Abstract
Aims
Cardiac miR-132 activation leads to adverse remodelling and pathological hypertrophy. CDR132L is a synthetic lead-optimized oligonucleotide inhibitor with proven preclinical efficacy and safety in heart failure (HF) early after myocardial infarction (MI), and recently completed clinical evaluation in a Phase 1b study (NCT04045405). The aim of the current study was to assess safety and efficacy of CDR132L in a clinically relevant large animal (pig) model of chronic heart failure following MI.
Methods and results
In a chronic model of post-MI HF, slow-growing pigs underwent 90 min left anterior descending artery occlusion followed by reperfusion. Animals were randomized and treatment started 1-month post-MI. Monthly intravenous (IV) treatments of CDR132L over 3 or 5 months (3× or 5×) were applied in a blinded randomized placebo-controlled fashion. Efficacy was evaluated based on serial magnetic resonance imaging, haemodynamic, and biomarker analyses. The treatment regime provided sufficient tissue exposure and CDR132L was well tolerated. Overall, CDR132L treatment significantly improved cardiac function and reversed cardiac remodelling. In addition to the systolic recovery, diastolic function was also ameliorated in this chronic model of HF.
Conclusion
Monthly repeated dosing of CDR132L is safe and adequate to provide clinically relevant exposure and therapeutic efficacy in a model of chronic post-MI HF. CDR132L thus should be explored as treatment for the broad area of chronic heart failure.
Keywords: Chronic heart failure, Contractile function, Translational studies, Myocardial infarction, Cardiac remodelling, MicroRNAs
Graphical Abstract
See page 202 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa870)
Introduction
With over 30% mortality in the first year after diagnosis and high re-hospitalization rates, the global burden of chronic heart failure (CHF) is steadily increasing despite improvements in the standard of care.1 MiRNAs serve as molecular master switches, directly coordinating the expression of multiple genes, often acting on the same pathological pathway.2 , 3 We previously identified miR-132 as a promising target in HF with a unique mode of action directly affecting multiple pathways in cardiomyocytes in the failing heart.4 Next, we developed and optimized a synthetic antisense oligonucleotide as a pharmacological inhibitor of miR-132 (CDR132L). Mode of action studies in vitro and in various animal models of heart failure revealed that reversal of cardiomyocyte hypertrophy, normalization of autophagy, and calcium signalling, as well as the reduction of cardiac fibrosis are CDR132Ls main effects.4 , 5 In a pig model of early HF post-myocardial infarction (MI), we demonstrated dose-dependent efficacy and thus strong clinical potential CDR132L treatment given twice monthly.5 Subsequent preclinical assessment, as part of a drug development programme, revealed that CDR132L is safe and well tolerated at a wide pharmacological dose range. Based on these results, a Phase 1b clinical trial with CDR132L in heart failure patients was conducted and recently completed.6
Translational perspective
Chronic heart failure affects millions of patients worldwide with increasing prevalence due to an aging population. Pharmacotherapies that limit, or reverse disease progression are utmost needed. Antisense oligonucleotides targeting microRNAs, key regulators in disease pathogenesis, open up fundamentally new treatment options. CDR132L is a synthetic antisense inhibitor of miR-132 with cardiac anti-remodelling effects early after post-myocardial infarction. Here, we demonstrate the efficacy and safety of monthly application of CDR132L, in a translational model of chronic heart failure, using clinically relevant endpoints (serial magnetic resonance imaging, haemodynamics, and biomarkers). The findings encourage the subsequent clinical testing of CDR132L in chronic heart failure patients.
The purpose of the present study was to evaluate the efficacy and clinical relevance of monthly CDR132L treatment in a large animal model of CHF. The study was carried out in a translational chronic porcine model of post-MI HF with 6 months of follow-up and serial magnetic resonance imaging (MRI). In this unique chronic model, the animals developed progressive systolic dysfunction and remodelling with diastolic involvement. Intravenous placebo (placebo group) or CDR132L treatment (5 mg/kg) starting 1 month after the initial MI was given monthly over 3 months (3× group) or 5 months (5× group) in a randomized placebo-controlled fashion. The impact of CDR132L treatment on chronic post-MI adverse remodelling was evaluated by cardiac MRI (cMRI), invasive haemodynamic measurements, analysis of clinical parameters, and biomarkers. Molecular, histological, and biochemical analysis of tissue and blood samples were used to further evaluate safety and therapeutic effects of CDR132L.
Methods
A detailed description of all methods is provided in the Supplementary material online.
Large animal model of post-myocardial infarction HF and analysis of cardiac function
All procedures involving large animals have been reviewed and approved by the Animal Welfare Committee of the University of Kaposvar, Hungary. For details see Supplementary material online. Sample size calculation was performed in accordance with the American Association for Laboratory Animal Science (IACUC). Based on preliminary results performed with the clinically relevant pig (mangalica) model of post-MI HF5 and assuming an α of 0.05 and a power of 80%, the required group size was estimated to be n = 9. Due to the estimated perioperative (e.g. lethal arrhythmia) and infarction-related loss of animals and animals that might not reach the inclusion criteria [ejection fraction (EF) < 40% post-MI at month 1], an additional number of 6 animals per group was included as a safety margin to a total number of 15 animals per group.
