Abstract
The effective utilization of okara (soybean residue) has become a considerable challenge in recent years. In this paper, the potential advantages of β-glucosidase production from okara fermented by Kluyveromyces marxianus were evaluated and the properties of the β-glucosidase were also characterized. The results showed that okara can significantly induce the production of β-glucosidase from K. marxianus. The β-glucosidase activity was up to 4.5 U/mg under optimized fermentation conditions. The optimal parameters were as follows: fermentation temperature 35 °C, cultivation time 98 h, inoculum concentration 10%, and 30 g/L of okara. After two steps of purification using ammonium sulfate precipitation and Sephadex G-75 column chromatography, the activity of β-glucosidase was 71.4 U/mg. The native enzyme was an approximately 66 kDa dimer consisting of two different subunits (22 and 44 kDa). The kinetic parameters of the K. marxianus β-glucosidase, using pNPG as substrate, were Vmax 8.34 μmol min−1 mg−1 and Km 7.42 mM. The β-glucosidase showed high thermostability and acid–alkali tolerance as well as low inhibition by DMSO (10–50%). In conclusion, this study supports the notion that okara fermentation by K. marxianus could be a useful process to produce β-glucosidase.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04550-y) contains supplementary material, which is available to authorized users.
Keywords: Okara, β-Glucosidase, Kluyveromyces marxianus, Fermentation, Purification, Characteristic
Introduction
Okara (soybean residue) is a byproduct of tofu or soymilk production. A considerable amount of okara is produced by the food industry, particularly in Asian countries. The soybean curd-manufacturing sectors of Korea, Japan, and China generate approximately 310,000, 800,000, and 2,800,000 tons of annual okara, respectively (Li et al. 2011). Large quantities of okara discarded as a waste seriously pollute the environment. However, okara still contains most of the carbohydrates, some of the protein and a small portion of the oil of soybean (van der Riet et al. 1989). Several strategies have been proposed to avoid the waste of nutrients in okara. Quitain et al. (2006) extracted oil from okara by physical methods. Yeast fermentation is also a low-cost method to increase the added-value of okara such as obtaining erythritol from okara by Y. lipolytica (Liu et al. 2017). Yeast Kluyveromyces marxianus strains isolated from a wide variety of dairy products, such as kefir grains, soft cheeses, and aged cheeses (Padilla et al. 2012; Fadda et al. 2017) are generally considered as safe as S. cerevisiae and Kluyveromyces lactis (Lopes et al. 2014). Kluyveromyces marxianus from different sources showed interesting traits, including the livability in multitudinous substrates and at high temperatures (Fonseca et al. 2008).
β-Glucosidases (EC 3.2.1.21) are enzymes that catalyze the hydrolysis of β-1,4-glycosidic linkages and partially hydrolyze β-1,1, β-1,2, β-1,3 and β-1,6 glycosidic linkages (Wallecha and Mishra 2003). Most of these enzymes hydrolytically bind to a terminal and nonreducing β-d-glycosidic linkages to release β-d-glucose and the corresponding aglycone, mediating transglycosylation reactions. β-glucosidases are widely used in the food industry and other fields. For example, β-glucosidase hydrolyzes aromatic precursors to debitter and enhance the aroma of wine (Belancic et al. 2003) and generates high-purity isoflavone aglycones. Microbes are commonly used to produce β-glucosidase, such as Aspergillus niger (Seidle and Huber 2005) and Aspergillus oryzae (Watanabe et al. 2016). Plants also can produce β-glucosidase, such as tea leaves (Sener 2015). However, the diverse applications of these enzymes warrant the continued searching for new β-glucosidase producers that could expand the availability of enzymes with certain specificities and be used under feasible operating conditions. Kluyveromyces marxianus can secrete extracellular enzymes including pectinases, polygalactoronases, inulinase, proteases (Foukis et al. 2012), and β-galactosidase (Padilla et al. 2012). Rajoka et al. (2004) found that K. marxianus can be an efficient producer of a thermostable β-glucosidase.
In our previous study, the nutritional values and processing characteristics of K. marxianus fermented okara was significantly improved (Hu et al. 2019), which may be related to the induction of β-glucosidase (Su et al. 2018), but it was not clear. Many reports have shown that fibrous substances can induce the production of β-glucosidase (Rajoka et al. 2004; Seidle and Huber 2005; Baffi et al. 2011), so okara may be a well considerable inducer for β-glucosidase. Therefore, we used okara as a matrix using the yeast K. marxianus fermentation to produce β-glucosidase in this study. The results of this study can provide a new way to utilize the abundant soybean byproduct and might have a significant contribution to the industrial production of β-glucosidase.
Materials and methods
Materials and reagents
Soybeans (Heihe 43, Heilongjiang, China) were kindly supplied by the Shandong Shengfeng Seeds Co., Ltd. (Shandong, China). One hundred grams of raw soybeans preserved at room temperature (25 °C) were soaked in a 5-fold amount of water for 12 h. Then the soaked soybeans were grounded into fine particles with a 9-fold amount of water (80 °C), at a low speed for 3 min using a soymilk machine (JYL-Y20, Joyang Co., Ltd., Shandong, China), and filtered through a 60-mesh sieve to obtain the okara. The okara was stored at − 20 °C until use.
Kluyveromyces marxianus from kefir grains was isolated and identified previously by colony morphology and sequence analysis of the 26S rDNA D1/D2 region (Su et al. 2018) and deposited at the China General Microbiological Culture Collection Center (CGMCC) under accession number 13907 (Beijing, China).
The bicinchoninic acid (BCA) and glucose oxidase/peroxidase (GOD-PAP) kits were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The sodium dodecyl sulfate (SDS) gel electrophoresis kit (12% pre-casted gel), cellobiose, and galactose were obtained from the Beijing Dingguo Changsheng Biotechnology Co., Ltd. (Beijing, China). Carboxymethyl cellulose, microcrystalline cellulose, and salicin were obtained from the Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Beef extract, peptone, and yeast extract were purchased from Beijing Aoboxing Bio-tech Co., Ltd. (Beijing, China). All other chemicals used in this study were of analytical grade and were purchased from Beijing Chemical Works (Beijing, China).
