Abstract
Fish oil (FO) is a rich source of long-chain omega-3 polyunsaturated fatty acids (ω-3 LCPUFA) which are important for human health. This research investigated the fortification of chicken nuggets with encapsulated FO-Garlic essential oil (GEO) as a possible way for delivery of ω-3 LCPUFA. Five different chicken nugget samples were prepared according to different treatments: Control sample (without fish oil and encapsulated FO-GEO), bulk fish oil samples (0.4% and 0.8%, w/w), and encapsulated FO-GEO samples (4% and 8%, w/w). The quality of the chicken nugget samples were monitored during a 20-day refrigerated storage. Results showed that the addition of encapsulated FO-GEO could significantly delay lipid oxidation and microbiological spoilage of the samples during refrigerated storage. This is reflected by the pH, PV, TBARS and TVBN data (P < 0.05). Samples fortified with encapsulated FO-GEO also showed significantly higher sensory quality and overall acceptability (P < 0.05). The use of 8% encapsulated FO-GEO gave the best antioxidative and antimicrobial properties during storage. However, the best sensory scores were observed in the 4% encapsulated FO-GEO up to 20 days of storage. This study demonstrated that the encapsulated FO-GEO could be used for fortifying and extending shelf-life of food products.
Keywords: Encapsulation, Fish oil, Garlic essential oil, Chicken nuggets, Fortification
Introduction
Long-chain omega-3 polyunsaturated fatty acids (ω-3 LCPUFA) including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have important health and nutritional benefits in human health (Delgado-Lista et al. 2012). Thus, sufficient intake of ω-3 LCPUFA from diet is important (Chen et al. 2013a). Fish and fish oil supplements are the main dietary sources of ω-3 LCPUFA. In many countries, the consumption of fish is far lower than the recommended minimum intake of two or three servings per week, which is equivalent to an approximately 200 mg per day of ω-3 LCPUFA (Sanders 2000). Enrichment of food products with fish oil could be the best way to increase the dietary intake of ω-3 LCPUFA (Velasco et al. 2009). Among food products, meat products seem to be a good target for fortification, due to the high consumption and their fatty acid profiles. Generally, meat has a low content of ω-3 LCPUFA but high in monounsaturated and saturated fatty acids (Givens et al. 2006). There are different methods of fortifying meat products, either by feeding the animals with enriched ω-3 fatty acids in the feedstuff (Corino et al. 2014) or by fortifying the products using technological approaches. For the latter case, three main methods can be applied. The simplest method is by adding the fish oil to food products (Valencia et al. 2008), and the second technique is by emulsification of fish oil (Salminen et al. 2013). Emulsification of fish oil can protect ω-3 LCPUFA from lipid oxidation during storage compared to simply adding fish oil to the food products. However, emulsification is unable to mask the undesirable odours of the fish oil (Jiménez-Colmenero 2007). The third technique involved the use of encapsulated fish oil for food fortification. Encapsulation is a process in which droplets are entrapped as ‘core’ materials within a homogenous or heterogeneous matrix as ‘wall’ (Chen et al. 2013b). Biopolymers e.g. chitosan and gums are stable during thermal processing and have good emulsifying properties that allowed their use as wall materials for encapsulation of fish oils (Klaypradit and Huang 2008; Raeisi et al. 2019). This technique provides good prevention from oxidation and rancidity in food products, thus preserving good sensory properties (Keenan et al. 2015). Previous research has demonstrated that co-encapsulation of fish oil with natural antioxidants can increase its shelf-life effectively (Chen et al. 2013b). Garlic (Allium sativum L.) essential oil has been proven to be useful to reduce fish oil oxidation when co-encapsulated with fish oil, due to its high antioxidant activity and flavor-enhancing characteristics (Raeisi et al. 2017; Sharifi-Rad et al. 2016).
Today’s busy lifestyle has driven the changes in food preparation methods and dietary habits of consumers. The increased demand of “ready-to-heat” products to save food preparation time has been evidenced (Xiao et al. 2011). Among the convenience products, chicken nuggets is one of the most common and popular one. Therefore, fortification of chicken nugget with ω-3 LCPUFA could be a promising strategy to increase the intake of these essential fatty acids. Enrichment of food products with fish oil has been widely studied. However, research on enrichment of food products using co-encapsulated fish oil with other bioactive components, e.g. garlic essential oil, has not been reported. The aim of present study was to investigate the fortification of chicken nuggets with encapsulated fish oil-garlic essential oil for shelf-life extension, and to study the associated quality changes in the products during storage at refrigerated conditions.
Materials and methods
Materials
Fish oil (FO) was obtained from Sigma-Aldrich (St Louis, MO, USA). Garlic essential oil (GEO) was sourced from the Barij Essence Pharmaceutical Company, Tehran, Iran. Chitosan with low molecular weight (75–85% deacetylation degree) was purchased from Sigma–Aldrich (St. Louis, MO, USA) and Persian gum obtained from a local herbal store (Shiraz, Iran) were selected as wall material of encapsulated FO-GEO. Chicken breast was purchased in a local market (Gorgan, Iran). Deionized water was used for preparation of all the solutions.