Myocardial infarction was induced by coronary artery occlusion by inflation with a balloon catheter for 90 min, followed by reperfusion via balloon deflation.5 , 7 Cardiac function was assessed by serial cMRI (Siemens Magnetom Vision 1.5 Tesla field strength equipment) at indicated time points (Supplementary material online, Figure S1). Only animals showing an EF of <40% at month 1 were included in the final statistical analysis. Left ventricular haemodynamics were assessed using intracardiac pressure–volume (PV) catheter (Ventri-Cath 507 using MPVS Ultra system, Millar Instruments). Animals were assigned randomly into treatment groups (placebo, 3× CDR132L or 5× CDR132L treatment) based on body weights in a blinded fashion receiving the first treatment 1 month post-MI by IV injection of a 5 mg/kg CDR132L or placebo (Supplementary material online, Figure S1).
Assessment of CDR132L concentrations
CDR132L levels from biological matrices were quantified at Axolabs GmbH (Kulmbach, Germany) using an high performance liquid chromatography (HPLC) hybridization assay with complementary 16-mer PNA-probes. For details see Supplementary material online.
Histology and fibrosis assessment
Fibrosis and cardiomyocyte surface areas were assessed in cardiac paraffin sections stained with picrosirius red (PSR) or wheat germ agglutinin (WGA), respectively, as indicated in the Supplementary material online.
Plasma sampling and laboratory diagnostics
Ethylenediaminetetraacetic acid (EDTA) plasma was sampled (see Supplementary material online) and laboratory diagnostics were performed by PraxisLab Kft (Budapest, Hungary). N-terminal pro-brain natriuretic peptide (NT-proBNP) was assessed in plasma samples using an Enzyme-linked Immunosorbent Assay (ELISA) kit (Cloud Clone Corp, Wuhan, China).
Gene expression array analysis
Total RNA was isolated using miRNeasy Mini Kit (Qiagen, Hilden, Germany). RNA was reverse transcribed into complementary cDNA and assessed by quantitative real-time PCR analysis or used for profiling of signalling pathways based on porcine TaqMan Gene Expression Array Cards. For details see Supplementary material online.
Statistics
The primary aim of the study was to compare Placebo vs. 5× treatment regimen, Placebo vs. 3× comparison is also reported. Therefore, P-values were calculated using unpaired two-sided Mann–Whitney U test. Data are shown as mean ± SD. Data were analysed using GraphPad Prism 8 software (GraphPad Software, La Jolla, CA, USA).
Results
Characterisation of a large animal model of chronic post-myocardial infarction HF with 6 months of follow-up
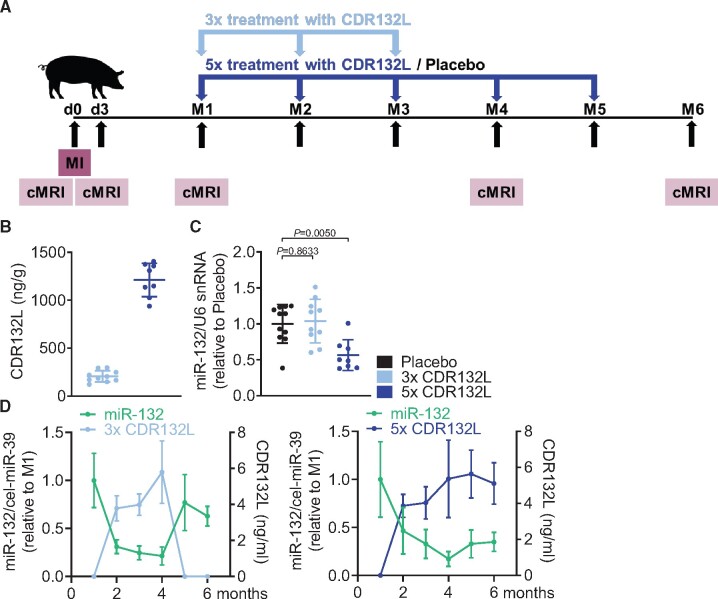
Here, we describe a novel large animal model of CHF. The study was carried out in slow-growing (‘mangalica’ breed) pig, allowing serial cMRI evaluations under chronic settings. After baseline imaging, the animals underwent 90 min transient left anterior descending artery occlusion followed by reperfusion (Figure 1A). This procedure resulted in large, sublethal MI. Scar size was similar in all treatment groups (placebo: 22.17 ± 1.17 mL, 3×: 20.19 ± 1.95 mL; 5×: 20.96 ± 1.12 mL). Out of 45 animals, three animals were lost post-MI prior to the initiation of treatment due to peri-procedural and MI-related complications (Supplementary material online, Figure S1). Overall, 42 pigs were randomly assigned into treatment groups 1 month post-MI. Out of those animals, due to biological and technical variability, seven surviving animals developed limited cardiac damage (EF ≥ 40%) and thus were excluded. One additional animal has been excluded due to unrelated illness. Thus, based on the above-defined criteria, 29 animals were included in the final evaluation (Supplementary material online, Figure S1). Following MI, left ventricular (LV) function progressively deteriorated (EF decrease from 35.10% at month 1 post-MI to 31.69% at the endpoint; Supplementary material online, Table S1 and Figure S2), giving a net decrease of 3.41% in EF over 6 months (Supplementary material online, Table S2) in the placebo group. Over time, the animals in the placebo group developed characteristic left ventricular adverse remodelling, as indicated by the increase of left ventricular volumes (Supplementary material online, Tables S1 and S2 and Figure S2). Chronic progression of HF was further evidenced by concomitant elevation of circulating NT-proBNP levels (Supplementary material online, Figure S3) in this cohort.
