Abstract
Aminoglycosides belong to a class of antibiotics now widely used in agriculture and veterinary medicine and expected to contaminate food products. In this study, a sensitive lateral flow immunoassay (LFIA) of an aminoglycoside neomycin (NEO) was developed. Two methods of immunochromatographic detection based on various techniques of gold nanoparticles (AuNPs) introduction as a label were compared. It was demonstrated that the indirect labeling (a conjugation of anti-species antibodies with a marker) allowed for an increase in assay sensitivity by 80 times. The test system was characterized by an instrumental limit of detection of 0.1 ng/mL and the cutoff level of 10 ng/mL; the assay duration was 15 min. Specificity only toward NEO was demonstrated. The developed LFIA has been tested to detect NEO in different foodstuffs. It has been demonstrated that 70–119% of NEO (coefficients of variations < 10%) can be determined in milk, turkey meat, honey, and eggs using simple procedures of preliminary sample preparation. Testing the samples showed the coincidence of the results for the developed lateral flow assay and for commercial ELISA kit.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04541-z) contains supplementary material, which is available to authorized users.
Keywords: Aminoglycosides, Antibiotics, Food safety, Lateral flow assay, Neomycin
Introduction
Providing the planet’s growing population with safe food is and will remain a critical task (Thompson and Darwish 2019). The efforts and means of many highly developed countries are directed to solving this problem. Among various approaches developed to overcome the crisis of the world food problem, the use of biologically active substances such as antibiotics, in particular, in the agricultural sector can be mentioned (Bacanli and Basaran 2019). Antimicrobial agents are added with food premixes for prophylaxis at critical moments in raising livestock animals, used for stopping and treating mild infections with the help of appropriate water-soluble antibiotics, and applied in injectable forms for severe and complicated animal diseases (Bacanli and Basaran 2019; Scott et al. 2019).
Among the antimicrobials widely used in animal husbandry, aminoglycosides should be noted (Jaimee and Halami 2016). Aminoglycosides are bactericidal antibiotics that are active against aerobic gram-negative bacteria and are used for the prevention and treatment of many diseases in livestock animals (van Duijkeren et al. 2019). Neomycin (NEO), an antibiotic from the aminoglycoside group, is a mixture of the neomycin A, B, and C antibiotics produced by Streptomyces fradiae and is commonly used in a sulfate or phosphate form (Zheng et al. 2019). NEO is often used to treat enteritis caused by microorganisms sensitive to this substance, some skin and eye diseases, vaginal infections, infected wounds locally, and other conditions (van Duijkeren et al. 2019). Adverse effects of NEO are associated with a high nephro- and ototoxicity (House and House 2014).
The common practice of using antimicrobials in livestock animals may lead to their transit through the food chain and the accumulation of residual amounts of antimicrobials in food products (Chen et al. 2019). Systematic intake of food containing antimicrobials carries a potential risk to human health, provoking the emergence of antibiotic-resistant strains of pathogens and the development of various pathological effects—allergic reactions, gastrointestinal tract diseases, candidiasis, and more (Chen et al. 2019; Perez-Rodriguez et al. 2019).The usage of antimicrobials in animal husbandry is now strictly regulated, and the minimal residue levels (MRLs) of antibiotics in food are established (https://ec.europa.eu/health/sites/health/files/files/mrl/regpdf/2001_04_25-0807_en.pdf). Thus, the MRLs of NEO are 500 ng/g for muscle, 1500 ng/g for milk, and 500 ng/g for eggs. Therefore, the need for mass and productive control of antibiotics’ residual amounts in foodstuffs causes an increasing demand for the development of simple, rapid, and inexpensive methods for their detection.
Conventional techniques of antibiotic detection in food products include microbiological methods that despite methodological simplicity are low specific and time-consuming (Wu et al. 2019). Instrumental analytical methods including LC–MS and HPLC are characterized by high precision, specificity, and sensitivity and commonly used as reference methods (Yang et al. 2017). The expensive equipment, highly qualified personnel, and complex procedures of food sample preparation limits point-of-care application of these approaches.
Immunoanalytical methods are based on the highly specific reaction of the analyte with antibodies. Among them, enzyme linked immunosorbent assay (ELISA) and lateral flow immunoassay (LFIA) seem to be rapid and inexpensive methods of mass screening for the presence of antibiotics in foodstuffs (Xu et al. 2015; Cháfer-Pericás et al. 2010; Parthasarathy et al. 2018; Lu et al. 2017). Both approaches are characterized by high sensitivity and specificity, but LFIA is more rapid (the results can be estimated within 10–20 min), simpler, less expensive, and more stable. Besides, it is suitable for point-of-care usage without using any additional equipment or portable devices.
