Abstract
Eukaryotic cells take up macromolecules and particles from the surrounding milieu and also internalize membrane proteins via a precise process of endocytosis. The role of endocytosis in diverse physiological processes such as cell adhesion, cell signaling, tissue remodeling, and healing is well recognized. The epithelial tight junctions (TJs), present at the apical lateral membrane, play a key role in cell adhesion and regulation of paracellular pathway. These vital functions of the TJ are achieved through the dynamic regulation of the presence of pore and barrier-forming proteins within the TJ complex on the plasma membrane. In response to various intracellular and extracellular clues, the TJ complexes are actively regulated by intracellular trafficking. The intracellular trafficking consists of endocytosis and recycling cargos to the plasma membrane or targeting them to the lysosomes for degradation. Increased intestinal TJ permeability is a pathological factor in inflammatory bowel disease (IBD), and the TJ permeability could be increased due to the altered endocytosis or recycling of TJ proteins. This review discusses the current information on endocytosis of intestinal epithelial TJ proteins. The knowledge of the endocytic regulation of the epithelial TJ barrier will provide further understanding of pathogenesis and potential targets for IBD and a wide variety of human disease conditions.
Keywords: tight junction, endocytosis, caveolae, clathrin, gut permeability
INTESTINAL TIGHT JUNCTION BARRIER STRUCTURE AND PHYSIOLOGICAL FUNCTION
Epithelial cell junctions occur at points of cell-cell contact. In vertebrates, cell-cell adhesion between 2 adjacent epithelial cells is mediated by tight junctions (TJs), adherens junctions (AJs), and desmosomes.1 The most apically located intercellular TJs are the principal regulator of paracellular permeability or passive diffusion of solutes and macromolecules in between adjacent cells (gate function). Tight junctions also polarize the epithelial cell membrane into apical and basolateral regions (fence function).2 By gate and fence function, TJs provide a gradient between the intestinal lumen and epithelial basolateral side that is important for active and passive ion transport and nutrient absorption. The TJs also act as a paracellular barrier and serve as a first line of defense against paracellular permeation of noxious antigens into the epithelia and subepithelial host tissue.3, 4
At TJs, an array of membrane-spanning proteins are linked to cytoplasmic plaque proteins, which in turn are attached to the actin cytoskeleton. The transmembrane proteins of TJs include single transmembrane domain proteins, junctional adhesion molecule (JAM), and coxsackievirus and adenovirus receptor (CAR); the triple transmembrane domain protein blood vessel epicardial substance (Bves); and the four-transmembrane domain proteins of the claudin and TAMP (tight junction-associated MARVEL proteins) families, which include occludin, tricellulin, and MarvelD3.5, 6 The combination of barrier-forming proteins (eg, occludin and claudin-1, -3, -4, -8), cationic pore-forming proteins (eg, claudin-2 and -15), and anionic pore-forming proteins (eg, claudin-10a and -17) primarily determine TJ permeability. Functionally, the TJs are known to have 2 pathways based on size and charge selectivity: a small, cation selective, high capacity “pore” pathway and a large, noncharge selective “leak” pathway. The pore pathway is defined by a subset of claudins, particularly pore-forming claudin-2, whereas occludin is involved in the regulation of leak pathway.7, 8 The expression of claudins varies in different intestinal segments and also along the crypt-villus axis, conferring regional gradients of size and charge selectivity.9, 10 Disturbances in this TJ composition play an important role in a variety of diseases including inflammatory bowel diseases, kidney diseases such as hypomagnesemia and hypercalciuria, autoimmune diseases, and dermal or retinal diseases.
MECHANISMS OF ENDOCYTOSIS
Endocytosis is a process that enables eukaryotic cells to take up any extracellular or membrane-bound molecules regardless of their size. Besides carrying extracellular molecules and particles into the cell, endocytic processes also internalize plasma membrane proteins. In epithelial cells, plasma membrane proteins are endocytosed mainly via 3 routes of clathrin-coated pits, lipid raft dependent caveolae, and macropinocytosis; 11 although, there are additional pathways mediated by GTPase regulator associated with focal adhesion kinase-1 (GRAF1) and adenosine diphosphate-ribosylation factor 6 (Arf6).12, 13 These endocytic pathways are defined by the morphology of the carriers, the cargo being transported, and the accessory regulatory cytosolic proteins that physically attach to the carriers.14 The internalized cargo is sorted in the endocytic compartments and can be either recycled back to the membrane through the early and recycling endosomes or targeted to the lysosomes for degradation through the late endosomes. Various modes of intracellular vesicular transport such as clathrin-mediated transport, caveolar transport, and macropinocytosis have been shown to regulate maintenance and function of TJs.15, 16 The TJ proteins such as occludin and claudin-2 were shown to be localized to lysosomes, and lysosomal inhibition increased total cellular TJ protein levels.17, 18 There is currently no evidence of retrograde recycling of TJ proteins from endosome to the trans-Golgi network.12
The clathrin-coated endocytic pathway is initiated by transient assembly of a complex protein machinery that selects and concentrates cargo molecules into a vesicle. Transferrin receptor (TfR) and epidermal growth factor receptor (EGFR) are known classical cargos that are endocytosed by the clathrin pathway. Distinct stages of nucleation, cargo selection, clathrin coat assembly, vesicle scission, uncoating, and clathrin component recycling have been identified in clathrin-mediated endocytosis.19 Studies have shown that pioneering molecules such as the BAR domain proteins F-BAR domain only protein 1 (FCHO1) and FCHO220 or the AP2 complex21 or comprehensive action of several proteins22 may initiate the endocytic process. These pioneer proteins organize interactions between cargo molecules, clathrin, and other accessory proteins.22 Dynamin, a 100-kDa GTPase, organizes itself around the neck of the newly formed vesicle and is needed for fission of the vesicles from the membrane in both clathrin-mediated and caveolar endocytosis.23 Several accessory proteins are critical for clathrin-coated endocytosis; for instance, AP180/CALM (clathrin recruitment24), amphiphysin (clathrin polymerization25), epsin (clathrin organization26), and auxilin (uncoating of clathrin vesicles27) are involved in clathrin-AP2 adaptin α-specific modules.
