Introduction
Tachycardia induction is an essential part of supraventricular tachycardia (SVT) ablation that helps to establish the accurate diagnosis, mechanism, and location of the arrhythmia. Several approaches are typically employed to induce SVT in the electrophysiology (EP) laboratory, including drug infusion (isoproterenol, caffeine, epinephrine, adenosine, etc), pacing maneuvers (multisite pacing, extrastimuli, burst pacing), and varying the degree of sedation.1,2 We describe a case of SVT that could be induced only after altering the patient’s position via reverse Trendelenburg (RT).
Case report
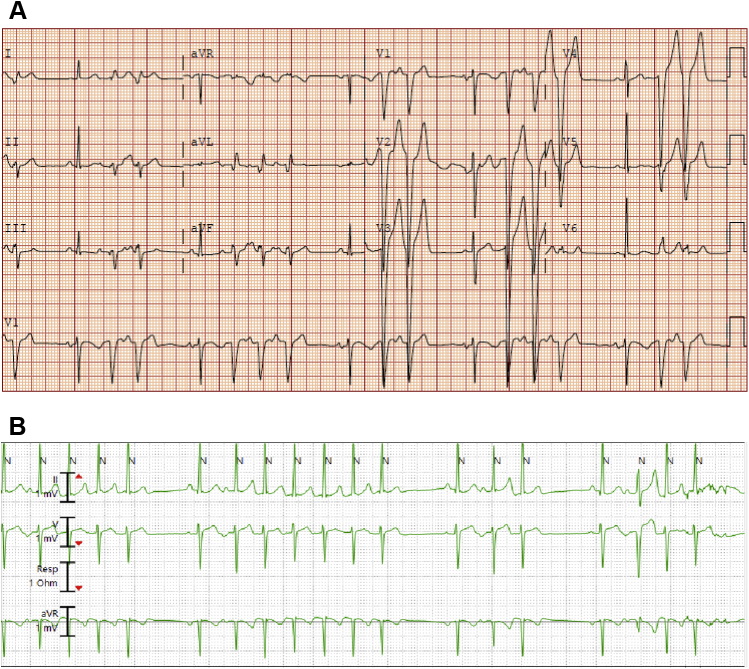
A 31-year-old man presented with history of schizoaffective disorder, polysubstance abuse (cocaine and marijuana), and frequent, symptomatic, nonsustained atrial arrhythmia. He previously underwent attempted SVT ablation at a referring facility 9 years prior to presentation to our institution (procedure detail not available). He was admitted via the emergency room to Vanderbilt University Medical Center with palpitations and shortness of breath. Initial work-up included negative urine drug screen and troponin level within normal limits. A 12-lead electrocardiogram showed reproducible bursts of SVT (Figure 1A). Telemetry monitoring over 72 hours revealed very frequent salvos of the same SVT, felt to be consistent with atrial tachycardia (AT) (Figure 1B).
Figure 1.
A: The patient’s presenting electrocardiogram, which shows salvos of supraventricular tachycardia that appear to be consistent with atrial tachycardia. B: Telemetry during the admission showed very frequent runs of the same atrial tachycardia.
A transthoracic echocardiogram revealed severely depressed left ventricular (LV) systolic function (ejection fraction 25%–35%) without significant valvular abnormalities. He subsequently underwent a coronary angiogram that revealed no significant disease and normal LV diastolic filling pressure. The patient’s systolic dysfunction was thought to be related to incessant tachycardia and the decision was made to proceed with an EP study and SVT ablation. Beta-blockers had been avoided, and calcium channel blockers were ineffective.
While the patient was supine on the EP lab table, baseline rhythm was sinus with no ectopy or spontaneous SVT. A propofol bolus was infused before vascular access was obtained. The procedure was performed entirely without fluoroscopy. CARTO 3 software (Biosense Webster, Diamond Bar, CA) and intracardiac echocardiography (ICE) were used to guide electroanatomic mapping and ablation.
Several approaches were unsuccessful in inducing the patient’s SVT. These included atrial burst and extrastimulus pacing from multiple sites (right atrium, right ventricle, parahisian, coronary sinus), complete washout of the anesthetic agent (propofol) with a 30-minute wait time, and high-dose isoproterenol infusion via a central catheter (2 mcg/min titrated to 20 mcg/min, max heart rate 135 beats/min). After a 20-minute isoproterenol washout period and no sedation, the following intravenous drugs were infused: epinephrine bolus (10 mcg); adenosine (6, 12, and 18 mg boluses), phenylephrine (3 boluses, 100 mcg each), metoprolol (5 mg), and esmolol infusion. SVT could not be induced.