Identification of β-glucosidase production
At first, the production of the β-glucosidase level on okara by K. marxianus fermentation was determined using the esculin medium. (Renchinkhandand et al. 2017). One hundred μL of K. marxianus suspension (107 cells/mL) was evenly coated to a plate (9.0 cm × 9.0 cm) with modified esculin medium (1 g/L esculin (6,7-dihydroxycoumarin 6-glucoside), 1 g/L ferric ammonium citrate, 1 g/L KH2PO4, 0.5 g/L MgSO4, 2 g/L agar, and 2.5 g/L peptone, 10 g/L yeast extract or 10 g/L okara, pH 5.0). A medium containing no yeast extract and okara was used as a control. During the incubation period at 30 °C for 0–120 h, the amount of β-glucosidase production was observed by observing the speed and depth color of the dark brown precipitate (Li et al. 2017).
Crude enzyme preparation
The K. marxianus was activated in basal medium (8 g beef powder, 10 g peptone, 4 g yeast extract, 20 g glucose, 2 g potassium hydrogen phosphate, 2 g diammonium hydrogen citrate, 5 g sodium acetate, 0.2 g magnesium sulfate, 0.04 g manganese sulfate, and 10 mL Tween 80 in 1000 mL water, pH 5.7 ± 0.2) and the cultures were incubated for 15 h at 30 °C and 120 rpm on an incubator shaker. After cultivation, the cells were centrifuged (5 050 g for 20 min at 4 °C), washed twice with distilled water to remove the medium, and then adjusted to a concentration of 107 cells/mL for inoculation.
Thirty grams of okara soaked in distilled water at a ratio of 1:5 (w/v) were placed in 500 mL Erlenmeyer flask and sterilized at 121 °C for 15 min. After cooling, K. marxianus inoculum solution was added at a ratio of 6% (v/w) in the okara medium. After fermentation, a clear supernatant containing β-glucosidase was obtained by centrifugation (5 050 g for 20 min at 4 °C) and used as the crude enzyme for further experiments.
β-Glucosidase activity assay
The β-Glucosidase activity was assayed according to a modified method (Belancic et al. 2003) using p-nitrophenyl-β-d-glucopyranoside (pNPG) as a substrate. A reaction mixture comprising 0.2 mL of 20 mM pNPG in 10 mM sodium acetate buffer (pH 5.0) and 0.2 mL of the enzyme fraction was incubated at 60 °C for 30 min. The reaction was terminated by the addition of 0.4 mL of 1 M Na2CO3, and the liberation of p-nitrophenol (pNP) was evaluated by measuring the absorbance at 405 nm. (Infinite M200, Tecan (Shanghai) Trading Co., Ltd., Shanghai, China). One unit (U) of β-glucosidase activity was defined as the amount of enzyme in sodium acetate buffer (10 mM, pH 5.0) that released 1 μmol pNP per minute at 60 °C. The protein concentration was estimated using the BCA kit (bovine albumin at a concentration of 1 mg/mL was used as calibrant).
Effect of carbon sources on β-glucosidase production
Different carbon sources for inducing the production of β-glucosidase were evaluated by previously described methods (Baffi et al. 2011; Harrer et al. 1983) with modification. The carbon sources added to the medium were monosaccharide (glucose and galactose), disaccharide (sucrose, cellobiose, and maltose), and polysaccharide (carboxymethyl cellulose) at a concentration of 5 g/L and 10 g/L (Table 1); they were separately added to the yeast media (YP medium, 10 g/L yeast extract, 20 g/L peptone, pH 5.0) and sterilized at 121 °C for 20 min. With the K. marxianus suspension inoculated, the medium was cultured at 30 °C for 48 h under aerobic conditions. The same concentrations of okara were added in the YP medium in the same manner. At the same time, a solution consisting of 30 g/L of pure okara was used to estimate β-glucosidase production. Following the crude β-glucosidase activity, the effect of different carbon sources on the β-glucosidase production was assessed.
Table 1.
The effects of various carbon sources on the induction of β-glucosidase from K.marxianus
| Carbon sources | Concentration (g/L) | |
|---|---|---|
| 5.0 | 10.0 | |
| Monosaccharide | ||
| Glucose* | 111.79 ± 2.83%f | 148.30 ± 3.17%f |
| Galactose* | 112.42 ± 3.25%f | 152.43 ± 2.52%e |
| Disaccharide | ||
| Sucrose* | 123.43 ± 2.66%d | 182.90 ± 1.36%c |
| Maltose* | 124.45 ± 2.37%c | 146.90 ± 3.76%g |
| Cellobiose* | 148.03 ± 4.02%a | 260.02 ± 3.83%a |
| Polysaccharides | ||
| Microcrystalline cellulose | 121.44 ± 2.85%e | 156.29 ± 2.36%d |
| Carboxymethyl cellulose* | 56.64 ± 2.84%g | 86.30 ± 1.65%h |
| Okara* | 136.53 ± 2.33%b | 230.15 ± 2.26%b |
| Okara** | 466.03 ± 4.29% | |
The relative activity was defined as 100% cultured in the YP medium (10 g/L yeast extract, 20 g/L peptone, pH 5.0)
*YP medium with different carbon sources
**Solution of pure okara at 30 g/L, natural pH
Different letters in the same row indicate significant differences at p < 0.05
Fermentation optimization and purification of β-glucosidase
Based on the single factor tests (Supplementary Fig. 1), three factors of cultivation temperature, cultivation time, and inoculum concentration were considered for response surface methodology (RSM) to optimize the β-glucosidase production in okara using Box–Behnken design (Bello et al. 2012). With the β-glucosidase activity being response value, three factors and three levels of response surface design were chosen for the optimization of the K. marxianus fermentation conditions (Table 2). The best conditions could be predicted according to the following quadratic models:
where Y is the predicted response, XiXj represents coded independent variables, β0 is the offset term, and βi, βii and βij are the interaction coefficients of the linear, quadratic and second-order terms, respectively.