Figure 1.
Pharmacokinetic/Pharmakodynamic (PK/PD) relationship in a large animal model of chronic HF. (A) Study outline of treatment regimens with CDR132L [after induction of myocardial infarction at day 0, animals were randomized at month 1, treated either with placebo or 5 mg/kg CDR132L for 3 or 5 months (3× or 5×) and cardiac function was monitored by magnetic resonance imaging]. (B) Tissue levels of CDR132L detected in the left ventricular remote region at endpoint (month 6). (C) Functional levels of miR-132 in the left ventricular remote region at endpoint (month 6). (D) Relation of circulating miR-132 level and CDR132L in plasma of CDR132L-treated animals over time. Data are mean ± SD; P-values: two-tailed Mann–Whitney U test (Placebo vs. treatment regimen).
Sufficient tissue exposure and successful target engagement in the heart
CDR132L treatment was given as a monthly IV injection either five or three times (Figure 1A). At the 6-month endpoint, tissue levels of CDR132L were detectable in all cardiac samples (LV MI remote region) in both treatment groups (Figure 1B). The different levels detected in the 5× and 3× groups reflect the longer elimination period for the 3× group (3 months vs. 1 month for the 5× group). The detected high cardiac tissue concentrations of CDR132L in the 5× group well correlated with the significant reduction of functional cardiac miR-132 levels, compared with placebo (Figure 1C). As expected, no such effect was seen in the 3× group, due to the longer wash-out period.
The silencing of miR-132 in cardiac tissue was accompanied with strong reduction of circulating miR-132 levels after monthly treatment with CDR132L. Both CDR132L concentrations and miR-132 levels in plasma remained constant in the 5× group at endpoint, 1 month after the last dose (Figure 1D, right panel). In the 3× group, CDR132L similarly remained constant for 1 month, following the last dose (month 4). After that, levels fell below limit of detection (months 5 and 6) (Figure 1D, left panel). miR-132 levels in plasma followed this timeline, with a rebound only after 2 months following the last dosing (months 5 and 6) (Figure 1D, left panel). These data provide additional evidence of CDR132L’s sustained pharmacological effect and long biological half-life.
Treatment with CDR132L improves cardiac function and reduces adverse remodelling in chronic post-myocardial infarction HF
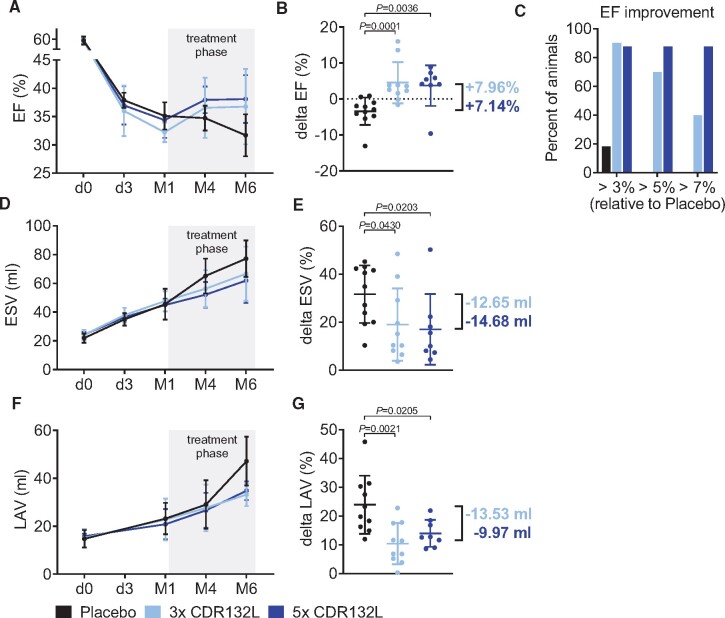
Changes in cardiac function were monitored by serial cMRIs (Figure 1A and Supplementary material online, Table S1 and Figure S2). At the end of the 6 months of follow-up, it was evident that CDR132L in both treatment arms (3× and 5×) effectively improved cardiac function. Compared with placebo treatment, there was a significant EF improvement in both treatment groups, with an EF absolute increase of 7.96% and 7.14% (3× and 5×, respectively; Figure 2A and B, Supplementary material online, Tables S1–S3 and Figure S2). To further demonstrate potential clinical utility, we also analysed the therapeutic response in all groups (Figure 2C). 40% of the 3× CDR132L group and 87.5% of the 5× group showed a delta EF of >7% at Month 6, while this was not the case in any of the 11 placebo animals. These data further underscore the prospective therapeutic value of the CDR132L treatment regime.
Figure 2.
Functional improvement in a large animal model of chronic HF. (A) Left ventricular ejection fraction over time for different treatment regimens with CDR132L. (B) Functional improvement indicated by ejection fraction change from month 1 to month 6 (delta ejection fraction; in absolute per cent points). (C) Responder analysis for different treatment regimens (values presented are corrected with the ejection fraction change in the placebo group). (D) End-systolic volume over time. (E) Changes in end-systolic volume from month 1 to month 6 (delta end-systolic volume). (F) Left atrial volume over time. (G) Changes in left atrial volume from month 1 to month 6 (delta left atrial volume). Data are mean ± SD; P-values: two-tailed Mann–Whitney U test (Placebo vs. treatment regimen). EF, ejection fraction; ESV, end-systolic volume; LAV, left atrial volume; LV, left ventricular.