Caveolae are cholesterol-enriched invaginations, 50 to 100 nm in size, arising from lipid raft areas in the plasma membrane. Caveolin, a 21-24-kDa protein, is a principal constituent of caveolae.28 Though the role and efficiency of caveolae in endocytosis is questioned by a few studies, caveolar endocytosis is widely accepted as a specific pathway for uptake of particular substances.29 The caveolin-1-binding motif ΦXXXXΦXXΦ or ΦXΦXXXXΦ (where Φ is aromatic amino acids), a peptide signature present in cargo proteins, is known to facilitate their binding to caveolin.28 However, indirect interaction of caveolin with cargo proteins is also possible through other intermediating proteins. In contrast to the clathrin pits, the caveolae formed at the membrane are thought to be relatively static, and their internalization rate is slow in the absence of endocytic stimuli. An interaction of a specific ligand with caveolin triggers the rapid internalization of caveolae. Caveolin phosphorylation, action of GTP-binding protein dynamin, and cytoskeletal rearrangements are critical for the caveolar pinching off from the plasma membrane. The cortical actin cytoskeleton stabilizes caveolae at the membrane while microtubules support inward movement of caveolae. Actin depolymerization is also thought to cluster cargo proteins within membrane subdomains for caveolae-mediated internalization. For instance, actin depolymerization may concentrate occludin within TJ subdomains that are adjacent to the lateral membrane from where occludin undergoes caveolae-mediated internalization.30 After internalization, caveolae may integrate into a classical endocytic pathway, where Rab5 and Rab7 regulate early and late endosomal sorting, respectively. Cargos endocytosed via caveolae are targeted to lysosomes for degradation or can be recycled back to the membrane.
Macropinocytosis is a process in which a large amount of fluid or macromolecule is engulfed via the actin-driven extension of plasma membrane ruffles. Similar to caveolae and clathrin-mediated pathways, macropinocytosis can internalize TJ proteins and target them to the early/recycling endosomes.31
REGULATION OF TIGHT JUNCTIONS BY ENDOCYTOSIS
Tight junction proteins are constitutively exchanged between the membrane and cytoplasmic pool, indicating importance of endocytosis for normal cellular functions of intestinal epithelial cells. Such normal functions of intestinal epithelial cells mediated by endocytosis of junctional proteins may include but are not limited to the formation of TJ domains,32, 33 tissue remodeling,34 normal shedding of epithelial cells at the tip of villi,35 cell polarity,36 intracellular signal transduction pathway,11 repairing of TJ complex by removal of defective TJ proteins,11 wound healing,37 and fortification of TJ barrier during the stress.17 The half-life of occludin ranged between 6 and 8 hours in intestinal epithelial cells.38, 39 About 25%–40% of surface biotinylated occludin was found to be internalized in an hour,18, 40 and half or more of the endocytosed occludin was quickly recycled back to the membrane.40 Therefore, there is significant and constant trafficking of occludin between the membrane and the cytoplasmic compartments. The half-life of claudins was found to be 90 minutes for claudin-5,41 2 hours for claudin-2,42 and 4 hours for claudin-4,42, 43 and their endocytosis and recycling dynamics can vary based on the cell type, endocytosis, recycling pathway and their regulators, and the degradation rate.40 There is also evidence of hemophilic and heterophilic crossover endocytosis of TJ proteins, particularly claudins, between adjacent cells.44, 45 Such crossover endocytosis seems to be similar to the internalization of gap junction proteins and suggests coupled and synchronized cellular responses. Besides these constitutive, physiological contexts, numerous cell culture and in vivo studies have demonstrated endocytosis of TJ proteins in response to pathological stimuli.
How is a specific TJ protein removed from the membrane during endocytosis? Many studies show internalization of a specific TJ protein under various experimental conditions, but few studies reveal concurrent endocytosis of more than one TJ protein. Bulk endocytosis of TJ proteins can also occur as a secondary event after primary barrier loss.30 Therefore, the differences between various events of TJ barrier loss are probably dependent on the specific stimuli, the cell model, and the complex interactions between various TJ proteins. The interactions between specific TJ protein domains and others including cytoplasmic plaque protein ZO-1 are thought to be critical for release of TJ protein into endocytic compartments.46 Although diffusion of occludin and other TJ proteins within the membrane represents rapid remodeling for acute accommodation of structural and functional needs, removal of TJ proteins from the membrane by endocytosis probably signifies sustained responses. Because loss of clathrin is lethal in multicellular and not unicellular organisms,19 clathrin-mediated endocytosis assumingly plays a significant role in tissue remodeling in multicellular organisms in which TJs and other junctional complexes help bind adjacent cells.
