Abstract
Purified enzymes of microbial origin are applied in the beverage industry since decades because of their ability to enhance products and processes with minimal side effects and low costs. Commercial enzymes are widely used during different wine making steps providing a broad range of effects, such as to maximise juice yield, improve aroma compounds, flavour enhancement, colour extraction in red wines, and contribute in the removal of dissolved unwanted colloidal particles and pectin substances during wine stabilization and filtration. This review presents a study of recent advances in the application of commercial enzymes in the wine making of red, white and sweet wines that have been made in essentially the last 13 years (2005–2018). Literature has been critically analysed to discover general rules about previous research. Special attention is paid to the safety of enzyme application due to allergic issues. Future research efforts should be concentrated on application of immobilizated enzymes and the use of microorganisms with potential enzymatic side activities during wine production.
Keywords: Enzymes, Wine, Wine making, Biotechnology
Introduction
Enzymes are proteins formed by long chains of amino acids with peptide bonds with particular structures produced by living cells, they are specific biological catalysts involved in different biochemical reactions. Enzymes are currently used during production in different food industries (dairy, meat, fruit juices, fish, tea and coffee processing, vegetable oil extraction and refining, starch processing, bakery, brewing and wine making), and other technological applications in sectors such as pharmaceuticals, cosmetics, animal feed, textile, paper, bioethanol, and detergent industries (Sarrouh et al. 2012). Most of enzymes for food industries are now derived from selected and optimized microorganisms grown at industrial scale, using microbial fermenters under strictly controlled conditions.
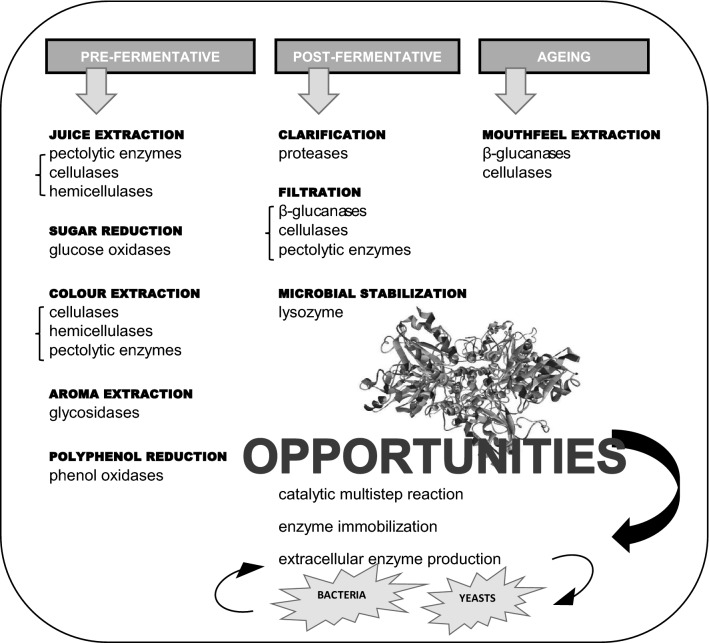
Consumers demand for quality wines besides providing safe and nutritional characteristics. As other food industries, wine producers were introducing different technological improvements based in biotechnology resources such as dry yeast, acid lactic bacteria starters or enzymes for offering enhanced performance, despite traditionalism of this beverage sector. There are different ingredients or additives (potassium sorbate, sulphur dioxide, etc.) and processing aids allowed to be added during production, and that vary depending on wine type, technological function or country legislation. In this context, the use of commercial enzymes in wine industry is related to a variety of goals, especially in the processing (maceration, extraction), stabilisation (clarification, filtration) and aging steps (maturation on lees) (Fig. 1). The activity of the enzymes naturally present in grapes and indigenous microflora is important during wine making processes. Unfortunately, the action of such endogenous enzymes is insufficient to obtain regular results to produce high-quality wines.
Fig. 1.
An overview of the main enzymes types used in wine making process and their phase application
Diverse reviews have been previously published about enzymes in wine making (Ugliano 2009; Gómez-Plaza et al. 2010; Rodriguez-Nogales et al. 2016; Claus and Mojsov 2018) most of them reviewed relevant studies published before the year 2000. The aim of this review is to provide an overview of the enzyme utilization in red, white and sweet wines. At the same time, offering a comprehensive overview on the recent advancements that could be useful to identify some considerations about the future needs of this specific biotechnological topic in wine production. Special attention was focused on safety issues related with the allergic properties of enzymes and its impact on wine consumption.
Effect of enzyme treatments on technological properties of must and wine
Several studies have investigated the effects of pectolytic enzymes preparations on technological parameters such as viscosity, turbidity and filterability of musts and wines. At the beginning, the positive economical repercussions (reducing costs) during these wine making steps have influenced on the decision against to use of enzymes in wineries. Pectins are structural polysaccharides in the middle lamella and primary cell walls of higher plants. Grape pectins joint to cellulose and hemicelluloses polysaccharides have important influence on clarification and filtration of must and wine, and possible related technological problems (Pinelo et al. 2006). The commercially available pectinase preparations are heterogeneous associations of polygalacturonases, pectin lyases and pectin methylesterases, in some cases are applied associated with other cell wall degrading enzymes as cellulases and hemicellulases, where the desired result is liquefaction. The mechanism of action of pectinases is to hydrolyse the pecto-cellulosic cell walls of the berry skin facilitating the liberation of liquid and other compounds. Consequently, this improves juice yield when enzymes are applied with a short maceration previously to pressing, this increase can go up to 10% (Gómez-Plaza et al. 2010). Besides shorter pressing times at lower pressure, there is also an increase in flocculation speed of the must before the alcoholic fermentation by breaking down pectins and macromolecules to smaller compounds removing matter in suspension (Ugliano 2009). In red wine making, the use of pectinase preparations produces higher juice during mechanical extraction either pumping over or punching down. This positive effect on clarification of the obtained juice is due to significant reduction of the protecting colloidal effect of macromolecules and viscosity reduction (Ugliano 2009). Numerous studies revealed that the addition of pectolytic enzymes has a positive effect on the clarification of must and consequently on related parameters such as viscosity and filterability, breaking up complex carbohydrate structures into more soluble oligomers, specially in grape varieties with higher pectin compounds (Samoticha et al 2017). In a recent study of Dal Magro et al. (2016) have been proved eight pectinase commercial preparations in the extraction of juice from Concord grape cultivar. These enzyme preparations improved juice yield up to 9% higher than control, especially with pectinolytic (pectin lyase) and cellulolytic enzyme preparations.
Polygalacturonases, pectinases, cellulases, hemicellulases and β-glucanases (EC 3.2.1.6) are used to improve filtration process after clarification of wine. Pectin and glucan type polysaccharides have both a high molecular weight, their hydrolysis avoid filter blockages, and reducing the turbidity (Fig. 2). Glucans are a cell wall component from yeasts, but glucans can be also produced by the mould Botrytis cinerea; in this case, β-glucans have high molecular weight (Rodriguez-Nogales et al. 2016). In addition, the use of pectinases and glucanases blend or cocktails at the end of alcoholic fermentation can help to stabilize and filter the wine. The filterability of wines, produced from grapes partially infected with Botrytis cinerea or from thermovinification systems, significantly increased after enzyme addition, particularly with β-glucanases, together with viable processing costs.
Fig. 2.
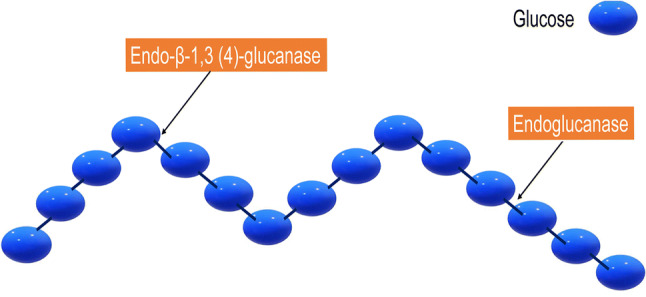
Schematic representation of the action of β-glucanases on β-glucans: molecules of linked 1,3–1,4-glucose
Effect of enzyme treatments on colour composition and mouthfeel of red wines
Table 1 presents an overview of relevant studies of the main enzyme applications in red wines found in the reviewed literature. In red wine making, colour extraction has also influenced by several factors such as grape variety, culture practices, maturation stage, environmental conditions during pre-harvest stage and different technological parameters (maceration time, temperature, frequency of pump-overs during fermentative maceration, etc.). It is well known that pectolytic (polygalacturonase, pectin methyl esterase and pectin lyase), cellulase, hemicellulase and acid protease activities are included in commercial maceration enzyme preparations (Fia et al. 2014), and next to xylanase and β-glucosidase activities can all degrade cell wall structures (Pinelo et al. 2006).