Table 2.
Box–Behnken experimental design and the results of β-glucosidase activity from K.marxianus in pure okara solution
| Run number | A | B | C | β-glucosidase activity (U/mg) |
|---|---|---|---|---|
| 1 | − 1 (30) | − 1 (72) | 0 (10) | 2.69 |
| 2 | 1 (40) | − 1 | 0 | 2.84 |
| 3 | − 1 | 1 (120) | 0 | 2.84 |
| 4 | 1 | 1 | 0 | 2.97 |
| 5 | − 1 | 0 (96) | − 1 (8) | 2.14 |
| 6 | 1 | 0 | − 1 | 2.41 |
| 7 | − 1 | 0 | 1 (12) | 2.09 |
| 8 | 1 | 0 | 1 | 2.2 |
| 9 | 0 (35) | − 1 | − 1 | 2.69 |
| 10 | 0 | 1 | − 1 | 3.07 |
| 11 | 0 | − 1 | 1 | 2.69 |
| 12 | 0 | 1 | 1 | 2.66 |
| 13 | 0 | 0 | 0 | 4.5 |
| 14 | 0 | 0 | 0 | 4.54 |
| 15 | 0 | 0 | 0 | 4.56 |
| 16 | 0 | 0 | 0 | 4.38 |
| 17 | 0 | 0 | 0 | 4.58 |
A, cultivation temperature (°C); B, cultivation time (h); C, inoculum concentration (%)
To improve the purification of β-glucosidase, the crude β-glucosidase was precipitated firstly with ammonium sulfate at a concentration range of 20–80% saturation at 4 °C overnight and the precipitated enzyme was collected after centrifugation at 5 050 g at 4 °C for 30 min. Then, the collections were dissolved in 20 mM acetate buffer (pH 5.0) and dialyzed in 0.1 M distilled water for one day in a cold room, with continuous stirring. The enzyme dialysate was applied to a Sephadex G-75 column (Jinan Bona Biological Technology, Co, Ltd., Shandong, China) (40 × 3.5 cm) and eluted with distilled water at a flow rate of 0.5 mL/min. The protein fractions were detected at 280 nm, collected, and analyzed for β-glucosidase activity.
Molecular weight determination
The active fraction was analyzed by 7% nondenaturing polyacrylamide gel electrophoresis (Native PAGE) and 12% SDS-PAGE (Lee et al. 2013). After electrophoresis, the two gels were stained with Coomassie blue. To determine the band of the β-glucosidase, these gels were also immersed in a solution containing 0.1 mM sodium acetate buffer solution (pH 5.0), 0.1% esculin, and 0.3% 0.1 mM FeCl3 at 60 °C for 30 min, and then soaked in a 10% glucose solution to stop the reaction. The position of a black band was used to determine the molecular mass of the β-glucosidase (Kwon et al. 1994).
Substrate specificity analysis
The relative activities of β-glucosidase against 10 kinds of substrates including aryl-glycosides (20 mM; pNPG, o-nitrophenyl-D-β-galactopyranoside (oNPG)), esculin, and salicin), disaccharides (20 mM; cellobiose, maltose, lactose, and sucrose), and polysaccharides (1%; carboxymethyl cellulose and starch) were tested (Mallek-Fakhfakh and Belghith 2016). The activities were determined by the glucose oxidase/peroxidase (GOD-PAP) method using a commercial kit except for pNPG and oNPG (Table S1).
Kinetic parameters
The kinetic parameters (Km and Vmax) for the β-glucosidase were obtained at 1.7–10 mM pNPG from the reaction rates (mmol mg−1 min−1). The Km and Vmax values were determined according to the Lineweaver–Burk plot.
Optimal temperature of β-glucosidase
The optimal temperature for the β-glucosidase activity was determined to range from 20 to 80 °C. To assess the thermal stability of the β-glucosidase, the purified enzyme was incubated at different temperatures (40–65 °C) for various amounts of time (1–5 h) and the residual activities were estimated according to the β-glucosidase activity assay.
Optimal pH of β-glucosidase
The optimal pH for the β-glucosidase activity was estimated ranging from pH 3.0 to 8.0 (citrate buffer, pH 3.0–3.5; sodium acetate buffer, pH 4.0–7.0; phosphate buffer, pH 7.5–8.0; 0.1 M). The pH stability was analyzed by preincubating 100 μL of β-glucosidase in 900 μL of the different buffers at 4 °C for 12 h or 24 h. The activities were estimated according to the β-glucosidase activity assay.
Effects of metal ions on β-glucosidase
The purified β-glucosidase was preincubated at 4 °C for 24 h at room temperature in the presence of different metal ions (K+, Ca2+, Na+, Mg2+, Mn2+, Ba2+, Fe3+, Zn2+, Ag+, and Cu2+) at 10 mM concentration, and β-glucosidase activity was evaluated according to the β-glucosidase activity assay.
Effects of organic solvents on β-glucosidase
The purified enzyme was used to investigate the effects of organic solvents, such as alcohols (methanol, ethanol, 1-propanol, 1-butanol, and 1-pentanol) at 1 M concentration and DMSO (10–50%) according to the β-glucosidase activity assay.
Statistical analysis
The least significant difference (LSD) was carried out using multiple regression analysis (ANOVA) with an SPSS 19.0 for Windows (SPSS Inc, Chicago, IL, USA), and p < 0.05 was considered to be statistically significant. The optimization of the culture conditions was analyzed with Design-Expert 8.0 (STAT-EASE Inc., MN, USA). All data are expressed as the means ± standard deviations (SD) and were calculated in triplicate.
