Abstract
Background
We aimed to investigate (1) the stability of inflammatory aspects of diet over 1 year among persons with inflammatory bowel disease (IBD) and (2) the impact of change in diet on changes in inflammation and IBD symptoms over 1 year.
Methods
Participants were recruited to the Manitoba Living with IBD Study and completed the Harvard Food Frequency Questionnaire (FFQ). The Dietary Inflammatory Index (DII) and the Empirical Dietary Inflammatory Index (EDII) were used to calculate the inflammatory potential of the diet. Inflammation was measured by fecal calprotectin (≥250 µg/g). Symptoms were measured by the IBD Symptom Inventory (IBDSI). All measures were obtained at baseline and 1 year. Dietary Inflammatory Index and Empirical Dietary Inflammatory Index scores >0 and <0 reflect pro- and anti-inflammatory diet, respectively. Variance components analyses were used to describe diet stability. Associations between changes in diet and changes in active inflammation and symptoms were assessed using ordinal logistic regression and multilevel linear regression modeling.
Results
One hundred thirty-five participants (66% CD) were included. Approximately one third of the variance in EDII (36%) and DII (33%) scores was explained by changes in diet over time. Each unit increase in the change in EDII (baseline to follow-up) was associated with a greater odds of FCAL, indicating active inflammation (>250 µg/g; odds ratio, 3.1; 95% confidence interval [CI], 1.02–9.93; P = 0.04) and with a rise in IBDSI of 6.7 (95% CI, 1.0–12.4; P = 0.022; theoretical IBDSI range, 0–81). There was no association between changes in DII and changes in FCAL or IBDSI.
Conclusion
The EDII, but not the DII, may have utility to identify the inflammatory potential of diet. This inflammatory potential can contribute to inflammation and/or disease symptoms in persons with IBD.
INTRODUCTION
Inflammatory bowel disease (IBD)–related inflammation and symptom severity have been linked to diet.1–6 Despite this, the stability of diet among people living with IBD is not well documented. People may try to manage their IBD-related symptoms by experimenting with diet changes.3 However, there is a lack of consistent data on whether dietary manipulation can affect the course of IBD.
Many diets are marketed as having anti-inflammatory properties based on certain components present in these diets that may regulate the inflammatory process. The concept of an anti-inflammatory diet has significant appeal to IBD patients and their caregivers who may be seeking nonpharmaceutical based therapies to control their symptoms and prevent intestinal inflammation. However, evidence is lacking from longitudinal studies about the degree to which people with IBD change their diets and the impact that diet change may have on inflammation and symptoms.
One of the challenges in assessing the impact of diet on various health outcomes is the many dimensions to a diet. One must cluster large numbers of dietary items based on various factors to create summary indices that can then be useful for determining the impact of diet on a specified health outcome. Two diet indices, the Dietary Inflammatory Index (DII),7 which categorizes by nutrients, and the Empirical Dietary Inflammatory Index (EDII),8 which categorizes by food types, have been developed as tools to measure the inflammatory potential of the diet. We sought to assess whether a change in dietary inflammatory potential was associated with changes in intestinal inflammation, disease symptoms, or flares. We also sought to assess the stability of the diet over 1 year by determining the stability of the DII and EDII over a 1-year period and the stability of the individual components of the DII and EDII.
METHODS
Recruitment and Eligibility
Recruitment and data collection were undertaken as part of the Manitoba Living with IBD Study between June 2015 and May 2017. Study participants were recruited through the University of Manitoba IBD Research Registry, regional gastroenterology clinics, posters in hospital and gastroenterology clinic offices, and information posted on the University of Manitoba IBD Clinical and Research Centre’s website (ibdmanitoba.org). All participants had a clinically confirmed diagnosis of IBD, experienced IBD-related symptoms (including bowel, systemic, social, and emotional symptoms) during the 2-year period before recruitment, and were at least 18 years old. Participants completed biweekly online surveys using Research Electronic Data Capture (REDCap) software from baseline until 1 year postbaseline. Dietary measures were completed only at the beginning (baseline) and at the end of the study (at 1 year). Reminders were sent to trigger survey completion. Stool samples were collected at baseline and at 1 year.
Diet Measurement
Diet was assessed using the 149-item Harvard 2007 Food Frequency Questionnaire (FFQ). Food frequency questionnaires have long been adopted as a standardized way to measure dietary intake.9–11 These questionnaires take a variety of forms. The Harvard FFQ is a general FFQ measure that has been used in a wide variety of settings,12–14 including in studies of people living with IBD.15, 16
For each food item included in the Harvard FFQ, study participants identified their frequency of consumption using 8 categories: “never or less than once per month,” “1 to 3 per month,” “1 per week,” “2 to 4 per week,” “5 to 6 per week,” “1 per day,” “2 to 3 per day,” “4 to 5 per day,” and “6 or more per day.” Participants completed the Harvard FFQ at week 2 (ie, baseline) and at week 50 (ie, 1 year follow-up). Diet stability was defined as a diet that did not vary from baseline to follow-up by more than 0.5 standard deviation. Diet stability was assessed DII and EDII globally and for the individual components of the DII and EDII.
Dietary Inflammatory Index
The DII was developed in 2014 as a measure of the inflammatory potential of a diet.7 It has since been validated17 and used in a variety of settings.18–20 The DII is calculated by first translating the consumption of common foods (such as chicken or milk) into daily servings of 45 macronutrients and micronutrients (such as anthocyanins, flavones, magnesium, and various types of fat) that are believed to have pro- or anti-inflammatory activity. Using the Harvard FFQ, we had data for 33 of the 45 food parameters included in the development of the DII score and used these 33 parameters to determine the DII score. Details of the calculation of the DII score using these 33 nutrients are provided elsewhere.7 To summarize, each nutrient was assigned a prespecified weight based on its inflammatory activity. The quantity of the nutrient consumed by a study participant was converted to a percentile based on a Z-score derived using the global mean and standard deviation of nutrient consumption. This percentile was then converted to a value that ranged between −1 and 1. A table of the global means, standard deviations, and weighting factors for all nutrients has been reported elsewhere.7 Finally, the overall DII score for each study participant was calculated as the sum of scores for the 33 nutrients. The more negative the DII score is, the more the diet is considered to be anti-inflammatory; conversely, the more positive it is, the more it is considered to be pro-inflammatory.7 An overall DII score near 0 was considered to be inflammatory-neutral.
Empirical Dietary Inflammatory Index
The EDII was developed and validated in 2016.8 Like the DII, the EDII has been used in a variety of settings as an indicator of the inflammatory potential of diet.21–23 The EDII groups foods into categories (such as red meat, refined grains, green leafy vegetables, dark yellow vegetables); the daily consumption of each food category is then quantified using FFQ data. Like the DII, the EDII aims to define the extent to which a diet is pro- or anti-inflammatory.
