Abstract
In this study, the effect of Hibiscus sabdariffa L. flowers marmalade (HM) addition at different ratios (0%, 15%, 20%) was determined on the certain quality properties, total phenolic contents, antioxidant activity, mineral composition and heavy metal content of stirred-type yogurts (C, HM15, and HM20). The marmalade addition increased dry matter, ash, titratable acidity and viscosity whereas decreased pH, fat and protein values. HM addition significantly increased the antioxidant properties of yogurt samples. 2,2-Diphenylpicrylhydrazyl radical-scavenging activity, Copper (II) reducing antioxidant capacity and total phenolic content were found to be in the range of 5.92–26.73 mg TE/100 g, 4.88–15.03 mg TE/100 g, and 5.57–14.69 mg GAE/100 g, respectively. There were no statistically differences between control and HM-added groups in terms of lactic acid bacteria (LAB) counts, also in all samples the total LAB count was above 6 Log cfu/g during the storage. Fe, Mn, B, and Ba mineral values of samples with HM were higher than control sample. Cd, As, Hg and Li heavy metals were not detected in any of the samples, consequently results were within reliable limits reported by JECFA (Joint FAO/WHO Expert Committee on Food Additives) and Turkish Food Codex. As a result of the sensory evaluation, the samples containing 20% HM generally received higher scores than the samples containing 15% HM. Considering all the parameters, it was concluded that HM yogurts can be used as a different type in the functional yogurt industry due to its pleasant and characteristic taste.
Keywords: Hibiscus sabdariffa L. flowers, Antioxidant, Total phenolic content, Mineral, Yogurt
Introduction
Recently, public interest in healthy foods has led to the development of functional dairy products that mainly provide health benefits. In general, the health benefits of fermented food products are related to their nutritional and physiological functions (Fazilah et al. 2018). Yogurt is one of the most popular fermented dairy products due to its unique taste and functional properties. Yoghurt, widely consumed worldwide (El-Said et al. 2014), is an acid-gel food obtained by the inoculation and fermentation of Streptococcus thermophilus and Lactobacillus bulgaricus in milk, which is of great importance in human nutrition (such as cow, buffalo, sheep and goat milk), produced by conventional and industrial methods under controlled conditions (Fazilah et al. 2018). Yogurt is similar to milk in terms of nutritional content including protein, Ca, riboflavin, B6, B12 vitamins with high biological value and is an excellent source of Ca (O’Sullivan et al. 2016; Fazilah et al. 2018).
Various probiotics, prebiotic and symbiotic components (Fazilah et al. 2018), antioxidant components such as omega-3 fatty acids, vitamins, minerals, polyphenols and carotenoids (O’Sullivan et al. 2016), dietary fibers and various bioactive components (El-Said et al. 2014) are widely used to improve nutritional and functional properties of yogurt. Also, the fruits, wild fruits, vegetables, medicinal-aromatic plants, and various bee products (such as honey, pollen, and propolis) which have potential in terms of these components can be used to develop functional yogurt formulations. Various studies have been conducted to develop new formulations and enhance the functional properties of yogurt (El-Said et al. 2014; Roy et al. 2015; O’Sullivan et al. 2016).
Fruit yogurts are made by addition of fruit, usually in the form of fruit preserves, puree or jam, to yogurt (Tamime and Deeth 1980). Fruits used in yogurt production give the final product a unique and original taste. Fruit ratio in fruit yogurt in fermented dairy products should be in the range of 5–15% according to FAO/WHO (Roy et al. 2015). Thanks to the many nutrient components they contain, fruits are among the foodstuffs of high importance for human health. Particularly, vitamins (such as vitamins A, C, and E), minerals, antioxidants, phenolic compounds and carotenoids (such as β-carotene, lycopene, and lutein) and dietary fibers (such as fructooligosaccharides) are important components for the healthy nutrition for individuals of all ages (Fernandez and Marette 2017). Peaches, oranges, strawberries, pineapples, cherries, apricots, blueberries (Fazilah et al. 2018), watermelon, papaya, banana, and grapes are frequently used fruits in the development of functional yogurt formulations (Roy et al. 2015). Also, medicinal-aromatic and wild plants that have become increasingly important in recent years can be used.
Hibiscus sabdariffa L. is a tropical wild plant from the Malvaceae family (Patel 2014). Today, it is widely cultivated in tropical and subtropical regions including India, Saudi Arabia, China, Malaysia, Indonesia, Philippines, Vietnam, Sudan, Egypt, Nigeria and Mexico (Mahadevan and Kamboj 2009; Patel 2014), although not much known and consumed in Turkey. The plant has a worldwide reputation and is also known by various local names (such as Bissap, Roselle and Ribena Malaysia) (Mohagheghi et al. 2011). Hibiscus sabdariffa L. flowers is similar to cranberry in flavor and it can be consumed in the form of jam, jelly, sauce, wine (Mohd-Esa et al. 2010), marmalade, pickles, spices, tea and cocktails (Patel 2014).
Hibiscus sabdariffa L. contains polyphenolic acid, flavonoids and anthocyanin with high antioxidant activity (Mohagheghi et al. 2011). This plant also contains 9.2% moisture, 1.145% protein, 2.61% fat, 12% fiber, 6.90% ash, 12.63 mg/100 g calcium, 273.2 mg/100 g phosphorus, 8.98 mg/100 g iron, 0.117 mg/100 g thiamine, 0.277 mg/100 g riboflavin and 3.765 mg/100 g niacin (Mahadevan and Kamboj 2009).
Many medical applications of this plant have been developed worldwide. In China, it is used in the treatment of hypertension, fever, liver damage and leukemia due to its high protocatechuic acid content (Mohd-Esa et al. 2010). Furthermore, antioxidant, hypocholesterolemic, antiobesity, hypotensive, antidiabetic, immunomodulatory, anticancer, hepatoprotective, antimicrobial, renoprotective, diuretic and antiurolytic effects of Hibiscus sabdariffa L. have been proven in clinical studies and its phytochemical profile has been reported in related studies (Riaz and Chopra 2018).
In this study, Hibiscus sabdariffa L. flowers marmalade (HM) which has a proven phytochemical profile was selected and it was aimed to produce an aromatic and functional yogurt. Accordingly, HM with a uniform sugar concentration was used in the production of yogurt at different concentrations (0%, 15% and 20%). The functional yogurts were analyzed in terms of various quality properties and the effect of HM addition on the certain quality properties, total phenolic contents, antioxidant activity, mineral composition and heavy metal content of stirred-type yogurts were determined.