Table 1.
Overview on studies relating to the impact of enzymes used in red wines
| Grape variety | Enzymatic activities | Treatment conditionsa | Resultsb | References |
|---|---|---|---|---|
| Monastrell | Pectinase–cellulase–hemicellulase | n.s.c | TPId ↑ colour intensity → anthocyanins → | Bautista-Ortín et al. (2005) |
| Öküzgӧzü | Pectinases | 192 h + 22 °C | TPI ↑; colour intensity ↑anthocyanins ↑ | Kelebek et al. (2007) |
| Kalecik Karasi | Pectinase–hemicellulase | 1 h | Anthocyanins ↗ colour intensity ↑ | Kelebek et al. (2009) |
| Cabernet Sauvignon | Pectinase–hemicellulase | 96 h + 25 °C | Anthocyanins ↑TPI ↑ | Puértolas et al. (2009) |
| Mencía | Pectin lyase–polygalacturonase–cinnamil–esterase | 288 h | Colour intensity ↑ anthocyanins ↑ | Soto-Vázquez et al. (2010) |
| Merlot | Pectinases-β-glucanases | 12 h + 12 °C | Colour intensity ↑ proanthocyanidins ↑ rhamnogalacturonan-II ↑ | Ducasse et al. (2010) |
| Merlot, Cabernet franc, Cabernet Sauvignon | Pectinase–protease–cellulase | n.s | Colour intensity ↑ TPI ↑ anthocyanins → | Di Profio et al. (2011) |
| Mencía | Pectinases | n.s | Colour intensity ↑tonality → | Ortega-Heras et al. (2012) |
| Öküzgӧzü | Pectinases | n.s | Anthocyanins ↓total phenolic acids ↓ | Bozaran and Bozan (2013) |
| Tannat, Syrah, Merlot | Pectinases | 168 h + 23–29 °C | Colour intensity ↑ anthocyanins ↑ proanthocyanidins ↑ methanol ↑ | González-Neves et al. (2013) |
| Monastrell | Pectinases-α-β-galactosidase | 240 h + 25 °C | Oligosaccharides ↑ | Apolinar-Valiente et al. (2014) |
| Cabernet Sauvignon, Nebbiolo | Pectin lyase–polygalacturonase–cellulase | 192 h + 25 °C | Anthocyanins ↑ | Río-Segade et al. (2015) |
| Nero di Troia | Pectinases | 1 h + 25 °C | Proanthocyanidins ↑ anthocyanins → | Baiano et al. (2016) |
| Cabernet Sauvignon | Pectinases | 384 h + 30 °C | Colour intensity ↑anthocyanins → | El Darra et al. (2016) |
| Monastrell | Pectinases | n.s | Proanthocyanidins ↑ | Castro-López et al. (2016) |
| Concord | Pectinase–cellulase | n.s | Anthocyanins ↑ | Dal Magro et al. (2016) |
| Cabernet Gernischt | Pectinases–β-d-glucosidase | 24 h + 15 °C | C6 compounds, terpenes, C13 norisoprenoids ↑ | Sun et al. (2018) |
aTime and temperature conditions at pre-treatment maceration or alcoholic fermentation
bResults obtained in enzyme treated wines in relation to controls
cNot specified
dTotal polyphenols index
Numerous studies have been focused on maceration enzymes that are able to enhance colour extraction in red wines. Bautista-Ortín et al. (2005) studied the chromatic and colour stability and sensory characteristics of Monastrell red wine using two maceration enzyme preparations (with cellulase and hemicellulase side activities besides polygalacturonase, pectin lyase and pectin esterase main activities). Enzyme treated wines showed higher values of total polyphenol index (OD at 280 nm) due to the phenolics compounds extracted from solids parts. On the other hand, non-significant results were obtained with respect to colour parameters (intensity and tint) and anthocyanin contents. Maceration enzymes have widely used in red grapes varieties with naturally low anthocyanin contents such as Pinot Noir and Öküzgӧzü from Turkey. Kelebek et al. (2007) studied anthocyanin composition of the red grape variety Öküzgӧzü. Long maceration times (8 days at 20–22 °C) with two different enzyme preparations at 3 g/100 kg were studied. Enzyme addition had significant effect on total polyphenol index, proanthocyanidins and colour intensity. Enzyme red wines contained higher monoglucoside derivatives, acylated, and p-coumarocylated anthocyanins compared to the control wines. In another study, Kelebek et al. (2009) found that the anthocyanin profile of Kalecik Karasi red grapes of enzyme treatments and control wines were similar at early stages, although final wines after alcoholic fermentation contained the highest amounts of anthocyanin and other phenolic compounds with increasing maceration time and the use of pectolytic enzymes. Puértolas et al. (2009) compared the effect of two macerating preparations with the application of pulsed electric fields on colour and phenolic compounds of Cabernet Sauvignon wine. The authors showed that both techniques promoted greater extraction than control wine but pulsed electric fields treatment was more effective than the pectinase enzymatic preparations used. Soto Vázquez et al. (2010) compared pre-fermentative maceration using procyanidin tannins, ellagic tannins, oak chips and pectolytic enzymes (cinnamyl-esterase, polygalacturonase and pectin lyase activies) in red Mencía grapes. The authors showed significant increase of colour, enhancing co-pigmentation reactions with greater anthocyanin contents when pectolytic enzymes were used at 2.4 g/hL dosage. Di Profio et al. (2011) compared the effect of two pectolytic enzymes on three red grapes varieties during three consecutive vintages. The effects of canopy manipulation and its interactions with enzyme treatments on colour indexes, anthocyanins and total phenols were studied. They observed that the colour of the obtained wines was depended on grape variety and vintage. The results suggested that enzymes led to increase the total phenolic concentrations and colour intensity, but not significant effect of these enzymes on anthocyanins was observed, possibly due to contamination by glycosidase secondary activities from enzyme preparations used. In another comparative study, Ortega-Heras et al. (2012) compared the use of maceration enzymes and cold- pre-fermentative maceration using dry ice in Mencía red wine during two vintages. A vintage effect was observed on colour stability and intensity. In another study, Romero-Cascales et al. (2012) observed that enzyme preparations could be more useful in short macerations for colour extraction in red wine making. The enzyme-treated wines showed the higher tannin content and total polyphenol index after 12 months of storage. On contrary, Borazan and Bozan (2013) showed lower monomeric anthocyanin and flavan-3-ol contents with the pectolytic enzyme addition than control in Öküzgӧzü red wine. In another study, significant differences in colour extraction were showed between the studied red grape varieties with pectolytic enzyme addition (González-Neves et al. 2013). The maceration time and temperature during enzyme action were not provided. The influence of wine making practices is conditioned by grape variety used and the grade of ripeness of the grapes, among other factors. In a recent experiment with Cabernet sauvignon and Nebbiolo grapes it was shown skin degradation after enzyme treatments (2 and 5 g/100 kg) at controlled conditions (during 192 h at 25 °C) (Río Segade et al. 2015). Anthocyanin extraction from the skins into a wine-like solution (without alcoholic fermentation) was increased with enzyme addition and speeded up the extraction yield (~ 40 h) of extractable anthocyanins. The effect of enzyme on the anthocyanin profile associated to the grape variety was only significant for Cabernet sauvignon variety, not in Nebbiolo variety. The latter finding could possibly reflect cultivar-specific differences between enzyme additions or not, also may illustrate the considerable influence of plant genetic profile. Baiano et al. (2016) compared four different maceration procedures using Nero di Troia red grapes. Only few differences were showed between standard maceration and enzyme preparation used (a mixture of pectine lyase, polygalacturanase and pectine methylesterase), dosage at 2 g per 100 kg for 1 h of maceration. Cryo-macerated and enzyme treated grapes showed higher proanthocyanidins values and in the experiment with pectolytic enzymes the ratio of large and small polymeric pigments was also higher. El Darra et al. (2016) compared pulsed electric field, maceration enzyme and thermovinification pre-treatments in Cabernet sauvignon grapes. All these pre-treatments cause breakdown of the skin cell walls. At the end of alcoholic fermentation, all pre-treatments showed higher colour intensity (at 520 nm) than control. The contents of native anthocyanins, phenolic acids and proanthocyanidins did not show significant differences compared to the control. On the contrary, flavonols from thermovinification and pulsed electric field treatments showed significantly higher values against control (97% and 48%, respectively). Temperature and time of enzymatic maceration pre-treatment were not provided in this study. Temperature and maceration time affect the anthocyanin extraction, chromatic parameters and colour stability in red wine (Kelebek et al. 2009; Río Segade et al. 2015). These apparently contradictory results can be attributed to natural variety differences, grape maturity, principal and secondary activities of used commercial enzymes and the different experimental conditions of the studies. In a recent study, Monastrell red grapes were used by Castro-López et al. (2016) to confirm the interactions between proanthocyanins and cell wall material from grapes with and without maceration enzyme addition. The authors showed that macerating enzymes increase proanthocyanidin content of must and wine by enhancing extraction from skin cell vacuoles and favouring lower adsorption on cell wall material. The adsorption of proanthocyanidin to cell walls decreased in the presence of enzyme, hence increasing colour stability and phenol contents. The binding capacity of the cell walls also depends of the maturity stage of the grapes. Enzymatic maceration seems to be influenced by degree of maturity of grapes, vintage and genetic characteristics of the variety among other factors.