To calculate the EDII score, each food from the FFQ was categorized into one of 17 food groups. An 18th group, organ meats, was not measured in the FFQ. Each food group was weighted by its pro- or anti-inflammatory potential, using values derived through reduced rank regression, associating food groups with inflammatory markers from the Nurses’ Health Study (NHS); details of the method and the food group weights are provided elsewhere.8 To obtain the EDII score, the weighted number of daily servings for each food group was summed. To reduce the magnitude of the scores and aid in interpretation of statistical analyses, we followed the guide of the developers of the EDII and rescaled the final score by dividing by 1000. The lower and upper limits for EDII scores in our study were −1.0 and +1.4, respectively. The more negative an EDII score, the more the diet is considered to be anti-inflammatory, and the more positive it is, the more the diet is considered to be pro-inflammatory.8 An EDII score near 0 is considered inflammatory-neutral.
Assessment of Intestinal Inflammation
The level of fecal calprotectin (FCAL) in a stool sample correlates with the presence and severity of gut inflammation and is well established as a valid biomarker of intestinal inflammation.24–26 Fecal calprotectin was measured at weeks 0 and 52 based on stool samples from participants provided at those time points. Consistent with previous studies in the IBD population, FCAL values ≥250 µg/g correspond to significant intestinal inflammation, whereas values less than this cutoff typically indicate no significant inflammation.27, 28 An index for the change between the 2 time points for FCAL was used in this study, where −1 indicated FCAL improving from high FCAL of ≥250 µg/g to low FCAL of <250 µg/g, 0 indicated no change to the other category, and +1 indicated FCAL worsening from <250 µg/g to ≥250 µg/g.
Assessment of IBD Symptom Activity
We used the IBD Symptom Index (IBDSI) to assess the burden of IBD-related symptoms. The IBDSI is a 35-item symptom inventory recently developed to improve on existing symptom indices.29 The IBDSI measures symptoms experienced in the past 7 days. Data were collected at 0 and 52 weeks. The IBDSI score can range from 0 to 95, with higher scores suggesting greater symptom severity. The cutoff for active symptoms was >24 for CD participants and >17 for UC participants to indicate active symptomatic disease.(32)
Self-reported Flares
Participants completed a single-item, 7-level symptom change indicator biweekly. Participants were considered in a flare if they reported “moderately” or “much worse” IBD symptoms when compared with the previous biweekly assessment. Participants who completed at least 60% of their biweekly assessments were included in the flare analysis.
Statistical Analysis
The analysis was limited to study participants who completed both the baseline and follow-up Harvard FFQ. Participants were described on the following characteristics collected at the baseline assessment using frequencies and percentages: demographics, smoking status (current, former, never), type of disease (CD, UC), and duration of disease (measured in years). These descriptive analyses were stratified by EDII and DII scores and by change in EDII and DII scores. The EDII scores were stratified into approximate tertiles. The DII scores were stratified in line with the approach used by the developers of the index, such that DII scores were stratified into approximately 25% with low scores (anti-inflammatory) and 25% with high scores (pro-inflammatory). The remaining middle 50% were neither high nor low. Differences in characteristics between strata were assessed using the Fisher exact test. Where multiple measures per person were analyzed (for example, when comparing FCAL [2 measures] to EDII [2 measures]), the Cochran-Mantel Haenzel test was used.
Variance components analysis with intraclass correlations were used to describe diet stability across the 2 time periods by examining the change in pro- and anti-inflammatory components of each measure (ie, EDII—food categories; DII—nutrient categories). Variance components analyses with the corresponding intraclass correlation have long been used to assess how much of the variance in an outcome measure is attributable to a factor of interest.30, 31 The intraclass correlation was used to describe the percentage of variance in diet explained by differences between individuals compared with the percentage of variance explained by differences within individuals across measurement occasions (ie, between FFQs for the same person).
Multivariable ordinal logistic regression models were used to test the association of a change in diet on change in (1) active inflammation as measured using FCAL and (2) active symptoms as measured using IBDSI. For the former outcome, change was categorized as: −1 (FCAL improved from ≥250 µg/g at baseline to <250 µg/g at 1-year follow-up), 0 (FCAL was ≥250 µg/g at baseline and 1-year follow-up, or <250 µg/g at baseline and follow-up), or 1 (FCAL worsened from <250 µg/g at baseline to ≥250 µg/g at follow-up). For the latter, change was categorized as −1 (active symptom status changed for the better), 0 (no change in active symptom status), or 1 (active symptom status changed for the worse). Odds ratios (ORs) for each level of the outcome (eg, worse outcome vs no change, or no change vs improvement) and 95% confidence intervals (95% CIs) are reported. Multilevel linear regression models, with random intercepts and slopes for each study participant, were used to assess the association between a change in diet and a change in IBDSI (on a continuous scale).
Multivariable logistic regression models were used to test the association between baseline EDII and DII scores and presence of a self-reported flare at any of the biweekly assessments. Odds ratios and 95% CIs are reported. In all multivariable models, we controlled for smoking status, gender, disease type, and disease duration.
RESULTS
Characteristics of Study Participants
Of the 155 participants who completed the baseline Harvard FFQ, 135 (87%) completed the 1-year follow up FFQ. Among these 135 completers, 108 (80%) had FCAL measures at both occasions, 132 (98%) had IBDSI measures at both occasions, and 133 (99%) completed at least 60% of the biweekly assessments to contribute to analyses of the association between change in diet and self-reported disease flare.
Among the 135 completers, 40 (30%) were male, 36 (27%) were younger than 35 years old, 32 (24%) were 55 years or older, with a mean age of 45 years (SD 1.5), and 70 (52%) were current or past smokers. A total of 89 (66%) completers had a confirmed diagnosis of CD, and the remainder had a confirmed diagnosis of UC. A total of 50 (37%) completers had received an IBD diagnosis less than 10 years ago, and 25 (19%) were had received an IBD diagnosis more than 25 years ago (Table 1).
TABLE 1.