Materials and Methods
Materials
Whole raw cow milk containing 3.0% fat was obtained Bayburt from the PeyKar Dairy and Agricultural Foods Co. Ltd., Bayburt, Turkey. Skimmed milk powder was supplied by the Pınar Dairy Products Co. (Izmir, Turkey). Commercial lyophilized direct vat set (DVS) starter culture (Streptococcus salivarius subsp. thermophilus and Lactobacillus delbrueckii subsp. bulgaricus) was supplied by Chr. Hansen (Istanbul, Turkey) and Hibiscus sabdariffa L. flowers marmalade made in Senegal was supplied by Racines S.A., Montpellier, France.
Yogurt Production
Cow’s milk was standardized with skim milk powder in such a way that the amount of dry matter was at least 16% and added starch (1.0%) and pectin (0.1%) to give consistency. It was then subjected to heat treatment at 90 °C for 15 min. The DVS culture inoculation (Str. salivarius ssp. thermophilus and Lb. delbrueckii ssp. bulgaricus) was performed at the rate recommended by the supplier at 43 ± 1 °C. The samples were incubated at the inoculation temperature until the pH reached 4.7 ± 0.1, and then they were stored at 4 °C for one day. Yogurt was divided into 3 equal portions. One part of the yogurt was used as the control (0% HM). HM was added into the second and third part of yogurt two different proportions (15% and 20%). In order to improve the color, 0.1% purple carrot concentrate (65°Brix) was added and mixed. The samples were transferred into sterile glass bottles in equal amounts and coded as C (control, %0 marmalade), HM15 (15% marmalade), and HM20 (20% marmalade). The production of yogurt was repeated twice. Then they were stored at 4 ± 1 °C for 21 days and analysis was carried on the 1, 7, 14, and 21 days of storage.
Physical and chemical analysis
The dry matter, ash, protein, pH, titratable acidity (anhydrous citric acid, %), water soluble dry matter and water activity (aw) of the HM were measured according to the method described by Cemeroglu (2010). The dry matter, protein (micro-Kjeldahl method) and fat content of the milk, skim milk and yogurt samples were performed according to AOAC (2005).. Titratable acidity as lactic acid was determined by titrimetric method (Bradley et al. 1992) and pH was measured using a pH meter (Mettler Toledo Seven Compact S220; Mettler-Toledo International Inc. Im Langacher Greifensee, Switzerland). Syneresis was determined using the method stated by Atamer and Sezgin (1986). The viscosity of yogurt was measured using digital Brookfield viscometer (Model DV-II, Brookfield Engineering Laboratories, Stoughton, MA, USA) at 4 ± 1 °C, after 15 s rotation at 50 rpm with spindle no 6. Physical and chemical analysis of the experimental yogurts were measured during 1, 7, 14, or 21 d of storage. The study was conducted in two replicates.
Microbiological analysis
For microbiological analyses, 10 g of yogurt samples were weighed under sterile conditions into jars and transferred into stomacher bags, and 90 ml of sterile physiological saline (0.85% NaCl) was added. Then, the samples were homogenized in the Stomacher (Interscience Bag Mixer® 400 France) for 2 min.
Standard Plate Count (SPC) on PCA, yeast and mould on Dichloran-Rose Bengal Chloramphenicol Agar (DRBCA, Merck) (Harrigan 1998), Lb. delbrueckii ssp. bulgaricus and Str. salivarius ssp. thermophilus on MRS agar (Dave and Shah 1996) were counted during storage. Coliform group bacteria numbers on VRB agar, Escherichia coli on Chromocult Tryptone Bile X Glucuronide Agar (CTBXA-Merck), and Staphylococcus aureus on Baird Parker Agar (BPA-Merck) (Harrigan 1998) were only counted on 1st of storage.
Mineral and heavy metal content
Determination of the multi-elements was done according to the method of Akinwande and Olatunde (2015). Aliquots of three replicates (about 0.5 g) from each of the samples were weighed into cleaned digestion tube (Pyrex 50 mL-culture tubes). Into each tube was added 2 mL concentrated redistilled Nitric acid (HNO3), covered with cling film and then left overnight at room temperature for cold digestion. The tubes were subsequently placed to a digestion block at 120 °C. Heating was continued and as the liquid dried off, additional 2 mL HNO3 was added and was eventually heated to dryness. This step was repeated until the sample no longer gave off reddish–brown (ferrous oxide) fumes and the solution was clear. Addition of 1 mL solution of Nitric acid and Perchloric acid (50/50) was done into the solution in the tubes and the temperature of the block was raised to 180 °C and digested for 2 h. There was further increase in temperature to 220 °C and the solution was heated to dryness. The tubes were then removed from digestion block and allowed to cool to room temperature. The ash obtained was re-dissolved in 1 mL concentrated HCl and 10 mL of 5% Nitric acid, mixed and transferred into plastic tubes for analysis. The sample ash solution was injected into ICP-MS (Agilent 7800) to determine the mineral content. Macro minerals were given in ppm (mg/kg) and micro minerals and trace elements were given ppb (µg/kg) in yogurt samples.
Determination of antioxidant activity and total phenolic content
Antioxidant activity of yogurt samples were determined two different methods (2,2-Diphenylpicrylhydrazyl method and cupric reducing antioxidant capacity method). Antioxidant and total phenolic analyses were performed on the first day of storage.
Preparation of the extracts
The dried and powdered 1 g of the sample was diluted with 10 ml of methanol (80%) and sonicated for 5 min (Feng et al. 2019). Then, samples were centrifuged at 560 rpm for 20 min at room temperature. The supernatants obtained were passed through a 0.45 μm membrane filter and the prepared extracts were transferred to the test tube and stored at − 20 °C until analysis.