Preparation of encapsulated FO-GEO
The encapsulated FO-GEO was prepared according to layer-by-layer deposition technique as described by Raeisi et al. (2019). Chitosan solution and FO-GEO was combined in the ratio 75:25%w/w, Tween 80 (0.1%) was added and mixed with a magnetic stirrer for 5 min. The mixture was homogenized by a rotor–stator homogenizer at 4000 rpm for 3 min, followed by sonication at amplitude of 100% for 4 min (UP 400 s, Dr. Hielscher, Germany). Persian gum solution was slowly added then homogenized by a rotor–stator homogenizer (3000 rpm for 0.5 min) followed by sonication (amplitude 80%, 0.5 min). Finally, the emulsions were frozen at − 70 °C overnight, freeze dried at − 51 °C and 0.120 mbar (Beta 1-8 LSCPlus, Martin Christ Gefriertrocknungsanlagen GmbH, Harz, Germany) for 72 h. Samples were stored in moisture impermeable plastic bags at − 18 °C in dark condition for future experiments.
Preparation of enriched chicken nuggets
Chicken nuggets, consisted of skinless chicken breast (86%), salt (1%), onion (12%), and pepper (1%), were prepared manually for this study. As the first step, chicken breast was minced using a meat mincer (Pars Khazar, Iran). The minced meat was then allocated for five different treatments: control sample (without bulk fish oil and encapsulated FO-GEO), samples enriched with bulk fish oil (0.4% and 0.8% w/w), and samples enriched with encapsulated FO-GEO (4% and 8% w/w). The samples were further mixed with spices to achieve a homogenous mixture. They were placed in a freezer (−18 °C) for 3 h before being cut into a dimension of 4.5 cm (L) × 2.6 cm (W) using a stainless steel mould. The cut samples were coated with a batter containing wheat flour, baking powder and salt, followed by coating with bread crumbs. Samples were immediately pre-fried in sunflower oil at 180 °C for 10 s. After that, they were removed from the fryer and placed on paper towel to get rid of excessive oil. Pre-fried samples were separately packaged in polyethylene bags and stored at refrigeration temperature (4 ± 1 °C). Chemical, microbiological and sensory properties of the samples were investigated at a 5-day interval for a period of 20 days.
Chemical analysis
Determination of pH
The pH values were measured by immersing a glass-calomel electrode in the homogenate of chicken nugget samples by using a pH meter (WTW, USA)
Determination of peroxide value (PV)
The PV of the chicken nuggets was determined using a ferric thiocyanate method outlined by Chapman and Mackay (Chapman and Mackay 1949). A standard curve using ferric iron solution was used to estimate the peroxide values in the lipid from the samples.
Determination of thiobarbituric acid reactive substances (TBARS)
The secondary oxidation changes in the samples were determined by measuring the thiobarbituric acid reactive substance (TBARS) value following the method of McDonald and Hultin (Mcdonald and Hultin 1987). Two milliliters of TBA reagent was placed in a test tube and 1 ml of the sample (5 mg of the nuggets in 1 ml of acetate buffer) was added. Test tubes were mixed completely by vortexing and incubated in a boiling water bath at 100 °C for 15 min, and after that, it is cooled for 10 min by placing in a cool water bath. Afterwards, the mixture was centrifuged for 15 min at 1000 rpm. The absorbance of supernatant was determined at 532 nm. TBARS was expressed as milligrams of malondialdehyde (MDA)/kg.
Determination of total volatile basic nitrogen (TVBN)
The TVBN values of the nugget samples were determined as described by Tecator (2002). TVBN values were measured with a Kjeltec 2300 (Auto analyzer, Foss Tecator AB, Hoganas, Sweden). TVBN values were expressed in mg nitrogen/100 g nugget sample.
Microbiological analysis
The nugget samples (10 g) were transferred into a stomacher bag containing 90 ml of 0.85% NaCl and homogenized. Then, decimal dilutions (10−2 to 10−8) were prepared from this mixture. Total viable count (TVC) and psychrophilic bacterial counts (PTC) were performed using the plate count agar (Merck, Darmstadt, Germany). For TVC determination, the plates were incubated at 25 °C for 2 days while for PTC estimation, the plates were incubated at 7 °C for 10 days (Raeisi et al. 2016). All counts were expressed as log colony-forming units (cfu)/g.
Sensory evaluation
The pre-fried chicken nugget samples were deep-fried at 180 °C for 5 min in sunflower oil. These samples were evaluated by a sensory panel consisted of ten experienced panelists (25–35 years old, male). The following sensory attributes were studied: color, appearance, taste, odor, and tenderness. A 10-point line scale was applied for the sensory evaluation as described by Kim et al. (2015), with score 1 for extremely undesirable and score 10 for extremely desirable.
Statistical analysis
All the experiments were performed in triplicate. Results were reported as mean value ± standard deviation. Analysis of variance was conducted on all the variables using the Statistical Analysis System (SPSS 11.5, IBM SPSS, New York, USA), followed by post hoc test (Duncan’s multiple range test) to determine statistical significant differences (P < 0.05) among the samples.
Results and discussion
pH
The pH of food products served as a good indicator for their quality (Khare et al. 2016). Figure 1 showed the changes of pH values in the chicken nuggets samples during storage. The pH of all the samples showed a decrease during part of the storage period (10 days for the control sample and samples contained bulk fish oil, and 15 days for the samples containing encapsulated FO-GEO). Then, the pH values increased until the end of the storage period. Results from this study are consistent with those of Hwang et al. (2013) that reported the pH of deep fried chicken nuggets treated by ganghwayakssuk (Artemisia princeps Pamp.) in combination with ascorbic acid decreased during the first 7 days of storage, and then increased until the end of storage. The pH reduction is mainly due to the accumulation of lactic acid caused by the growth of lactic acid bacteria (Suárez Mahecha et al. 2014). After certain period of storage, the increase of pH values may be related to the accumulation of alkaline compounds, such as ammonia compounds and trimethylamine, mainly due to microbial actions (Duman and Özpolat 2015).