Characteristics of Study Participants, Stratified by EDII Score
| Baseline EDII Score | Change in EDII Score | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic (n, %) | <−0.18 | −0.18 to +0.15 | >0.15 | P a | <−0.18 | −0.18 to +0.15 | >0.15 | P a |
| Characteristics with 1 measurement per person | ||||||||
| Sex | 0.23 | 0.74 | ||||||
| Male (40, 30%) | 30% | 35% | 35% | 23% | 50% | 28% | ||
| Female (95, 70%) | 29% | 48% | 22% | 20% | 57% | 23% | ||
| Age (mean 45.0 years) | 0.10 | 0.20 | ||||||
| 18–34 (36, 27%) | 39% | 39% | 22% | 14% | 67% | 19% | ||
| 35–54 (67, 50%) | 25% | 40% | 34% | 29% | 46% | 25% | ||
| 55–71 (32, 24%) | 28% | 59% | 13% | 13% | 59% | 28% | ||
| Smoking Status | 0.43 | 0.48 | ||||||
| Current smoker (20, 15%) | 25% | 40% | 35% | 35% | 45% | 20% | ||
| Past smoker (50, 37%) | 30% | 42% | 28% | 20% | 52% | 28% | ||
| Never smoked (65, 48%) | 29% | 52% | 19% | 17% | 60% | 23% | ||
| Disease Type | 0.003 | 0.25 | ||||||
| Crohn’s disease (89, 66%) | 27% | 38% | 35% | 25% | 50% | 25% | ||
| Ulcerative colitis (46, 34%) | 35% | 57% | 9% | 13% | 63% | 24% | ||
| Disease Duration (mean 16.7 years) | 0.73 | 0.53 | ||||||
| <10 years (50, 37%) | 32% | 48% | 20% | 14% | 58% | 28% | ||
| 10–24 years (60, 44%) | 28% | 40% | 32% | 27% | 53% | 20% | ||
| 25+ years (25, 19%) | 28% | 48% | 24% | 20% | 52% | 28% | ||
| Number of flares | 0.46 | 0.19 | ||||||
| 0 (71, 54%) | 22% | 48% | 30% | 27% | 53% | 20% | ||
| 1–2 (33, 25%) | 36% | 43% | 21% | 15% | 61% | 24% | ||
| 3+ (29, 22%) | 38% | 41% | 21% | 10% | 52% | 38% | ||
| EDII at time of measurement below (baseline or 1-year follow-up) | Comparing change in EDII to baseline measurement below | |||||||
| Characteristics with 2 measurements per person | ||||||||
| Fecal Calprotectin (FCAL) | 0.01c | P = 0.56 | ||||||
| <250 µg/g (138, 57%) | 34% | 44% | 22% | 18% | 56% | 26% | (n = 73) | |
| ≥250 µg/g (104, 43%) | 18% | 51% | 31% | 25% | 53% | 22% | (n = 59) | |
| IBD Symptom Inventory (IBDSI) Score | 0.08c | P = 0.36 | ||||||
| No active symptomsb (163, 62%) | 31% | 48% | 21% | 17% | 60% | 23% | (n = 72) | |
| Active symptomsb (101, 38%) | 24% | 46% | 30% | 25% | 48% | 27% | (n = 60) | |
| DII Score | 0.62c | P = 0.57 | ||||||
| <−2 (64, 24%) | 38% | 38% | 25% | 23% | 47% | 30% | (n = 30) | |
| −2 to +2 (138, 51%) | 28% | 44% | 28% | 23% | 54% | 23% | (n = 73) | |
| > +2 (68, 25%) | 23% | 59% | 18% | 12% | 66% | 22% | (n = 32) | |
aFisher exact test
bIBDSI range is 0–81. IBDSI >24 (CD) and IBDSI >17 (UC) is considered active disease.
cDue to 2 measures per person, P values from Cochran-Mantel-Haenzel test.
Diet Stability
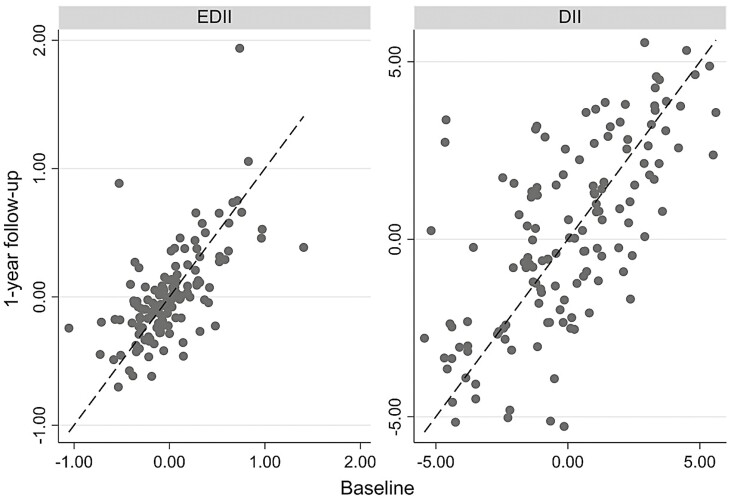
For most of the study participants, diet habits as described by EDII and DII scores were relatively consistent from baseline to follow-up (Fig. 1). We found that 59% of diets could be classified as stable based on EDII scores, and 48% could be classified as stable based on DII scores. Pearson correlation coefficient was 0.64 for baseline and follow-up EDII scores (P < 0.001) and 0.67 for baseline and follow-up DII scores (P < 0.001).
FIGURE 1.
Diet Stability (DII and EDII).
The intraclass correlation coefficient was estimated as 0.64 and 0.67 for the EDII and DII scores, respectively (Appendix 1), indicating that for both measures, approximately two thirds of the variance in diets were due to variation between individuals. Variance components analyses and respective intraclass correlations show that the variance between individuals explains more than 50% of total variance in the consumption of most of the 17 components that comprise the EDII and most of the 33 components that comprise the DII (Appendix 1). That said, some of the variance was still explained by differences between the 2 FFQ measurements within individuals (>40% for many consumption items, and even >50% for a few), suggesting that individuals may be altering their intake of foods based on circumstances that can include symptom management, disease activity, or simply personal preference over time. For example, the intraclass correlation for consumption of snacks was only 30%, which means that the variance in snacks consumed within individuals over time (between FFQs) explains almost 70% of total variance in snacks consumed in our population.
Characteristics of Study Participants by EDII Scores, DII Scores, and Change in Scores
The EDII scores ranged from −1.1 to +1.9 (mean 0.0, SD 0.4), whereas change in EDII scores ranged from −1.0 to +1.4 (mean 0.0, SD 0.3). The DII scores ranged from −5.4 to +5.5 (mean 0.1, SD 2.6), whereas change in DII scores ranged from −5.1 to +8.0 (mean 0.1, SD 2.1). Study participant characteristics, stratified by baseline EDII scores and change in EDII score from baseline to follow-up, are described in Table 1. Similarly, study participant characteristics, stratified by DII score at baseline and by change in DII score from baseline to follow-up, are described in Table 2. Disease type was strongly associated with both EDII and DII scores. Almost 4 times as many CD participants had a high (pro-inflammatory) EDII score than UC participants (P = 0.003, Table 1), and almost 3 times as many CD participants had a higher DII score than UC participants (P = 0.015, Table 2). In addition, participants with a high FCAL measure suggesting active inflammation were more likely to have a high EDII score than participants with a low FCAL <250 µg/g (31% vs 22%; P = 0.011, Table 1). Study participants with active IBD symptoms (IBDSI) were more likely to have high EDII scores than participants without active symptoms (30% vs 21%, P = 0.08). There was no evidence to suggest a similar association between DII scores and FCAL or symptoms (Table 2). There was no statistically significant association between the 2 diet inflammatory potential measures, the DII and EDII (bottom 3 rows of Table 1).
TABLE 2.