Majority of authors showed a significant increase in the anthocyanin contents and colour intensity attribute due to the action of enzymatic preparations, while others have found the opposite effect (Bozaran and Bozan 2013) or not significant results (Bautista-Ortín et al. 2005). The effect of maceration enzyme preparations on the anthocyanin contents and final colour intensity in red wines is controversial according to the different studies. It is not clear the impact of maceration enzymes on macromolecular extraction (tannins, polysaccharides and proteins) and colour stability after vinification. To identify the potential mechanism by which extracted polyphenols may be lost from obtained wines seems to be a goal not yet achieved. These disagreements are probably due to the different enzyme preparations used (Capounová and Drdák 2002), as well as to different non-controlled factors in the experimental trials. Phenolic compounds extracted during microvinification at laboratory scale with small samples (between 1.5 and 5 kg) can be lower than in commercial fermentations and maceration time should be longer due to delayed extraction of phenolic compounds (Sampaio et al. 2007). The lack of studies with semi-industrial trials cause that some results not obtain high confidence and reliability, at least from the wine technician’s point of view.
Because not only colour compounds are released from skins, side activities of maceration enzyme preparations (cellulases, proteases or hemicellulases) may help to extraction in red vinification and it take an important positive role in wine structure and mouthfeel sensations due to grape polysaccharides dissolution. In a study of Doco et al. (2007) pectinolytic enzyme preparations were compared against to flash release to enhance aroma, phenolic and polysaccharide compounds in red wine. The importance of the maceration time for the complete extraction was revealed. The use of enzymes modified the composition and structure of pectic polysaccharides rich in arabinose and galactose. Ducasse et al. (2010) studied colour parameters, polyphenol and polysaccharide composition of Merlot wine over three vintages. The effect of enzyme treatment on tannin and rhamnogalacturonan II was vintage dependent. Enzyme treatment wines resulted in increased polyphenol extraction and higher colour stability during wine aging (20 months). Apolinar-Valiente et al. (2014) studied the oligosaccharide structures in red grapes of Monastrell cultivar from four different “terroirs”. The results confirmed a significant effect of the commercial enzyme (5 g/100 kg) on released oligosaccharide composition; on contrary, β-galactosidase enzyme addition (1 g/100 kg) had no evident effect on oligosaccharide composition against control wines. At the same time, the results indicated an interaction between enzymatic treatments and grape origin (terroir influence). Zietsman et al. (2015) studied the cell wall monosaccharide composition of grape berries from Pinotage variety. The ripeness level of berries had a significant effect on the cell walls and consequently in the action of the endogenous and exogenous enzymes. This study shows that it was difficult to distinguish between the action of the endogenous ripening enzymes and the maceration enzymes added. In this context, the intra-vineyard ripeness variation factor was studied, and different maceration enzyme preparations were evaluated on polysaccharides at the cell wall polymer level with Cabernet sauvignon red grapes (Gao et al. 2016). This study showed that all used enzyme preparations reduced cell wall variation via de-pectination increasing the uniformity of the grape pomace. All enzymes opened up the hemicellulose fraction enabling access to cell wall polymers, especially effective in ripe grapes probably by endogenous enzymes, which enhanced the impact from exogenous maceration enzymes.
Effect of enzyme treatments on aroma compounds
Extraction of aroma compounds and their precursors from grapes is one of main objectives during wine production. In recent years, most attention has been placed on using glycosidases to increase the aroma of white wines. The action of endogenous and exogenous enzymes produces that odourless glycosidically-bound precursors are converted into aromatic compounds. Glycosidases operate by releasing aromas that have bound to sugars (glucose or disaccharides) and carbohydrate residues to form odorless glycosides, mainly amongst monoterpene-rich varieties (Maicas and Mateo 2005). Monoterpenes, benzene derivatives, C13-norisoprenoids and aliphatic alcohols are aromatic compounds that were glycosylated inside the grape berry cells and can be released by enzyme hydrolysis action (Cabaroglu et al. 2003). Volatile thiols compounds can also produce from odourless precursors in white and red grapes varieties. In this sense, Sun et al. (2018) revealed higher varietal compounds with β-d-glucosidase addition in Cabernet Gernischt red wine.
According to the step of vinification proccess, different enzymatic preparations were used in white wines to increase or release aroma compounds mainly due to β-glucosidase, rhamnosidase, pectinase and glycosyl hydrolase activities (Pogorzelski and Wilkowska 2007). Table 2 shows an overview of reviewed studies of the main enzyme applications in white wines. Due to inhibitory effect on glycosidases by glucose, these preparations give best results when it added after alcoholic fermentation (Maicas and Mateo 2005). Volatile composition of white wines from Maria Gomes and Bical Portuguese grape varieties was studied using a commercial enzyme preparation with β-glucosidase, pectinase, arabinosidase and rhamnosidase activities (1 g/100 L), used after the alcoholic fermentation (Rocha et al. 2005). This work showed that the potential volatile profile development due to enzyme addition is dependent on the varietal aroma of the grape variety. Therefore, enzymatic treatment increased the amounts of monoterpenoids (+ 32%), terpendiols and aromatic alcohols (+ 19%) in Maria Gomes grape variety, but on contrary, this enzymatic treatment did not promote any improvement in the Bical wine aroma profile. A study of Masino et al. (2008) showed white wines with higher 4-vinylphenol concentrations when musts were treated with pectinase preparations. A β-glucanase commercial preparation was used during aging on lees. The enzyme addition further enhanced different volatile compounds such as 2-phenylethanol, fatty acids and ethyl ester compounds due to yeast-cell wall degradation. Different pectolytic enzyme preparations and one with β-glycosidase activity were studied in Gewürztraminer variety (Rusjan et al. 2009). This study revealed that the wines from musts treated with enzymes had higher monoterpenes (nerol, geraniol, α-terpineol and linalool) against to the control. In this case, an extensive maceration time (8 h) at 17 °C was applied to aroma extraction. Monoterpenes concentrations were higher, when β-glycosidase preparation was used. Another study carried out by Armada et al. (2010) investigated the application of pectolytic maceration enzymes and one clarificant enzyme preparation in Albariño white wine aromatic profile. Maceration enzymes produce liberation of the free forms of terpenes and C13-norisoprenoids (β-pinene, 1,8-cineol, nerol, geraniol and β-damascenone), but the combination of maceration and clarification enzymes together was not effective to increase aromatic precursors extraction.