Equally important to the endocytic process, the exocytic pathway is also known to affect TJ assembly and structure through recycling of previously endocytosed proteins or through the biosynthetic pathway. Recycling endosomes in conjunction with small GTPase Rab11 and Rab13 have been shown to be involved in assembly of TJs.47, 48 The biosynthetic pathway, which delivers newly synthesized proteins from the endoplasmic reticulum (ER) and the Golgi to the membrane, is also involved in the regulation of paracellular barrier. Reticulon-4B, a protein required for formation and stabilization of endoplasmic reticulum, is downregulated in Crohn’s disease and experimental colitis, and its downregulation is associated with the defects in paracellular barrier and reduced expression of occludin. The ER stress and disruption of ER and Golgi during intestinal inflammation may also cause defects in TJ assembly.49, 50 Disruption of trafficking between the ER and the Golgi or within the Golgi complex and fragmentation of the Golgi is known to compromise TJ assembly and epithelial TJ barrier.51–53
Endocytosis of Occludin
The role of occludin as a barrier-forming TJ protein is established by several in vitro and in vivo studies.54–57 Overexpression of occludin has been shown to reduce TJ permeability,56 whereas depletion of occludin increased macromolecular TJ permeability.54, 57 Steady-state endocytosis, recycling of occludin,36, 58 and its diffusion within the plasma membrane59 have been demonstrated previously. Several studies have pointed out caveolae as a primary route for endocytosis of occludin. A series of elegant studies from Turner and coworkers have demonstrated rapid, actomyosin-dependent, caveolae-mediated occludin endocytosis, correlating with the functional loss of TJ barrier, after T-cell activation and direct administration of recombinant tumor necrosis factor (TNF) or lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells (LIGHT) in mouse intestine.60–63 Activation of small GTPase RhoA by cytotoxic necrotizing factor-1, a toxin from Escherichia coli, has also been shown to cause caveolae-mediated endocytosis of occludin.64 Furthermore, extraction of cholesterol, which enriches caveolae, has been shown to prevent occludin internalization caused by actin depolymerization.30 In our studies, we have observed an inverse relationship between caveolin-1 and occludin protein levels. Intestinal Caco-2 cells genetically manipulated to have reduced levels of caveolin-1 showed increased level of occludin and reduced rate of occludin endocytosis.65 We also found that exogenous cell permeant caveolin-1 scaffolding domain was sufficient to downregulate the occludin protein level in Caco-2 cells.65 In the same way, intestine specific disruption of von Hippel-Lindau tumor suppressor (VHL), an E3 ubiquitin protein ligase, has been shown to increase caveolin-1 expression in mice colon via hypoxia-inducible factor (HIF) activation and cause reduction in occludin expression.66 In the same study, knockdown of caveolin-1 in colonic HCT116 cells increased occludin expression, and exogenous expression of caveolin-1 decreased occludin expression. On a similar note, increased caveolin-1 expression precedes the loss of occludin expression during blood-brain barrier breakdown.67 Although reduced caveolin-1 levels have been shown to cause diminished caveolae formation and reduced targeting of occludin to lysosomes,65 Rab13-dominant active mutant inhibited postendocytic recycling of occludin to the membrane, suggesting the role of Rab13 in occludin recycling to the membrane.36
The mechanism of occludin removal from the membrane during endocytosis is not entirely clear. The C-terminal coiled-coil occludin/ELL domain (OCEL) was shown to be critical in case of TNF-α-induced endocytosis of occludin.62, 68 Though occludin and caveolin-1 were coimmunoprecipitated, deletion of C‐terminal 150 amino acids of occludin did not affect its interaction with caveolin-1, suggesting that caveolin-1 might interact with occludin indirectly through another protein.69 Moreover, the fact that occludin does not have consensus caveolin-binding motif69 raises the possibility that caveolae-mediated endocytosis of occludin is orchestrated by other caveolae-regulating proteins.65 In our recent studies, autophagy-related ATG6/beclin-1, which is involved in multiple vesicle trafficking and endocytosis pathways, was found to form a complex with occludin, providing an additional mechanism of occludin endocytosis.18 Activation of beclin-1 stimulated the rate of occludin endocytosis, increased colocalization of occludin to caveolae, and increased targeting of occludin to lysosomes, resulting in reduced total occludin levels.18 Among the accessory proteins involved in occludin endocytosis, the role of small GTPases Rab5 is particularly remarkable. Rab5 controls intracellular vesicle docking and fusion and is closely involved in the movement of occludin between cytoplasmic vesicular and membrane domains. The exogenous expression of dominant negative Rab5 trapped occludin at the tight junction, and constitutively active Rab5 led to the retention of occludin in the cytoplasmic vesicles.70 Caveolin-1 can directly bind and activate Rab5, resulting in increased caveolar endocytosis.71 Although, caveolae are not an exclusive route for occludin endocytosis. Additionally, Ca+ depletion has been shown to cause clathrin-mediated endocytosis of occludin,72 whereas IFN-γ induces endocytosis of occludin via a macropinocytosis-like process, sorting it to the early recycling endosomal compartment.31 Also, poly-L-arginine treatment of Caco-2 cells induced transient clathrin-mediated internalization of occludin and increased paracellular permeability.73
Endocytosis of Claudins
Claudins have diverse expression patterns and functions. The claudin family includes claudins that form cation pores (claudin-2, -10b, and -15), anion pores (claudin-10a and -17), and barrier-forming members (claudin-1, -3, -4, -5, -6, -8, -12, -18, and -19).6 Integration of claudins into the TJs may also vary; for instance, claudin-1 has less mobile fraction within the TJ membrane compared with occludin.59 Moreover, though many claudins are restricted to TJ, some such as claudin‐7 are also localized to the lateral plasma membrane.74 All these factors may influence the endocytic process for claudins under physiological or pathological conditions. A peculiar “eat each other” kind of endocytosis has been described for claudins,44 wherein during cellular remodeling, GFP-claudin-3 was endocytosed together with endogenous claudins. The claudin aggregates on 2 apposed membranes of TJs were not separated but co-endocytosed together into one of the adjacent cells. Moreover, other TJ proteins including occludin, JAM, and ZO-1 were dissociated from the claudins aggregates before the endocytosis. Wounding of the monolayers, which triggers cellular motility, increased the rate of claudin endocytosis, suggesting a crucial role for claudin internalization during the cell remodeling. The claudins are the key components of the TJ strands, which are visible on the freeze fracture electron microscopy as the contact points between 2 adjacent cells. The intracellular trafficking must play a principal role in the continuous renewal of the TJ strands. Indeed, a recent model speculated polymerization and incorporation of newly synthesized claudins at the strand breaks or free edges of strands on the basal side of TJs and removal of old claudins via endocytosis, explaining continous renewal of TJ strands without losing the cell-to-cell contact and the paracellular barrier.75 In contrast, occludin is thought to be able to associate with preformed TJ strands within the apical TJ complex.75