Currently, a number of studies on the LFIA of NEO can be found in the literature (Shi et al. 2018a, b; Peng et al. 2016; Jin et al. 2006). All the described test systems used a direct LFIA method where specific antibodies were conjugated with gold nanoparticles (AuNPs) as a label. It was demonstrated that cutoff levels of NEO varied from 7 to 100 ng/mL; instrumental LODs were in the range of 0.2 pg/mL–7 ng/mL. In all of these studies, the determination of NEO was carried out only in milk as a food matrix with recovery values ranging from 86 to 121%.
The aim of this study is to develop an indirect immunochromatographic AuNPs-based test system with colorimetric detection for the determination of NEO in a panel of foodstuffs (milk, turkey meat, chicken eggs, and honey). The novelty of the proposed method is that it is based on another approach of a gold marker introduction, namely, the conjugation of AuNPs not with specific antibodies (as in the direct LFIAs described above) but with anti-species ones. The advantage of the indirect LFIA is the increased sensitivity (Urusov et al. 2016, 2014; Hendrickson et al. 2018; Berlina et al. 2018). The proposed test system based on an easy-to-obtain and stable gold label, which can be visually detected, allows for a significant gain in sensitivity simply by changing its componentry. Besides, because labeling of specific antibodies is not required the necessary componentry for the detection of different food contaminants can be formed without additional syntheses.
Materials and methods
Reagents
Neomycin trisulphate salt hydrate (NEO), streptomycin (STR), tobramycin (TOB), amikacin (AMI), clindamycin (CLIN), ofloxacin (OFL), clinafloxacin (CLI), tylosin (TYL), ampicillin (AMP), tetracycline (TET), cephalosporin C (CEPH), penicillin V (PEN V), bacitracin (BAC), amoxicillin (AMO), penicillin G (PEN G), chloramphenicol (CAP), sodium azide, 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB), bovine serum albumin (BSA), chloroauric acid, Triton X-100, and Tween-20 (Sigma-Aldrich, St. Louis, MO, USA) were applied in this study. Anti-NEO monoclonal antibodies and NEO–BSA conjugate were purchased from EximioBiotec (Hangzhou, Zhejiang, China). Goat antibodies against mouse immunoglobulins (GAMI) and donkey antibodies against goat immunoglobulins (DAGI) were used (Arista Biologicals, Allentown, PA, USA). Peroxidase-labeled antibodies against rabbit IgG (Jackson ImmunoResearch, Cambridgeshire, UK) were also used. All auxiliary reagents (salts, acids, alkalis, and organic solvents) were of analytical or chemical purity.
Solutions for the synthesis of AuNPs and their conjugates with immunoglobulins as well as NEO stock solution (100 μg/mL) were prepared with water deionized using a Milli-Q unit (Millipore, Burlington, MA, USA).
The ELISA was performed in 96-well transparent Costar 9018 polystyrene microplates (Corning Costar, Tewksbury, MA, USA).
Competitive ELISA of NEO
Competitive ELISA of NEO was carried out as described by Hendrickson et al. 2018. For this, a solution of NEO–BSA conjugate (1 μg/mL) in 50 mM K-phosphate buffer, pH 7.4, containing 0.1 M NaCl (PBS) was immobilized in the microplate wells for 16 h at 4 °C. After that, the microplate was washed four times with PBS containing 0.05% of Triton X-100 (PBST). Then, 50 μL of NEO solutions (5 × 103; 1.7 × 103; 556; 185; 62, 21, 6.9; 2.3; 0.76; 0.25; 85 × 10−3; 28 × 10−3; 9.4 × 10−3; 3.1 × 10−3; 1 × 10−3 ng/mL) and 50 μL of anti-NEO antibodies (40 ng/mL) in PBST were added to the microplate wells and incubated for 1 h at 37 °C. After washing the microplate, 100 μL of peroxidase-labeled antibodies against mouse IgG (diluted 1:5,000 in PBST) was added to the wells and incubated for 1 h at 37 °C. The microplate was washed and then the peroxidase activity was measured. For this purpose, 100 μL of the substrate solution (0.42 mM TMB and 1.8 mM H2O2 in 0.1 M Na-citrate buffer, pH 4.0) was incubated in the wells for 10 min. The reaction was stopped by adding 50 μL of 1 M H2SO4 per well. The optical density (OD) was measured at 450 nm, with a Zenyth 3100 photometer (Anthos Labtec Instruments, Salzburg, Austria).