Fig. 1.
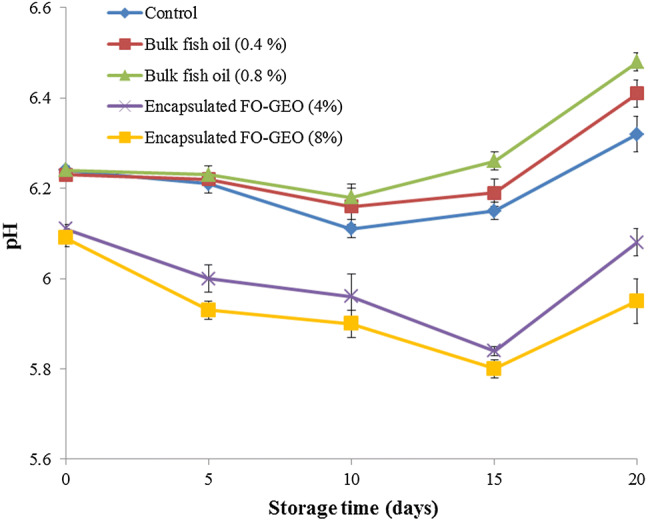
Changes in the pH values of the enriched chicken nugget samples during storage
Peroxide values (PV)
The changes in the peroxide value (PV) of chicken nugget samples are shown in Fig. 2. The PVs significantly increased as storage time increased for all samples (P < 0.05). The increase of PVs in samples could be attributed to the increase in aldehydes during storage (Alghazeer et al. 2008). It was observed that the control and samples treated with bulk fish oil (0.4 and 0.8%) had higher PVs compared to those treated with encapsulated FO-GEO. These results suggested that there were positive influence in the samples containing encapsulated FO-GEO perhaps due to the antioxidant activities of chitosan (Ngo and Kim 2014) and garlic essential oil (Sharifi-Rad et al. 2016). The acceptability limit of PVs is reported to be 10–20 meq O2 kg−1 oil for foodstuffs (Raeis et al. 2016). For the control and all samples treated with bulk fish oil (0.4% and 0.8%), the PVs reached the upper limit of 20 meq O2 kg−1 oil after 15 days of storage. In contrast, it took 20 days for the samples treated with 4% encapsulated FO-GEO to reach the same value. The PVs for samples treated with 8% encapsulated FO-GEO were less than 20 meq O2 kg−1 oil during the storage period, indicating better oxidative stability and acceptability for human consumption. Similar results were reported by Khare et al. (2016), who found a significant decrease of PV in chicken nuggets coated by chitosan enriched with cinnamon oil under refrigeration storage.
Fig. 2.
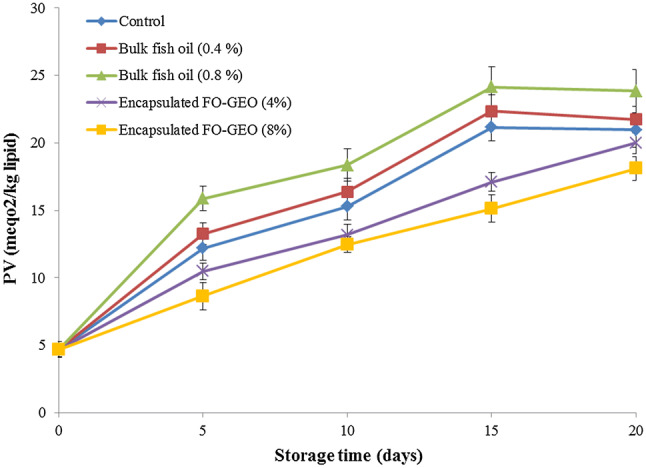
Changes in the peroxide value (PV) of the enriched chicken nugget samples during storage
TBA reactive substances (TBARS)
Thiobarbituric acid Reactive Substances (TBARS) is a widely used index for the evaluation of lipid oxidation based on the estimation of Malondialdehyde (MDA) content. MDA is formed by hydroperoxides, which are the product of the initial reaction of polyunsaturated fatty acids with oxygen (Raeisi et al. 2017). Changes in TBA values are shown in Fig. 3. The TBA values of samples treated with encapsulated FO-GEO were significantly lower than other samples (P < 0.05). These results were consistent with the findings of Jiménez-Martín et al. (2016). The initial TBARS of the nugget samples were 0.05 ± 0.01 mg MDA per kilogram of tissue. In all samples, the TBARS values increased with increase in the storage period (P < 0.05). As reported, the acceptability limit of TBA value during storage is 1–2 mg MDA per kg tissue (Byun et al. 2003). In this study, TBARS were lower than the above values up to 15 days of storage for the control and samples treated with bulk fish oil (both 0.4% and 0.8%). For samples treated with encapsulated FO-GEO (4%), the acceptable limit was up to day 20 of storage. In contrast, the TBA value of the samples treated with 8% encapsulated FO-GEO was less than the recommended limit until end of storage period. These results strongly implied that the encapsulated FO-GEO gave good antioxidant activity to protect the chicken nugget perhaps through scavenging of free radicals, chelating metal ions or donating hydrogen atoms (Pokorny et al. 2001).