Characteristics of Study Participants, Stratified by DII Scores
| Baseline DII Score | Change in DII Score | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | <−2 | −2 to +2 | > +2 | P a | <−1.5 | −1.5 to +1.5 | > +1.5 | P a |
| Characteristics with 1 measurement per person | ||||||||
| Sex | 0.96 | 0.56 | ||||||
| Male | 23% | 53% | 25% | 28% | 55% | 17% | ||
| Female | 22% | 55% | 23% | 21% | 55% | 24% | ||
| Age (years) | 0.20 | 0.76 | ||||||
| 18–34 | 36% | 47% | 17% | 20% | 58% | 22% | ||
| 35–54 | 18% | 54% | 28% | 27% | 49% | 24% | ||
| 55–71 | 16% | 62% | 22% | 19% | 62% | 19% | ||
| Smoking Status | 0.07 | 0.46 | ||||||
| Current smoker | 10% | 70% | 20% | 25% | 55% | 20% | ||
| Past smoker | 16% | 64% | 20% | 30% | 46% | 24% | ||
| Never smoked | 31% | 42% | 28% | 17% | 62% | 21% | ||
| Disease Type | 0.01 | 0.13 | ||||||
| Crohn’s disease | 17% | 53% | 30% | 28% | 52% | 20% | ||
| Ulcerative colitis | 33% | 56% | 11% | 13% | 61% | 26% | ||
| Disease Duration | 0.35 | 0.45 | ||||||
| <10 years | 24% | 54% | 22% | 28% | 54% | 18% | ||
| 10–24 years | 24% | 58% | 19% | 17% | 55% | 28% | ||
| 25+ years | 16% | 44% | 40% | 28% | 56% | 16% | ||
| Number of flares | 0.30 | 0.16 | ||||||
| 0 | 17% | 53% | 30% | 28% | 47% | 25% | ||
| 1–2 | 24% | 58% | 18% | 18% | 67% | 15% | ||
| 3+ | 35% | 48% | 17% | 10% | 66% | 24% | ||
| DII at time of measurement below (baseline or 1-year follow-up) | Comparing change in DII to baseline measurement below | |||||||
| Characteristics with 2 measurements per person | ||||||||
| Fecal Calprotectin (FCAL) | 0.88c | 0.74 | ||||||
| <250 | 23% | 50% | 27% | 22% | 53% | 25% | ||
| ≥250 | 21% | 53% | 26% | 24% | 57% | 19% | ||
| IBD Symptom Inventory (IBDSI) Scores | 0.46c | 0.25 | ||||||
| No active diseaseb | 26% | 47% | 26% | 18% | 63% | 19% | ||
| Active diseaseb | 18% | 58% | 24% | 27% | 48% | 25% | ||
| EDII Score | 0.62c | 0.27 | ||||||
| <-0.18 | 31% | 49% | 20% | 17% | 60% | 23% | ||
| -0.18 to +0.15 | 19% | 49% | 32% | 23% | 60% | 17% | ||
| > +0.15 | 24% | 58% | 18% | 29% | 40% | 31% | ||
aFisher exact test.
bIBDSI range is 0–95. IBDSI>24 (CD) and IBDSI>17 (UC) is considered active disease.
cDue to 2 measures per person, p-values obtained using Cochran-Mantel-Haenzel test.
There were no statistically significant associations between participant characteristics and change in DII or EDII scores, nor between FCAL or IBDSI at baseline and change in DII or EDII scores (Tables 1 and 2). There was some evidence that the number of self-reported flares over the year was associated with change in eating habits from baseline to 1 year in ways that impacted EDII scores. For example, 38% of study participants with 3+ flares during the 1-year study period had an increase in EDII scores by more than 0.15, whereas only 20% of those with no flares had EDII scores that were greater than 0.15 (Table 1). However, this finding was not statistically significant (P = 0.198).
Associations Between Diet and Inflammation and Between Diet and Symptoms
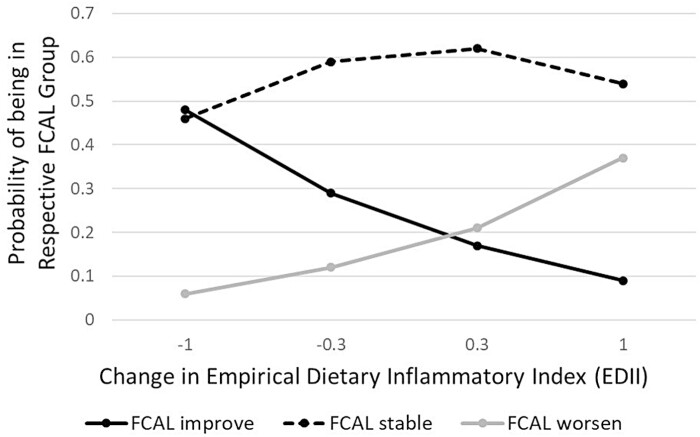
Controlling for smoking status, sex, disease type, and disease duration, a change towards a more pro-inflammatory diet per the EDII score was associated with worse FCAL values (Table 3). The opposite direction could also be stated—a change in diet toward a more anti-inflammatory one was associated with an improvement in FCAL values. Specifically, results from the multivariable ordinal logistic regression models indicated that each unit change in EDII was associated with 3.12 greater odds of worsening FCAL values from baseline to 1-year follow-up (95% CI, 1.02–9.93). When this model is translated into probabilities (Figure 2), it can be seen, for example, that just 6% of people with EDII scores that improved by −1.0 (a decrease in EDII means a movement toward anti-inflammatory) were estimated to have a FCAL that worsened, but 48% of them were estimated to have a FCAL that improved. Study participants for whom EDII changed relatively little (ie, between −0.3 to +0.3 score change) were also most likely to experience no change in FCAL values.
TABLE 3.
Odds Ratios and 95% Confidence Intervals for Multivariable Ordinal Logistic Regression Models of Change in Inflammation and Symptom Activity
| Outcome: Change in Inflammation Possible Outcomes: −1, 0, or +1a | Outcome: Change in Symptom Activity Possible outcomes: −1, 0, or +1b | |||||||
|---|---|---|---|---|---|---|---|---|
| DII Model (N = 108) | EDII Model (N = 108) | DII Model (N = 129) | EDII Model (N = 129) | |||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Change in DIIc | 1.08 (0.89–1.31) | 0.430 | — | — | 1.03 (0.86–1.24) | 0.710 | —— | — |
| Change in EDIId | — | — | 3.12 (1.02–9.93) | 0.04 | 1.86 (0.33–5.57) | 0.48 | ||
| Current smoker (Ref = not current smoker) | 1.01 (0.34–2.95) | 0.989 | 0.99 (0.34–2.84) | 0.97 | 0.65 (0.22–1.90) | 0.433 | 0.65 (0.24–1.74) | 0.39 |
| Female (Ref = male) | 1.32 (0.57–3.04) | 0.514 | 1.37 (0.63–2.97) | 0.42 | 0.84 (0.36–1.97) | 0.695 | 0.87 (0.37–2.01) | 0.73 |
| Crohn’s Disease (Ref = Ulcerative Colitis) | 1.01 (0.42–2.44) | 0.980 | 0.98 (0.41–2.37) | 0.96 | 1.00 (0.41–2.43) | 0.998 | 0.99 (0.42–2.31) | 0.97 |
| Disease duration in years | 1.01 (0.97–1.04) | 0.744 | 1.00 (0.96–1.04) | 0.97 | 1.00 (0.96–1.04) | 0.941 | 1.00 (0.96–1.05) | 0.99 |
aIntestinal inflamation measured by FCAL ≥ 250 µg/g. Change from baseline to 1-year follow-up can be one of the following:
−1 (FCAL > 250 µg/g at baseline and <250 µg/g at follow-up),
0 (FCAL > 250 µg/g at baseline and follow-up, or <250 µg/g at baseline and follow-up),
+1 (FCAL < 250 µg/g at baseline and > 250 µg/g at follow-up).
bActive symptoms status measured by IBD Symptom Inventory (IBDSI). Change from baseline to follow-up can be one of the following:
−1 (active symptoms per IBDSI at baseline [CD > 24; UC > 17] and not active at 1-year follow-up),
0 (active symptoms per IBDSI at baseline and follow-up, or not active at baseline and follow-up),
+1 (not active symptoms per IBDSI at baseline and active at 1-year follow-up).
cChange in DII score from baseline to 1-year follow-up: range −5.1 to +8.0.
dChange in EDII score from baseline to 1-year follow-up: range −1.0 to +1.4.