Determination of antioxidant activity (2,2-Diphenylpicrylhydrazyl (DPPH) method)
The hydrogen atoms or electron-donating ability of the corresponding extracts and butylated hydroxytoluene (BHT) was determined from the bleaching of purple-colored methanol solution of DPPH. This spectrophotometric assay uses the stable radical DPPH as a reagent (Lin et al. 1999). Fifty microliters of various concentrations of the extracts in methanol was added to 5 mL of a 0.004% methanol solution of DPPH in a 10 mL test tube. After a 30 min incubation period at room temperature, the absorbance was read against a blank at 517 nm. Inhibition free radical DPPH in percent (I%) was calculated as.
where Ablank is the absorbance of the control reaction (containing all of the reagents except the test compound) and Asample is the absorbance of the test compound. Extract concentration providing 50% inhibition (IC50) was calculated from the graph plotted as inhibition percentage against extract concentration. Tests were carried out in triplicate.
Cupric reducing antioxidant capacity (CUPRAC method)
Cupric reducing antioxidant capacity of yogurt samples was determined using colorimetric method by Apak et al. (2004). Trolox was used as the reference standard. Results calculate the millimolar Trolox Equivalent (TEAC) values with respect to the standard for 100 g of dry weight (TE/100 g).
Determination of total phenolic contents
The total phenolic constituents of the samples extracts were determined using the literature methods involving Folin-Ciocalteu reagent and Gallic acid as standard (r2 = 0.998) (Spanos and Wrolstad 1990). The extract solution (0.1 mL) containing 1000 µg of extract was put in a volumetric flask, 46 mL of distilled water and 1 mL of Folin-Ciocalteu reagent were added, and the flask was shaken thoroughly. After 3 min, 3 mL of 2% Na2CO3 was added, and the mixture was allowed to stand for 2 h with intermittent shaking. The absorbance was measured at 760 nm. The same procedure was repeated for all of the standard Gallic acid solutions (0–1000 µg/0.1 ml), and the standard curve was determined using the equation.
Sensory analysis
In the determination of the sensory parameters, the score card described by TS 1330 (Turkish Standards Institution, 2006) was modified and used (Bakırcı and Arslaner 2007). For this purpose, the sensory properties of the yogurt samples, such as the appearance, consistency with a spoon, consistency by mouth, smell and taste were evaluated using a scale 1 (extremely poor) to 5 (extremely excellent) by ten trained panelists from the Department of Food Engineering of Bayburt University on days 1, 7, 14 and 21 of storage at 4 ± 1 °C.
Statistical analysis
Samples were analyzed twice on days 1, 7, 14, and 21 of storage analysis of variance (ANOVA) was employed to establish statistical differences were evaluated using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) statistical software. Different groups were analyzed by Duncan multiple comparison.
Results and Discussion
Physical and chemical properties
The mean values of some properties of the raw materials used in yogurt production are given Table 1. Total non-fat dry matter, protein, fat, titratable acidity and pH of cow’s milk were 9.64%, 3.8%, 3.0%, 0.14%, and 6.66, respectively and total dry matter, protein, fat, titratable acidity and pH of skim milk powder were 95.59%, 36.00%, 1.25%, 0.10%, and 6.75. Total dry matter, protein, fat, pH, anhydrous citric acid, total sugar, water soluble dry matter (°Brix), aw, ash and fiber were found 67.72%, 0.5%, 0.3%, 2.29, 8.53%, 58.0%, 63.76, 0.825, 4.73% and 0.2% in HM, respectively.
Table 1.
Some physicochemical properties of raw materials
| Cow milk | Skim milk powder | HM | |
|---|---|---|---|
| Physical and chemical properties | |||
| pH | 6.66 ± 0.01 | 6.75 ± 0.00 | 2.29 ± 0.00 |
| Titratable acidity (%) | 0.14 ± 0.00 | 0.10 ± 0.00 | 8.53a ± 0.07 |
| Non-fat dry matter (%) | 9.64 ± 0.06 | 95.59 ± 0.05 | 67.72 ± 1.21 |
| Protein (%) | 3.8 ± 0.01 | 36.00 ± 0.07 | 0.50 |
| Fat (%) | 3.00 ± 0.00 | 1.25 ± 0.00 | 0.30 |
| Total sugar (%) | – | – | 58.00 |
| WSDM (ºBrix) | – | – | 63.76 ± 0.05 |
| aw | – | – | 0.825 ± 0.00 |
| Ash (%) | – | – | 4.73 ± 0.00 |
| Fibre (%) | – | – | 0.20 |
| Microbiological analysis (log cfu/g) | |||
| TAMB | 2.70 ± 0.02 | 4.20 ± 0.01 | 5.00 ± 0.10 |
| Yeast-mold | < 2 | < 2 | < 2 |
| Coliform group | < 1 | < 1 | < 1 |
aanhydrous citric acid (ACA %)
HM addition significantly affected the physicochemical properties of yogurt samples (p < 0.01). As seen in Table 2, pH 3.84–4.31, 1.00–1.15% titratable acidity (in terms of lactic acid) in yoghurt samples, 14.77–24.54% total dry matter, 2.43–3.20% fat, 2.76–3.82% protein and 0.623–0.683% ash values were obtained. The addition of HM at increasing concentrations increased total dry matter, ash and titratable acidity in all yogurt samples whereas decreased the pH, fat and protein values. During the storage period, it was determined that titratable acidity increased whereas pH decreased (p < 0.01). During the formation of yogurt, the high metabolic activity of yogurt bacteria decreases with cooling whereas enzymatic activity continues. Therefore, an increase in lactic acid values and a decrease in pH values are observed during storage after incubation is ended (Tamime and Deeth 1980). Similar results were also reported by Bakirci et al. (2017) in yogurt samples with cranberry paste and pumpkin fiber addition.
Table 2.