Fig. 3.
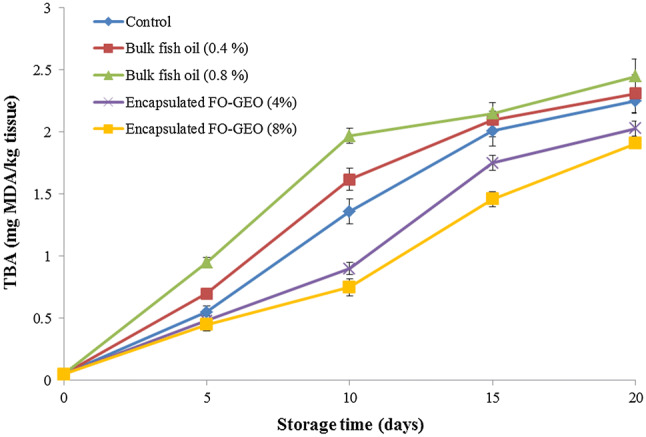
Changes in the TBA reactive substances of the enriched chicken nugget samples during storage
Total volatile basic nitrogen (TVBN)
Total volatile basic nitrogen (TVBN) content is an important index for evaluating the freshness of meat products which measure mainly trimethylamine (TMA), ammonia and dimethylamine (DMA). The level of TVBN can increase with either enzymatic or bacterial degradations (Urmila et al. 2015). The changes in the TVBN of chicken nugget samples are shown in Fig. 4. Results showed that the TVBN values increased significantly during the 20-day storage period for all samples (P < 0.05), with more rapid increases in the control and the samples treated with bulk fish oil. According to the results, using the encapsulated FO-GEO (4% and 8%) in the chicken nuggets gave better efficacy in delaying the rate of TVBN formation during the storage period. The recommended limit of TVBN for palatability of meat products is at 20 mg N/100 g sample (Byun et al. 2003). The TVBN values surpassed the recommended limit after 15 days of storage as observed in three samples: control (21.96 ± 0.5), and samples treated with 0.4% bulk fish (23.8 ± 1.2) and 0.8% bulk fish oil (26.3 ± 1.5). For the samples treated with 4% encapsulated FO-GEO, the recommended TVBN limits were surpassed after 20 days of storage (22.86 ± 0.4). In contrast, the TVBN value (19.90 ± 0.7) of the samples treated with 8% encapsulated FO-GEO was less than the recommended limit until end of storage period. The decrease rate of TVBN formation in the samples treated with encapsulated FO-GEO can be attributed to a decrease in bacterial population or the reduce in bacteria capacity for oxidative deamination of non-protein nitrogen compound or a combination of both parameters (Frangos et al. 2010) caused by antimicrobial properties of chitosan (Khare et al. 2016) and garlic essential oil (Sharifi-Rad et al. 2016).
Fig. 4.
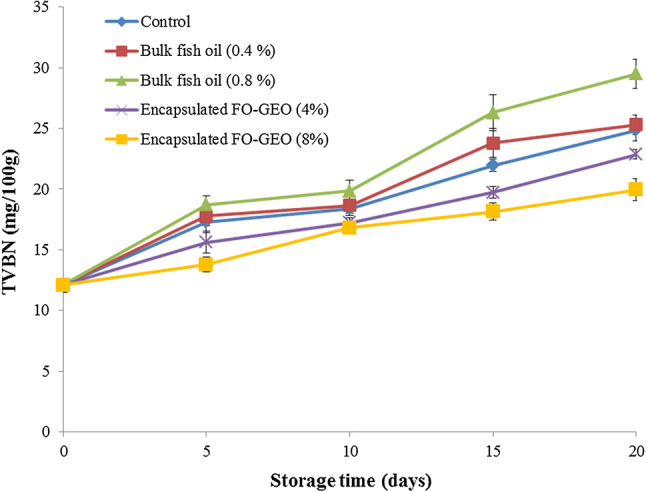
Changes in the total volatile basic nitrogen (TVBN) of the enriched chicken nugget samples during storage
Microbiological analysis
Total viable count (TVC) is an useful method for assessing quality of food products and post-processing contamination (Duman and Özpolat 2015). The initial TVC (log10 cfu/g) of the samples ranged from 3.74 log10 cfu/g in the samples treated with encapsulated FO-GEO (8%) to 3.98 log10 cfu/g in the samples treated with bulk fish oil (0.8%) (Fig. 5a). Based on the recommended safety limit of 7 log10 cfu/g (ICMSF 1986), these samples have shown acceptable quality. During storage, the TVC values increased in all samples. The control and the samples treated with bulk fish oil (0.4% and 0.8%) reached the upper acceptability limit after 15 days of storage. However, it took 20 days for the samples treated with encapsulated FO-GEO (4%) to reach the safety limit. The samples with 8% encapsulated FO-GEO has the best performance as their TVC values remain lower than the recommended safety limit.
Fig. 5.