FIGURE 2.
Probability of FCAL improving, remaining stable, or worsening1, depending on amount and direction of change in EDII. FCAL measures were categorized into ≥250 µg/g (suggesting intestinal inflammation), or <250 µg/g. Therefore, “FCAL improve” = FCAL measure ≥250 µg/g at baseline and <250 µg/g at 1-year follow-up, “FCAL stable” = FCAL measure ≥250 µg/g at baseline and follow-up, or <250 µg/g at baseline and follow-up, “FCAL worsen” = FCAL measure <250 µg/g at baseline and ≥250 µg/g at 1-year follow-up.
It is possible that a change in EDII may result in a change in IBD-related symptoms. Although not statistically significant, the OR was 1.86 (95% CI, 0.33–5.57), suggesting that each unit increase was associated with 1.86 greater odds of moving from nonsymptomatic disease to active symptomatic disease, rather than moving in the other direction (from symptomatic disease to nonsymptomatic) or an unchanged IBDSI status. In the multilevel regression model in which the outcome was the continuous IBDSI score (ranging from 0 to 81), and with random effects for both the intercept and slope of EDII, the association between EDII and IBDSI was statistically significant. Within individuals, each unit increase in EDII was associated with an increase of 6.7 in IBDSI scores (95% CI, 1.0–12.4; P = 0.024; data not shown).
There was no evidence of an association between a change in DII score from baseline to 1-year follow-up or a change in either FCAL (≥250 µg/g or <250 µg/g) or IBDSI (active symptomatic disease or nonsymptomatic disease).
Associations Between Diet and IBD-related Flares
The multivariable logistic regression model for self-reported flares demonstrated that each unit increase in the change in EDII score from baseline to 1-year follow-up was associated with substantially greater odds of having at least 1 flare during the year (OR 4.08; 95% CI, 1.10–15.16; Table 4). There was no significant association between a change in DII from baseline to follow-up and having at least 1 reported flare during the year.
TABLE 4.
Odds ratios and 95% Confidence Interval for Logistic Regression Models of a Self-Reported Flare
| DII Model | EDII Model | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Change in DIIa | 1.05 (0.89–1.24) | 0.597 | — | — |
| Change in EDIIb | — | — | 4.08 (1.10–15.16) | 0.03 |
| Current smoker | 1.07 (0.39–2.94) | 0.88 | 1.05 (0.37–2.95) | 0.92 |
| Female | 1.35 (0.63–2.88) | 0.44 | 1.43 (0.66–3.10) | 0.36 |
| Crohn’s Disease | 1.15 (0.52–2.53) | 0.73 | 1.17 (0.53–2.58) | 0.69 |
| Disease duration in years | 1.02 (0.98–1.05) | 0.31 | 1.01 (0.98–1.05) | 0.41 |
aChange in DII score from baseline to 1-year follow-up: range −5.1 to +8.0.
bChange in EDII score from baseline to 1-year follow-up: range −1.0 to +1.4.
DISCUSSION
In this study of diet stability over time, we concentrated on the inflammatory aspects of diet, using 2 different measurement approaches: the 17 food groups comprising the EDII and the 33 nutrient components comprising the DII. Using these parameters, we first investigated the degree to which people living with IBD change their diets over a 1-year period; then we examined the potential association between a change in the inflammatory nature of diet and a change in IBD inflammation and symptoms.
In our cohort of adults living with IBD, the diets that make up these inflammatory indices are relatively stable for most people. That said, some people reported large diet changes (varies more than ½ SD from baseline) over the course of 1 year, and some aspects of diet (such as amount of snacks consumed) were more prone to change than other aspects. We found that when using the EDII, nearly 60% of persons had a stable diet over 1 year, whereas using the DII, nearly 50% had a stable diet over 1 year. For the approximate 40%–50% of participants whose diets were “less stable,” this was not surprising; it is not uncommon for persons with IBD to have strong beliefs about the role diet may play on the exacerbation or management of symptoms,2 and undoubtedly some may have changed their diets in response to having active symptoms. We found that EDII was associated with active inflammation and active symptoms, but the DII was not associated with either outcome.
The 2 measurements, approximately a year apart for each of diet, inflammation, and gastrointestinal symptoms, allowed for examination of the association between change in diet (EDII, DII) and changes in inflammation (FCAL) and symptoms (IBDSI). Because the FFQ measures diet in the recent past, whereas FCAL measures current inflammation and IBDSI measures current symptoms, it is reasonable to assume a temporal association between diet and these outcomes at baseline, suggesting we cannot infer causality. It would be valuable to both individuals living with IBD and clinicians to provide sound, detailed dietary advice as to whether a food item or nutrient may “cause” intestinal inflammation and/or symptoms. A dietary study that is prospective in nature that measures the intake of inflammatory foods (as identified in EDII) with concurrent measures of inflammation and symptoms could infer causality between diet and outcomes. A study of this type could be conducted by using monthly monitoring of FFQs along with detailed food diaries, FCAL, and symptom measurements collected at the same time as the diet data.
We also examined the association between the inflammatory potential of diet at baseline and subsequently experiencing IBD-related flares. The study found that each unit increase in EDII at baseline was associated with over 4-times greater likelihood of having at least 1 reported flare during the year. This finding suggests that increases in the inflammatory potential of the diet, for example, an increase in consumption of processed meat, red meat, or high energy beverages (ie, regular sugar-laden colas/carbonated beverages)—all foods within the EDII associated with an increase in inflammatory potential—may play a role in the likelihood of having a an IBD flare-up. As with any diet-related research, it is difficult to ascertain if there is a direct link to an increase in inflammatory foods and the onset of a flare. Ideally, implementing an FFQ, or a 3 to 7 day food recall to capture specific food intake at the time of the self-reported flare, would have provided more insight into the diet changes (if any) implemented by the individual. This is a topic to be explored in our future work.
As the EDII and the DII were developed by 2 different groups of investigators but were purportedly measuring a similar concept—the inflammatory potential of a given diet—it was expected there would be some relationship between the 2 measures. However, there appeared to be no association suggesting either that these 2 measures are not measuring the same underlying concept or that the specific FFQ employed in this study provided a better estimate of one of these measures over the other. This finding, that DII and EDII were not correlated, was unexpected by our group but nevertheless interesting. This is the first study to our knowledge that compares the DII and the EDII with each other, using the FFQ among individuals with IBD. Both the EDII and the DII were developed based on the modulating effects of specific aspects of diet on levels of inflammatory markers interleukin (IL)-6, tumor necrosis factor (TNF)-alpha and C reactive protein.7, 8, 32 The DII is derived from specific food parameters and nutrients, whereas the EDII is based on food groups. It is difficult to identify what aspects of the DII and EDII led to the discordant results. The majority of the studies that evaluate the inflammatory potential of the diet generally employ only 1 method to determine the inflammatory score (DII, EDII or other) and have not compared them directly. Our study is novel in the use of both scores among an IBD cohort and raises questions about the summative value of the literature in this area given the lack of comparability of 2 key measures used to assess dietary inflammatory potential.