Results and discussion
Okara induced the production of β-glucosidase
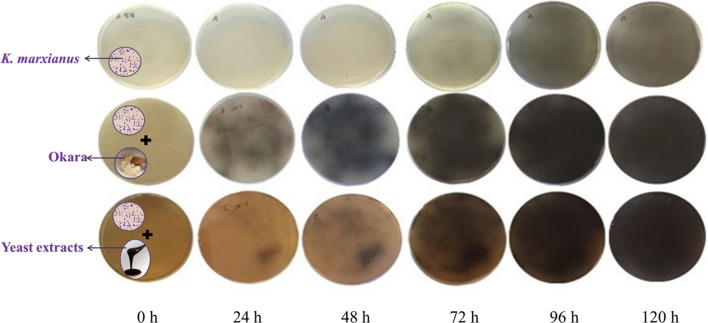
The production of β-glucosidase can be demonstrated by the color depth of a dark brown region (Renchinkhandand et al. 2017) using esculin medium. As shown in Fig. 1, β-glucosidase secretion showed different patterns on the esculin medium with or without okara. In 24 h, a dark brown region appeared on the esculin plates with okara, which means that β-glucosidase was secreted rapidly, and the color of dark brown region get more intense in 48–96 h. In contrast, the plate supplemented with yeast extract didn’t appear similar color until 72 h incubation, and the control medium in the first line didn’t appear a dark brown region until 96 h. The color difference on these three lines fully demonstrated that okara could significantly promote the secretion of β-glucosidase from K. marxianus.
Fig. 1.
Okara induces the production of extracellular β-glucosidase. Esculin iron agar plates with unsupplemented (Up), supplemented with okara (Middle) and with yeast extract (Down), were observed at 30 °C during 0–120 h in the presence of K. marxianus
β-Glucosidase is a type of cellulase, which can be induced by the addition of carbohydrates using microorganism-driven production (Rajoka et al. 2004). To further investigate the ability of okara to induce β-glucosidase from K. marxianus, the effects of various carbon sources on β-glucosidase induction were investigated in more detail. The results showed that except for carboxymethyl cellulose, most of the carbohydrates (monosaccharides, disaccharides, polysaccharides, and okara) used in this study exerted a positive effect on the induction of β-glucosidase expression (Table 1). Among them, the cellobiose showed a very strong β-glucosidase induction. In the presence of cellobiose at 5 g/L and 10 g/L, the β-glucosidase activity was 1.48-fold and 2.60-fold higher than that of control, respectively. Cellobiose has shown the strongest induction effect (Baffi et al. 2011), possibly because β-glucosidase can convert cellobiose and cellotriose into glucose upon hydrolyzing cellulose, which can participate in cell metabolism, or because cellobiose is important in the regulation of β-glucosidase-encoding genes (Seidle and Huber 2005). The effects of different carbon sources for the production of β-glucosidase by K. marxianus fermentation had the following order: cellobiose > okara > sucrose > microcrystalline cellulose > galactose > glucose > maltose. Rajoka et al. (2004) had also reported that cellobiose and sucrose greatly improved β-glucosidase production.
It is worth noting that the amount of β-glucosidase produced in pure okara solution was much higher than those reached in other carbon sources. This was a very favorable consequence. It has recently been reported that K. marxianus cultured in wheat bran, which contains 46.4% of total dietary fiber, can significantly increase the β-glucosidase secretion (Zhang et al. 2019), indicating the potential application of the cellulose-rich material in the production of β-glucosidase. The okara contains more than 50% dietary fiber (Li et al. 2011; Hu et al. 2019), and may contribute to the production of large amounts of β-glucosidase; thus, the fermentation conditions of K. marxianus in pure okara solution need to be optimized to obtain more β-glucosidase.
Optimization and purification of β-glucosidase from fermentation okara
According to the multiple regression analysis of RSM, a second-order polynomial equation describing the correlation of the β-glucosidase production and the relevant independent variables (X1, X2 and X3) is described as below:
where Y represents the β-glucosidase activity and X1, X2 and X3 represent the experimental values of cultivation temperature, cultivation time and inoculum concentration, respectively.
As presented in Table 3, model terms with values of P > F (P < 0.0001) are considered to be highly significant The lack of fit F-value of 0.8603 suggests that the lack of fit is not significant. The correction coefficient R2 (0.9979) indicated that the model could explain 99.79% of the variation of response value. Thus, the result of ANOVA analysis implied that the model of RSM can be taken. The maximal activity of β-glucosidase was measured as 4.5 U/mg and reached at Design-Expert 8.0 predicted conditions of cultivation: fermentation temperature 35 °C, cultivation time 98 h, inoculum concentration 10%, and 30 g/L of okara. Rajoka et al. (2004) also reported that the temperature of 35 °C was most favorable for the secretion of β-glucosidase from K. marxianus.
Table 3.