CDR132L additionally showed beneficial effect on adverse left ventricular remodelling. Indeed, CDR132L treatment in both treatment arms significantly attenuated the post-MI enlargement of LV end-systolic volume (ESV) over the 6 months of follow-up, compared with placebo (Figure 2D and E and Supplementary material online, Tables S1–S3 and Figure S2).
Imaging of the left atrium (LA) revealed that post-MI chronic atrial remodelling has also been reduced by CDR132L treatment. When compared with placebo CDR132L in both treatment arms effectively reduced the increase of the LA volume (LAV) over time and at 6 month endpoint (Figure 2F and G and Supplementary material online, Tables S1–S3 and Figure S2). Beneficial changes in ventricular and atrial indices (LVESV Index, LVEDV Index, LAV index) were confirmed when normalized to body surface area (Supplementary material online, Table S1 and Figure S2).
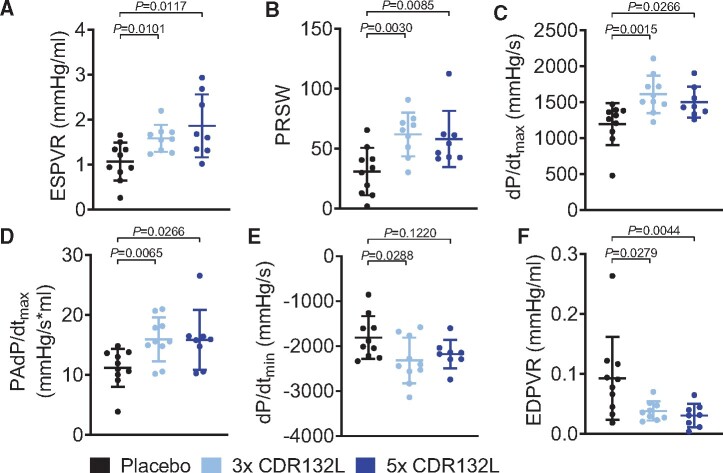
We also found that systolic function and contractility were significantly improved by CDR132L treatment in the post-MI failing heart, assessed by invasive haemodynamic measurement at the 6-month endpoint (Supplementary material online, Table S4). Detailed analysis of load-independent parameters revealed a CDR132L treatment-dependent improvement of myocardial contractility (ESPVR, end-systolic PV relationship and PRSW, preload recruitable stroke work) (Figure 3A and B), which clearly translated into better overall systolic function, assed by dP/dtmax (maximum rate of pressure change in the ventricle) and by the more sensitive preload-adjusted dP/dtmax (PAdP/dtmax) (Figure 3C and D).
Figure 3.
Haemodynamic measurements of left ventricular function in a large animal model of chronic HF. (A) End-systolic pressure–volume relationship for different treatment regimens with CDR132L at endpoint (month 6). (B) Preload recruitable stroke work. (C) Maximum rate of pressure change in the ventricle (dP/dtmax). (D) Preload-adjusted maximal change in pressure over time (PAdP/dtmax). (E) Minimum rate of pressure change in the ventricle (dP/dtmin). (F) End-diastolic pressure–volume relationship (EDPVR). Data are mean ± SD; P-values: two-tailed Mann–Whitney U test (Placebo vs. treatment regimen).
Left atrial remodelling is closely associated with diastolic dysfunction. Intriguingly, CDR132L treatment significant improved diastolic function, in line with the effect on LAV. The global diastolic parameter dP/dtmin (minimum rate of pressure change in the ventricle) and the load-independent parameter of EDPVR (end-diastolic PV relationship), a sensitive marker of cardiac stiffness and capacitance, were both improved by CDR132L treatment (Figure 3E and F). Additional parameters of left ventricular function are summarized in Supplementary material online, Tables S1–S3 and Figure S2. There was no difference in heart rate or blood pressure among the treatment groups (Supplementary material online, Tables S1 and S4 and Figure S2).
In summary, CDR132L given monthly, effectively improved remodelling, cardiac contractility, and diastolic function in a chronic post-MI HF model.
CDR132L treatment reduces the HF biomarker N-terminal pro-brain natriuretic peptide
Due to its high translational relevance, we assessed circulating NT-proBNP to validate the treatment response in the CHF model (Supplementary material online, Figure S3). As indicated, NT-proBNP was significantly elevated in the placebo group after 6 months compared with baseline. In addition to the functional improvements, CDR132L treatment also reduced NT-proBNP in both treatment groups, reaching statistical significance in the 5× treatment group, suggesting reduced LV wall stress by CDR132L treatment.
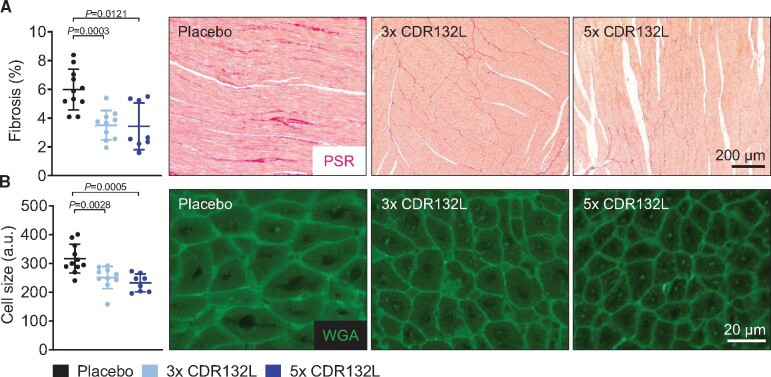
CDR132L reduces fibrosis and maladaptive cardiomyocyte hypertrophy
The functional improvements and efficacy of CDR132L treatment are further supported by histological data from the myocardium. Analysis of fibrosis in PSR stained cardiac slices (LV MI remote region) revealed that the degree of interstitial fibrosis was significantly reduced in both CDR132L treatment groups, compared with placebo (Figure 4A). The average cardiomyocyte size in remote regions in the heart was also significantly reduced in both CDR132L groups (Figure 4B).