Table 2.
Overview on studies relating to the impact of enzymes used in white wines
| Grape variety | Enzymatic activities | Treatment conditionsa | Resultsb | References |
|---|---|---|---|---|
| Maria Gomes, Bical | Pectinase–arabinosidase–rhamnosidase–β-glucosidase | afc | Terpenes ↑aromatic alcohols ↑esters ↑ | Rocha et al. (2005) |
| Bombino bianco | Pectinase–β-glucanase | 24 h + 4 °C | Ethyl esters ↑ hexanol ↑ trans-3-hexenol ↑ methanol → | Masino et al. (2008) |
| Gewürztraminer | Pectinases–β-glucosidase | 8 h + 17 °C | Monoterpenes ↑ | Rusjan et al. (2009) |
| Albariño | Pectinase–β-glucosidase | 6 h + 8 °C | Terpenes ↑ C13-norisoprenoids ↑ C6-alcohols ↑ | Armada et al. (2010) |
| Pedro Ximenez | Pectinases–cellulase | 3 h + 28 °C | TPI → total juice yield ↑ total soluble solids ↑ | Espejo and Armada (2010) |
| Furmint | Pectinase–β-glucosidase–rhamnosidase–arabinosidase | 8 h + 17 °C | Linalool → | Rusjan et al. (2012) |
| Roscetto | β-glucanase–pectinases | af | Amino acids ↑ | Torresi et al. (2014) |
| Solaris | Pectinases | 1 h + 23 °C | Phenolic acids ↑pH ↑ total juice yield ↑ | Samoticha et al. (2017) |
aTime and temperature conditions at pre-treatment maceration or alcoholic fermentation
bResults obtained in enzyme treated wines in relation to controls
cAfter alcoholic fermentation
At this point, it is important to note that studies with negative results were also published relating to white wines. For instance, Rusjan et al. (2012) studied the effect of seven enzyme preparations on terpene profile in non-aromatic Furmint white variety. Grape pomace (300 kg of grapes) was macerated during 8 h at 17 °C before fermentation. Linalool was the only monoterpene compound quantified in this experiment. The enzyme preparations did not significantly influence on linalool contents of the resulted wines and the sensory evaluation confirmed these results. The presence of α-rhamnosidase, α-arabinosidase and β-glucosidase activities are necessary for the hydrolysis of glycosides for aroma release. The limitations of using poorly characterized commercial preparations at batch production level might lead to invalid conclusions when comparing the effectiveness of different treatments.
The research of enzymes applied to sweet wines has been mainly linked to the effects on filterability of the wines and the extraction of aroma. In this sense, a study of Espejo and Armada (2010) used two pectolytic enzyme preparations in sun-dried grapes of the Pedro Ximenez white variety to obtain sweet wine without fermentation. Enzymatic dynamic maceration during 3 h at variable temperature between 28 and 36 °C prior to pressing was performed, comparing the effects of enzymatic trials with control sweet wine. Total juice yield and total soluble solids were significantly increased after enzyme addition with dynamic maceration possibly by cellulose side action. On the contrary, total polyphenol index and other chemical parameters were not affected. The risk of extraction of polyphenolic compounds increases with high maceration times. Sensorial trials confirmed a higher qualitative level in enzymatically obtained sweet wines. The effect of different enzyme preparations on wine flavour, aroma and quality parameters on sweet wines remain unclear due to the lack of comparative research.
To date most studies have focused on study aroma enhancement in white wines from aromatic varieties, less information is available regarding enzymes addition during ageing. Commercial β-glucanases are actually available for applications of filtration, clarification and aging of wines. Glucanase-based preparations can be used to release yeast intracellular compounds such as polysaccharides, mannoproteins, amino acids and peptides. Basically, β-glucanases have been added to musts affected by Botrytis cinerea to hydrolyze glucans avoiding subsequent colour and filtration problems (Fig. 2). These enzymes preparations are produced by strains of Trichoderma sp. and speed up yeast autolysis during maturation on lees mainly in white wines at the end of alcoholic fermentation (Gómez-Plaza et al. 2010). In this sense, a study of Torresi et al. (2014) in sparkling wine revealed that β-glucanase improved autolysis degradation of the cell wall of yeast strains studied. The results also indicated that β-glucanase addition influenced the free amino acid profile and probably with positive consequences on aroma compounds and mouthfeel characteristics. Also, residual acid proteases contained in commercial enzyme preparations can help to release all these compounds. When glycosidases or glucanases are used for aroma and mouthfeel extraction compounds, one inconvenient is the impossibility to control the hydrolisis process, which only can be stopped with bentonite.
Others enzyme types
Lysozyme (EC 3.2.1.17) is a muramidase enzyme derived from egg white proteins used as antimicrobial on Gram-positive bacteria in white and red wines. Its antibacterial action is due to lytic activity on the β(1,4)-glycosidic linkages between N-acetylmuramic acid and N-acetyl-D-glucamide residues in the peptidoglycan of the bacterial cell wall (Liburdi et al. 2014). Main applications of lysozyme in wines are the prevention or total inhibition of malolactic fermentation and microbial stabilization after malolactic fermentation. For those products, allergic reactions against treated wines could not be discarded; detectable residual amounts of lysozyme are possible to produce adverse reactions in allergic persons. In this case, it not could be assumed that used enzymes may completely remove during the production process, and then wines treated with lysozyme do require to be labelled on the wine bottle “contains egg”.
Instable protein fractions in white wine have been the target of studies applying proteolytic enzymes to avoid protein haziness and turbidity instead of the traditional fining with bentonite. Bentonite adsorption is not specific, removing aroma and flavour compounds besides of proteins. Acidic proteases of fungal origin were used in early studies with disappointing results in white wines. This subclass, inside of hydrolases enzymes, has been utilized in protein hydrolysis. The hydrolysis of peptide bonds by proteases through nucleophilic attack reduces wine protein haze formation. The selection of the most appropriate protease is essential since not all proteases are suitable for use in wine. Appropriate proteases must be act under restricted wine conditions of pH, alcohol contents or temperature. The use of a fungal endoprotease (EC 3.4.21.26) as an alternative to bentonite treatment was studied in musts and white wines to stabilize unstable proteins (Marangon et al. 2012). In this study, the need for flash pasteurization of the juice together with proteolysis by Aspergilloglutamic peptidase addition limits its viability at industrial scale. There is increasing interest in evaluating proteases hoping to find a suitable protease for wine stabilization and making possible to reduce or replace bentonite fining in the future. On the other hand, proteolytic enzymes are potential ingestive allergens and its allergenic power due to occupational exposure has been studied in many industrial processes of detergent, food and pharmaceutical industries (Schweigert et al. 2000). Two families of proteases contain allergenic proteins (the papain-like cysteine and subtilisin-like serine proteases) (Breiteneder and Radauer 2004). This suggests that proteases could produce allergenic residues in final products when the control of the process and the final treatments in wine are not suitable. It has to consider that bentonite binds with proteins such as enzymes and eliminates them from wine through precipitation.