Synthesis and characterization of AuNPs
AuNPs were obtained by the technique described by Frens (1973). The dimensional characteristics of the synthesized AuNPs were determined by transmission electron microscopy (TEM) using an electron microscope (JEM-100C, JEOL, Japan), as described by Hendrickson et al. 2018.
Preparation of antibody–AuNPs conjugates
The selection of antibodies’ concentration required for conjugation with AuNPs and the synthesis of antibody–AuNPs conjugates was carried out as described by Hendrickson et al. (2018). GAMI were dialyzed against 10 mM Tris–HCl buffer, pH 8.5. Then, 50 μL of GAMI solutions (0; 1; 2; 3; 4; 5; 6; 8; 10; 12; 14; 16; 18; 20; 22 μg/mL) were mixed with 0.5 mL of AuNPs (OD at 520 nm = 1) adjusted to pH 8.9 by 0.1 M K2CO3 and incubated for 10 min at room temperature. After that, 50 μL of a 10% NaCl solution was added. After stirring, OD520 was measured. A 6 μg/mL concentration of GAMI was selected for conjugation.
For conjugation, GAMI were added to AuNPs (OD520 = 1). The mixture was incubated with stirring for 45 min at room temperature, after which BSA was added to a final concentration of 0.25% and incubated for 10 min. GAMI–AuNPs conjugate was separated by centrifugation at 9,000 g for 15 min. The supernatant was removed, and the pellet was resuspended in 10 mM Tris buffer, pH 8.5, containing 1% BSA, 1% sucrose and 0.1% sodium azide (TBSA).
Production of immunochromatographic test systems
Test strips were produced using membrane sets (Mdi Easypack, Advanced Microdevices, India). A test strip was fabricated from a plastic support with a GFB-R4 sample pad, an AP045 adsorption pad, a PT-R7 fiberglass pad, and a working CNPC nitrocellulose membrane with a pore size of 15 μm. The test strip construction is presented in Fig. 1S. To apply the reagents onto the working membrane, an automatic dispenser (Iso-Flow, Imagene Technology, USA) was used. The antibody–AuNPs conjugate was applied manually with a loading of 32 μL per 1 cm of the fiberglass membrane. After applying all the reagents, the membranes were dried for at least 16 h at room temperature and 1 h at 37 °C. Then, a multimembrane composite was assembled and cut using an automatic guillotine cutter (Index Cutter-1, A-Point Technologies, USA) for test strips with a width of 3 mm.
Direct LFIA. The conjugate of anti-NEO antibodies and AuNPs in TBSA containing 0.05% Tween-20 (TTBSA) were applied onto the fiberglass membrane in a dilution corresponding to OD520 = 2. The NEO–BSA conjugate (0.25 mg/mL in PBS) was applied onto the working membrane to form the analytical zone. The control zone of the working membrane was formed by immobilization of the GAMI (0.25 mg/mL in PBS).
Indirect LFIA. The analytical and control zones were formed by applying the NEO–BSA conjugate (0.5 mg/mL in PBS) and DAGI (0.2 mg/mL in PBS), respectively. The GAMI–AuNPs conjugate in TTBSA was applied to a fiberglass membrane in a dilution corresponding to OD520 = 1.5. The procedures of drying, cutting, and packing the test strips was performed as described in Hendrickson et al. 2019. The test strips were hermetically sealed in bags of laminated aluminum foil with silica gel as a desiccant using a mini-conveyor (FR-900, Wenzhoudingli Packing Machinery, China). Cutting and packing were carried out at a relative humidity of no more than 30% at 20–22 °C.
Studying stability of the test strips stored at room temperature in closed packs with a desiccant demonstrated that neither the sensitivity of the analysis nor the intensity of the formed colored bands underwent significant changes during storage of the test strips for 12 months.
Preparation of food samples
The four foodstuffs used in the study (turkey meat, chicken eggs, honey, and cow milk with 2.5% fat content) were purchased at local supermarkets. For NEO detection, undiluted milk was tested. Sample preparation for eggs, honey, and turkey was carried out according to the technique used in the commercial ELISA kits “RIDASCREEN®” (R-Biopharm AG, Darmstadt, Germany, www.r-biopharm.com).