Changes in some physicochemical and microbiological properties of yogurt samples
| Properties | Storage period (day) | Yogurt samples | ||
|---|---|---|---|---|
| C | HM15 | HM20 | ||
| pH | 1 | 4.31 ± 0.00a, A | 4.07 ± 0.00b, A | 3.95 ± 0.01c, A |
| 7 | 4.25 ± 0.01a, B | 4.03 ± 0.01b, B | 3.91 ± 0.01c, B | |
| 14 | 4.20 ± 0.01a, C | 4.03 ± 0.01b, B | 3.87 ± 0.01c, C | |
| 21 | 4.16 ± 0.01a, D | 3.99 ± 0.00b, C | 3.84 ± 0.01c, D | |
| Titratable acidity (%) | 1 | 1,00 ± 0.02c, C | 1.05 ± 0.00b, B | 1.08 ± 0.01a, D |
| 7 | 1.04 ± 0.01c, B | 1.06 ± 0.01b, B | 1.10 ± 0.01a, C | |
| 14 | 1.07 ± 0.02b, A | 1.08 ± 0.01b, A | 1.13 ± 0.01a, B | |
| 21 | 1.08 ± 0.01b, A | 1.08 ± 0.01b, A | 1.15 ± 0.01a, A | |
| Total solid (%) | 1 | 14.91 ± 0.33c, A | 22.27 ± 0.60b, A | 24.40 ± 0.09a, AB |
| 7 | 15.11 ± 0.37c, A | 22.16 ± 0.14b, A | 24.54 ± 0.43a, A | |
| 14 | 14.77 ± 0.34c, A | 21.65 ± 0.56b, AB | 23.80 ± 0.52a, B | |
| 21 | 15.30 ± 0.61c, A | 21.39 ± 0.26b, B | 23.06 ± 0.42a, C | |
| Fat (%) | 1 | 3.06 ± 0.06a, AB | 2.68 ± 0.07b, A | 2.48 ± 0.01c, AB |
| 7 | 3.10 ± 0.08a, AB | 2.61 ± 0.02b, AB | 2.52 ± 0.05b, A | |
| 14 | 2.97 ± 0.07a, B | 2.53 ± 0.07b, B | 2.43 ± 0.05b, B | |
| 21 | 3.20 ± 0.13a, A | 2.61 ± 0.03b, AB | 2.43 ± 0.05c, B | |
| Protein (%) | 1 | 3.38 ± 0.19a, B | 3.31 ± 0.04b, A | 2.76 ± 0.58c, D |
| 7 | 3.82 ± 0.16a, A | 3.15 ± 0.03b, B | 2.96 ± 0.01c, C | |
| 14 | 3.79 ± 0.08a, A | 3.44 ± 0.01b, A | 3.19 ± 0.02c, B | |
| 21 | 3.52 ± 0.04a, B | 3.13 ± 0.06b, B | 3,00 ± 0.08c, A | |
| Ash (%) | 1 | 0.624 ± 0.01c, A | 0.651 ± 0.03b, A | 0.683 ± 0.01a, A |
| 7 | 0.623 ± 0.00c, A | 0.657 ± 0.01b, A | 0.676 ± 0.01a, A | |
| 14 | 0.627 ± 0.05c, A | 0.651 ± 0.05b, A | 0.677 ± 0.02a, A | |
| 21 | 0.631 ± 0.01c, A | 0.642 ± 0.02b, A | 0.682 ± 0.00a, A | |
| SPC | 1 | 8.91 ± 0.02a, A | 8.37 ± 0.11b, A | 8.29 ± 0.01b, A |
| 7 | 8.76 ± 0.01a, B | 8.27 ± 0.01b, B | 8.26 ± 0.03b, AB | |
| 14 | 8.35 ± 0.08b, C | 8.23 ± 0.01ab, B | 8.12 ± 0.14b, B | |
| 21 | 8.20 ± 0.06a, D | 7.33 ± 0.01b, C | 7.22 ± 0.12b, C | |
| LAB | 1 | 9.05 ± 0.01a, A | 8.89 ± 0.11b, A | 8.83 ± 0.04b, A |
| 7 | 8.99 ± 0.09a, A | 8.75 ± 0.03b, B | 8.58 ± 0.09c, B | |
| 14 | 8.65 ± 0.30a, B | 8.59 ± 0.06a, C | 8.17 ± 0.10b, C | |
| 21 | 8.50 ± 0.01a, B | 7.97 ± 0.03b, D | 7.90 ± 0.04c, D | |
*Different lower letters (between samples at the same storage day) in the same column and different capital letters (during storage days) in the same line indicate significant differences (p < 0,01)
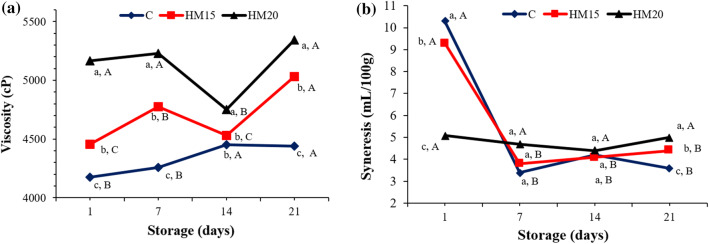
Viscosity is a parameter that affects structure and texture significantly in yogurt. The changes in viscosity values in the yogurt samples during the storage period is given in Fig. 1. The addition of HM significantly increased the viscosity (p < 0.01). During the storage period, the lowest viscosity value was determined in the control sample (C) whereas the highest value was determined in the yogurt sample containing 20% HM (HM20). Viscosity continuously increased in the control sample whereas was variable in HM-added samples during storage. The increasing effect of the addition of HM on viscosity can be associated with its content and the high water-binding capacity of the fibers contained in its composition. Similarly, Bakirci et al. (2017) found that viscosity increased with increasing pumpkin fiber concentration.
Fig. 1.
Viscosity (a) and Syneresis (b) change in yogurt samples during storage. Different lower letters (between samples at the same storage day) and different capital letters (during storage days) given on the chart indicate significant differences (p < 0,01)
Serum separation, which is an important parameter in determining the textural properties of yogurt, determines the property of curd stability in yogurt. Serum separation is defined as the separation of water or serum from the gel structure without any external effect in the set-type yogurt (Lucey 2002). The addition of HM significantly (p < 0.01) affected the serum separation and values varied between 3.40 and 10.29 mL/100 g. The effect of storage on serum separation was significant (p < 0.01). The addition of HM reduced serum separation only on day 1 of storage, whereas serum separation increased with the increasing concentration of HM on 7, 14 and 21 days of storage (Fig. 1). This may have facilitated the removal of water from the structure by dissolving the sugar in the yogurt serum over time, possibly with increasing marmalade concentration. Bakirci et al. (2017) have reported that serum separation decreased with the increased pumpkin fiber concentration.
Microbiological properties
Both HM addition and storage period significantly affected (p < 0.01) the total aerobic bacteria and lactic acid bacteria counts of the yogurt samples (Table 2). The total aerobic mesophilic bacteria count in all yogurt samples during storage were in the range of 7.22–8.91 log cfu/g. The addition of HM generally reduced the total aerobic mesophilic bacteria counts and lactic acid bacteria in yogurt samples. Similarly, a decrease in this group of microorganisms was observed during storage. Although the addition of HM reduced the lactic acid bacteria (LAB) count, there were no statistically differences between control and HM-added groups, also in all samples the total LAB count was above 6 Log cfu/g during the storage. The lowest lactic acid bacteria count was detected in the HM20 sample (7.90 log cfu/g) on the 21 day of storage whereas the highest lactic acid bacteria count was found in the control sample (9.05 log cfu/g) on the first day of storage. This was associated with a decrease in pH and an increase in sugar and acidity in the samples, related to the increased concentration of marmalade.