Figure 4.
Hallmarks of cardiac remodelling in a large animal model of chronic HF. (A) Quantification and representative micrographs of picrosirius red (PSR) staining of the left ventricular remote regions for different treatment regimens at endpoint (6 months) (scale bar = 200 µm). (B) Quantification and representative micrographs of wheat germ agglutinin (WGA) staining for cardiac cell size measurement of the left ventricular remote region (scale bar = 20 µm). Data are mean ± SD; P-values: two-tailed Mann–Whitney U test (Placebo vs. treatment regimen).
These histological findings further support our hypothesis regarding the anti-remodelling effects of CDR132L at cardiomyocyte level, as the basis of the overall improvement of LV function in chronic post-MI HF.
Chronic CDR132L treatment is safe and well tolerated
Animals were carefully monitored for drug-related adverse effects during the study. Repeated dosing of CDR132L was safe and well tolerated and did not affect animal growth (Supplementary material online, Figure S4). Haematology and laboratory chemistry parameters were continuously monitored from blood samples (Supplementary material online, Figure S5A). At baseline, all measured parameters were in the normal range. Infarct induced changes could be observed in both placebo and treatments groups, and were similar in nature, measured at day 3 and month 1 post-MI. After treatment start, no differences were found between the placebo and the CDR132L treatment groups over time. In addition, there was no treatment-related effect on safety-relevant electrocardiographic parameters such as the heart rate corrected QT-interval (QTc) (Supplementary material online, Figure S5B).
Overall, no therapy-related adverse events or changes in haematology or laboratory chemistry were observed, further supporting the safety profile of the drug.
Effect of CDR132L on cardiac signalling pathways
We also evaluated the effect of chronic miR-132 inhibition on intracellular pathways which may contribute to the mode of action in our heart failure model. For that, we specifically designed custom TaqMan Gene Expression Array Cards with porcine probes for 384 genes related to the pathogenesis of heart failure and compared samples from the left ventricular remote region of animals treated five times with CDR132L to animals receiving placebo. We found that the expression levels of 14 mRNAs were significantly altered in the CDR132L-treated animals 6 months of post-MI (P < 0.05; Supplementary material online, Figure S6A and B and Table S5). Most of the affected genes are directly or indirectly involved in extracellular matrix biology and fibrogenesis (CD44,8 extracellular matrix protein 1,9 and GATA binding protein 310) and cardiac hypertrophy (reticulon 4,11 leukaemia inhibitory factor receptor alpha,12 myocyte enhancer factor 213), as well as vasculogenesis and vascular function (bone morphogenetic protein receptor type II,14 adrenoceptor alpha 1D15). Interestingly, angiotensin-converting enzyme 2, which has been recently shown to be a functional entry receptor for SARS-CoV-2,16 was also CDR132L treatment responsive and down-regulated.
Discussion
The aim of this study was to evaluate the therapeutic efficacy, safety, and tolerability of monthly CDR132L treatment in CHF using a clinically relevant large animal model over 6-month post-MI. During pathological cardiac conditions, expression of the miR-212/132 family in cardiomyocytes is increased.5 Transgenic mice overexpressing the miR-212/132 cluster develop pathological cardiac remodelling and die prematurely from progressive HF.4 Further studies by our group showed that miR-132 is both necessary and sufficient to drive the pathological growth of cardiomyocytes and we proposed to develop a therapeutic strategy for heart failure based on pharmacologic miR-132 inhibition.5 We thus developed CDR132L, an optimized miR-132 antisense oligonucleotide inhibitor.5 We previously demonstrated sufficient tissue exposure and target engagement in cardiac tissue by IV application.5 We also showed dose-dependent improvement in cardiac function and the reversal of adverse cardiac remodelling 2 months of post-MI in a large animal model.5 The pleiotropic effects of cardiac miR-132 inhibition affect well-defined pathways that contribute to adverse remodelling of the left ventricle and the progression of heart failure,3 , 4 by reduction of hypertrophy, fibrosis, normalization of autophagy, and calcium signalling. Hence, we designed a study to explore the effects of the miR-132 inhibitor CDR132L in progressive, chronic HF setting using a large animal model of post-MI chronic HF.
Novel large animal model of chronic HF
Consistent and thoroughly characterized large animal models of CHF are a critical translational tool for drug development.17 , 18 Likewise, adequate techniques and valid analytic tools are necessary to analyse clinically relevant parameters of cardiac function and LV structural remodelling in order to investigate therapeutic response for novel strategies. Since the most significant proportion of HF aetiology is of ischaemic origin, we used a preclinical large animal model of pig post-MI HF.18 Our study is the first to characterize CHF conditions 6 months of post-MI HF in a large animal model with serial MRI. Pigs are a human-relevant model for studying post-MI HF. The clinical signs of ensuing HF in pigs [e.g. functional (LV volumes, EF), molecular, and histological changes in heart] are very similar to the human disease setting.7 This study was conducted in a special pig breed ‘mangalica’. As opposed to the commonly used breeds, this pig has a limited growth, making the model closer to human and allowing serial MRI evaluations under chronic settings.