It seems that clathrin pits are the main route for claudins internalization. Claudin-2 is known to be endocytosed via MEK/ERK pathway–mediated activation of clathrin pit after EGF treatment. Moreover, the clathrin pits carrying claudin-2 are known to be positive for adaptin α, a key constituent of clathrin adaptor protein AP-2 complex that links cargo proteins to the clathrin coat and the accessory proteins regulating coat assembly and disassembly.76 Inhibition of clathrin-mediated endocytosis also increases surface expression of claudin-16 L203X, a mutant with entire C-terminal cytosolic domain deletion,77 supporting clathrin pits as a constitutive endocytic route for claudin-16.78 Familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC)–related mutations in claudin-16 have been shown to affect intracellular trafficking of claudin-16 and its ability to facilitate paracellular Mg2+ transport.79 Lipid kinase PIKfyve promotes claudin-1 and -2 endocytic recycling,80 whereas Rab14a regulates endocytic recycling of claudin-2.81 Infectious agents such as Hepatitis C and West Nile virus have been shown to cause clathrin and dynamin-dependent endocytosis of claudin-1.82, 83 Also, claudin-1-derived peptide C1C2 and the endogenous claudin-1 were found to be mainly internalized via clathrin pathway.84 Interactions of phosphorylated claudin-4 with β-arrestin 2, which regulates signal transduction at G protein-coupled receptors, have been shown to cause clathrin-dependent claudin-4 internalization.85 Claudins can be endocytosed via caveolae, too. In an oxygen and glucose deprivation (OGD) model of blood-brain barrier, knockdown of caveolin-1 with siRNA prevented the degradation of claudin-5. Nitric oxide promoted the caveolar endocytosis of claudin-5 and its subsequent lysosomal degradation under ischemic conditions.86
Phosphorylation is a major mechanism among the post-translational modifications that affect localization of TJ proteins. Claudin-1 and -2 are dephosphorylated during their clathrin-dependent internalization and lysosomal degradation in response to hypotonic stress. However, hypotonic stress failed to reduce expression levels of T191E phosphorylated claudin-1 and S208E phosphorylated claudin-2 mutant.87 But the phosphorylation effect on claudin localization can vary among different claudins. The cAMP-dependent protein kinase–mediated phosphorylation at T192 redistributed claudin-3; 88 but EPH receptor A2 (EphA2), which induced a specific phosphorylation of Tyr-208 in the cytoplasmic tail of claudin-4, caused a delay in relocalization of claudin-4 to the TJs after the calcium switch.89 Claudins are also known to be endocytosed and colocalized to late endosome marker Rab7 or the lysosome enzyme cathepsin D during polyubiquitination. The p80 isoform of the E3 ubiquitin ligase ligand of Numb-protein X1 (LNX1p80) directly binds to claudin-1 and promotes its proteasome-independent degradation through polyubiquitination.90
TIGHT JUNCTION BARRIER DEFECTS IN INFLAMMATORY BOWEL DISEASES
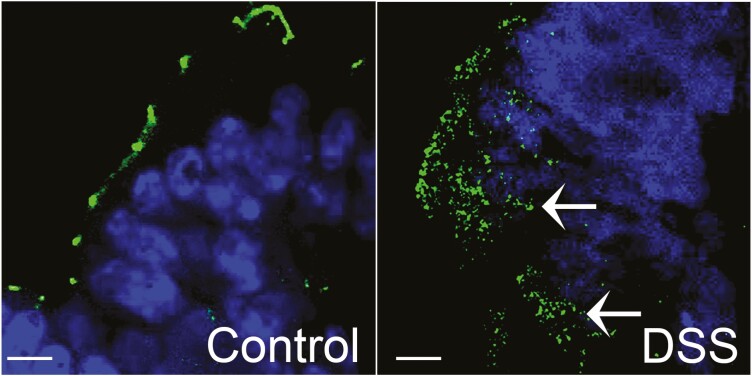
The defects in intestinal TJ barrier are believed to allow increased antigenic penetration, resulting in amplified inflammatory response in intestinal tissue. Several lines of evidence indicate the importance of TJ barrier defects in the pathogenesis of IBD. Structural and morphological abnormalities in the strands of the TJs and increased intestinal permeability have been described in Crohn’s disease, ulcerative colitis, and celiac disease patients.91–94 Moreover, the TJ composition in terms of pore-forming and barrier-forming proteins is altered in Crohn’s disease,95 ulcerative colitis,96–98 and microscopic colitis.99, 100 In contrast to the distinct, dot-like presence of occludin and JAM-A at the apical region of the lateral plasma membrane in epithelial cells of normal mucosa, occludin and JAM-A were found to be internalized and present in subapical vesicle-like structures in mucosal biopsies from patients with actively inflamed ulcerative colitis.31 In addition, numerous studies have demonstrated increased pro-inflammatory cytokines in IBD mucosal samples, and these cytokines have been shown to alter TJ permeability in cell or animal IBD models. For instance, in live imaging of mouse intestine, TNF-α-mediated activation of myosin light chain kinase (MLCK) has been shown to trigger caveolin-1-dependent endocytosis of occludin within 90 minutes.62 Proteins that stabilize TJ barrier-forming occludin at the TJs have been shown to have reduced levels in IBD tissue, and their genetic deficiency exaggerates experimental colitis.38, 101 We have previously shown that in dextran sulfate sodium (DSS) colitis, a mouse model of IBD, occludin expression shifts from detergent insoluble to detergent soluble fraction on sucrose density gradient,101 indicating its redistribution from the TJs. In experimental DSS colitis, presence of occludin away from the apical membrane of colonic epithelium can be seen commonly (Fig. 1). Similarly, in a trinitrobenzenesulfonic acid (TNBS) colitis mouse model, TJ proteins have been shown to be redistributed in membrane micro domains.102 Myosin light chain kinase–induced occludin endocytosis has been shown to mediate intestinal epithelial barrier dysfunction during anoxia/reoxygenation injury.103 In a mouse model of necrotizing enterocolitis (NEC), internalization of enterocyte claudin-4 and occludin occurs at early time points.104 Similarly, claudin-3 in enterocytes has been shown to be remarkably internalized in a neonatal rat NEC model. Based on these studies, it is quite understandable that altered intracellular trafficking or increased endocytosis of TJ barrier proteins can disrupt TJ barrier in human diseases.50 Autophagy, a cell survival and degradation pathway, is closely aligned with endocytic processes. The genome wide association studies have identified mutations in autophagy related genes ATG16L1 and IRGM as substantiated risk factors for Crohn’s disease. In our previous studies, autophagy enhanced TJ barrier via degradation of claudin-2. Induction of autophagy increasingly removed claudin-2 from the membrane and targeted it to the lysosomes.17 Though specifics of the role of autophagy in TJ barrier are not yet known, association of autophagy with intracellular trafficking machinery, including early endosomes,105 clathrin pits,106 caveolin-1,107 cell signaling involved in intracellular trafficking,108 and overlap and intersection of endocytotic and autophagic pathways,109, 110 is expected to play an important role in the homeostasis of TJ protein trafficking in health and disease.