ANOVA for the quadratic model of the β-glucosidase activity from K. marxianus in pure okara solution
| Variable | Sum | Degree of freedom | Mean square | F value | P |
|---|---|---|---|---|---|
| Model | 13.96 | 9.0 | 1.55 | 362.60 | < 0.0001 |
| A | 0.054 | 1 | 0.054 | 12.72 | 0.0091 |
| B | 0.050 | 1 | 0.050 | 11.59 | 0.0114 |
| C | 0.056 | 1 | 0.056 | 13.11 | 0.0085 |
| AB | 1.000E−004 | 1 | 1.000E−004 | 0.023 | 0.8828 |
| AC | 6.400E−003 | 1 | 6.400E−003 | 1.50 | 0.2659 |
| BC | 0.042 | 1 | 0.042 | 9.82 | 0.0165 |
| A2 | 5.30 | 1 | 5.30 | 1239.21 | < 0.0001 |
| B2 | 1.30 | 1 | 1.30 | 302.80 | < 0.0001 |
| C2 | 5.86 | 1 | 5.86 | 1369.45 | < 0.0001 |
| Residual | 0.030 | 7 | 4.279E−003 | ||
| Lack of fit | 4.675E−003 | 3 | 1.558E−003 | 0.25 | 0.8603 |
| Pure error | 0.025 | 4 | 6.320E−003 | ||
| Cor total | 13.99 | 16 | |||
| R2 = 0.9979 | |||||
A, cultivation temperature (°C); B, cultivation time (h); C, inoculum concentration (%)
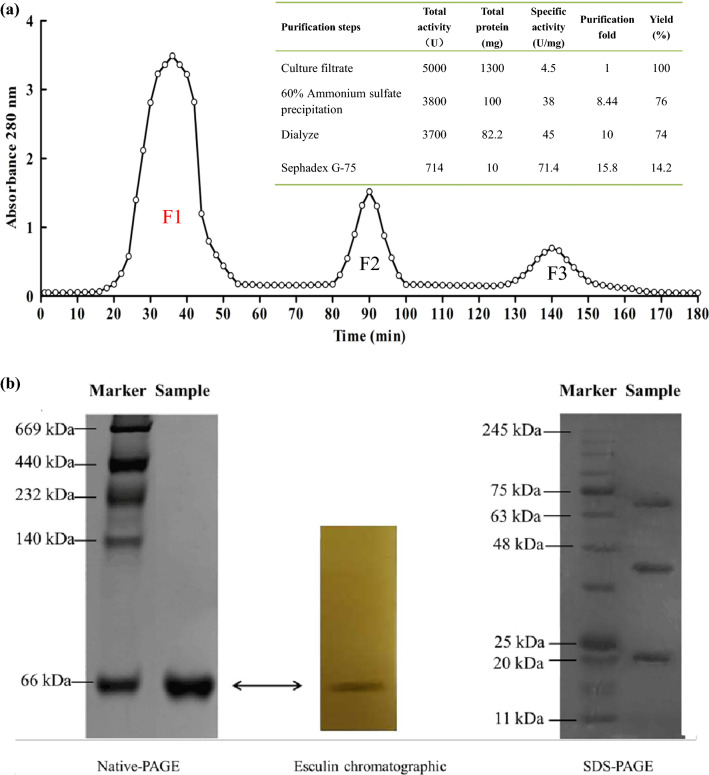
For the first step of purification, an ammonium sulfate solution of different saturation was used to precipitate the enzyme protein, and 60% saturation ammonium sulfate was selected (Supplementary Fig. 2), the β-glucosidase was purified by 8.44 times. Then, the Sephadex G-75 chromatography was used as the second purification step, and the β-glucosidase activity was only detected in F1 fraction (Fig. 2a). After two steps of purification, the activity of β-glucosidase was 71.4 U/mg corresponding to 15.87-fold purification and 14.28% of yield.
Fig. 2.
Purification of β-glucosidase activities with gel filtration columns. a Sephadex G-75; b Apparent molecular mass identification. Native PAGE (Left), Esculin chromatograph (Middle) and SDS-PAGE (Right)
To further confirm the purity of the β-glucosidase in F1 fraction, Native PAGE, esculin chromogenic assay, and SDS-PAGE with Coomassie blue staining were used (Fig. 2b). The purified enzyme showed a single protein band on the Native PAGE with an apparent molecular mass of approximately 66 kD with a black band indicating β-glucosidase activity when esculin chromogenic assay was developed. Three bands appeared on SDS-PAGE at 22, 44 and 66 kDa, respectively, and no band showed β-glucosidase activity. The best explanation was that the enzymatic activity of 22 and 44 kDa under denaturing conditions (SDS, reducing agent) were prohibited and the β-glucosidase was an apparent molecular mass of approximately 66 kDa containing two subunits (approximately 22 and 44 kDa). As for the SDS-PAGE 66 kDa band, no enzyme activity was appeared, which may be related to the changes in the spatial structure of the enzyme molecule caused by SDS reduction, which needs further research and explanation. The molecular mass of β-glucosidases from various microbial sources (Yeom et al. 2012; Kuo et al. 2018; Watanabe et al. 2016; Baffi et al. 2011; Yoshida et al. 2010) usually range from 30 to 500 kDa as monomers or multimers (dimers to octamers). β-Glucosidases from Dekkera bruxellensis yeast (Kuo et al. 2018) and Agaricus bisporus fungi (Ašić et al. 2015) exhibited a dimeric structure. β-Glucosidases from K. marxianus reported by Yoshida et al. (2010) is a tetramer. These results have shown that the β-glucosidase obtained in this study has a different structure, which may be due to the induction of okara.
As is known to all, the cost and the loss of activity of enzymes has always been a problem that must be solved in isolation and purification. Many chromatographies such as anion exchange (Mallek-Fakhfakh and Belghith 2016), hydrophobic interaction (Hernandez-Guzman et al. 2016), and size-exclusion chromatography (Ašić et al. 2015; Mallek-Fakhfakh and Belghith 2016) have been applied to the isolation of β-glucosidases. Most of the purifications include two or more chromatography steps. However, the purification process developed in this study was evenly simple and effective, which could be attributable to the crude enzyme solution containing few protein impurities and the effectiveness of the ammonium sulfate purification step, which increased the efficacy of the subsequent dialysis and gel filtration. The use of simple purification technologies significantly contributes to cost savings in enzyme preparations.
Substrate specificity and kinetic parameters of β-glucosidase
Most microbial β-glucosidases are classified into glycoside hydrolase family 3 (GH3) based on their amino acid sequence and substrate specificity, which is characterized by broad substrate specificity. (Guo et al. 2015). The substrate specificity of the β-glucosidase from K. marxianus was summarized in Table S1. In the aryl glycoside substrate, β-glucosidase had the highest activity with pNPG, followed by esculin and salicin, but it was inactive with oNPG. In the disaccharide substrate, β-glucosidase showed the highest hydrolysis activity with cellobiose, followed by maltose. No β-glucosidase activity was detected in substrates of lactose, sucrose, and polysaccharides. Because the substituent group and its position on the phenyl ring greatly impact the electron-withdrawing ability (Seidle and Huber 2005), this factor may have contributed to the observation that esculin was a better substrate than salicin. We also observed that the enzyme hydrolyzed glucosidic bonds of cellobiose (β-1,4) and maltose (α-1,4) but not β-1,4 glucosidic bonds in carboxymethyl cellulose. These results indicated that β-glucosidase from K. marxianus belongs to GH3 (Krogh et al. 2010).