The animals developed cardiac dysfunction during the early reperfusion post-MI and the LV function progressively deteriorated during the 6 months of follow-up period. Over time, cardiac dysfunction was accompanied by progressive adverse cardiac remodelling with characteristic increase of LVESV and elevated circulating NT-proBNP levels. Thus, this chronic pig model of post-MI HF represents a highly suitable model to evaluate therapy responses.
Monthly systemic CDR132L treatment provides sufficient cardiac exposure in pigs
Monthly dosing of CDR132L led to adequate cardiac tissue exposure. As with other systemic antisense oligonucleotides, the absorption is rapid and the elimination half‐life in target tissue is several weeks, supporting infrequent dosing. Indeed, we previously found fast absorption and bi-phasic elimination of the compound with a rapid alpha phase and a long beta phase after single-dose application.5 The half-life of single-dose CDR132L treatment in the cardiac tissue was found to be ∼3 weeks. In line with this and literature data, here we found the terminal elimination of CDR132L to be over 1 month, after repeated dose administration.2 , 19 Interestingly, the cardiac tissue levels after five monthly treatments are comparable to tissue levels in our previous study with two monthly treatments of the same dose, indicating the absence of tissue accumulation effects over time after multiple administrations.5 This is further supported by the absence of therapy-related adverse events or changes in haematology or laboratory chemistry parameters. CDR132L levels were also detectable in plasma for at least 1 month after the last treatment. Tissue exposure was associated with target engagement at the cardiac tissue level. We found potent miR-132 silencing in the in cardiac tissue from the 5× group, 1 month after last dose. The effect in cardiac tissue was accompanied with the strong reduction of circulating miR-132 levels. Both plasma CDR132L levels and circulating miR-132 levels in plasma remained constant 1 month after the last dose. Thus, CDR132L-related assessment of circulating miR-132 levels could be used in future studies during clinical development and beyond to titrate levels of CDR132L in patients. In addition to the pharmacological context, we found significant correlation of miR-132 plasma levels in general and the severity of HF in patients tested in the GISSI cohort.20 Thus, circulating miR-132 levels as a biomarker in general may predict outcome in HF patients.
CDR132L improves cardiac function and reduces adverse remodelling in chronic post-myocardial infarction HF
The results of the study provide clear evidence that monthly CDR132L administration effectively improved cardiac function in a clinically relevant CHF model (Take home figure). We demonstrated significant improvement in delta EF (7–8% net increase of EF, placebo corrected EF change between month 1 and 6), based on gold-standard cMRI measurements, in animals receiving monthly IV treatments of CDR132L. The potential therapeutic utility was further demonstrated by a high treatment response rate. Based on invasive PV loop analysis, we found a clear improvement of myocardial contractility (ESPVR and PRSW) and better overall systolic function. Remarkably, CDR132L treatment also enhanced diastolic function. In particular, the load-independent parameter of EDPVR, a marker of cardiac stiffness and capacitance was strongly improved.
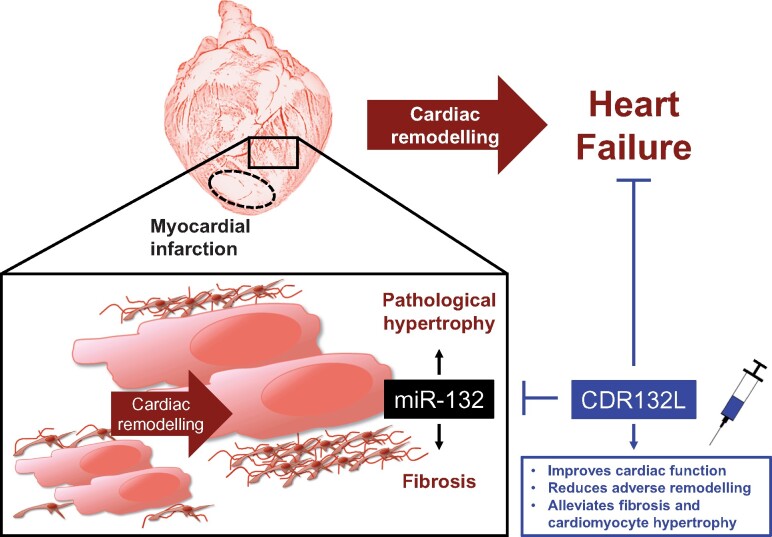
Take home figure.
Repeated CDR132L treatment is safe, improves systolic and diastolic cardiac function and reduces both ventricular as well as atrial remodelling in a clinically relevant post-MI large animal model of chronic heart failure.
The treatment regime provided persistent, clinically highly relevant therapeutic effect on cardiac function. These benefits of CDR132L in progressive adverse cardiac remodelling are explainable by the anti-hypertrophic and anti-fibrotic effects of the selective antisense inhibitor of miR-132 (Take home figure).4,5 This is further reinforced by our porcine-specific RNA array study using tissue samples 6 months of post-MI from placebo and CDR132L-treated animals.
Even though the clear effect on cell size did not translate to significant changes in cMRI-based LV mass, a clear treatment-related trend in reduction could be observed (Supplementary material online, Table S1 and Figure S2). Due to the different nature of these data sets, substantial cellular changes in certain specific regions of the LV may not be reflected in changes of the LV mass. Also, global imaging parameters have a high potential for measuring errors and biological variability and compensatory growth of other LV regions may affect the overall volume development over time.
