbp.2019.109702. https://doi.org/10.1016/j.pnpbp.2019.109702 . [DOI] [PubMed] [Google Scholar]
- Querol-Vilaseca M, Colom-Cadena M, Pegueroles J, Nuñez-Llaves R, Luque-Cabecerans J, Muñoz-Llahuna L, et al. Nanoscale structure of amyloid-β plaques in Alzheimer's disease. Sci Rep. 2019;9:5181. doi: 10.1038/s41598-019-41443-3. https://doi.org/10.1038/s41598-019-41443-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi M, Moradi S, Azadbakht L, Rezaei M, Hojati N. Adherence to healthy diet is related to better linear growth with open growth plate in adolescent girls. Nutr Res. 2020;76:29–36. doi: 10.1016/j.nutres.2020.02.002. [DOI] [PubMed] [Google Scholar]
- Samadi M, Moradi S, Moradinazar M, Mostafai R, Pasdar Y. Dietary pattern in relation to the risk of Alzheimer's disease: a systematic review. Neurol Sci. 2019;40:2031–2043. doi: 10.1007/s10072-019-03976-3. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009a;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006a;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009b;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006b;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeram NP. Bioactive polyphenols from foods and dietary supplements: challenges and opportunities. In: Wang M, Sang S, Hwang LS, Ho CT, editors. Herbs: Challenges in Chemistry and Biology. Vol 92. ACS Publications; Washington, DC, USA: 2006. pp. 25–38. [DOI] [Google Scholar]
- Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer's disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. 2017;59:815–849. doi: 10.3233/JAD-170248. [DOI] [PubMed] [Google Scholar]
- Swain JF, McCarron PB, Hamilton EF, Sacks FM, Appel LJ. Characteristics of the diet patterns tested in the optimal macronutrient intake trial to prevent heart disease (OmniHeart): options for a heart-healthy diet. J Am Diet Assoc. 2008;108:257–265. doi: 10.1016/j.jada.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney CC, Staffileno BA, Rasmussen HE. Healthy eating: how do we define it and measure it? What's the evidence? J Nurse Pract. 2017;13:e7–e15. doi: 10.1016/j.nurpra.2016.08.026. [DOI] [Google Scholar]
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLOS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. https://doi.org/10.1371/journal.pmed.0040297 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilaki M, Aakre JA, Syrjanen JA, Mielke MM, Geda YE, Kremers WK, et al. Mediterranean diet, its components, and amyloid imaging biomarkers. J Alzheimers Dis. 2018;64:281–290. doi: 10.3233/JAD-171121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylilauri MP, Voutilainen S, Lönnroos E, Mursu J, Virtanen HE, Koskinen TT, et al. Association of dietary cholesterol and egg intakes with the risk of incident dementia or Alzheimer disease: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2017;105:476–484. doi: 10.3945/ajcn.116.146753. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]