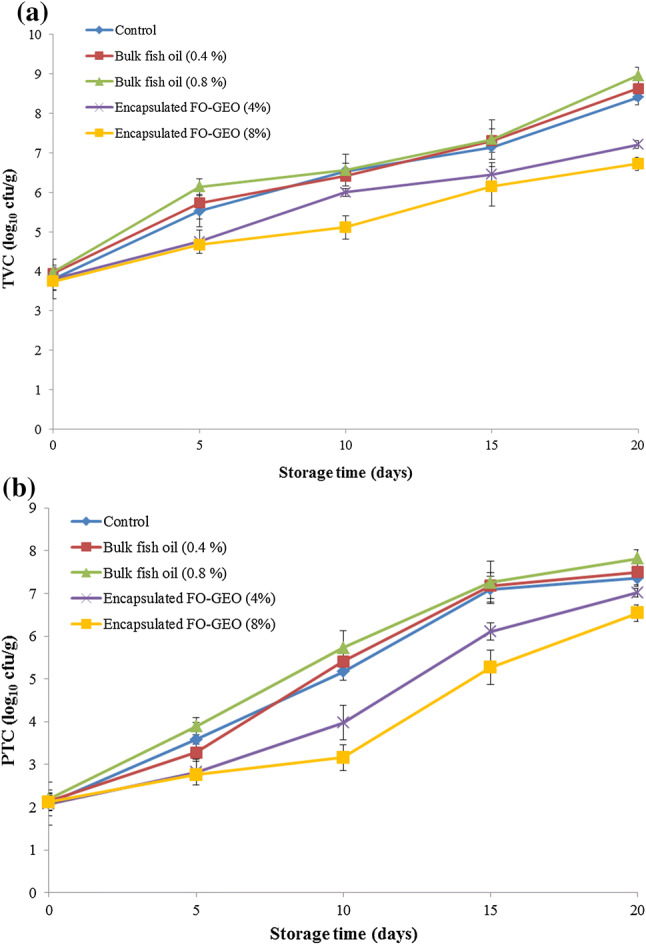
Microbiological analysis of the enriched chicken nugget samples during storage a: changes in the microbiological total viable count (TVC) b: changes in the psychrophilic bacterial counts (PTC)
As a main group of microorganisms, the psychrotrophic bacteria (PTC) are responsible for aerobic spoilage of fresh meat during refrigerated storage (Sallam 2007). In all samples, the PTC was significantly increased during the storage (P < 0.05) (Fig. 5b). The samples treated with encapsulated FO-GEO have the lower PTC compared to other samples. The results also revealed that the PTC decreased with an increase in the concentration of encapsulated FO-GEO present in the chicken nugget samples, showing a positive relationship between antibacterial activity and the concentration of encapsulated FO-GEO.
Sensory evaluation
The acceptability of food products during refrigerated storage is associated with the changes in their sensory properties. As evaluated by the sensory panel, the scores for color, appearance, taste, odor, tenderness and overall acceptability of the chicken nugget samples reduced significantly (P < 0.05) during the storage period (Table 1). The results showed that the samples fortified with encapsulated FO-GEO have significantly better sensory scores (for all characteristics including overall acceptability) than those fortified with bulk fish oil (P < 0.05). The samples with 0.8% bulk fish oil has a lowest overall acceptability among all samples, implying that fortification of food higher level of bulk fish oil is undesirable. For enrichment of fish oil in food products, the concentration of fish oil added is usually limited because the highly unsaturated oil could affect the sensory properties and palatability of the final products (Jiménez-Martín et al. 2016). The co-encapsulated fish oil and garlic essential oil in the samples have likely helped to mask the fishy odors or off-flavor associated with lipid oxidation, therefore giving higher sensory scores that samples fortified with bulk fish oil. Considering both samples fortified with the encapsulated FO-GEO, samples containing a higher level of encapsulated FO-GEO (8%) consistently gave better sensory qualities than those fortified with lower level of encapsulated FO-GEO (4%) except for the taste, odor, and tenderness characteristics. The results of sensory evaluation were in agreement with the microbial and chemical quality analyses. The results also consistent with previous research where good correlations among sensory attributes with microbial and chemical quality were reported (Ghollasi-Mood et al. 2017). Overall, the sensory results showed that the addition of encapsulated FO-GEO gave better sensory properties for the chicken nugget samples due to the antioxidant and antimicrobial activity of garlic essential oil.
Table 1.