Furthermore, chronic CDR132L treatment was safe and well tolerated. No drug-induced adverse events or changes in haematology or laboratory chemistry were observed in the chronic setting, further supporting the previously demonstrated safety profile of the drug. We also would like to point out that the compound was recently tested in stable CHF patients (Phase 1b clinical trial6). The regulatory approval was based on successful demonstration of preclinical efficacy and favourable non-clinical safety profile from a large good laboratory practice (GLP) safety toxicology programme in two species (unpublished data).
An additional therapeutic consideration in CHF is the frequent presence of comorbidities that are associated with miR-132 dysfunction. Both, non-alcoholic fatty liver disease and chronic kidney disease are often associated with CHF. Indeed, it was shown that pharmacologic inhibition of miR-132 was beneficial in relevant animal models of such HF-comorbidities.21 , 22
As an important clinical consideration, we expect meaningful synergistic effects of CDR132L, given in combination with the current ‘standard of care’ therapies, owning to the unique mode of action of CDR132L. This positive effect is expected due to the complementarity of the individual therapeutic approaches, as CDR132L counteracts cardiac remodelling through modulation of genetic pathways, which is distinct from all current treatments of HF.
Overall, monthly repeated administration of CDR132L is adequate to provide clinically relevant exposure and therapeutic efficacy in the porcine model of chronic post-MI HF. Therefore, a treatment regime of CDR132L with monthly frequency could be the basis for its potential clinical development in the broad area of CHF. The simultaneous improvement of both cardiac systolic and diastolic functions suggests a broad applicability of CDR132L in chronic HF patients.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank C. Albrecht (Cardior) and M. Schuster (Axolabs) for their excellent technical support. We further thank the whole team from Medicopus Nonprofit Ltd and the team of Experimental and Clinical Cardiology Lab of the Medical University of Vienna for their great commitment and their excellent work in the large animal study.
Funding
The study was supported by Cardior Pharmaceuticals GmbH.
Conflict of interest: S.B. and T.T. are co-founders and hold shares of Cardior Pharmaceuticals GmbH. T.T. and S.B. filed and licensed patents through the Hannover Medical School to Cardior Pharmaceuticals GmbH. S.B., S.R., T.B., C.G., and J.V. are full-time employees of Cardior Pharmaceuticals GmbH.
Contributor Information
Sandor Batkai, CARDIOR Pharmaceuticals GmbH, Feodor-Lynen-Str. 15, Hannover 30625, Germany.
Celina Genschel, CARDIOR Pharmaceuticals GmbH, Feodor-Lynen-Str. 15, Hannover 30625, Germany.
Janika Viereck, CARDIOR Pharmaceuticals GmbH, Feodor-Lynen-Str. 15, Hannover 30625, Germany.
Steffen Rump, CARDIOR Pharmaceuticals GmbH, Feodor-Lynen-Str. 15, Hannover 30625, Germany.
Christian Bär, Institute of Molecular and Translational Therapeutic Strategies (IMTTS), Hannover Medical School, Carl-Neuberg-Str. 1, Hannover 30625, Germany; REBIRTH Center for Translational Regenerative Medicine, Hannover Medical School, Carl-Neuberg-Str. 1, Hannover 30625, Germany.
Tobias Borchert, CARDIOR Pharmaceuticals GmbH, Feodor-Lynen-Str. 15, Hannover 30625, Germany.
Denise Traxler, Division of Cardiology, Medical University of Vienna, Waehringer Guertel 18-20, Vienna 1090, Austria.
Martin Riesenhuber, Division of Cardiology, Medical University of Vienna, Waehringer Guertel 18-20, Vienna 1090, Austria.
Andreas Spannbauer, Division of Cardiology, Medical University of Vienna, Waehringer Guertel 18-20, Vienna 1090, Austria.
Dominika Lukovic, Division of Cardiology, Medical University of Vienna, Waehringer Guertel 18-20, Vienna 1090, Austria.
Katrin Zlabinger, Division of Cardiology, Medical University of Vienna, Waehringer Guertel 18-20, Vienna 1090, Austria.
Ena Hašimbegović, Division of Cardiology, Medical University of Vienna, Waehringer Guertel 18-20, Vienna 1090, Austria.
Johannes Winkler, Division of Cardiology, Medical University of Vienna, Waehringer Guertel 18-20, Vienna 1090, Austria.
Rita Garamvölgyi, Department of Diagnostic Imaging and Oncoradiology, University of Kaposvár, Guba S. Street 40, Kaposvár 7400, Hungary.
Sonja Neitzel, Axolabs GmbH, Fritz-Hornschuch-Straße 9, Kulmbach 95326, Germany.
Mariann Gyöngyösi, Division of Cardiology, Medical University of Vienna, Waehringer Guertel 18-20, Vienna 1090, Austria.
Thomas Thum, CARDIOR Pharmaceuticals GmbH, Feodor-Lynen-Str. 15, Hannover 30625, Germany; Institute of Molecular and Translational Therapeutic Strategies (IMTTS), Hannover Medical School, Carl-Neuberg-Str. 1, Hannover 30625, Germany; REBIRTH Center for Translational Regenerative Medicine, Hannover Medical School, Carl-Neuberg-Str. 1, Hannover 30625, Germany.