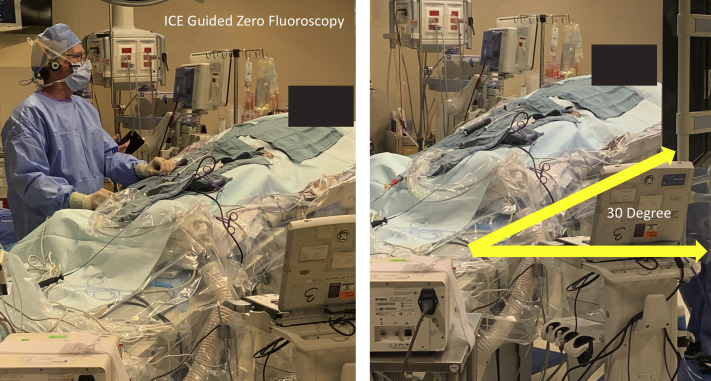
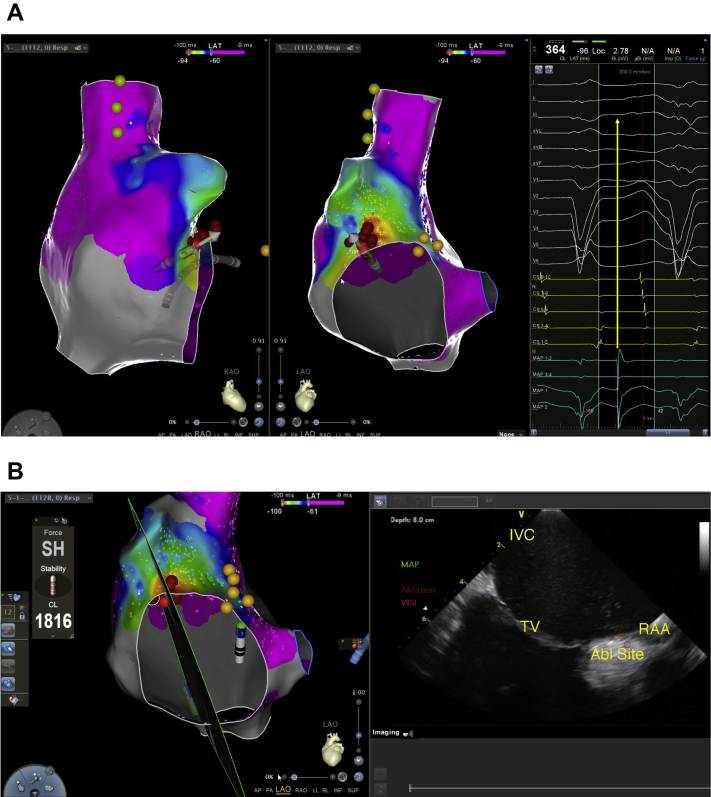
After a 1-hour wait in the supine position, the patient was placed in RT position to mimic the 72 hours of clinical observation of incessant bursts of AT while the patient was sitting upright or partially upright. The SVT was then observed spontaneously within 2 minutes after adjusting the table to 30 degrees RT position, with no additional drug infusion, change in sedation, or pacing maneuvers (Figure 2). Mapping using a 20-pole mapping catheter (PentaRay; Biosense Webster) and entrainment confirmed right AT. The earliest local electrogram during AT was mapped to the anterior tricuspid annulus (35 ms pre–surface P wave). The site of origin was successfully ablated at 35 W with a 3.5 mm ThermoCool SmartTouch DF ablation catheter (Biosense Webster) with attention to catheter tissue contact using ICE (Figure 3). No further spontaneous AT or regional ectopy was noted after a 30-minute wait time, both while supine and while in RT position. A Holter monitor placed after ablation showed no SVT and a follow-up echocardiogram revealed normalization of LV systolic function.
Figure 2.
A picture from the ablation procedure that shows the ∼30-degree reverse Trendelenburg position, which readily induced the patient’s supraventricular tachycardia. The ablation procedure was done with intracardiac echocardiography (ICE) guidance and no fluoroscopy, which made achieving this position easier.
Figure 3.
A: The final activation map in right anterior oblique (left) and left lateral (middle) projections. The red dots indicate the site of ablation that successfully terminated tachycardia. The light yellow dots in the supraventricular tachycardia correspond to the phrenic nerve location and the darker yellow dots by the tricuspid valve indicate the His signal location. The right side of the panel shows the early local activation at the location of the mapping catheter (turquoise color) as compared to the surface P wave (yellow arrow). B: Intracardiac echocardiography (ICE)–guided ablation, with the left side indicating the section of the right atrium through the ablation site that is represented by the ICE image on the right. ICE was used to check the ablation catheter location and confirm surface contact with tissue throughout the procedure. Abl = ablation; IVC = inferior vena cava; RAA = right atrial appendage; TV = tricuspid valve.