Although its use is still not allowed as a food additive in wine, enzymes active against polyphenols (oxidases, hydrolases and transferases) could be used during early wine making steps in white wine production. To reduce polyphenolic compounds previously to alcoholic fermentation that could react and produce chromatic evolution and instability. To this end, it can generally use physical–chemical fining adsorbent agents such as bentonite, polyvinylpyrrolidone (PVPP) and others inorganic clarificants. Copper-containing enzymes such as tyrosinase (EC 1.14.18.1) and laccase (EC 1.10.3.2) catalyze a wide spectrum of phenolic compounds with characteristics similar to a p-diphenol due to low substrate specificity. Similarly, tannase (EC 3.1.1.20) only acts on hydrolyzable tannins and gallic acid esters (Aguilar and Gutiérrez-Sánchez 2001). Laccases are typically extracellular glycoproteins with the ability to reduce molecular oxygen to water as by-product, accompanied by the oxidation of phenolic and non-phenolic substrates via one-electron removal. Phenolic compounds are firstly oxidized, polymerized in non-enzymatic reactions forming high molecular weight compounds (oligomeric or polymeric products) (Alcalde 2007), which later eliminated during settling of the must or after vinification. Diverse early studies concluded that laccase can eliminate instability in white wines caused by oxidizable polyphenols. Servili et al. (2000) investigated the removal of phenolic compounds from white musts using fungal laccase immobilized on cooper-chelate substrate. The process exploits the ability of laccase to remove phenolic compounds (epicatechin, ferulic and o-coumaric acids) due to oxidative coupling forming insoluble polymeric products which can be removed from must or wine as a precipitate. In a study conducted by Minussi et al. (2007), the impact of laccase from Trametes versicolor was evaluated on white must with positive results, showing higher reduction in total phenol than in the antioxidant potential, on contrary in red wine laccase affected phenolic compounds responsible for the antioxidant potential. Phenol degradation was very fast for catechins, but slowly for stilbenes (cis- and trans-resveratrol) and derivatives of cinnamic (ferulic and caffeic) and benzoic (syringic, vanillic, and gallic) acids. Moreover, wine stabilization, laccases could also be used as oxygen scavengers to improve storage life avoiding unwanted oxidation and off-flavours development after bottling. Laccase is able to reduce the oxygen content significantly in the headspace of bottles, lowering detrimental chemical changes during its shelf life, and its efficiency has been demonstrated in the brewing industry (Alcalde 2007). A controversial issue may arise from the use of phenoloxidases, the possible reduction of free-radical scavaging of phenolic compounds and its antioxidant properties could be altered with the use of these enzymes, especially in red wines. Several compounds relating with sensorial characteristics could be altered. In the future, different oxidoreductase enzymes need to be tested in diverse types of wine to have a more accurate vision of their real efficiency for this specific application.
A relatively new recent biological technique to produce reduced alcohol wines is glucose oxidase (EC 1.1.3.4) utilized in pre-fermentative processes. Until now, the use of this enzyme in wine making is not permitted in some countries. This aerobic glycoprotein catalyzes the oxidation of β-d-glucose to d-glucono-1,5-lactone (d-gluconic acid δ-lactone) and hydrogen peroxide as a by-product. Simultaneously, d-gluconic acid δ-lactone hydrates forming gluconic acid (Schmidtke et al. 2012). This stable organic acid cannot to be fermented by yeasts; consequently, alcohol levels in resulted wines are lower compared to untreated wines. There are some drawbacks with this technique: need of higher dissolved oxygen concentrations in the must, optimum glucose oxidase activity at high pH levels from 5.5 to 6.0, in consequence deacidification of the grape juice is needed to guarantee an efficient treatment, and finally high levels of gluconic acid in finished wines. Besides, hydrogen peroxide produced can result in inhibition of glucose oxidase and acts as an antimicrobial agent. Glucose oxidation inefficiency produces alcohol decrease from less than 4–40% (Schmidtke et al. 2012). In a recent work, Röcker et al. (2016) studied the use of glucose oxidase and catalase to reduce glucose in white must with high production of no-fermentable gluconic acid. The results showed treated wines more acidic and less fruitiness than controls. An alcohol reduction of 2% v/v was obtained with the procedure applied (30 h of aeration, pH 3.5, 30 kU/L of glucose oxidase and 15 kU/L of catalase). Alteration of desirable organoleptic qualities and possible off-flavour development are important limitations.
Principal drawbacks of enzyme use
It is important to stress out that enzymes can have some drawbacks to being considered. Because of the nature of enzyme preparations, it is not always possible to check the specific main activity and side activities provided by the supplier to compare between commercial products or to check its efficiency and possible detrimental effects. For instance, cinnamoyl esterase (EC 3.1.1.1) activity can produce free phenolic acids such as vinyl-4-phenol and vinyl-4-guayacol in white and red wines due to hydrolysis of p-coumaric and ferulic acids by yeast fermentative cinnamate decarboxylase action (Fia et al. 2014). These volatile phenols can confer undesirable off-odours such as “horsey” or “farmyard” odours to red wines and nail polish odour to white wines and is also responsible for premature loss of fruitiness and freshness in white wines, thus affecting negatively the volatile composition of resulted wines.
Another undesirable side activity is anthocyanase (β-glucosidases) which could induce a loss of colour in red wine production due to unstable anthocyanidin formation; breaking the bond between the glucose and the anthocyanidin-3-glucosides (Romero-Cascales et al. 2012). While this fact is beneficial in white wine production, but not to red or rosé wines, due to red colour degradation and colourless pigment formation.
At early stages of commercial enzyme preparations used for clarification, its main drawback was the methanol production. There are strong evidences proving that pectolytic enzymes produce methanol, with a higher level in red wines (Ugliano 2009). Pectin methyl esterases release methanol and polygalacturonic acid from pectin substances. No production or low level of methanol is considered a goal to promote enzyme application in beverages industry. Some studies have showed methanol increased due to pectolytic enzymes addition, but within maximum acceptable limits (Romero-Cascales et al. 2012; González-Neves et al. 2013). Nowadays, to keep these problems under control, producers commercialized enzyme preparations with no undesirable activities or kept at negligible levels. In any case, industrial purification processes to remove these undesirable activities and other toxic secondary metabolites produce simultaneously a loss of the main activity of the enzyme preparations (Guérin et al. 2009).
Different grape and wine compounds may play an important role in wine allergy and intolerance reactions in sensitized individuals such as biogenic amines, sulphites or ethanol. Some proteins have allergenic potential and their residues in the final product could represent a risk for allergic consumers sensitive to the protein used (Wüthrich 2011). In particular, animal proteins are widely used in the clarification of wines (caseinates, fish gelatine, egg white proteins), besides other grape and microbiological origin proteins may be involved as well in the sensitization such as β-1.3-glucanase, endochitinase 4 and thaumatin-like proteins (Pastorello et al. 2003; Wüthrich 2011). Actually, other fining agents produced from vegetal proteins (e.g. glutens, lupin, pea proteins) are used in wines instead of animal proteins, also presenting a potential risk to sensitised individuals (Verma et al. 2013). In principle, it can be assumed that residues of these substances are not present in the final commercial wines. Verhoeckx et al. (2015) reviewed the effect of processing on the allergenicity of proteins in peanuts, tree nuts, cows’ milk, hens’ eggs, wheat, mustard and soy. The studies available showed that only microbial fermentation and enzymatic or acid hydrolysis may have the potential to reduce the allergenic role of these proteins. Nevertheless, for each allergen, treatments during wine processing might eliminate or reduce the allergen contents at secure levels for patients with food allergies. The use of technological procedures such as sheet filtration or bentonite addition leads to wines containing no detectable amounts of hidden allergens and low risk of immunoreactivity. Different studies have reported low or negative allergic response to ovalbumin in white and red wines after bentonite application or sheet filtration both in conjunction with sterile filtration (Kirschner et al. 2009). Some studies suggested that wines treated with bentonite and filtered present a negligible risk of inducing a clinically significant adverse reaction in subjects with confirmed allergy to the used proteins (Vassilopoulou et al. 2011). Until recent years, the allergic effects of enzymes have been studied mainly regarding their uptake by inhalation. It is well documented allergic and irritative effects after directly contact with a high concentration of pure enzyme, especially in the production and handling of pulverized enzymes in occupational and industrial contexts (Schweigert et al. 2000). A few cases of oral allergy to fungal enzymes are described in literature (Rizzi et al. 2016). In wine industry, the enzymes are applied during wine production as processing agents and not in the final products. It should be kept in mind that wine is processed before consumption. Most commonly used physical treatments are cold and heat treatments, depth and sterile filtration or centrifugation among others. Biochemical processes such as microbial fermentation (alcoholic and malolactic), aging and clarification treatments are extensively applied to wines. All these stabilization protocols and treatments are depending on the characteristics of the produced wines. After enzyme performance, the enzymes are denatured by bentonite treatment, then precipitated and finally removed by at least two filtration steps.