No coliform bacteria (< 1 log cfu/g), E. coli (< 1 log cfu/g), S. aureus (< 2 log cfu/g) and yeast-mold (< 2 log cfu/g) were detected as a result of the analyses carried out to determine the microbiological quality of ice cream samples on the 1st day of the storage.
Mineral and heavy metal content
Yogurt, which is a dairy product, constitutes a good source of minerals for human nutrition (De La Fuente et al. 2003). Macro and micro mineral contents of yogurt samples are given in Table 3. HM addition significantly affected the mineral composition of yogurt samples (p < 0.05). Ca is an important mineral for human health and is absorbed in the intestinal tract. Calcium is used for many basic functions in the body such as muscle, nervous and immune systems (Gharibzahedi and Jafari 2017). The Ca values of yogurt samples varied between 739.24 and 1255.23 mg/kg and HM addition significantly reduced the Ca levels (p < 0.01). De La Fuente et al. (2003) have reported that the Ca levels in commercial yogurt samples changed in the range of 1090–2050 mg/kg.
Table 3.
Mineral and heavy metal content of yogurt samples*
| Yogurt Samples | |||
|---|---|---|---|
| C | HM15 | HM20 | |
| Macro minerals (mg/kg) | |||
| Ca | 1255.23 ± 89.57a | 826.28 ± 6.78b | 739.24 ± 1.87b |
| K | 12,123.46 ± 104.34a | 8124.96 ± 80.38b | 7274.50 ± 8.73b |
| P | 7335.94 ± 133.82a | 4569.65 ± 40.74b | 3569.11 ± 57.48c |
| Na | 3450.60 ± 276.67a | 2167.13 ± 33.80b | 1995.11 ± 5.14b |
| Mg | 771.87 ± 73.87a | 590.81 ± 0.12b | 560.68 ± 9.07b |
| Micro minerals (µg/kg)* | |||
| Cu | 845.13 ± 79.30a | 408.76 ± 37.89a | 241.80 ± 20.48a |
| Fe | 4543.85 ± 752.72c | 7996.04 ± 100.25b | 11,162.16 ± 1413.56a |
| Mn | 766.72 ± 127.30c | 3351.01 ± 10.78b | 3861.12 ± 177.69a |
| Si | 99,220.14 ± 5066.22a | 104,496.88 ± 6766.71a | 129,651.42 ± 2127.31a |
| Zn | 24,948.39 ± 100.94a | 17,881.25 ± 259.03b | 13,655.0 ± 575.35c |
| Rb | 10,130.86 ± 63.62a | 7196.02 ± 28.23a | 8259.90 ± 22.83a |
| Sr | 6537.80 ± 432.52a | 6022.38 ± 2.47a | 6307.40 ± 202.72a |
| B | 2206.35 ± 48.18b | 2288.85 ± 159.48b | 2921.41 ± 104.94a |
| Ba | 368.02 ± 191.60c | 1716.34 ± 153.48b | 2301.27 ± 14.89a |
| Ga | 37.02 ± 1.81a | 32.52 ± 0.42a | 34.06 ± 1.05a |
| In | 3360.83 ± 203.05a | 1370.27 ± 121.75a | 2052.58 ± 133.90a |
| Cs | 97.62 ± 8.69a | 62.78 ± 1.67ab | 29.52 ± 1.94b |
| Heavy metals (µg/kg)* | |||
| As | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Al | 4248.76 ± 432.48a | 5606.38 ± 376.87a | 8987.73 ± 1185.95a |
| Ag | 1144.78 ± 67,21a | 8710.36 ± 87,18b | 8987.73 ± 85,95b |
| Cr | 495.25 ± 94.45a | 484.04 ± 20.55a | 439.24 ± 0.88a |
| Cd | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Hg | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Li | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Ni | 61.99 ± 6.27b | 170.48 ± 58.0b | 363.77 ± 32.24a |
| Pb | 99.69 ± 2.39a | 42.75 ± 1.15a | 7.59 ± 1.73a |
| V | 36.58 ± 5.73a | 22.50 ± 3.82a | 7.53 ± 1.65a |
*Mean values followed by different letters in the same column indicate significant differences (p < 0,05)
Fresh and dried fruits and vegetables, whole grains, and dairy products are important sources of K. K is known to have a very important role in various physiological functions of the human body (fluid balance, nerve conduction, muscle contraction, and proper maintenance of blood pressure) (Gharibzahedi and Jafari 2017). The lowest K value (7274.50 mg/kg) in the yogurt samples was detected in HM20 sample whereas the highest K value (12,123.46 mg/kg) was found in the C sample. As in Ca, the addition of HM reduced the K levels compared to those of the C sample (p < 0.01). The results of the study were substantially higher than those (1547–2630 mg/kg) reported by De La Fuente et al. (2003).
P values in yogurt samples varied between 3569.11 and 7335.94 mg/kg. The addition of HM significantly reduced the P values and this was also observed in samples containing increased concentrations of HM (p < 0.01). De La Fuente et al. (2003) found the P levels were in the range of 878–1560 mg/kg in their yogurt samples.
Na is an essential mineral for many functions in metabolism including nerve conduction, muscle contraction, electrolyte and fluid balance (Gharibzahedi and Jafari 2017). The Na values were determined to be 3450.60 mg/kg, 2167.13 mg/kg and 1995.11 mg/kg in C, HM15, and HM20 samples, respectively. Safe and adequate intake of Na for adults was reported 500 mg/day (Nabrzyski 2007). Milk and dairy products are a good source of Mg. Mg is an essential mineral for protein formation, muscle contraction, immune system health and nerve conduction (Gharibzahedi and Jafari 2017). The lowest (560.68 mg/kg) and highest (771.87 mg/kg) values of Mg in yogurt samples were determined in HM20 and C sample, respectively. HM addition significantly reduced the Na and Mg values in the samples (p < 0.05). Compared to the samples containing HM, higher values of Ca, K, P, Na, and Mg were determined in the C sample, indicating that these macro minerals are of milk origin. De La Fuente et al. (2003) determined the Na and Mg values in commercial yogurt samples to be 476–777 mg/kg and 101–177 mg/kg, respectively.