References
- 1. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016;13:368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van RE, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res 2012;110:496–507. [DOI] [PubMed] [Google Scholar]
- 3. Lu D, Thum T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol 2019;16:661–674. [DOI] [PubMed] [Google Scholar]
- 4. Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 2012;3:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foinquinos A, Batkai S, Genschel C, Viereck J, Rump S, Gyöngyösi M, Traxler D, Riesenhuber M, Spannbauer A, Lukovic D, Weber N, Zlabinger K, Hašimbegović E, Winkler J, Fiedler J, Dangwal S, Fischer M, J de la R, Wojciechowski D, Kraft T, Garamvölgyi R, Neitzel S, Chatterjee S, Yin X, Bär C, Mayr M, Xiao K, Thum T. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat Commun 2020;11:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical Study to Assess Safety, PK and PD Parameters of CDR132L—No Study Results Posted—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/results/NCT04045405?18 view=results (18 September 2020).
- 7. Gyögyöi M, Blanco J, Marian T, Trón L, Petneházy OR, Petrasi Z, Hemetsberger R, Rodriguez J, Font G, Pavo IJ, Kertész I, Balkay L, Pavo N, Posa A, Emri M, Galuska L, Kraitchman DL, Wojta J, Huber K, Glogar D. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ Cardiovasc Imaging 2008;1:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang L-W, Qin D-Z, James E, McKallip RJ, Wang N-P, Zhang W-W, Zheng R-H, Han Q-H, Zhao Z-Q. CD44 deficiency in mice protects the heart against angiotensin ii-induced cardiac fibrosis. Shock 2019;51:372–380. [DOI] [PubMed] [Google Scholar]
- 9. Hardy SA, Mabotuwana NS, Murtha LA, Coulter B, Sanchez-Bezanilla S, Al-Omary MS, Senanayake T, Loering S, Starkey M, Lee RJ, Rainer PP, Hansbro PM, Boyle AJ. Novel role of extracellular matrix protein 1 (ECM1) in cardiac aging and myocardial infarction. PLoS One 2019;14:e0212230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang M, Song L, Wang L, Yukht A, Ruther H, Li F, Qin M, Ghiasi H, Sharifi BG, Shah PK. Deficiency of GATA3-positive macrophages improves cardiac function following myocardial infarction or pressure overload hypertrophy. J Am Coll Cardiol 2018;72:885–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Huang Y, Cantalupo A, Azevedo PS, Siragusa M, Bielawski J, Giordano FJ, Di Lorenzo A. Endothelial Nogo-B regulates sphingolipid biosynthesis to promote pathological cardiac hypertrophy during chronic pressure overload. JCI Insight 2016; 1:e85484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, Vernallis AB, Heath JK, Pennica D, Wood WI, Chien KR. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem 1996;271:9535–9545. [DOI] [PubMed] [Google Scholar]
- 13. Kim Y, Phan D, Rooij E, van Wang D-Z, McAnally J, Qi X, Richardson JA, Hill JA, Bassel-Duby R, Olson EN. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest 2008;118:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 2004;63:423–432. [DOI] [PubMed] [Google Scholar]
- 15. Jensen BC, Swigart PM, Laden M-E, DeMarco T, Hoopes C, Simpson PC. The alpha-1D is the predominant alpha-1-adrenergic receptor subtype in human epicardial coronary arteries. J Am Coll Cardiol 2009;54:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Groß S, Jahn C, Cushman S, Bär C, Thum T. SARS-CoV-2 receptor ACE2-dependent implications on the cardiovascular system: from basic science to clinical implications. J Mol Cell Cardiol 2020;144:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dixon JA, Spinale FG. Large animal models of heart failure. Circ Hear Fail 2009;2:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCall FC, Telukuntla KS, Karantalis V, Suncion VY, Heldman AW, Mushtaq M, Williams AR, Hare JM. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc 2012;7:1479–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rottiers V, Obad S, Petri A, McGarrah R, Lindholm MW, Black JC, Sinha S, Goody RJ, Lawrence MS, deLemos AS, Hansen HF, Whittaker S, Henry S, Brookes R, Najafi-Shoushtari SH, Chung RT, Whetstine JR, Gerszten RE, Kauppinen S, Naar AM. Pharmacological inhibition of a microRNA family in nonhuman primates by a seed-targeting 8-mer antimiR. Sci Transl Med 2013;5:212ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masson S, Batkai S, Beermann J, Bär C, Pfanne A, Thum S, Magnoli M, Balconi G, Nicolosi GL, Tavazzi L, Latini R, Thum T. Circulating microRNA-132 levels improve risk prediction for heart failure hospitalization in patients with chronic heart failure. Eur J Heart Fail 2018;20:78–85. [DOI] [PubMed] [Google Scholar]
- 21. Bijkerk R, de Bruin RG, van Solingen C, van Gils JM, Duijs JMGJ, van der Veer EP, Rabelink TJ, Humphreys BD, van Zonneveld AJ. Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney Int 2016;89:1268–1280. [DOI] [PubMed] [Google Scholar]
- 22. Hanin G, Yayon N, Tzur Y, Haviv R, Bennett ER, Udi S, Krishnamoorthy YR, Kotsiliti E, Zangen R, Efron B, Tam J, Pappo O, Shteyer E, Pikarsky E, Heikenwalder M, Greenberg DS, Soreq H. miRNA-132 induces hepatic steatosis and hyperlipidaemia by synergistic multitarget suppression. Gut 2018;67:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.