A key finding was the association between change in diet and change in inflammation and symptoms. The odds of a negative FCAL at baseline (<250 µg/g) being positive at 1-year follow-up (≥250 µg/g) was 3 times higher for each unit increase change in the EDII score. Because the higher the EDII score the greater the purported pro-inflammatory nature of the diet, it is possible that a shift in diet elements to one that is more pro-inflammatory (per EDII) may have a real biologic effect (increased FCAL). For gastrointestinal symptoms, the study found that for each unit increase in EDII, there was a nearly 2-fold increase in the likelihood that the IBDSI score would shift from below to above threshold for active symptoms; multilevel analysis demonstrated that each unit increase in EDII was associated with an almost 7 unit increase in IBDSI. However, these relationships were not statistically significant.
There were no significant relationships when conducting parallel analyses substituting change in DII for change in EDII as the predictor of change in inflammation and symptoms. This, along with the fact that EDII and DII were not correlated in our study, suggests that DII may not adequately measure the inflammatory nature of a diet relevant for those with IBD. Alternatively, it may be that the DII score was insufficiently realized, as there were only data for 33 of the 45 recommended food parameters as part of the DII. Though this in fact is a larger number of items than many other studies have been able to measure,33–36 it nonetheless means that approximately one quarter of the 45 items intended to compose the DII were not used in this study’s DII. It is possible, therefore, that even if the full version of the DII is a good marker of the inflammatory potential of a diet, the ability to measure all or most of the 45 items may not be practical.
An advantage of the EDII over the DII, in addition to the more practical ability to gather the diet components needed to calculate it, is its utility for patients. Although the components of the DII are primarily nutrients, many of which are unfamiliar to the general public, the components of the EDII were primarily food items such as fish, red meat, and green leafy vegetables. In short, individuals with IBD may more readily understand and be able to implement advice about changes in food items than changes in nutrients.
STRENGTHS AND LIMITATIONS
The main strengths of the study included the well-characterized participant sample, the longitudinal design, and the use of validated self-report and biologic measures. The design facilitated examination of a temporal association between a change in diet and a change in a biological measure of inflammation (FCAL ≥ 250 µg/g) and between a change in diet and a change in active symptoms per the IBDSI.
A limitation of the study was that although the information on changes in diet, inflammation, and symptoms were obtained 1 year apart, there was no information on changes that may have occurred throughout the year. Ideally, implementing the FFQ at the time of the self-reported flare would have provided more direct linkages of diet changes (if any) implemented by the individual. We also are aware of the limiting nature of our results in that the measures of inflammation using FCAL are 1 year apart and the obvious possibility that FCAL can fluctuate over the course of 1 year. In our study, we are not suggesting that diet is associated with inflammation throughout the year. We simply identified 2 specific time points, 12 months apart, and studied the association of diet and inflammation.
It is important to underscore the fact that both the DII and EDII have been validated in previous studies against inflammatory biomarkers, namely, pro-inflammatory mediators such as IL-1, IL-6, and TNF-alpha.7, 8 In our study, we did not measure these specific biomarkers and chose instead to use a novel, more intestine-specific marker for inflammation (FCAL). Understanding changes on FCAL levels due to diet is a more applicable and relevant inflammatory “marker” for individuals with IBD; managing inflammation of the gut is critical in the management of IBD. Nevertheless, future studies that include both measurements of circulating and tissue inflammatory cytokines, in addition to intestinal inflammation, would be of interest and would substantially add to the understanding of how the inflammatory potential of the diet impacts all levels of inflammation.
We also acknowledge the limitation in our work regarding calculation of the DII score. As indicated in the methodology, we only had data for 33 of the 45 food parameters included in the development of the DII score. Not included in the DII score were Eugenol, garlic, ginger, saffron, selenium, turmeric, vitamin E, green/black tea, isoflavones, pepper, thyme/oregano, and rosemary, as these were not able to be quantified by the Harvard FFQ. Similarly, to calculate the EDII score, we used 17 of the 18 food groups that originally comprised the development of the EDII; the 18th group (organ meats) was not measured in the FFQ.
Furthermore, it was not practical or feasible to collect the missing data after the fact from the participants, and we acknowledge this as a limitation of our work. However, although we acknowledge this as a limitation, other studies have reported significant findings using a similar number of nutrients/food groups, and in fact, some studies used even fewer nutrients.34, 36 Nevertheless, a prospective dietary study that incorporates all elements of the DII and EDII to fully assess the inflammatory potential of the diet is undoubtedly of interest to pursue. The combined use of an FFQ alongside a specific questionnaire to collect data on all the DII and EDII food and nutrient components would be of interest in a prospective dietary study.
Another novel future dietary trial could also include data collection on consumption of food additives and preservatives. There is a growing body of evidence that food additives and preservatives may play a potential role as triggers of IBD symptoms or inflammation.37 The FFQ used in our study was not designed to capture this information but would be of great interest to pursue in the future.
As with all diet studies, there is the potential limitation related to recall bias. In our work, patients are asked to report their usual intake on a lengthy number of food items and report their “usual” consumption, reflecting over the past year. In an ideal, controlled diet study, FFQs combined with daily food journals and frequent monitoring from a dietitian would provide more accurate information. However, this requires a larger burden of time on both the subjects involved and those conducting the research study. Nevertheless, it can be noted from our study that patients with IBD tend to follow a consistent diet and consume similar foods as a means of controlling symptoms. Therefore, despite the limitations surrounding diet recall limitations, perhaps the FFQ may be appropriate within IBD population, considering their more consistent diet patterns.
CONCLUSIONS
There may be a role for a targeted anti-inflammatory diet for persons living with IBD, with a potential impact on inflammation and symptoms. The EDII shows promise as a relevant measure to inform about diet elements; DII has some practical challenges to implement and does not appear to be as sensitive. Future studies using an experimental design to control intake of foods with inflammatory potential by introducing this diet in remission may help to determine how that diet impacts disease course.