Sensory properties of the enriched chicken nugget samples during storage time
| Storage time (days) | Control | Bulk fish oil (0.4%) | Bulk fish oil (0.8%) | Encapsulated FO-GEO (4%) | Encapsulated FO-GEO (8%) |
|---|---|---|---|---|---|
| Color | |||||
| 0 | 9.70 ± 0.02B | 9.64 ± 0.02C | 9.64 ± 0.04C | 9.75 ± 0.00A | 9.75 ± 0.02A |
| 5 | 6.63 ± 0.01I | 6.25 ± 0.01 K | 5.56 ± 0.03 N | 8.83 ± 0.02E | 8.54 ± 0.00D |
| 10 | 5.74 ± 0.04 M | 5.11 ± 0.00P | 4.67 ± 0.03Q | 7.71 ± 0.01G | 8.24 ± 0.02F |
| 15 | 4.31 ± 0.01R | 4.11 ± 0.03S | 3.94 ± 0.01T | 6.52 ± 0.02 J | 7.34 ± 0.04H |
| 20 | 3.91 ± 0.03U | 3.50 ± 0.04 V | 3.12 ± 0.04 W | 5.43 ± 0.06O | 5.82 ± 0.01L |
| Appearance | |||||
| 0 | 9.75 ± 0.02B | 9.68 ± 0.00C | 9.61 ± 0.02D | 9.84 ± 0.02A | 9.84 ± 0.03A |
| 5 | 7.11 ± 0.02I | 6.51 ± 0.03L | 6.44 ± 0.01 K | 8.72 ± 0.00F | 8.86 ± 0.04E |
| 10 | 5.75 ± 0.00 M | 5.64 ± 0.00 N | 5.37 ± 0.00P | 7.53 ± 0.01H | 8.14 ± 0.00G |
| 15 | 4.82 ± 0.04Q | 4.47 ± 0.00R | 4.10 ± 0.03T | 5.36 ± 0.02P | 6.72 ± 0.03 J |
| 20 | 3.77 ± 0.00U | 3.61 ± 0.01 V | 3.16 ± 0.01 W | 4.27 ± 0.00S | 5.58 ± 0.05O |
| Taste | |||||
| 0 | 9.53 ± 0.04C | 6.58 ± 0.01L | 6.11 ± 0.02 N | 9.86 ± 0.03A | 9.64 ± 0.01B |
| 5 | 8.31 ± 0.03G | 6.14 ± 0.02 M | 5.93 ± 0.01O | 9.12 ± 0.00D | 8.94 ± 0.02E |
| 10 | 7.10 ± 0.01 J | 4.26 ± 0.04R | 4.21 ± 0.01S | 8.56 ± 0.00F | 8.11 ± 0.04H |
| 15 | 5.12 ± 0.03Q | 3.41 ± 0.00U | 3.26 ± 0.01 V | 7.41 ± 0.01I | 6.85 ± 0.01 K |
| 20 | 4.17 ± 0.04T | 2.54 ± 0.01 W | 2.32 ± 0.01X | 6.11 ± 0.02 N | 5.72 ± 0.03P |
| Odor | |||||
| 0 | 9.62 ± 0.00B | 6.83 ± 0.03I | 6.72 ± 0.01 J | 9.86 ± 0.00A | 9.85 ± 0.02A |
| 5 | 8.58 ± 0.01E | 6.17 ± 0.02 N | 6.10 ± 0.00O | 8.73 ± 0.03C | 8.67 ± 0.01D |
| 10 | 7.33 ± 0.04H | 5.14 ± 0.01R | 5.12 ± 0.00S | 7.47 ± 0.00F | 7.41 ± 0.01G |
| 15 | 6.63 ± 0.04 K | 4.10 ± 0.02T | 3.91 ± 0.00U | 6.38 ± 0.00L | 6.31 ± 0.02 M |
| 20 | 5.11 ± 0.00S | 2.84 ± 0.00 V | 2.51 ± 0.04 W | 5.31 ± 0.02P | 5.26 ± 0.04Q |
| Tenderness | |||||
| 0 | 9.52 ± 0.00B | 9.46 ± 0.02C | 9.30 ± 0.00D | 9.72 ± 0.01A | 9.71 ± 0.00A |
| 5 | 7.47 ± 0.00I | 6.92 ± 0.00 J | 6.73 ± 0.02 K | 8.62 ± 0.02F | 8.57 ± 0.01G |
| 10 | 6.45 ± 0.03L | 5.31 ± 0.02P | 5.26 ± 0.04Q | 7.83 ± 0.05E | 7.71 ± 0.04H |
| 15 | 5.69 ± 0.00O | 4.27 ± 0.04U | 4.18 ± 0.02 V | 6.16 ± 0.00 M | 6.10 ± 0.01 N |
| 20 | 4.55 ± 0.02T | 3.81 ± 0.00 W | 3.73 ± 0.03X | 5.23 ± 0.00R | 5.19 ± 0.04S |
| Overall acceptability | |||||
| 0 | 9.81 ± 0.03C | 6.62 ± 0.04 J | 6.51 ± 0.01 K | 9.92 ± 0.04A | 9.88 ± 0.00B |
| 5 | 8.33 ± 0.05F | 5.61 ± 0.02 N | 5.56 ± 0.00O | 8.87 ± 0.02D | 8.84 ± 0.03E |
| 10 | 7.43 ± 0.00I | 4.37 ± 0.03S | 4.30 ± 0.01T | 7.64 ± 0.00G | 7.57 ± 0.02H |
| 15 | 5.53 ± 0.01P | 3.86 ± 0.02U | 3.12 ± 0.02 W | 6.21 ± 0.03L | 6.16 ± 0.02 M |
| 20 | 3.82 ± 0.01 V | 2.65 ± 0.04X | 2.50 ± 0.00Y | 5.10 ± 0.01Q | 4.96 ± 0.00R |
Different letters showed significant difference (P < 0.05) according to Duncan’s multiple range test for each sensory characteristic
Conclusion
This work reported for the first time, the enrichment of chicken nuggets with co-encapsulated fish oil and garlic essential oil. The results showed that fortification of chicken nugget with encapsulated FO-GEO could efficiently delay chemical deterioration, decelerate microbial growth, maintain or improve sensory properties and extend the shelf-life of the samples during refrigerated storage. Therefore, this could be a promising way for enrichment of meat products with fish oil, for extending shelf-life and preserving the sensory properties of the enriched products. This kind of enrichment will promote a higher intake of fish oil in the daily diet to promote beneficial health effects.
Acknowledgements
The authors would like to acknowledge the Gorgan University of Agricultural Sciences and Natural Resources for funding this research.
Compliance with ethical standards
Conflict of interest
The authors declare no financial or other conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sara Raeisi, Email: Sarah.reisi69@gmail.com.
Siew Young Quek, Email: sy.quek@auckland.ac.nz.