However, only limited data are known about how food processing can affect allergic sensitization and about the degradation of proteinogenic fining agents used in wine clarification which may undergo allergenic residues in the final wines, similarly little is known about how wine making steps may affect allergic sensitization and subsequent elicitation of adverse reactions to enzymes in sensitized individuals (Rizzi et al. 2016). In principle, the possible concentration of enzymes in final commodities is very low; hence irritating properties are not considered a risk for final consumers (Spök 2006). Nevertheless, the resulting detectable sub-trace amounts of enzymes able to induce a clinical reaction by oral route in sensitized adults are probably negligible, if existent (Bindslev-Jensen et al. 2006). On the other hand, there are not enough studies about if enzymes used in food products are also potential ingestive allergens or not (EFSA 2009). Hence conclusive data are not currently available in respect, probably due to the lack of sensitive and accurate routine analytical methods to characterize and quantify these residual proteins in different food matrices. At present, advanced proteomic methods using mass spectrometry provide results of hidden allergenic food proteins, particularly absolute quantification of multiplex allergens (Kirsch et al. 2009). In this context, the complete characterization at molecular level of enzymes naturally present and added to wines, including identification of suitable marker peptides for development of analytical methods is needed. Therefore, while everything suggests that enzyme residues in the final products following filtration are low or negligible in all cases. In view of published studies, it is difficult to conclude whether wine, in which enzymes were utilized, is harmful to sensitized consumers or not, and at what levels of consumption.
Future directions
In the field of enzyme optimization, different biotechnological tools would be studied in wine such as immobilized and embedded enzymes. Immobilization of enzymes to solid supports was utilized to improve operational stability, offer better activity, and to reduce costs at the same time (Bleve et al. 2016). The use of immobilized enzymes is a much safer approach, being easier to remove residual proteins from the process flow of the must and wine, at the same time could be used repeatedly depending on the intended purpose and avoiding potentially allergenic residual proteins in final products. The selection of support material and the method of enzyme immobilization, which cover both economic and technical requirements for large-scale application, are the key factors when most wines are of low added commercial value. Despite multiple advantages associated with immobilization of enzymes, nowadays there are few successful examples of immobilized enzymes for food processing used industrially. Then, the ability to scale up this technique or the robustness still needs to be demonstrated in different industrial scenarios. In recent years, several studies have investigated the use of different supports for immobilized yeasts (Bleve et al. 2016), lactic acid bacteria (Agouridis et al. 2005), and enzymes (González-Pombo et al. 2014); but there is a lack of enough published studies regarding immobilized enzymes used in wine (Spagna et al. 2002; González-Pombo et al. 2014). An alternative to the attachment of the enzyme to a solid inert support may be to use cross-linked enzyme aggregates by conventional non-denaturing protein precipitation techniques (Ahumada et al. 2016). This study used this immobilization technique with glycosidases in Muscat wine, showing higher concentrations of linalool, nerol, geraniol and benzyl alcohol than in the control. Simultaneous co-immobilization of yeast and bacteria cells has also been evaluated with positive results, obtaining acceptable quality wines with significant decrease in time needed (Bleve et al. 2016).
New strategies may develop when wine making process requires the participation of various enzymes in sequence, therefore the application of immobilized multi-enzymes or catalytic cascade process can be investigated. Another research direction can be investigating endogenous enzymes in grapes and yeasts to develop practices that enhanced its activities during the different processing steps, becoming a promising alternative for exogenous enzymes addition. It is important to highlight that biotechnology tools are associated with a negative consumer’s perception because they have very limited knowledge of these production practices and its consequences in product’s quality (Søndergaard et al. 2005). In this sense, the use of non-Saccharomyces yeasts and lactic acid bacteria with extracellular enzyme production could represent one viable alternative to exogenous enzymes addition. Important activities have been observed in lactic acid bacteria (esterase, lipase, protease, β-glucanase, cellulase, glucosidase and tannase), and in non-Saccharomyces yeasts (pectinase, protease, β-glucanase, cellulase, xylanase, glucosidase) under wine-like conditions (Maicas and Mateo 2005; Escribano et al. 2017). Finally, there are multiple molecular engineering strategies for improving food enzymes or to rationally modify its properties, used by enzyme manufacturers, such as directed evolution, protein engineering or de novo designing biocatalysts (Yang et al. 2014). Future manipulated and customized microorganisms may express capacity to produce extracellular enzymes during wine production processes.
Conclusion
Enzyme application in wine making has gained much attention of industry during the last few decades. In general, enzymes are widely accepted and well establish in wine industry. Providing further technological and economic advantages and helping to solve the insufficient activity of endogenous enzymes in grapes and wines. As described in this review, studies of different enzyme preparations have been gradually accumulating valuable information for technicians that can lead to considerable improvements in strategic decisions for wine making management. Other aspects such as enzyme immobilization, multi-enzyme complex preparations are probably to be a focus of future research in wine. Differences between studies can be attributed to the fact that grape varieties and enzyme preparations employed were not the same. Nonetheless, due to the complex matrix studied the obtained results were closely related to a large number of intrinsic and extrinsic factors that are relevant (variety, grape maturity, time and temperature of enzymatic maceration, enzymatic preparation profile, and so on). Consumers demand for minimally processed foods with “invisible” ingredients or clean labels which have apparent more healthy perception. This review can serve to help oenologists to make an informed choice and judicious application, at the same time advising consumers about enzyme preparations properties in wine and to increase their knowledge about the product safety perception. In this context, it can be expected that consumers will not be exposed to enzyme residues from wine consumption, but there is still a lack of adequate information regarding potential enzyme residues on the final products and its possible risk for sensitisation. In any case, further scientific research will be necessary to know these and other unanswered questions.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agouridis N, Bekatorou A, Nigam P, Kanellaki M. Malolactic fermentation in wine with Lactobacillus casei cells immobilised on de-lignified cellulosic material. J Agric Food Chem. 2005;53(7):2546–2551. doi: 10.1021/jf048736t. [DOI] [PubMed] [Google Scholar]
- Aguilar CN, Gutiérrez-Sánchez G. Review: sources, properties, applications and potential uses of tannin acyl hydrolase. Food Sci Technol Int. 2001;7(5):373–382. [Google Scholar]