Appendix
Appendix 1A. Variance Components Analysis
| Aspect of Diet | Variance in Diet Explained by Differences in Diets Between Peoplea | Variance in Diet Explained by Differences Within People Over Time (ie, different consumption recorded on their baseline and follow up FFQs)a | Intraclass Correlation (The percentage of Variance Explained by Differences in Diets Between People) |
|---|---|---|---|
| EDII (in “servings”) | |||
| Pro-Inflammatory per EDII: | |||
| High energy beverages | 0.945 | 0.437 | 68.4% |
| Low energy beverages | 0.577 | 0.089 | 86.6% |
| Fish | 0.022 | 0.033 | 40.2% |
| Other vegetables | 0.903 | 0.590 | 60.5% |
| Processed meat | 0.290 | 0.191 | 60.3% |
| Red meat | 0.133 | 0.068 | 66.1% |
| Refined grains | 0.937 | 0.374 | 71.4% |
| Tomatoes | 0.025 | 0.032 | 43.9% |
| Anti-Inflammatory per EDII: | |||
| Beer | 0.064 | 0.074 | 46.6% |
| Coffee | 1.426 | 0.325 | 81.4% |
| Dark Yellow Vegetables | 0.158 | 0.129 | 55.1% |
| Fruit Juice | 0.218 | 0.159 | 57.9% |
| Leafy Green Vegetables | 0.440 | 0.208 | 67.9% |
| Pizza | 0.008 | 0.006 | 59.4% |
| Snacks | 0.018 | 0.041 | 30.1% |
| Tea | 0.650 | 0.538 | 54.7% |
| Wine | 0.059 | 0.025 | 70.1% |
| Mean intraclass correlation for EDII items | 60.0% | ||
| EDII score | 0.083 | 0.046 | 64.3% |
aFor example, the variance in consumption of high energy beverages was 0.945 + 0.437 = 1.382. This variance is partitioned into two components—variance explained by different consumption habits across different people (variance = 0.945, first column), and variance explained by people changing their consumption habits over the 2 timepoints (variance = 0.437, second column).
Bold rows indicate the aspects of diet in which more than half of the variance is explained by people changing their eating habits (variance within people).
Appendix 1B. Variance Components Analysis
| DII Components (in grams unless otherwise indicated) | Variance in Diet Dxplained by differences in Diets Between Peoplea | Variance in Diet Explained by Differences Within People Over Time (ie, different consumption recorded on their baseline and follow up FFQs)a | Intraclass Correlation (The percent of variance explained by differences between people) |
|---|---|---|---|
| Pro-Inflammatory per DII | |||
| Vitamin B12 (mcg) | 18.21 | 9.80 | 65.0% |
| Calories (kcal) | 423,938.1 | 272,236.1 | 60.9% |
| Carbohydrates | 7952.41 | 5354.41 | 59.8% |
| Cholesterol | 10,826.34 | 7166.33 | 60.2% |
| Iron (mg) | 39.08 | 52.89 | 42.5% |
| Protein | 926.41 | 727.72 | 56.0% |
| Saturated Fat | 101.59 | 68.84 | 59.6% |
| Total Fat | 859.56 | 425.19 | 66.9% |
| Trn11 | 0.360 | 0.232 | 60.9% |
| Anti-Inflammatory per DII | |||
| Alcohol | 60.71 | 20.53 | 74.7% |
| Fiber | 68.88 | 37.16 | 65.0% |
| Thiamin (mg) | 0.431 | 0.405 | 51.6% |
| Riboflavin (mg) | 0.609 | 0.538 | 53.1% |
| Pyridoxine (mg) | 9.32 | 4.87 | 65.7% |
| Beta Carotene (mcg) | 16,000,000 | 13, 000,000 | 55.7% |
| Caffeine | 16,878.13 | 5571.73 | 75.2% |
| Folic Acid (mcg) | 16,006.48 | 18,033.66 | 47.0% |
| Magnesium (mg) | 22,408.01 | 23,855.95 | 48.4% |
| Monounsaturated Fat | 142.13 | 52.76 | 72.9% |
| Niacin | 264.48 | 170.61 | 60.8% |
| Onions (grams) | 1307.61 | 2988.32 | 30.4% |
| Omega 3 | 0.285 | 0.397 | 41.8% |
| Omega 6 | 43.54 | 18.83 | 69.8% |
| Polyunsaturated Fat | 50.53 | 24.32 | 67.5% |
| Vitamin A (mcg) | 282,392.8 | 419,465.8 | 40.2% |
| Anthocyanidins | 1397.35 | 1141.31 | 55.0% |
| Flavan-3-ols | 24,615.95 | 19,693.13 | 55.6% |
| Flavanone | 492.46 | 168.64 | 74.5% |
| Flavone | 0.418 | 0.217 | 65.8% |
| Flavonol | 221.48 | 202.29 | 52.3% |
| Vitamin C (mcg) | 2254.30 | 1139.00 | 66.4% |
| Vitamin D (IU) | 24,067.47 | 22,949.12 | 51.2% |
| Zinc (mg) | 27.87 | 32.74 | 46.0% |
| Mean intraclass correlation | 58.1% | ||
| DII score | 4.41 | 2.22 | 66.5% |
aFor example, the variance in mcg of Vitamin B12 was 18.21 + 9.80 = 28.01. This variance is partitioned into 2 components—variance explained by different consumption habits across different people (variance = 18.21, first column), and variance explained by people changing their consumption habits over the 2 timepoints (variance = 28.01, second column).
Bold rows indicate the aspects of diet in which more than half of the variance is explained by people changing their eating habits (variance within people).
Appendix 2. Mean Daily Servings of the EDII Food Categories at Baseline
| Aspect of diet | CD | UC | FCAL <250 | FCAL >250 | Activea | Nonactivea | Everybody |
|---|---|---|---|---|---|---|---|
| Pro-Inflammatory per EDII: | |||||||
| High energy beverages | 0.61 | 0.58 | 0.63 | 0.59 | 0.89 | 0.37 | 0.60 |
| Low energy beverages | 0.32 | 0.07 | 0.27 | 0.19 | 0.23 | 0.22 | 0.23 |
| Fish | 0.22 | 0.20 | 0.21 | 0.23 | 0.22 | 0.20 | 0.21 |
| Other vegetables | 1.13 | 1.58 | 1.35 | 1.19 | 1.20 | 1.35 | 1.28 |
| Processed meat | 0.61 | 0.42 | 0.55 | 0.56 | 0.50 | 0.51 | 0.55 |
| Red meat | 0.61 | 0.49 | 0.57 | 0.58 | 0.50 | 0.55 | 0.57 |
| Refined grains | 1.26 | 1.05 | 0.95 | 1.50 | 1.38 | 1.04 | 1.19 |
| Tomatoes | 0.18 | 0.32 | 0.22 | 0.24 | 0.23 | 0.24 | 0.23 |
| Anti-Inflammatory per EDII: | |||||||
| Beer | 0.15 | 0.13 | 0.16 | 0.12 | 0.08 | 0.19 | 0.14 |
| Coffee | 1.37 | 1.27 | 1.29 | 1.43 | 1.38 | 1.22 | 1.34 |
| Dark Yellow Vegetables | 0.45 | 0.69 | 0.55 | 0.49 | 0.45 | 0.61 | 0.53 |
| Fruit Juice | 0.40 | 0.26 | 0.25 | 0.49 | 0.36 | 0.37 | 0.35 |
| Leafy Green Vegetables | 0.77 | 1.20 | 0.99 | 0.81 | 0.89 | 0.90 | 0.92 |
| Pizza | 0.11 | 0.12 | 0.13 | 0.11 | 0.11 | 0.13 | 0.12 |
| Snacks | 0.22 | 0.19 | 0.20 | 0.22 | 0.23 | 0.19 | 0.21 |
| Tea | 0.52 | 0.61 | 0.58 | 0.51 | 0.71 | 0.42 | 0.55 |
| Wine | 0.16 | 0.17 | 0.19 | 0.15 | 0.18 | 0.16 | 0.17 |
aActive disease defined as a score on IBD Symptom Inventory Index (IBDSI) >24 for CD participants, and >17 for UC participants. Theoretical range of IBDSI 0–81.