References
- Alghazeer R, Saeed S, Howell NK. Aldehyde formation in frozen mackerel (Scomber scombrus) in the presence and absence of instant green tea. Food Chem. 2008;108(3):801–810. doi: 10.1016/j.foodchem.2007.08.067. [DOI] [PubMed] [Google Scholar]
- Byun JS, Min JS, Kim IS, Kim JW, Chung MS, Lee M. Comparison of indicators of microbial quality of meat during aerobic cold storage. J Food Prot. 2003;66(9):1733–1737. doi: 10.4315/0362-028x-66.9.1733. [DOI] [PubMed] [Google Scholar]
- Chapman R, Mackay K. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J Am Oil Chem Soc. 1949;26(7):360–363. doi: 10.1007/bf02651444. [DOI] [Google Scholar]
- Chen Q, McGillivray D, Wen J, Zhong F, Quek SY. Co-encapsulation of fish oil with phytosterol esters and limonene by milk proteins. J Food Eng. 2013;117(4):505–512. doi: 10.1016/j.jfoodeng.2013.01.011. [DOI] [Google Scholar]
- Chen Q, Zhong F, Wen J, McGillivray D, Quek SY. Properties and stability of spray-dried and freeze-dried microcapsules co-encapsulated with fish oil, phytosterol esters, and limonene. Dry Technol. 2013;31(6):707–716. doi: 10.1080/07373937.2012.755541. [DOI] [Google Scholar]
- Corino C, Rossi R, Cannata S, Ratti S. Effect of dietary linseed on the nutritional value and quality of pork and pork products: systematic review and meta-analysis. Meat Sci. 2014;98(4):679–688. doi: 10.1016/j.meatsci.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr. 2012;107(S2):S201–S213. doi: 10.1017/S0007114512001596. [DOI] [PubMed] [Google Scholar]
- Duman M, Özpolat E. Effects of water extract of propolis on fresh shibuta (Barbus grypus) fillets during chilled storage. Food Chem. 2015;189:80–85. doi: 10.1016/j.foodchem.2014.08.091. [DOI] [PubMed] [Google Scholar]
- Frangos L, Pyrgotou N, Giatrakou V, Ntzimani A, Savvaidis I. Combined effects of salting, oregano oil and vacuum-packaging on the shelf-life of refrigerated trout fillets. Food Microbiol. 2010;27(1):115–121. doi: 10.1016/j.fm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Ghollasi-Mood F, Mohsenzadeh M, Housaindokht MR, Varidi M. Microbial and chemical spoilage of chicken meat during storage at isothermal and fluctuation temperature under aerobic conditions. Iran J Veterinary Sci Technol. 2017;8(1):38–46. doi: 10.22067/veterinary.v8i1.54563. [DOI] [Google Scholar]
- Givens D, Kliem KE, Gibbs RA. The role of meat as a source of n − 3 polyunsaturated fatty acids in the human diet. Meat Sci. 2006;74(1):209–218. doi: 10.1016/j.meatsci.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Hwang KE, Choi YS, Choi SM, Kim HW, Choi JH, Lee MA, Kim CJ. Antioxidant action of Ganghwayakssuk (Artemisia Princeps Pamp.) in combination with ascorbic acid to increase the shelf life in raw and deep fried chicken nuggets. Meat Sci. 2013;95:593–602. doi: 10.1016/j.meatsci.2013.05.035. [DOI] [PubMed] [Google Scholar]
- ICMSF . Microorganisms in foods 2. Sampling for microbiological analysis: Principles and specific applications. Toronto: University of Toronto Press; 1986. [Google Scholar]
- Jiménez-Colmenero F. Healthier lipid formulation approaches in meat-based functional foods Technological options for replacement of meat fats by non-meat fats. Trends Food Sci Technol. 2007;18(11):567–578. doi: 10.1016/j.tifs.2007.05.006. [DOI] [Google Scholar]
- Jiménez-Martín E, Pérez-Palacios T, Carrascal JR, Rojas TA. Enrichment of chicken nuggets with microencapsulated omega-3 fish oil: effect of frozen storage time on oxidative stability and sensory quality. Food Bioproc Tech. 2016;9(2):285–297. doi: 10.1007/s11947-015-1621-x. [DOI] [Google Scholar]
- Keenan DF, Resconi VC, Smyth TJ, Botinestean C, Lefranc C, Kerry JP, Hamill RM. The effect of partial-fat substitutions with encapsulated and unencapsulated fish oils on the technological and eating quality of beef burgers over storage. Meat Sci. 2015;107:75–85. doi: 10.1016/j.meatsci.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Khare AK, Robinson J, Rao VA, Babu RN, Ruban W. Effect of chitosan coating enriched with cinnamon oil (Cinnamomum zeylanicum) on storage stability of refrigerated chicken meat nuggets. J Anim Res. 2016;6(2):1–14. doi: 10.5958/2277-940x.2015.00160.6. [DOI] [Google Scholar]
- Kim HY, Kim KJ, Lee JW, Kim GW, Choe JH, Kim HW, Yoon Y, Kim CJ. Quality evaluation of chicken nugget formulated with various contents of chicken skin and wheat fiber mixture. Korean J Food Sci Anim Resour. 2015;35(1):19–26. doi: 10.5851/kosfa.2015.35.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaypradit W, Huang YW. Fish oil encapsulation with chitosan using ultrasonic atomizer. LWT Food Sci Technol. 2008;41(6):1133–1139. doi: 10.1016/j.lwt.2007.06.014. [DOI] [Google Scholar]