FIGURE 1.
Occludin (green) is commonly seen within the cytoplasm (arrows) in DSS colitis compared with the distinct punctate appearance at the tight junctions on the membrane in control mouse colon. Nuclei: blue. Bar = 7.5 µm.
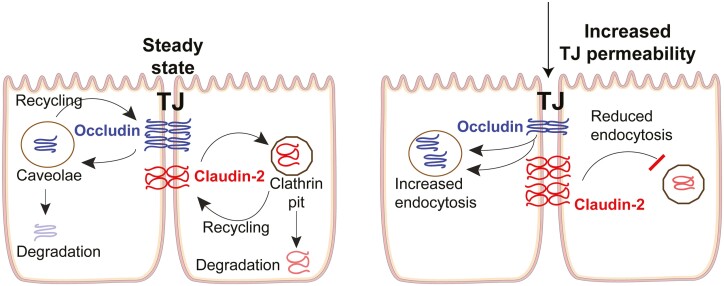
It is also noteworthy that the rate of endocytosis of membrane proteins is balanced by the rate of exocytosis or recycling of proteins to the membrane. Therefore, reduced rate of recycling or exocytosis of TJ barrier proteins can have similar disruptive effects on TJ barrier as those of increased endocytosis. For pore-forming TJ proteins such as claudin-2, increased endocytosis or decreased exocytosis would reduce TJ permeability (Fig. 2). Indeed, redistribution of sealing TJ proteins occludin, claudin-5, and -8 away from the TJs has been demonstrated in active Crohn’s disease,95 whereas internalization of claudin-4 was characteristic for refractory celiac disease.111 Zonulin (also identified as the precursor for haptoglobin-2 [pre-HP2]), whose levels are increased in the intestinal mucosa of celiac disease patients, is also known to increase intestinal permeability via activation EGF receptor and proteinase-activated receptor 2 (PAR2).112 The effect of alteration in TJ composition is, however, context dependent. For instance, an increase in cation and water permeable claudin-2 pores causes excessive water efflux into the lumen, causing diarrheal symptoms; such water efflux can also help clearance of the enteric pathogens.113 Numerous other human diseases including metabolic, neurodegenerative, and a variety of dominant myopathies and muscular dystrophies are linked to the defects in intracellular transport process; and in view of the vital importance of the core endocytic machinery, common mutations in endocytic or intracellular trafficking genes are not likely to be found.13, 114 Therefore, it is critical to understand how endocytic processes contribute to the cell and organismal health and how their dysregulation contributes to the human diseases.
FIGURE 2.
In steady state, the TJ permeability is balanced by the rate of endocytosis and recycling of pore- and barrier-forming TJ proteins. Accelerated endocytosis of barrier-forming occludin or reduced endocytosis of pore-forming claudin-2 under various pathological conditions lead to increased TJ permeability.
CONCLUSIONS
Various intracellular trafficking processes finely regulate epithelial TJs to maintain cell homeostasis and impart dynamicity to the TJ barrier. Intracellular trafficking of TJ proteins is also an efficient way to cope and communicate with external environment. At times, such as after Ca2+ chelation, the endocytic processes could internalize TJ proteins in a bulk but synchronized way, whereas other stimuli can alter the trafficking of specific TJ proteins and shape the TJ barrier. The insertion and removal of TJ proteins, to and from the membrane, also helps in tissue remodeling, wound healing, and fortification of the TJ barrier during stress. Though pathological insults can result into internalization of barrier-forming TJ proteins, the TJ barrier can be healed quickly through recycling of TJ proteins back to the membrane. Most importantly, cells can adjust the TJ barrier via intracellular trafficking under various physiological or pathological conditions without expending time and energy on new protein synthesis. The mechanisms and extent of TJ protein endocytosis and recycling varies based on the cell type and the stimuli. Further studies are warranted to define the commonalities and the specifics of TJ protein trafficking under various physiological and pathological conditions. The endocytic mechanisms of TJ barrier disruption during clinical mucosal inflammation are poorly understood currently, and there is a need for phenomenological or mechanistic research investigating the role of this important mechanism in intestinal inflammation and mucosal healing. The advances in the understanding of the molecular mechanisms involved in intracellular trafficking of TJ proteins will help rationalize and identify therapeutic targets against IBD and other autoimmune and infectious diseases.