The kinetic parameters for pNPG were calculated using Lineweaver–Burk plots and the linear regression (Supplementary Fig. 3). The Vmax and Km values determined for pNPG were 8.34 μmol min−1·mg−1 and 7.42 mM, respectively. The activity of the purified enzyme was 71.4 U/mg, being higher than that of the extracellular β-glucosidase activity (6.98 U/g) from K. marxianus reported by Zhang et al. (2019).
Effects of temperature, pH, metal ions, and organic solvents on β-glucosidase
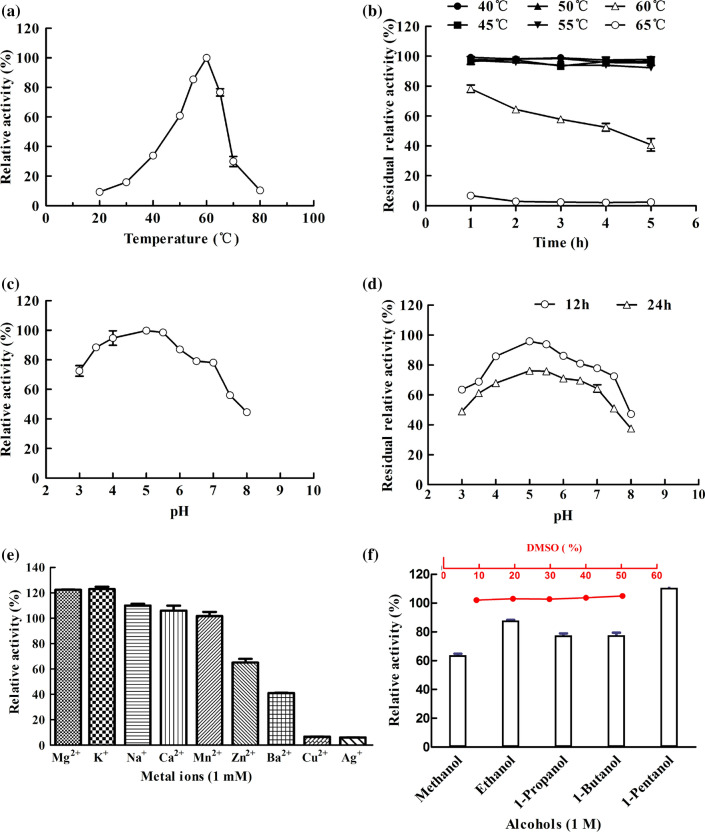
The effects of temperature on the stability of the β-glucosidase were shown in Fig. 3a, b. The β-glucosidase produced by okara using K. marxianus showed maximal catalytic activity at 60 °C that was higher than those previously reported for enzymes from fungi (Belancic et al. 2003; Watanabe et al. 2016; Belancic et al. 2003; Ašić et al. 2015) and bacteria (Bello et al. 2012). And it remained stable and retained almost full activity after 5 h incubation at 55 °C. The use of this enzyme in reactions at elevated temperatures would increase the solubility of the reactants and products, which would be conducive to the enzymatic hydrolysis of greater amounts of biomass.
Fig. 3.
Characteristics of the β-glucosidase from K. marxianus. a Effect of temperature on β-glucosidase activity, with a relative activity of 100% at 60 °C; b Thermal stability of the β-glucosidase after incubation for different amounts of time; the control was incubated at 4 °C; c Effect of pH on β-glucosidase activity, with a relative activity of 100% at pH 5.0; d pH stability of β-Glucosidase after incubation in citrate (pH 3.0–4.0), sodium acetate (pH 5.0–6.0), and phosphate (pH 6.0–8.0) for different amounts of time. The relative activity was defined as 100% at pH 5.0; e Effect of metal ions on β-glucosidase activity. The relative activity was defined as 100% without any additive (Control); f Effects of DMSO and alcohols on β-glucosidase. The relative activity was defined as 100% without any additive (Control). The values are shown as the mean ± SD of three independent experiments
The purified β-glucosidase showed maximal catalytic activity at pH 5.0 (Fig. 3c, d). The stability assay results showed that the enzyme was active over a wide range of pH values (from pH 3.0 to 8.0), as more than 38% of maximal enzyme activity was retained after 24 h. Notably, the enzyme retained almost 60% of its initial activity after 12 h at 4 °C and pH 3.0 and retained 42% of its initial activity at pH values of 7.0 to 7.5, providing further evidence of its acid–alkali tolerance under lingocellulose saccharification conditions (Ma et al. 2015).
As shown in Fig. 3e, the β-glucosidase activity was increased by approximately 20% in the presence of Mg2+ and K+ while Ba2+, Fe3+, Cu2+, Zn2+, and Ag+ significantly inhibited the enzyme.
In terms of tolerance to organic solvents, the β-glucosidase activity remained nearly constant at 10–50% DMSO. In contrast, almost all assayed alcohols inhibited the β-glucosidase, including methanol and 1-butanol. Interestingly, 1-pentanol slightly activated the enzyme, possibly through its transglycosylation, leading to the combination of pNPG and 1-pentanol accelerating the reaction(Watt et al. 1998). DMSO is generally used to promote the dissolution of low water-soluble substrates in industrial processing. However, most of the previously reported β-glucosidases were inhibited by DMSO (Mallek-Fakhfakh and Belghith 2016). Thus, the K. marxianus β-glucosidase obtained in this study has wide broad industrial applications.
Conclusion
This paper focused on the advantage of okara in the production of the β-glucosidase by K. marxianus fermentation. The results fully demonstrated that the fermentation of K. marxianus with a small amount of okara or pure okara can produce a large amount of β-glucosidase. The β-glucosidase from K. marxianus after only two purification steps can reach a high purity with a resistance of high temperature, pH, and wide range of DMSO concentrations. In conclusion, the research provides conditions for the improvement of the β-glucosidase production using okara as substrate and a practical technical solution for the added-value processing of okara.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Jilin Province Innovation Center for Biological Food and Manufacturing Technology for their excellent technical assistance. Financial support was provided by the Earmarked Fund for Ministry of Science and Technology of the People Republic of China (2017YFE0105400), Modern Agro-industry Technology Research System (CARS-04), the Jilin Provincial Science and Technology Department (20170312022ZX) Changchun Science and Technology Bureau (17DY013) and the Education Department of Jilin Province (JJKH20170302KJ).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Min Su and Yang Hu have contributed equally to this work.