- McDonald RE, Hultin HO. Some characteristics of the enzymic lipid peroxidation system in the microsomal fraction of flounder skeletal muscle. J Food Sci. 1987;52(1):15–21. doi: 10.1111/j.1365-2621.1987.tb13964.x. [DOI] [Google Scholar]
- Ngo DH, Kim SK. Antioxidant effects of chitin, chitosan, and their derivatives. Adv Food Nutr Res. 2014;73:15–31. doi: 10.1016/B978-0-12-800268-1.00002-0. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Yanishlieva N, Gordon M. Antioxidants in food. Practical applications. Abington: Woodhead Publishing Limited, Abington Hall; 2001. [Google Scholar]
- Raeis S, Quek SY, Ojagh SM, Alishahi AR. Effects of Cumin (Cuminum Cyminum L.) Seed and Wild Mint (Mentha Longifolia L.) Leaf Extracts on the Shelf Life and Quality of Rainbow Trout (Oncorhynchus Mykiss) Fillets Stored at 4 °C ± 1. J Food Saf. 2016;36(2):271–281. doi: 10.1111/jfs.12240. [DOI] [Google Scholar]
- Raeisi S, Sharifi-Rad M, Quek SY, Shabanpour B, Sharifi-Rad J. Evaluation of antioxidant and antimicrobial effects of shallot (Allium ascalonicum L.) fruit and ajwain (Trachyspermum ammi (L.) Sprague) seed extracts in semi-fried coated rainbow trout (Oncorhynchus mykiss) fillets for shelf-life extension. LWT Food Sci Technol. 2016;65:112–121. doi: 10.1016/j.lwt.2015.07.064. [DOI] [Google Scholar]
- Raeisi S, Ojagh SM, Sharifi-Rad M, Sharifi-Rad J, Quek SY. Evaluation of Allium paradoxum (MB) G. Don. and Eryngium caucasicum trauve. Extracts on the shelf‐life and quality of silver carp (Hypophthalmichthys molitrix) fillets during refrigerated storage. J Food Saf. 2017;37(3):12321. doi: 10.1111/jfs.12321. [DOI] [Google Scholar]
- Raeisi S, Ojagh SM, Quek SY, Pourashouri P, Salaün F. Nano-encapsulation of fish oil and garlic essential oil by a novel composition of wall material: persian gum-chitosan. LWT Food Sci Technol. 2019;116:108494. doi: 10.1016/j.lwt.2019.108494. [DOI] [Google Scholar]
- Sallam KI. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control. 2007;18(5):566–575. doi: 10.1016/j.foodcont.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen H, Herrmann K, Weiss J. Oil-in-water emulsions as a delivery system for n-3 fatty acids in meat products. Meat Sci. 2013;93(3):659–667. doi: 10.1016/j.meatsci.2012.11.035. [DOI] [PubMed] [Google Scholar]
- Sanders TA. Polyunsaturated fatty acids in the food chain in Europe. Am J Clin Nutr. 2000;71(1):176s–178s. doi: 10.1093/ajcn/71.1.176s. [DOI] [PubMed] [Google Scholar]
- Sharifi-Rad J, Mnayer D, Tabanelli G, Stojanović-Radić ZZ, Sharifi-Rad M, Yousaf Z, Vallone L, Setzer WN, Iriti M. Plants of the genus Allium as antibacterial agents: from tradition to pharmacy. Cell Mol Biol. 2016;62(9):57–68. doi: 10.14715/cmb/2016.62.9.10. [DOI] [PubMed] [Google Scholar]
- Suárez Mahecha H, Jiménez Toquica Á, Díaz Moreno AC. Physicochemical evaluation of Cachama fillets (Piaractus brachypomus) preserved with propolis during storage. Rev Fac Nac Agron Medellin. 2014;67(1):7229–7236. doi: 10.15446/rfnam.v67n1.42653. [DOI] [Google Scholar]
- Tecator F (2002) Determination of total volatile basic nitrogen of fresh fish and frozen fish. Application Sub Note 8. Denmark
- Urmila K, Li H, Chen Q, Hui Z, Zhao J. Quantifying of total volatile basic nitrogen (TVB-N) content in chicken using a colorimetric sensor array and nonlinear regression tool. A Anal Methods. 2015;7(13):5682–5688. doi: 10.1039/C5AY00596E. [DOI] [Google Scholar]
- Valencia I, O’Grady M, Ansorena D, Astiasarán I, Kerry J. Enhancement of the nutritional status and quality of fresh pork sausages following the addition of linseed oil, fish oil and natural antioxidants. Meat Sci. 2008;80(4):1046–1054. doi: 10.1016/j.meatsci.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Velasco J, Dobarganes C, Holgado F, Márquez-Ruiz G. A follow-up oxidation study in dried microencapsulated oils under the accelerated conditions of the Rancimat test. Food Res Int. 2009;42(1):56–62. doi: 10.1016/j.foodres.2008.08.012. [DOI] [Google Scholar]
- Xiao S, Zhang WG, Lee EJ, Ma CW, Ahn DU. Lipid and protein oxidation of chicken breast rolls as affected by dietary oxidation levels and packaging. J Food Sci. 2011;76(4):C612–C617. doi: 10.1111/j.1750-3841.2011.02137.x. [DOI] [PubMed] [Google Scholar]