In the studies, the recommended daily intake amounts of Ca, K, P and Mg from macro minerals for adults are as follows; 800–1200 mg/day, 2000 mg/day, 800–1200 mg/day and 280–350 mg/day and Fe, Mn and Zn from micro minerals recommended daily intake amounts has been reported as 10–15 mg/day, 2–3 mg/day and 12–15 mg/day, respectively (Nabrzyski 2007). As a result of the comparison of the mean values of these reference values with the related mineral quantities determined in the samples examined; it was observed that the amounts of Ca, K, P and Mg in 100 g of product corresponded to 7.39–12.55%, 36.27–60.62%, 35.69–73.36%, and 17.80–24.50% of the reference values, respectively.
Micro minerals Cu, Fe, Mn, Si, Zn, Rb, Sr, B, Ba, Ga, In and Cs were determined as 241.80–845.13 11,162.16–4543.85 µg/kg, 3861.12–766.72 µg/kg, 129,651.42–99,220.14 µg/kg, 24,948.39–13,655.00 µg/kg, 7196.02–10,130.86 µg/kg, 6022.38–6537.80 µg/kg, 2206.35–2921.41 µg/kg, 368.02–2301.27 µg/kg, 32.52–37.02 µg/kg, 1370.27–3360.83 µg/kg, 29.52–97.62 µg/kg in yogurt samples, respectively (Table 3). HM addition significantly affected the Fe, Mn, Zn, B, Ba and Cs values (p < 0.05) whereas had no significant effects on Cu, Si, Rb, Sr, Ga and In values (p > 0.05). Fe and Cu are important for human health; however high concentrations may cause poisoning in live cells. Zn, which is an important component in many enzymes, is an essential micro-mineral with many functions on human physiology (Gharibzahedi and Jafari 2017). HM addition decreased the Zn and Cs values whereas significantly increased Fe, Mn, B and Ba values (p < 0.05). De La Fuente et al. 2003) found the Zn levels in the range of 4.0–5.9 mg/kg in their yogurt samples. Souza et al. (2019) have reported that Fe values in yogurt samples were in the range of 0.00–0.01 mg/g.
Although milk and dairy products are the sources of various minerals, they can also contain high concentrations of pollutants (such as heavy metals) due to increased environmental pollution caused by high industrial, urban and agricultural emissions (Capcarova et al. 2017). As, Al, Cr, Cd, Hg, Li, Ni, Pb, and V the heavy metals were determined in the yogurt samples and the analysis results are given in Table 3. The values of Al, Ag, Cr, Ni, Pb and V in the yogurt samples were 4248.76–8987.73 µg/kg, 1144.78–8987.73 µg/kg, 439.24–495.25 µg/kg, 61.99–363.77 µg/kg, 7.59295.84 µg/kg and 7.53–36.58 µg/kg, respectively. HM addition did not affect Al, Cr, Pb and V values statistically (p > 0.05), however, it increased Al and Ni values (p < 0.05). Cadmium is a metal that causes toxic effects on kidney, lung damage and tumor development. Similarly, lead shows toxic effects on the brain and kidneys (Souza et al. 2019). As, Cd, Hg and Li were not detected in any of the yogurt samples. Souza et al. (2019) found Cd (21.4 µg/kg) and Pb (28.8 µg/kg) in yogurt samples below the detectable limit values.
Tolerable uptake levels of some heavy metals for adults by JECFA for P, Cd, Hg, As, Cu, Al and Sn are 0.025 mg/kg/week, 0.007 mg/kg/week, 1.6 µg/kg/week, 15 µg/kg/week, 0.5 mg/kg/day, 2 mg/kg/week and 14 mg/ kg/week, respectively (JECFA, 2010). The maximum permissible heavy metal limits in certain foods in the relevant regulations by Turkish Food Codex are 0.02–0.5 mg/kg for P, 0.05–1.0 mg/kg for Cd, 0.5–1.0 mg for Hg and 50–200 mg/kg for Sn (Anonymous 2011). According to the results of heavy metal analysis showed that yogurt samples were within reliable limits reported by JECFA and Turkish Food Codex.
Antioxidant activity and total phenolic compounds
Antioxidant activity and total phenolic analysis results of yogurt samples are given in Table 4. The addition of HM significantly affected the antioxidant activity and total phenolic content of the yogurt samples (p < 0.01). As shown in Table 4, DPPH radical scavenging activity and Copper (II) reducing antioxidant capacity (CUPRAC) in yogurt samples were in the range of 5.92–26.73 mg TE/100 g and 4.88–15.03 mg TE/100 g, respectively. Total phenolic contents were found to be 5.57, 12.42 and 14.69 mg GAE/100 g in K, B15, and B20 samples, respectively. The addition of HM increased the antioxidant activity and total phenolic content in all yogurt samples (p < 0.01). Various studies have reported that the antioxidant activity and phenolic content of Hibiscus sabdariffa L. are very high (Christian and Jackson 2009; Riaz and Chopra 2018). Mohd-Esa et al. (2010), have reported that, the total phenolic contents of the Hibiscus sabdariffa L. seeds, leaves, flower and stem tissues of in the methanol extract were were 4.87, 2.91, 2.20 and 1.31 mg GAE/g, respectively, and the total antioxidant activity values were 78.7, 61.5, 54.9 and 22.1%, respectively. In another study, the total phenolic content of dried Hibiscus sabdariffa L. was 4.73–23.12 mg GAE/g, and the antioxidant activity was in the range of 69–79% (Christian and Jackson 2009). Leyva Daniel et al. (2013), determined the total phenolic content of the yogurt produced by adding Hibiscus sabdariffa L. to be 15.21 mg GAE/100 g.
Table 4.