- Ahumada K, Martínez-Gil A, Moreno-Simunovic Y, Illanes A, Wilson L. Aroma release in wine using co-immobilized enzyme aggregates. Molecules. 2016;21:1485. doi: 10.3390/molecules21111485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalde M. Laccases: biological functions, molecular structure and industrial applications. In: Polaina J, MacCabe AP, editors. Industrial enzymes: structure, function and applications. Dordrecht: Springer; 2007. pp. 461–476. [Google Scholar]
- Apolinar-Valiente R, Williams P, Mazerolles G, Romero-Cascales I, Gómez-Plaza E, López-Roca JM, Ros-García JM, Doco T. Effect of enzyme additions on the oligosaccharide composition of Monastrell red wines from four different wine-growing origins in Spain. Food Chem. 2014;156:151–159. doi: 10.1016/j.foodchem.2014.01.093. [DOI] [PubMed] [Google Scholar]
- Armada L, Fernández E, Falqué E. Influence of several enzymatic treatments on aromatic composition of white wines. LWT Food Sci Technol. 2010;43:1517–1525. [Google Scholar]
- Baiano A, Previtali MA, Viggiani I, De Gianni A. Maceration procedures alternative to the standard vinification in red: the case of Nero di Troia wine. Eur Food Res Technol. 2016;242:825–835. [Google Scholar]
- Bautista-Ortín AB, Martínez-Cutillas A, Ros-García JM, López-Roca JM, Gómez-Plaza E. Improving colour extraction and stability in red wines: the use of maceration enzymes and enological tannins. Int J Food Sci Technol. 2005;40:867–878. [Google Scholar]
- Bindslev-Jensen C, Skov PS, Roggen EL, Hvass P, Brinch DS. Investigation on possible allergenicity of 19 different commercial enzymes used in the food industry. Food Chem Toxicol. 2006;44:1909–1915. doi: 10.1016/j.fct.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Bleve G, Tufariello M, Vetrano C, Mita G, Grieco F. Simultaneous alcoholic and malolactic fermentations by Saccharomyces cerevisiae and Oenococcus oeni cells co-immobilized in alginate beads. Front Microbiol. 2016;7:943. doi: 10.3389/fmicb.2016.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozaran AA, Bozan B. The influence of pectolytic enzyme addition and prefermentative mash heating during the winemaking process on the phenolic composition of Okuzgozu red wine. Food Chem. 2013;138(1):389–395. doi: 10.1016/j.foodchem.2012.10.099. [DOI] [PubMed] [Google Scholar]
- Breiteneder H, Radauer C. A classification of plant food allergens. J Allergy Clin Immunol. 2004;113(5):821–830. doi: 10.1016/j.jaci.2004.01.779. [DOI] [PubMed] [Google Scholar]
- Cabaroglu T, Selli S, Canbas A, Lepoutre JP, Günata Z. Wine flavor enhancement through the use of exogenous fungal glycosidases. Enzyme Microb Technol. 2003;33(5):581–587. [Google Scholar]
- Capounová D, Drdák M. Comparison of some commercial pectic enzyme preparations applicable in wine technology. Czech J Food Sci. 2002;20(4):131–134. [Google Scholar]
- Castro-López L, Gómez-Plaza E, Ortega-Regules A, Lozada D, Bautista-Ortín AB. Role of cell wall deconstructing enzymes in the proanthocyanidin-cell wall adsorption-desorption phenomena. Food Chem. 2016;196:526–532. doi: 10.1016/j.foodchem.2015.09.080. [DOI] [PubMed] [Google Scholar]
- Claus H, Mojsov K. Enzymes for wine fermentation: current and perspective applications. Fermentation. 2018;4:52. [Google Scholar]
- Dal Magro L, Goetze D, Ribeiro CT, Paludo N, Rodrigues E, Hertz PF, Klein MP, Rodrigues RC. Identification of bioactive compounds from Vitis labrusca L. variety Concord grape juice treated with commercial enzymes: improved yield and quality parameters. Food Bioprocess Technol. 2016;9(2):365–377. [Google Scholar]
- Di Profio F, Reynolds AG, Kasimos A. Canopy management and enzyme impacts on Merlot, Cabernet franc, and Cabernet Sauvignon. II. Wine composition and quality. Am J Enol Vitic. 2011;62(2):152–168. [Google Scholar]
- Doco T, Williams P, Cheynier V. Effect of flash release and pectinolytic enzyme treatments on wine polysaccharide composition. J Agric Food Chem. 2007;55(16):6643–6649. doi: 10.1021/jf071427t. [DOI] [PubMed] [Google Scholar]
- Ducasse MA, Canal-Llauberes RM, de Lumley M, Williams P, Souquet JM, Fulcrand H, Doco T, Cheynier V. Effect of macerating enzyme treatment on the polyphenol and polysaccharide composition of red wines. Food Chem. 2010;118:369–376. [Google Scholar]
- EFSA Scientific opinion: guidance of the Scientific Panel of Food contact materials, enzymes, flavourings and processing aids (CEF) on the submission of a dossier on food enzymes for safety evaluation by the Scientific Panel of Food contact material, enzymes, flavourings and processing aids. EFSA J. 2009;1305:1–26. [Google Scholar]
- El Darra N, Turk MF, Ducasse MA, Grimi N, Maroun RG, Louka N, Vorobiev E. Changes in polyphenol profiles and color composition of freshly fermented model wine due to pulsed electric field, enzymes and thermovinification pretreatments. Food Chem. 2016;194:944–950. doi: 10.1016/j.foodchem.2015.08.059. [DOI] [PubMed] [Google Scholar]
- Escribano R, González-Arenzana L, Garijo P, Berlanas C, López-Alfaro I, López R, Gutiérrez AR, Santamaría P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J Food Sci Technol. 2017;54(6):1555–1564. doi: 10.1007/s13197-017-2587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo F, Armada S. Effect of enzyme addition in the making of Pedro Ximenez sweet wines using dynamic pre-fermentative maceration. S Afr J Enol Vitic. 2010;31(2):133–142. [Google Scholar]
- Fia G, Canuti V, Rosi I. Evaluation of potential side activities of commercial enzyme preparations used in winemaking. Int J Food Sci Technol. 2014;49(8):1902–1911. [Google Scholar]
- Gao Y, Fangel JU, Willats W, Vivier MA, Moore JP. Effect of commercial enzymes on berry cell wall deconstruction in the context of intra-vineyard ripeness variation under winemaking conditions. J Agric Food Chem. 2016;64(19):3862–3872. doi: 10.1021/acs.jafc.6b00917. [DOI] [PubMed] [Google Scholar]
- Gómez-Plaza E, Romero-Cascales I, Bautista-Ortín AB. Use of enzymes for wine production. In: Bayindirli A, editor. Enzymes in fruit and vegetable processing. Chemistry and engineering applications. Boca Ratón: CRC Press; 2010. pp. 215–243. [Google Scholar]
- González-Neves G, Gil G, Favre G, Baldi C, Hernández N, Traverso S. Influence of winemaking procedure and grape variety on the colour and composition of young red wines. S Afr J Enol Vitic. 2013;34(1):138–146. [Google Scholar]
- González-Pombo P, Fariña L, Carrau F, Batista-Viera F, Brena BM. Aroma enhancement in wines using co-immobilizated Aspergillus niger glucosidases. Food Chem. 2014;143:185–191. doi: 10.1016/j.foodchem.2013.07.107. [DOI] [PubMed] [Google Scholar]
- Guérin L, Sutter D-H, Demois A, Chereau M, Trandafir G. Determination of activity profiles of the main commercial enzyme preparations used in winemaking. Am J Enol Vitic. 2009;60(3):322–331. [Google Scholar]
- Kelebek H, Canbas A, Cabaroglu T, Selli S. Improvement of anthocyanin content in the cv. Öküzgӧzü wines by using pectolytic enzymes. Food Chem. 2007;105:334–339. [Google Scholar]
- Kelebek H, Canbas A, Selli S. Effects of different maceration times and pectolytic enzyme addition on the anthocyanin composition of Vitis vinifera cv. Kalecik karasi wines. J Food Process Preserv. 2009;33:296–311. [Google Scholar]
- Kirsch S, Fourdrilis S, Dobson R, Scippo M-L, Maghuin-Rogister G, De Pauw E. Quantitative methods for food allergens: a review. Anal Bioanal Chem. 2009;395:57–67. doi: 10.1007/s00216-009-2869-7. [DOI] [PubMed] [Google Scholar]
- Kirschner S, Belloni B, Kugler C, Ring J, Brockow K. Allergenicity of wine containing processing aids: a double-blind, placebo-controlled food challenge. J Investig Allergol Clin. 2009;19:210–213. [PubMed] [Google Scholar]
- Liburdi K, Benucci I, Esti M. Lysozyme in wine: an overview of current and future applications. Compr Rev Food Sci Food Saf. 2014;13(5):1062–1073. [Google Scholar]
- Maicas S, Mateo JJ. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: a review. Appl Microbiol Biotechnol. 2005;67(3):322–335. doi: 10.1007/s00253-004-1806-0. [DOI] [PubMed] [Google Scholar]