Supported by: This work is supported in part by the National Institute Of Diabetes And Digestive And Kidney Diseases R01DK114024 and K01DK100562 to PN and 2R01 DK64165 to TM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: The authors have no potential conflicts of interest.
REFERENCES
- 1. Giepmans BN, van Ijzendoorn SC. Epithelial cell-cell junctions and plasma membrane domains. Biochim Biophys Acta. 2009;1788:820–831. [DOI] [PubMed] [Google Scholar]
- 2. Mandel LJ, Bacallao R, Zampighi G. Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions. Nature. 1993;361:552–555. [DOI] [PubMed] [Google Scholar]
- 3. Podolsky DK. Healing the epithelium: solving the problem from two sides. J Gastroenterol. 1997;32:122–126. [DOI] [PubMed] [Google Scholar]
- 4. Ma TY, Anderson JM.. Tight Junction and Intestinal Barrier. Textbook of Gastrointestinal Physiology. Johnson LR, ed. Philadelphia, PA: Elsevier Health Sciences; 2006:1559–1594. [Google Scholar]
- 5. Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma Thomas NP, Al-sadi Rana. Tight Junctions and the Intestinal Barrier. Physiology of the Gastrointestinal Tract. Said H, ed. 6th ed. Academic Press; 2018:587–640. [Google Scholar]
- 7. Shen L, Weber CR, Raleigh DR, et al. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Itallie CM, Holmes J, Bridges A, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. [DOI] [PubMed] [Google Scholar]
- 9. Fujita H, Chiba H, Yokozaki H, et al. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J Histochem Cytochem. 2006;54:933–944. [DOI] [PubMed] [Google Scholar]
- 10. Mariadason JM, Nicholas C, L’Italien KE, et al. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology. 2005;128:1081–1088. [DOI] [PubMed] [Google Scholar]
- 11. Ivanov AI, Nusrat A, Parkos CA. The epithelium in inflammatory bowel disease: potential role of endocytosis of junctional proteins in barrier disruption. Novartis Found Symp. 2004;263:115–124; discussion 124. [PubMed] [Google Scholar]
- 12. Stamatovic SM, Johnson AM, Sladojevic N, et al. Endocytosis of tight junction proteins and the regulation of degradation and recycling. Ann N Y Acad Sci. 2017;1397:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. [DOI] [PubMed] [Google Scholar]
- 14. Hinze C, Boucrot E. Endocytosis in proliferating, quiescent and terminally differentiated cells. J Cell Sci. 2018;131:jcs216804. [DOI] [PubMed] [Google Scholar]
- 15. Terry SJ, Zihni C, Elbediwy A, et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. [DOI] [PubMed] [Google Scholar]
- 17. Nighot PK, Hu CA, Ma TY. Autophagy enhancement of intestinal epithelial tight junction barrier function by targeting claudin-2 degradation. J Biol Chem. 2015;290:7234–7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong M, Ganapathy AS, Suchanec E, et al. Intestinal epithelial tight junction barrier regulation by autophagy related protein ATG6/beclin 1. Am J Physiol Cell Physiol. 2019;316:C753–C765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. [DOI] [PubMed] [Google Scholar]
- 20. Henne WM, Boucrot E, Meinecke M, et al. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cocucci E, Aguet F, Boulant S, et al. The first five seconds in the life of a clathrin-coated pit. Cell. 2012;150:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19:313–326. [DOI] [PubMed] [Google Scholar]
- 23. Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ford MG, Pearse BM, Higgins MK, et al. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. [DOI] [PubMed] [Google Scholar]
- 25. Peter BJ, Kent HM, Mills IG, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. [DOI] [PubMed] [Google Scholar]
- 26. Ford MG, Mills IG, Peter BJ, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. [DOI] [PubMed] [Google Scholar]
- 27. McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. [DOI] [PubMed] [Google Scholar]
- 28. Couet J, Li S, Okamoto T, et al. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. [DOI] [PubMed] [Google Scholar]
- 29. Kiss AL. Caveolae and the regulation of endocytosis. Adv Exp Med Biol. 2012;729:14–28. [DOI] [PubMed] [Google Scholar]
- 30. Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruewer M, Utech M, Ivanov AI, et al. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. Faseb J. 2005;19:923–933. [DOI] [PubMed] [Google Scholar]
- 32. Mruk DD, Lau AS, Conway AM. Crosstalk between Rab GTPases and cell junctions. Contraception. 2005;72:280–290. [DOI] [PubMed] [Google Scholar]
- 33. Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim Biophys Acta. 2008;1778:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Charrier LE, Loie E, Laprise P. Mouse Crumbs3 sustains epithelial tissue morphogenesis in vivo. Sci Rep. 2015;5:17699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guan Y, Watson AJ, Marchiando AM, et al. Redistribution of the tight junction protein ZO-1 during physiological shedding of mouse intestinal epithelial cells. Am J Physiol Cell Physiol. 2011;300:C1404–C1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morimoto S, Nishimura N, Terai T, et al. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem. 2005;280:2220–2228. [DOI] [PubMed] [Google Scholar]
- 37. Fletcher SJ, Poulter NS, Haining EJ, et al. Clathrin-mediated endocytosis regulates occludin, and not focal adhesion, distribution during epithelial wound healing. Biol Cell. 2012;104:238–256. [DOI] [PubMed] [Google Scholar]
- 38. Chen Y, Zhang HS, Fong GH, et al. PHD3 stabilizes the tight junction protein occludin and protects intestinal epithelial barrier function. J Biol Chem. 2015;290:20580–20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dokladny K, Ye D, Kennedy JC, et al. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: regulatory role of heat shock factor-1. Am J Pathol. 2008;172:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dukes JD, Fish L, Richardson JD, et al. Functional ESCRT machinery is required for constitutive recycling of claudin-1 and maintenance of polarity in vertebrate epithelial cells. Mol Biol Cell. 2011;22:3192–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mandel I, Paperna T, Volkowich A, et al. The ubiquitin-proteasome pathway regulates claudin 5 degradation. J Cell Biochem. 2012;113:2415–2423. [DOI] [PubMed] [Google Scholar]