Antioxidant activity and total phenolic content of yogurt samples
| Antioxidant properties | Yogurt samples | ||
|---|---|---|---|
| C | HM15 | HM20 | |
| DPPH (mg TE/100 g) | 5.92 ± 0.60c | 21.54 ± 1.16b | 26.73 ± 1.75a |
| CUPRAC (mg TE/100 g) | 4.88 ± 0.15c | 12.50 ± 0.34b | 15.03 ± 0.51a |
| TPC (mg GAE/100 g) | 5.57 ± 0.56c | 12.42 ± 0.58b | 14.69 ± 0.45a |
TE trolox equivalent, GAE Gallic acid equivalent
*Mean values followed by different letters in the same column indicate significant differences (p < 0.01)
Sensory properties
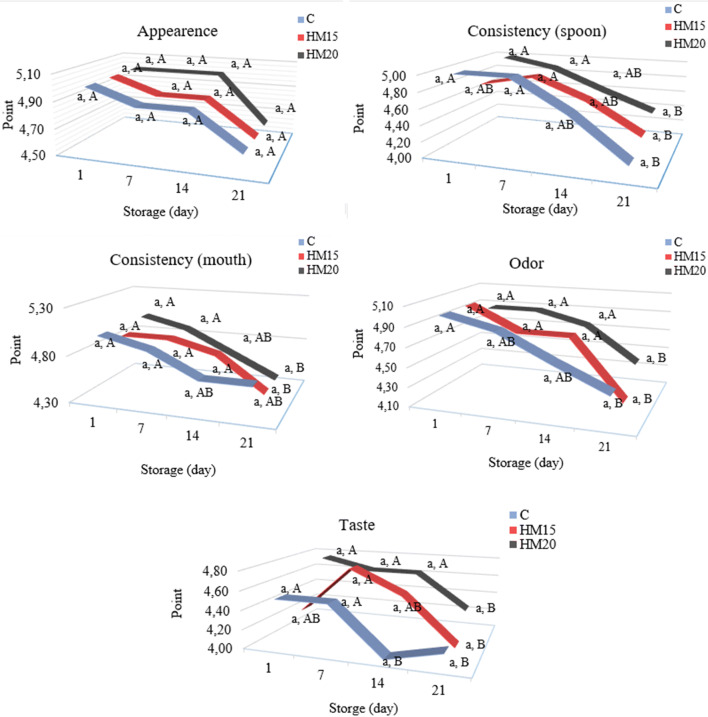
Sensory properties are an important criterion in the selection or rejection of food by the consumer. It also plays an important role in the market status of the food, its sale, and its market competition. The sensory analysis results of yogurt samples are given in Fig. 2. The effect of the addition of HM on sensory scores was statistically not significant, however, the effect of the storage period was statistically significant (p < 0.05). In general, the sensory scores of all yogurts decreased during storage. All received high scores by the panelists over 5 points in terms of sensory parameters. Although the effect of the addition of HM on sensory properties was statistically not significant, it was found that samples containing 20% HM generally received higher scores than the samples containing 15% HM.
Fig. 2.
Sensory properties change in yogurt samples during storage. Different lower letters (between samples at the same storage day) and different capital letters (during storage days) given on the chart indicate significant differences (p < 0,01)
Conclusion
The fruit yogurt industry, in which yogurt is made more attractive for all individuals, especially children, by the addition of fruit, is an important sector whose share in the food products market is growing day by day. In this research, Hibiscus sabdariffa L. flowers marmalade was used to produce functional yogurt and various quality properties, mineral composition and antioxidant activity of yogurt samples were determined. As a result of the research, the addition of HM did not have a negative effect on the physical, chemical, microbiological and sensory properties of the yogurt samples. Recent studies have shown an increase in interest in natural antioxidant sources. The addition of HM increased the antioxidant activity and total phenolic contents in all yogurt samples. In addition, HM significantly affected the mineral composition of yogurt samples. Fe, Mn, B, and Ba minerals significantly increased. Heavy metals Cd, Hg, and As were not detected in the yogurt samples. According to the results of heavy metal analysis showed that yogurt samples were within reliable limits reported by JECFA (Joint FAO/WHO Expert Committee on Food Additives) and Turkish Food Codex. Considering all the quality parameters, it was concluded that HM yogurts can be used as a different type in the functional yogurt industry due to its pleasant and characteristic taste.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akinwande BA, Olatunde SJ. Comparative evaluation of the mineral profile and other selected components of onion and garlic. Int Food Res J. 2015;22(1):332–336. [Google Scholar]
- Anonymous (2011) Turkish Food Codex Contaminants Regulation (Official Gazette Numbered 28157). Retrieved on August 16, 2019 from FSIS Website: https://www.mevzuat.gov.tr/Metin.Aspx?MevzuatKod=7.5.15692&MevzuatIliski=0
- AOAC . Official Methods of Analysis of AOAC International. 18. Gaithersburg, MD: AOAC International; 2005. [Google Scholar]
- Apak R, Güçlü K, Özyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 2004;52(26):7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Atamer M and Sezgin E (1986) Yoğurtlarda, kurumadde artırımının pıhtının fiziksel özellikleri üzerine etkisi. Gıda 11(6):327–331. https://dergipark.org.tr/tr/pub/gida/issue/6803/91480
- Bakırcı I, Arslaner A. The effects of partially skim milk powder with whey powder in set type yogurt manufacture. Milchwissenschaft. 2007;62:434–438. [Google Scholar]
- Bakirci S, Dağdemir E, Boran OS, Hayaloğlu AA. The effect of pumpkin fibre on quality and storage stability ofreduced-fat set-type yogurt. Int J Food Sci Tech. 2017;52(1):180–187. doi: 10.1111/ijfs.13264. [DOI] [Google Scholar]
- Bradley RL, Arnold E, Barbano DM, Semerad RG, Smith DE, Vines BK. Chemical and physical methods. In: Marshall RT, editor. Dairy products: standard methods for the examination of dairy products. 15. Washington DC: American Public Health Association; 1992. pp. 433–529. [Google Scholar]
- Capcarova M, Harangozo L, Toth T, Schwarczova L, Bobkova A, Stawarz R, Guidi A, Massanyi P. Detection of selected trace elements in yogurt components. J Environ Sci Health B. 2017;52(12):858–863. doi: 10.1080/03601234.2017. [DOI] [PubMed] [Google Scholar]
- Cemeroglu B. General methods in food analysis. In: Cemeroglu B, editor. Food Analysis. 2. Ankara: Food Tech Assoc Pub; 2010. pp. 87–93. [Google Scholar]
- Christian KR, Jackson JC. Changes in total phenolic and monomeric anthocyanin composition and antioxidant activity of three varieties of sorrel (Hibiscus sabdariffa) during maturity. J Food Compos Anal. 2009;22(7–8):663–667. doi: 10.1016/j.jfca.2009.05.007. [DOI] [Google Scholar]