- Marangon M, Van Sluyter SC, Robinson EM, Muhlack RA, Holt HE, Haynes PA, Godden PW, Smith PA, Waters EJ. Degradation of white wine haze proteins by Aspergillopepsin I and II during juice flash pasterurizatin. Food Chem. 2012;135:1157–1165. doi: 10.1016/j.foodchem.2012.05.042. [DOI] [PubMed] [Google Scholar]
- Masino F, Montevecchi G, Arfelli G, Antonelli A. Evaluation of the combined effects of enzymatic treatment and aging on lees on the aroma of wine from Bombino bianco grapes. J Agric Food Chem. 2008;56(20):9495–9501. doi: 10.1021/jf8015893. [DOI] [PubMed] [Google Scholar]
- Minussi RC, Rossi M, Bologna L, Rotilio D, Pastore GM, Durán N. Phenols removal in musts: strategy for wine stabilization by laccase. J Mol Catal B Enzym. 2007;45(3–4):102–107. [Google Scholar]
- Ortega-Heras M, Pérez-Magariño S, González-Sanjosé ML. Comparative study of the use of maceration enzymes and cold pre-fermentative maceration on phenolic and anthocyanic composition and colour of a Mencía red wine. LWT Food Sci Technol. 2012;48(1):1–8. [Google Scholar]
- Pastorello E, Farioli L, Pravettoni V, Ortolani C, Fortunato D, Giuffrida M, Garoffo L, Calamari A, Frenna O, Conti A. Identification of grape and wine allergens as endochitinase 4, a lipid-transfer protein, and a thaumatin. J Allergy Clin Immunol. 2003;111(2):350–359. doi: 10.1067/mai.2003.35. [DOI] [PubMed] [Google Scholar]
- Pinelo M, Arnous A, Meyer AS. Upgrading of grape skins: significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci Technol. 2006;17(1):579–590. [Google Scholar]
- Pogorzelski E, Wilkowska A. Flavour enhancement through the enzymatic hydrolysis of glycosidic aroma precursors in juices and wine beverages: a review. Flavor Fragr J. 2007;22(4):251–254. [Google Scholar]
- Puértolas E, Saldaña G, Condón S, Álvarez I, Raso J. A comparison of the effect of macerating enzymes and pulsed electric fields technology on phenolic content and color of red wine. J Food Sci. 2009;74(9):C647–C652. doi: 10.1111/j.1750-3841.2009.01343.x. [DOI] [PubMed] [Google Scholar]
- Río Segade S, Pace C, Torchio F, Giacosa S, Gerbi V, Rolle L. Impact of maceration enzymes on skin softening and relationship with anthocyanin extraction in wine grapes with different anthocyanin profiles. Food Res Int. 2015;71:50–57. [Google Scholar]
- Rizzi C, Mainente F, Pasini G, Simonato B. Hidden exogenous proteins in wine: problems, methods of detection and related legislation-a review. Czech J Food Sci. 2016;34(2):93–104. [Google Scholar]
- Rocha SM, Coutinho P, Delgadillo I, Dias A, Cardoso M, Coimbra M. Effect of enzymatic aroma release on the volatile compounds of white wines presenting different aroma potentials. J Sci Food Agric. 2005;85(2):199–205. [Google Scholar]
- Röcker J, Schmitt M, Pasch L, Ebert K, Grossmann M. The use of glucose oxidase and catalase for the enzymatic reduction of the potential ethanol content in wine. Food Chem. 2016;210:660–670. doi: 10.1016/j.foodchem.2016.04.093. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Nogales JM, Fernández-Fernández E, Vila-Crespo J. Enzymes in winemaking. In: Ray RC, Rosell CM, editors. Microbial enzyme technology in food applications. Boca Raton: CRC Press; 2016. pp. 315–332. [Google Scholar]
- Romero-Cascales I, Ros-García JM, López-Roca JM, Gómez-Plaza E. The effect of a commercial pectolytic enzyme on grape skin cell wall degradation and colour evolution during the maceration process. Food Chem. 2012;130(3):626–631. [Google Scholar]
- Rusjan D, Srlič M, Košmer T, Prosen H. The response of monoterpenes to different enzyme preparations in Gewürztraminer (Vitis vinifera L.) wines. S Afr J Enol Vitic. 2009;30(1):56–64. [Google Scholar]
- Rusjan D, Strlič M, Košmerl T, Prosen H. Contribution of enzyme preparations to the linalool content of wines made from the non-aromatic grapevine variety Furmint (Vitis vinifera L.) J Int Sci Vigne Vin. 2012;46(2):139–143. [Google Scholar]
- Samoticha J, Wojdylo A, Chmielewska J, Politowicz J, Szumny A. The effects of enzymatic pre-treatment and type of yeast on chemical properties of white wine. LWT Food Sci Technol. 2017;79:445–453. [Google Scholar]
- Sampaio TL, Kennedy JA, Vasconcelos MC. Use of microscale fermentations in grape and wine research. Am J Enol Vitic. 2007;58(4):534–539. [Google Scholar]
- Sarrouh B, Santos TM, Miyoshi A, Dias R, Azevedo V. Up-to-date insight on industrial enzymes applications and global market. J Bioprocess Biotech S. 2012;4:002. [Google Scholar]
- Schmidtke LM, Blackman JW, Agboola SO. Production technologies for reduced alcoholic wines. J Food Sci. 2012;71(1):R25–R41. doi: 10.1111/j.1750-3841.2011.02448.x. [DOI] [PubMed] [Google Scholar]
- Schweigert MK, Mackenzie DP, Sarlo K. Occupational asthma and allergy associated with the use of enzymes in the detergent industry-a review of the epidemiology, toxicology and methods of prevention. Clin Exp Allergy. 2000;30:1511–1518. doi: 10.1046/j.1365-2222.2000.00893.x. [DOI] [PubMed] [Google Scholar]
- Servili M, De Stefano G, Piaquadio P, Sciancalepore V. A novel method for removing phenols from grape must. Am J Enol Vitic. 2000;51(4):357–361. [Google Scholar]
- Søndergaard HA, Grunert KG, Scholderer J. Consumer attitudes to enzymes in food production. Trends Food Sci Technol. 2005;16(10):466–474. [Google Scholar]
- Soto Vázquez E, Río Segade S, Orriols Fernández I. Effect of the winemaking technique on phenolic composition and chromatic characteristics in young red wines. Eur Food Res Technol. 2010;231:789–802. [Google Scholar]
- Spagna G, Barbagallo RN, Greco E, Manenti I, Pifferi PG. A mixture of purified glycosidases from Aspergillus niger immobilised by inclusion in chitosan gels for oenological application. Enzyme Microb Technol. 2002;30(1):80–89. [Google Scholar]
- Spök A. Safety regulations of food enzymes. Food Technol Biotechnol. 2006;44(2):197–209. [Google Scholar]
- Sun W-X, Hu K, Zhang J-X, Zhu X-L, Tao Y-S. Aroma modulation of Cabernet Gernischt dry red wine by optimal enzyme treatment strategy in winemaking. Food Chem. 2018;245:1248–1256. doi: 10.1016/j.foodchem.2017.11.106. [DOI] [PubMed] [Google Scholar]
- Torresi S, Frangipane MT, Garzillo AM, Massantini R, Contini M. Effects of a β-glucanase enzymatic preparation on yeast lysis during aging of traditional sparkling wines. Food Res Int. 2014;55:83–92. [Google Scholar]
- Ugliano M. Enzymes in winemaking. In: Moreno-Arribas MV, Polo MC, editors. Wine chemistry and biochemistry. New York: Springer; 2009. pp. 103–126. [Google Scholar]
- Vassilopoulou E, Karathanos A, Siragakis G, Glavi S, Sinaniotis A, Douladiris N, Fernandez-Rivas M, Clausen M, Papadopoulos NG. Risk of allergic reactions to wine, in milk, egg and fish-allergic patients. Clin Transl Allergy. 2011;1:10. doi: 10.1186/2045-7022-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeckx KC, Vissers YM, Baumert JL, Faludi R, Feys M, Flanagan S, Herouet-Guicheney C, Holzhauser T, Shimojo R, van der Bolt N, Wichers H, Kimber I. Food processing and allergenicity. Food Chem Toxicol. 2015;80:223–240. doi: 10.1016/j.fct.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Verma AK, Kumar S, Das M, Dwivedi PD. A comprehensive review of legume allergy. Clin Rev Allergy Immunol. 2013;45:30–46. doi: 10.1007/s12016-012-8310-6. [DOI] [PubMed] [Google Scholar]
- Wüthrich B. Allergic and intolerance reactions to wine. Allergologie. 2011;34(8):427–436. doi: 10.5414/ALX01420E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Li J, Shin HD, Du G, Liu L, Chen J. Molecular engineering of industrial enzymes: recent advances and future prospects. Appl Microbiol Biotechnol. 2014;98(1):23–29. doi: 10.1007/s00253-013-5370-3. [DOI] [PubMed] [Google Scholar]
- Zietsman AJ, Moore JP, Fangel JU, Willats WG, Trygg J, Vivier MA. Following the compositional changes of fresh grape skin cell walls during the fermentation process in the presence and absence of maceration enzymes. J Agric Food Chem. 2015;63(10):2798–2810. doi: 10.1021/jf505200m. [DOI] [PubMed] [Google Scholar]