- 42. Capaldo CT, Farkas AE, Hilgarth RS, et al. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell. 2014;25:2710–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Itallie CM, Colegio OR, Anderson JM. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J Membr Biol. 2004;199:29–38. [DOI] [PubMed] [Google Scholar]
- 44. Matsuda M, Kubo A, Furuse M, et al. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci. 2004;117:1247–1257. [DOI] [PubMed] [Google Scholar]
- 45. Gehne N, Lamik A, Lehmann M, et al. Cross-over endocytosis of claudins is mediated by interactions via their extracellular loops. Plos One. 2017;12:e0182106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turner JR, Buschmann MM, Romero-Calvo I, et al. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol. 2014;36:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamamura R, Nishimura N, Nakatsuji H, et al. The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions. Mol Biol Cell. 2008;19:971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guichard A, Cruz-Moreno B, Cruz-Moreno BC, et al. Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions. Cell Host Microbe. 2013;14:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McGuckin MA, Eri R, Simms LA, et al. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–113. [DOI] [PubMed] [Google Scholar]
- 50. Lechuga S, Ivanov AI. Disruption of the epithelial barrier during intestinal inflammation: quest for new molecules and mechanisms. Biochim Biophys Acta Mol Cell Res. 2017;1864:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Naydenov NG, Brown B, Harris G, et al. A membrane fusion protein αSNAP is a novel regulator of epithelial apical junctions. Plos One. 2012;7:e34320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Naydenov NG, Harris G, Brown B, et al. Loss of soluble N-ethylmaleimide-sensitive factor attachment protein α (αSNAP) induces epithelial cell apoptosis via down-regulation of Bcl-2 expression and disruption of the Golgi. J Biol Chem. 2012;287:5928–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu M, Yang S, Qiu Y, et al. Par-3 modulates intestinal epithelial barrier function through regulating intracellular trafficking of occludin and myosin light chain phosphorylation. J Gastroenterol. 2015;50:1103–1113. [DOI] [PubMed] [Google Scholar]
- 54. Al-Sadi R, Khatib K, Guo S, et al. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1054–G1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Balda MS, Whitney JA, Flores C, et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McCarthy KM, Skare IB, Stankewich MC, et al. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109 (Pt 9):2287–2298. [DOI] [PubMed] [Google Scholar]
- 57. Mir H, Meena AS, Chaudhry KK, et al. Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice. Biochim Biophys Acta. 2016;1860:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nighot PK, Blikslager AT. Chloride channel ClC-2 modulates tight junction barrier function via intracellular trafficking of occludin. Am J Physiol Cell Physiol. 2012;302:C178–C187. [DOI] [PubMed] [Google Scholar]
- 59. Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clayburgh DR, Barrett TA, Tang Y, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Clayburgh DR, Musch MW, Leitges M, et al. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Marchiando AM, Shen L, Graham WV, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schwarz BT, Wang F, Shen L, et al. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hopkins AM, Walsh SV, Verkade P, et al. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116:725–742. [DOI] [PubMed] [Google Scholar]
- 65. Nighot PK, Leung L, Ma TY. Chloride channel ClC- 2 enhances intestinal epithelial tight junction barrier function via regulation of caveolin-1 and caveolar trafficking of occludin. Exp Cell Res. 2017;352:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xie L, Xue X, Taylor M, et al. Hypoxia-inducible factor/MAZ-dependent induction of caveolin-1 regulates colon permeability through suppression of occludin, leading to hypoxia-induced inflammation. Mol Cell Biol. 2014;34:3013–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nag S, Venugopalan R, Stewart DJ. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood-brain barrier breakdown. Acta Neuropathol. 2007;114:459–469. [DOI] [PubMed] [Google Scholar]
- 68. Buschmann MM, Shen L, Rajapakse H, et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell. 2013;24: 3056–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Itallie CM, Anderson JM. Caveolin binds independently to claudin-2 and occludin. Ann N Y Acad Sci. 2012;1257:103–107. [DOI] [PubMed] [Google Scholar]
- 70. Coyne CB, Shen L, Turner JR, et al. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hagiwara M, Shirai Y, Nomura R, et al. Caveolin-1 activates Rab5 and enhances endocytosis through direct interaction. Biochem Biophys Res Commun. 2009;378:73–78. [DOI] [PubMed] [Google Scholar]
- 72. Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yamaki T, Kamiya Y, Ohtake K, et al. A mechanism enhancing macromolecule transport through paracellular spaces induced by Poly-L-Arginine: poly-L-Arginine induces the internalization of tight junction proteins via clathrin-mediated endocytosis. Pharm Res. 2014;31:2287–2296. [DOI] [PubMed] [Google Scholar]
- 74. Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Van Itallie CM, Lidman KF, Tietgens AJ, et al. Newly synthesized claudins but not occludin are added to the basal side of the tight junction. Mol Biol Cell. 2019;30:1406–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ikari A, Takiguchi A, Atomi K, et al. Epidermal growth factor increases clathrin-dependent endocytosis and degradation of claudin-2 protein in MDCK II cells. J Cell Physiol. 2011;226:2448–2456. [DOI] [PubMed] [Google Scholar]
- 77. Müller D, Kausalya PJ, Meij IC, et al. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: blocking endocytosis restores surface expression of a novel Claudin-16 mutant that lacks the entire C-terminal cytosolic tail. Hum Mol Genet. 2006;15:1049–1058. [DOI] [PubMed] [Google Scholar]