Contributor Information
Min Su, Email: 15243290823@163.com.
Yang Hu, Email: huyang920501@163.com.
Yang Cui, Email: m18844101835@163.com.
Yuhua Wang, Email: yuhua-ww@163.com.
Hansong Yu, Email: yuhansong@163.com.
Junmei Liu, Email: spring430817@163.com.
Weichang Dai, Email: 17139070@qq.com.
Chunhong Piao, Email: piaochunhong9111@163.com.
References
- Ašić A, Bešić L, Muhović I, Dogan S, Turan Y. Purification and characterization of β-glucosidase from Agaricus bisporus, (white button mushroom) Protein J. 2015;34:453–461. doi: 10.1007/s10930-015-9640-z. [DOI] [PubMed] [Google Scholar]
- Baffi MA, Tobal T, Henrique J, Lago G, Leite RS, Boscolo M, Gomes E, Da-Silva R. A novel β-glucosidase from Sporidiobolus pararoseus: characterization and application in winemaking. J Food Sci. 2011;76:997–1002. doi: 10.1111/j.1750-3841.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- Belancic A, Gunata Z, Vallier MJ, Agsin E. β-Glucosidase from the grape native yeast Debaryomyces vanrijiae: purification, characterization, and its effect on monoterpene content of a muscat grape juice. J Agric Food Chem. 2003;51:1453–1459. doi: 10.1021/jf025777l. [DOI] [PubMed] [Google Scholar]
- Bello XV, Devesa-Rey R, Cruz JM, Moldes AB. Study of the synergistic effects of salinity, pH, and temperature on the surface-active properties of biosurfactants produced by Lactobacillus pentosus. J Agric Food Chem. 2012;60:1258–1265. doi: 10.1021/jf205095d. [DOI] [PubMed] [Google Scholar]
- Fadda ME, Mossa V, Deplano M, Pisano MB, Cosentino S. In vitro screening of Kluyveromyces strains isolated from Fiore Sardo cheese for potential use as probiotics. LWT—Food Sci Technol. 2017;75:100–106. doi: 10.1016/j.lwt.2016.08.020. [DOI] [Google Scholar]
- Fonseca GG, Heinzle E, Wittmann C, Gombert AK. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl Microbiol Biotechnol. 2008;79:339–354. doi: 10.1007/s00253-008-1458-6. [DOI] [PubMed] [Google Scholar]
- Foukis A, Stergiou PY, Theodorou LG, Papagianni M, Papamichael EM. Purification, kinetic characterization and properties of a novel thermo-tolerant extracellular protease from Kluyveromyces marxianus IFO 0288 with potential biotechnological interest. Bioresour Technol. 2012;123:214–220. doi: 10.1016/j.biortech.2012.06.090. [DOI] [PubMed] [Google Scholar]
- Guo Y, Yan Q, Yang Y, Yang S, Liu Y, Jiang Z. Expression and characterization of a novel β-glucosidase, with transglycosylation and exo-β-1,3-glucanase activities, from Rhizomucor miehei. Food Chem. 2015;175:431–438. doi: 10.1016/j.foodchem.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Harrer W, Kubicek CP, RÖhr M, Wurth H, Marihart J. The effect of carboxymethyl-cellulose addition on extracellular enzyme formation in Trichoderma pseudokoningii. Appl Microbiol Biotechnol. 1983;17:339–343. doi: 10.1007/BF00499500. [DOI] [Google Scholar]
- Hernandez-Guzman A, Flores-Martinez A, Ponce-Noyola P, Villagomez-Castro JC. Purification and characterization of an extracellular β-glucosidase from Sporothrix schenckii. FEBS open bio. 2016;6:1067–1077. doi: 10.1002/2211-5463.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Piao CH, Chen Y, Zhou YN, Wang D, Yu HS, Xu BJ. Soybean residue (okara) fermentation with the yeast Kluyveromyces marxianus. Food Biosci. 2019;31:1–9. doi: 10.1016/j.fbio.2019.100439. [DOI] [Google Scholar]
- Krogh KBRM, Harris PV, Olsen CL, Johansen KS, Hojer-Pedersen J, Borjesson J, Olsson L. Characterization and kinetic analysis of thermostable GH3 β-glucosidase from Penicillium brasilianum. Appl Microbiol Biotechnol. 2010;86:143–154. doi: 10.1007/s00253-009-2181-7. [DOI] [PubMed] [Google Scholar]
- Kuo HP, Wang R, Huang CY, Lai JT, Lo YC, Huang ST. Characterization of an extracellular β-glucosidase from Dekkera bruxellensis for resveratrol production. J Food Drug Anal. 2018;26:163–171. doi: 10.1016/j.jfda.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KS, Lee J, Kang HG, Hah YC. Detection of β-glucosidase activity in polyacrylamide gels with esculin as substrate. Appl Environ Microbiol. 1994;60:4584–4586. doi: 10.1016/0922-338X(95)92742-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GW, Yoo MH, Shin KC, Kim KR, Kim YS, Lee KW, Oh DK. β-Glucosidase from Penicillium aculeatum, hydrolyzes exo-, 3-o-, and 6-o -β-glucosides but not 20-o-β-glucoside and other glycosides of ginsenosides. Appl Microbiol Biotechno l. 2013;97:6315–6324. doi: 10.1007/s00253-013-4828-7. [DOI] [PubMed] [Google Scholar]
- Li B, Qiao M, Lu F. Composition, nutrition and utilization of okara (soybean residue) Food Rev Int. 2011;28:231–252. doi: 10.1080/87559129.2011.595023. [DOI] [Google Scholar]