- Dave RI, Shah NP. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus, and Bifidobacteria. J Dairy Sci. 1996;79(9):1529–1536. doi: 10.3168/jds.S0022-0302(96)76513-X. [DOI] [PubMed] [Google Scholar]
- De La Fuente MA, Montes F, Guerrero G, Juarez M. Total and soluble contents of calcium, magnesium, phosphorus and zinc in yoghurts. Food Chem. 2003;80(4):573–578. doi: 10.1016/S0308-8146(02)00505-8. [DOI] [Google Scholar]
- El-Said MM, Haggag HF, Fakhr El-Din HM, Gad AS, Farahat AM. Antioxidant activities and physical properties of stirred yoghurt fortified with pomegranate peel extracts. Ann Agric Sci. 2014;59(2):207–212. doi: 10.1016/j.aoas.2014.11.007. [DOI] [Google Scholar]
- Fazilah NF, Ariff AB, Khayat ME, Rios-Solis L, Halim M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J Funct Foods. 2018;48:387–399. doi: 10.1016/j.jff.2018.07.039. [DOI] [Google Scholar]
- Feng C, Wang B, Zhao A, Wei L, Shao Y, Wang Y, Cao B, Zhang F. Quality characteristics and antioxidant activities of goat milk yogurt with added jujube pulp. Food Chem. 2019;277:238–245. doi: 10.1016/j.foodchem.2018.10.104. [DOI] [PubMed] [Google Scholar]
- Fernandez MA, Marette A. Potential health benefits of combining yogurt and fruits based on their probiotic and prebiotic properties. Adv Nutr. 2017;8(1):155–164. doi: 10.3945/an.115.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibzahedi SMT, Jafari SM. The importance of minerals in human nutrition: bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci Technol. 2017;62:119–132. doi: 10.1016/j.tifs.2017.02.017. [DOI] [Google Scholar]
- Harrigan WF. Laboratory methods in food microbiology. London: Academic Press; 1998. [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) (2010) Codex Alimentarius International Foods Standards, General Standard For Contaminants And Toxins In Food and Feed, CXS pp 193–1995, https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/
- Leyva Daniel DE, Barragan Huerta BE, Vizcarra Mendoza MG, Sosa IA. Effect of drying conditions on the retention of phenolic compounds, anthocyanins and antioxidant activity of roselle (Hibiscus sabdariffa L.) added to yogurt. Int J Food Sci Tech. 2013;48(11):2283–2291. doi: 10.1111/ijfs.12215. [DOI] [Google Scholar]
- Lin J, Opoku AR, Geheeb-Keller M, Hutchings AD, Terblanche SE, Jager AK, van Staden J. Preliminary screening of some traditional Zulu medicinal plants for antiinflammatory and anti-microbial activities. J Ethnopharmacol. 1999;68(1–3):267–274. doi: 10.1016/s0378-8741(99)00130-0. [DOI] [PubMed] [Google Scholar]
- Lucey JA. Formation and physical properties of milk protein gels. J Dairy Sci. 2002;85(2):281–294. doi: 10.3168/jds.S0022-0302(02)74078-2. [DOI] [PubMed] [Google Scholar]
- Mahadevan N, Kamboj SP. Hibiscus sabdariffa Linn: an overview. Nat Prod Rad. 2009;8(1):77–83. [Google Scholar]
- Mohagheghi A, Maghsoud S, Khashayar P, Ghazi-Khansari M. The effect of Hibiscus Sabdariffa on lipid profile, creatinine, and serum electrolytes: a randomized clinical trial. ISRN Gastroenterol. 2011;2011:1–4. doi: 10.5402/2011/976019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Esa N, Hern FS, Ismail A, Yee CL. Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 2010;122(4):1055–1060. doi: 10.1016/j.foodchem.2010.03.074. [DOI] [Google Scholar]
- Nabrzyski M. Functional role of some minerals in foods. In: Szefer P, Nriagu JO, editors. Mineral components in foods. 1. New York: CRC Press; 2007. pp. 123–161. [Google Scholar]
- O’Sullivan AM, O’Grady MN, O’Callaghan YC, Smyth T, O’Brien NM, Kerry JP. Seaweed extracts as potential functional ingredients in yogurt. Innov Food Sci Emerg Technol. 2016;37:293–299. doi: 10.1016/j.ifset.2016.07.031. [DOI] [Google Scholar]
- Patel S. Hibiscus sabdariffa: an ideal yet under-exploited candidate for nutraceutical applications. Biomed Prev Nutr. 2014;4(1):23–27. doi: 10.1016/j.bionut.2013.10.004. [DOI] [Google Scholar]
- Riaz G, Chopra R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed and Pharmacother. 2018;102:575–586. doi: 10.1016/j.biopha.2018.03.023. [DOI] [PubMed] [Google Scholar]
- Roy DKD, Saha T, Akter M, Hosain M, Khatun H, Roy MC. Quality evaluation of yogurt supplemented with fruit pulp (banana, papaya, and water melon) Int J Food Sci Nutr. 2015;4(6):695–699. doi: 10.11648/j.ijnfs.20150406.25. [DOI] [Google Scholar]
- Spanos GA, Wrolstad RE. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J Agric Food Chem. 1990;38:1565–1571. doi: 10.1021/jf00097a030. [DOI] [Google Scholar]
- Souza TSP, Luna AS, Barros DB, Pimentel TC, Pereira EPR, Guimaraes JT, Esmerino EA, Freitas MQ, Costa RGB, Silva MC, Quterio SL, Raices RSL, Cruz AG. Yogurt and whey beverages available in Brazilian market: mineral and trace contents, daily intake and statistical differentiation. Food Res Int. 2019;119:709–714. doi: 10.1016/j.foodres.2018.10.050. [DOI] [PubMed] [Google Scholar]
- Tamime AY, Deeth HC. Yogurt: technology and biochemistry. J Food Prot. 1980;43(12):939–977. doi: 10.4315/0362-028X-43.12.939. [DOI] [PubMed] [Google Scholar]
- Turkish Standards Institution–TSE . Yoghurt Standard (TSE–TS 1330) Ankara: Turkish Standards Institution; 2006. [Google Scholar]