- 78. Khailova L, Dvorak K, Arganbright KM, et al. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G940–G949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kausalya PJ, Amasheh S, Günzel D, et al. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Invest. 2006;116:878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dukes JD, Whitley P, Chalmers AD. The PIKfyve inhibitor YM201636 blocks the continuous recycling of the tight junction proteins claudin-1 and claudin-2 in MDCK cells. PLoS One. 2012;7:e28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu R, Johnson DL, Stewart L, et al. Rab14 regulation of claudin-2 trafficking modulates epithelial permeability and lumen morphogenesis. Mol Biol Cell. 2014;25:1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Farquhar MJ, Hu K, Harris HJ, et al. Hepatitis C virus induces CD81 and claudin-1 endocytosis. J Virol. 2012;86:4305–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xu Z, Waeckerlin R, Urbanowski MD, et al. West Nile virus infection causes endocytosis of a specific subset of tight junction membrane proteins. Plos One. 2012;7:e37886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Staat C, Coisne C, Dabrowski S, et al. Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers. Biomaterials. 2015;54:9–20. [DOI] [PubMed] [Google Scholar]
- 85. Cong X, Zhang Y, Li J, et al. Claudin-4 is required for modulation of paracellular permeability by muscarinic acetylcholine receptor in epithelial cells. J Cell Sci. 2015;128:2271–2286. [DOI] [PubMed] [Google Scholar]
- 86. Liu J, Weaver J, Jin X, et al. Nitric oxide interacts with caveolin-1 to facilitate autophagy-lysosome-mediated claudin-5 degradation in oxygen-glucose deprivation-treated endothelial cells. Mol Neurobiol. 2016;53:5935–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fujii N, Matsuo Y, Matsunaga T, et al. Hypotonic stress-induced down-regulation of claudin-1 and -2 mediated by dephosphorylation and clathrin-dependent endocytosis in renal tubular epithelial cells. J Biol Chem. 2016;291:24787–24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. D’Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233–26240. [DOI] [PubMed] [Google Scholar]
- 89. Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J Biol Chem. 2005;280:42375–42382. [DOI] [PubMed] [Google Scholar]
- 90. Takahashi S, Iwamoto N, Sasaki H, et al. The E3 ubiquitin ligase LNX1p80 promotes the removal of claudins from tight junctions in MDCK cells. J Cell Sci. 2009;122:985–994. [DOI] [PubMed] [Google Scholar]
- 91. Marin ML, Geller SA, Greenstein AJ, et al. Ultrastructural pathology of Crohn’s disease: correlated transmission electron microscopy, scanning electron microscopy, and freeze fracture studies. Am J Gastroenterol. 1983;78:355–364. [PubMed] [Google Scholar]
- 92. Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. [DOI] [PubMed] [Google Scholar]
- 93. Das P, Goswami P, Das TK, et al. Comparative tight junction protein expressions in colonic Crohn’s disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch. 2012;460:261–270. [DOI] [PubMed] [Google Scholar]
- 94. Landy J, Al-Hassi HO, Ronde E, et al. Innate immune factors in the development and maintenance of pouchitis. Inflamm Bowel Dis. 2014;20:1942–1949. [DOI] [PubMed] [Google Scholar]
- 95. Zeissig S, Bürgel N, Günzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. [DOI] [PubMed] [Google Scholar]
- 97. Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. [DOI] [PubMed] [Google Scholar]
- 98. Krug SM, Bojarski C, Fromm A, et al. Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 2018;11:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bürgel N, Bojarski C, Mankertz J, et al. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433–443. [DOI] [PubMed] [Google Scholar]
- 100. Tagkalidis PP, Gibson PR, Bhathal PS. Microscopic colitis demonstrates a T helper cell type 1 mucosal cytokine profile. J Clin Pathol. 2007;60:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nighot P, Young K, Nighot M, et al. Chloride channel ClC-2 is a key factor in the development of DSS-induced murine colitis. Inflamm Bowel Dis. 2013;19:2867–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li Q, Zhang Q, Zhang M, et al. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. Febs J. 2008;275:411–420. [DOI] [PubMed] [Google Scholar]
- 103. Jin Y, Blikslager AT. Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury. Am J Physiol Cell Physiol. 2016;311:C996–C1004. [DOI] [PubMed] [Google Scholar]
- 104. Bergmann KR, Liu SX, Tian R, et al. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol. 2013;182:1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fraser J, Simpson J, Fontana R, et al. Targeting of early endosomes by autophagy facilitates EGFR recycling and signalling. EMBO Rep. 2019;20:e47734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tian Y, Chang JC, Fan EY, et al. Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer’s APP-CTF for terminal degradation via autophagy. Proc Natl Acad Sci U S A. 2013;110:17071–17076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chen ZH, Cao JF, Zhou JS, et al. Interaction of caveolin-1 with ATG12-ATG5 system suppresses autophagy in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2014;306:L1016–L1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang J, Whiteman MW, Lian H, et al. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating beclin 1. J Biol Chem. 2009;284:21412–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tooze SA, Abada A, Elazar Z. Endocytosis and autophagy: exploitation or cooperation? Cold Spring Harb Perspect Biol. 2014;6:a018358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nighot P, Ma T. Role of autophagy in the regulation of epithelial cell junctions. Tissue Barriers. 2016;4:e1171284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schumann M, Kamel S, Pahlitzsch ML, et al. Defective tight junctions in refractory celiac disease. Ann N Y Acad Sci. 2012;1258:43–51. [DOI] [PubMed] [Google Scholar]
- 112. Tripathi A, Lammers KM, Goldblum S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A. 2009;106:16799–16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tsai PY, Zhang B, He WQ, et al. IL-22 upregulates epithelial claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe. 2017;21:671–681.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. [DOI] [PubMed] [Google Scholar]