- Li B, Lu F, Nan H, Liu Y. Isolation and structural characterisation of okara polysaccharides. Mol. 2012;17:753–761. doi: 10.3390/molecules17010753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yu X, Xia J, Lv J, Xu J, Dai B, Xu X, Xu J. Erythritol production by Yarrowia lipolytica from okara pretreated with the in-house enzyme pools of fungi. Bioresour Technol. 2017;244:1089–1095. doi: 10.1016/j.biortech.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Lopes MR, de Souza CJ, Rodrigues MQ, Costa DA, Dos Santos AF, De Oliveira LL, Ramos HJ, Guimaraes VM, Silveira WB, Passos FM, Fietto LG. Production and characterization of β-glucanase secreted by the yeast Kluyveromyces marxianus. Appl Biochem Biotechnol. 2014;172:2412–2424. doi: 10.1007/s12010-013-0683-3. [DOI] [PubMed] [Google Scholar]
- Ma Y, Liu X, Yin Y, Zou C, Wang W, Zou S, Hong J, Zhang M. Expression optimization and biochemical properties of two glycosyl hydrolase family 3 β-glucosidases. J Biotechnol. 2015;206:79–88. doi: 10.1016/j.jbiotec.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Mallek-Fakhfakh H, Belghith H. Physicochemical properties of thermotolerant extracellular β-glucosidase from Talaromyces thermophilus and enzymatic synthesis of cello-oligosaccharides. Carbohydr Res. 2016;419:41–50. doi: 10.1016/j.carres.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Padilla B, Ruiz-Matute AI, Belloch C, Cardelle-Cobas A, Corzo N, Manzanares P. Evaluation of oligosaccharide synthesis from lactose and lactulose using β-galactosidases from Kluyveromyces isolated from artisanal cheeses. J Agric Food Chem. 2012;60:5134–5141. doi: 10.1021/jf300852s. [DOI] [PubMed] [Google Scholar]
- Quitain AT, Oro K, Katoh S, Moriyoshi T. Recovery of oil components of okara by ethanol-modified supercritical carbon dioxide extraction. Bioresour Technol. 2006;97:1509. doi: 10.1016/j.biortech.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Rajoka MI, Khan S, Latif F, Shahid R. Influence of carbon and nitrogen sources and temperature on hyperproduction of a thermotolerant β-glucosidase from synthetic medium by Kluyveromyces marxianus. Appl Biochem Biotechnol. 2004;117:75–92. doi: 10.1385/ABAB:117:2:075. [DOI] [PubMed] [Google Scholar]
- Renchinkhandand G, Cho SH, Urgamal M, Park YW, Nam JH, Bae HC, Song GY, Nam MS. Characterization of Paenibacillus sp. MBT 213 isolated from raw milk and its ability to convert ginsenoside Rb1 into ginsenoside Rd from panax ginseng. Korean J Food Sci Anim Resour. 2017;37:735–742. doi: 10.5851/kosfa.2017.37.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidle HF, Huber RE. Transglucosidic reactions of the Aspergillus niger family 3 β-glucosidase: qualitative and quantitative analyses and evidence that the transglucosidic rate is independent of pH. Arch Biochem Biophys. 2005;436:254–264. doi: 10.1016/j.abb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Şener A. Extraction, partial purification and determination of some biochemical properties of β-glucosidase from Tea Leaves (Camellia sinensis L.) J Food Sci Technol. 2015;52:8322–8328. doi: 10.1007/s13197-015-1915-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Piao CH, Yang DC, Chu Q, Wang YH, Wang S, Chen Y, Hu Y, Huo Y. Screening and identification of β-glucosidase-producing yeast and its application in the bioconversion of ginsenoside Rg3. Food Sci. 2018;39:172–178. doi: 10.7506/spkx1002-6630-201814026. [DOI] [Google Scholar]
- Van der Riet WB, Weight AW, Cilliers JJL, Datel JM. Food chemical investigation of tofu and its byproduct okara. Food Chem. 1989;34:193–202. doi: 10.1016/0308-8146(89)90140-4. [DOI] [Google Scholar]
- Wallecha A, Mishra S. Purification and characterization of two β-glucosidases from a thermo-tolerant yeast Pichia etchellsii. BBA—Proteins and Proteomics. 2003;1649:74–84. doi: 10.1016/S1570-9639(03)00163-8. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Suzuki M, Ujiie S, Gomi K. Purification and enzymatic characterization of a novel β-1,6-glucosidase from Aspergillus oryzae. J Biosci Bioeng. 2016;121:259–264. doi: 10.1016/j.jbiosc.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Watt DK, Ono H, Hayashi K. Agrobacterium tumefaciens β-glucosidase is also an effective β-xylosidase, and has a high transglycosylation activity in the presence of alcohols. Bba Bioenergetics. 1998;1385:78–88. doi: 10.1016/S0167-4838(98)00046-6. [DOI] [PubMed] [Google Scholar]
- Yeom SJ, Kim BN, Kim YS, Oh DK. Hydrolysis of isoflavone glycosides by a thermostable β-glucosidase from Pyrococcus furiosus. J Agric Food Chem. 2012;60:1535–1541. doi: 10.1021/jf204432g. [DOI] [PubMed] [Google Scholar]
- Yoshida E, Hidaka M, Fushinobu S, Koyanagi T, Minami H, Tamaki H, Kitaoka M, Katayama T, Kumagai H. Role of a PA14 domain in determining substrate specificity of a glycoside hydrolase family 3 β-glucosidase from Kluyveromyces marxianus. Biochem J. 2010;431:39–49. doi: 10.1042/BJ20100351. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yang WD, Wang F, Jacob OO, Liu RS, Huang JX, Zhang L, Zou QB, Huang WN, Li SL. Use of Kluyveromyces marxianus prefermented wheat bran as a source of enzyme mixture to improve dough performance and bread biochemical properties. Cereal Chem. 2019;96:142–153. doi: 10.1002/cche.10125. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.