Discussion
We describe an unusual case of AT induction during an ablation procedure using the RT position after failing to induce the clinical tachycardia with the traditional pharmacologic and pacing approaches. To our knowledge, we are the first to report such a maneuver to induce AT in the EP lab.
The mechanism of AT induction with posture change is not entirely clear, but alterations in vagal tone are suspected to play a central role. The REVERT trial3 showed that in patients with SVT, a modified Valsalva maneuver that includes a Trendelenburg position was far more successful in terminating SVTs than the modified Valsalva maneuver alone. The proposed mechanism for this observation involves a vagally mediated, cardioinhibitory reflex that is triggered by the stimulation of cardiac stretch receptors (Bezold-Jarisch reflex).4 The opposite mechanism—ie, decreased vagal stimulation with RT position—could have induced AT in our patient. An argument against the role of sympathetic stimulation in this case, however, is the inability to trigger AT with high-dose catecholamine agents in the awakened state. At no point had the patient received adrenergic blocking medications pre-procedure, and a robust sympathetic response was achieved.
It is possible that a direct mechanical effect of changes in the right atrial volume might have had an effect on the atrial cells forming the automatic focus. Decreasing venous return by placing the patient in an RT position may decrease the mechanical effect of stretch on the automatic focus, which somehow triggered the AT, though previous studies favor a proarrhythmic effect of stretch.5
It is unclear if this positional change is also capable of affecting other SVT types such as AV nodal reentrant tachycardia. It is also unclear what role, if any, the presence of cardiomyopathy and systolic heart failure played in mediating the effect of position change on tachycardia.
The RT position in this case was attempted owing to the patient’s report and direct physician observation of repeated bursts of tachycardia when he was upright or tilted up in bed, but not while supine. This case changed our approach to SVT ablation in general such that position modulation is considered for patients with a positional component to their symptoms. This is especially true when other maneuvers such as pacing and drug infusion fail to achieve the objective. It should be noted, however, that changing the position of the patient to RT requires the procedure bed to be equipped with this function, which is not commonly available in EP laboratories (our EP hybrid lab is equipped with Siemens Model #10141018). The position change is much easier to perform without interference from the image intensifier using zero fluoroscopy methods.
Conclusion
We demonstrate the potential utility of RT position to induce posture-sensitive arrhythmias in the EP lab. In this case, an incessant AT could not be induced with the patient in a supine position despite pacing maneuvers, drug infusion, and cessation of sedation. Palpitations that occur in upright positions may be an important clue to positional dependence.
Key Teaching Points.
-
•
Automatic atrial arrhythmia induction in the electrophysiology (EP) lab (premature atrial contractions, atrial tachycardia [AT]) can be challenging given changes that occur in central autonomic tone following anesthetic induction.
-
•
Positional triggering for automatic AT can be mimicked in the setting of zero-fluoroscopy supraventricular tachycardia ablation using Trendelenburg functionality on the EP lab table when available.
-
•
Positional modulation can be considered when clinical history suggests a positional component to arrhythmia symptoms, or when traditional methods to elicit AT have been exhausted.
Footnotes
Funding Sources: The authors have no funding sources to disclose. Conflict of Interest: The authors have no conflicts to disclose relevant to this manuscript.
References
- 1.Brownstein S.L., Hopson R.C., Martins J.B. Usefulness of isoproterenol in facilitating atrioventricular nodal reentry tachycardia during electrophysiologic testing. Am J Cardiol. 1988;61:1037–1041. doi: 10.1016/0002-9149(88)90121-x. [DOI] [PubMed] [Google Scholar]
- 2.Hermann R., Vettermann J. Change of ectopic supraventricular tachycardia to sinus rhythm during administration of propofol. Anesth Analg. 1992;75:1030–1032. doi: 10.1213/00000539-199212000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Appelboam A., Reuben A., Mann C. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): a randomised controlled trial. Lancet. 2015;386:1747–1753. doi: 10.1016/S0140-6736(15)61485-4. [DOI] [PubMed] [Google Scholar]
- 4.Mark A.L. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1:90–102. doi: 10.1016/s0735-1097(83)80014-x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson D.M., Antoons G. Arrhythmogenic mechanisms in heart failure: Linking β-adrenergic stimulation, stretch, and calcium. Front Physiol. 2018;9:1453. doi: 10.3389/fphys.2018.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]