Supported by: Project grant awarded by Canadian Institutes of Health Research (Grant number 130539) awarded to Dr. C. N. Bernstein.
KV, LAS, KW, LET, CH, LAG, KAS, LL, MS, and CNB contributed to planning and/or conducting the study and reviewed and approved the final manuscript. LV, LAS, KW LET, CH, MS, and CNB contributed to collecting and/or interpreting data. KV and LAS drafted the manuscript.
Conflicts of interest: CNB has served on advisory boards or consulted to Abbvie Canada, Ferring Canada, Janssen Canada, Pfizer Canada, Shire Canada, Takeda Canada, and Mylan Pharmaceuticals and has received unrestricted educational grants from Abbvie Canada, Janssen Canada, Pfizer Canada, Shire Canada and Takeda Canada; he has been on the speaker’s bureau for Takeda Canada, Abbvie Canada, Janssen Canada, and Medtronic Canada. LET has served on the advisory boards for Pfizer Canada, Takeda Canada, Abbvie Canada, and Janssen Canada and on speaker’s panels for Janssen Canada, Takeda Canada, and Pfizer Canada and has received grant support from Pfizer Canada and Abbvie Canada. All other authors have no conflicts to declare.
REFERENCES
- 1. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40:833–842. [DOI] [PubMed] [Google Scholar]
- 2. Hou JK, Lee D, Lewis J. Diet and inflammatory bowel disease: review of patient-targeted recommendations. Clin Gastroenterol Hepatol. 2014;12:1592–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zallot C, Quilliot D, Chevaux JB, et al. Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:66–72. [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto T, Nakahigashi M, Saniabadi AR. Diet and inflammatory bowel disease–epidemiology and treatment. Aliment Pharmacol Ther. 2009;30:99–112. [DOI] [PubMed] [Google Scholar]
- 5. Neuman MG, Nanau RM. Inflammatory bowel disease: role of diet, microbiota, life style. Transl Res. 2012;160:29–44. [DOI] [PubMed] [Google Scholar]
- 6. Racine A, Carbonnel F, Chan SS, et al. Dietary patterns and risk of inflammatory bowel disease in Europe: results from the EPIC study. Inflamm Bowel Dis. 2016;22:345–354. [DOI] [PubMed] [Google Scholar]
- 7. Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and validation of an empirical dietary inflammatory index. J Nutr. 2016;146:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. [DOI] [PubMed] [Google Scholar]
- 10. Hu FB, Rimm EB, Stampfer MJ, et al. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. [DOI] [PubMed] [Google Scholar]
- 11. Sevak L, Mangtani P, McCormack V, et al. Validation of a food frequency questionnaire to assess macro- and micro-nutrient intake among South Asians in the United Kingdom. Eur J Nutr. 2004;43:160–168. [DOI] [PubMed] [Google Scholar]
- 12. McNutt S, Zimmerman TP, Hull SG. Development of food composition databases for food frequency questionnaires (FFQ). J Food Compos Anal. 2008;21:S20–S26. [Google Scholar]
- 13. Shahar D, Fraser D, Shai I, et al. Development of a food frequency questionnaire (FFQ) for an elderly population based on a population survey. J Nutr. 2003;133:3625–3629. [DOI] [PubMed] [Google Scholar]
- 14. Shahar D, Shai I, Vardi H, et al. Development of a semi-quantitative Food Frequency Questionnaire (FFQ) to assess dietary intake of multiethnic populations. Eur J Epidemiol. 2003;18:855–861. [DOI] [PubMed] [Google Scholar]
- 15. Lopes MB, et al. Restriction of dairy products; a reality in inflammatory bowel disease patients. Nutr Hosp. 2014;29:575–581. [DOI] [PubMed] [Google Scholar]
- 16. Wu GD, Bushmanc FD, Lewis JD. Diet, the human gut microbiota, and IBD. Anaerobe. 2013;24:117–120. [DOI] [PubMed] [Google Scholar]
- 17. Tabung FK, Steck SE, Zhang J, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wirth MD, Burch J, Shivappa N, et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. 2014;56:986–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shivappa N, Hébert JR, Rietzschel ER, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. 2015;113:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wood LG, Shivappa N, Berthon BS, et al. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin Exp Allergy. 2015;45:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tabung FK, Liu L, Wang W, et al. Association of dietary inflammatory potential with colorectal cancer risk in men and women. JAMA Oncol. 2018;4:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tabung FK, Smith-Warner SA, Chavarro JE, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr. 2017;147:1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tabung FK, Huang T, Giovannucci EL, et al. The inflammatory potential of diet and ovarian cancer risk: results from two prospective cohort studies. Br J Cancer. 2017;117:907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the simple endoscopic score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. [DOI] [PubMed] [Google Scholar]
- 25. Abraham BP, Kane S. Fecal markers: calprotectin and lactoferrin. Gastroenterol Clin North Am. 2012;41:483–495. [DOI] [PubMed] [Google Scholar]
- 26. Manz M, Burri E, Rothen C, et al. Value of fecal calprotectin in the evaluation of patients with abdominal discomfort: an observational study. BMC Gastroenterol. 2012;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abej E, El-Matary W, Singh H, et al. The utility of fecal calprotectin in the real-world clinical care of patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2016;2016:2483261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sunde K, et al. Analytical Performance of a Fecal CALPROTECIN (fCAL) PETIA Test. Paris: Poster EuroMedLab; Poster No. M089. 2015. [Google Scholar]
- 29. Witges K, Targownik LE, Haviva C, et al. Living with inflammatory bowel disease: protocol for a longitudinal study of factors associated with symptom exacerbations. JMIR Res Protoc. 2018;7:e11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Donner A,, Wells G. A comparison of confidence interval methods for the intraclass correlation coefficient. Biometrics. 1986;42:401–412. [PubMed] [Google Scholar]
- 31. Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. Am J Epidemiol. 1999;149:876–883. [DOI] [PubMed] [Google Scholar]
- 32. Nagle CM, Ibiebele T, Shivappa N, et al. ; Australian Ovarian Cancer Study . The association between the inflammatory potential of diet and risk of developing, and survival following, a diagnosis of ovarian cancer. Eur J Nutr. 2019;58:1747–1756. [DOI] [PubMed] [Google Scholar]
- 33. Abe M, Shivappa N, Ito H, et al. Dietary inflammatory index and risk of upper aerodigestive tract cancer in Japanese adults. Oncotarget. 2018;9:24028–24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shivappa N, Hébert JR, Rashvand S, et al. Inflammatory potential of diet and risk of ulcerative colitis in a case-control study from Iran. Nutr Cancer. 2016;68:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shivappa N, et al. Inflammatory potential of diet and risk of colorectal cancer: a case–control study from Italy. Br J Nutr. 2015;114:152–158. [DOI] [PubMed] [Google Scholar]
- 36. Wirth MD, et al. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Br J Nutr. 2015;113:1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shuk-Mei H, et al. Challenges in IBD research: environmental triggers. Inflamm Bowel Dis. 2019.25:S13–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]