For eggs, the sample (100 g) was homogenized using a mixer. Ethyl acetate (12 mL) was added to a homogenized sample (2 g) and mixed intensively for 10 min for NEO extraction. Then the mixture was centrifuged at 3,000 g for 10 min at room temperature. After that, an ethyl acetate supernatant (6 mL, corresponding to 1 g of the sample) was transferred to a vial and evaporated to dryness at 60 °C. The dried residue was dissolved in n-hexane (1 mL). Then PBS (1 mL) was added to this solution and mixed intensively for approx. 1 min. The resultant mixture was centrifuged at 3,000 g for 10 min at room temperature. The lower aqueous phase was used for the analysis.
For turkey, the sample (100 g) was homogenized using a mixer. Then distilled water (3 mL) and ethyl acetate (6 mL) were added to 3 g of the homogenized meat. The resultant mixture was mixed intensively for 10 min for NEO extraction. Then the mixture was centrifuged at 3,000 g for 10 min at room temperature. Ethyl acetate supernatant (4 mL, corresponding to 2 g of the sample) was transferred to a vial and evaporated to dryness at 60 °C. The dried residue was dissolved in n-hexane (1 mL), and PBS (0.5 mL) was added to this solution. The mixture was mixed intensively for approx. 1 min and then centrifuged at 3,000 g for 10 min at room temperature. The lower aqueous phase was used for the analysis.
For honey, the sample (2 g) was diluted in distilled water (4 mL). Then, ethyl acetate (4 mL) was added to the sample and mixed intensively for 10 min for NEO extraction. After that, the mixture was centrifuged at 3,000 g for 10 min at room temperature. Ethyl acetate supernatant (1 mL, corresponding to 0.5 g of the sample) was transferred to a vial and evaporated to dryness at 60 °C. The dried residue was dissolved in PBS (0.5 mL) and used for the analysis.
LFIA of NEO
Direct LFIA. A series of NEO solutions (100 μL, 104; 3.3 × 103; 1.1 × 103; 370; 123; 41.2; 13.7; 4.6; 1.5; 0.5; 0.17 ng/mL) in PBST was added to microplate wells. The test strip was immersed vertically in the well, and after 15 min of incubation the assay results were detected.
Indirect LFIA. A series of NEO solutions (50 μL, 103; 333; 111; 37; 12; 4.1; 1.4; 0.46; 0.15; 0.05 ng/mL) in PBST or extracts of turkey meat, eggs, and honey were added to microplate wells. Then NEO-specific antibodies (50 μL) in a concentration of 0.73 µg/mL in PBST were added and incubated for 10 min at room temperature. The test strip was immersed vertically in the well, and after 15 min of incubation the assay results were detected.
Detection of the LFIA results
For scanning the test strips, a scanner (Cano Scan LiDE 90, Canon, Japan) was used. The obtained images were processed using the Total Lab software (Total Lab TL120 1D v2009, Nonlinear Dynamics, United Kingdom) to assess the color intensity of the analytical and control zones.
Processing of the LFIA and ELISA results
The dependence of the detected signal (optical density for the ELISA or color intensity for the LFIA) on the analyte concentration was approximated by four-parameter sigmoid function using the Origin software (Origin Pro 9.0.0, Origin Lab Corporation, USA) as described by Hendrickson et al. 2019. The interval of the analyte concentrations causing decrease of the signal of 20% to 80% from its maximal value was considered as the working range of the assay basing on common practice for competitive immunotechniques (Uhrovcik 2014). The visual detection limit in the LFIA (cutoff) was interpreted as the minimum NEO concentration, which caused a complete disappearance of the colored band in the analytical zone (i.e. the absence of visually or instrumentally detected coloration). The LOD of the ELISA and the instrumental LOD of the LFIA was calculated as the antibiotic concentration causing 10% inhibition (IC10) of the detected signal (Uhrovcik 2014). Good accordance of the LOD calculations based on IC10 and on three sigma rule was demonstrated.
Testing of the LFIA specificity
The solutions of STR, TOB, AMI, OFL, CLI, TYL, AMP, TET, STR, CEPH, PEN V, BAC, AMO, PEN G, and CAP (50 μL, 103; 333; 111; 37; 12; 4.1; 1.4; 0.46; 0.15; 0.05 ng/mL) in PBST were added to microplate wells. Then NEO-specific antibodies (50 μL) in a concentration of 0.73 µg/mL in PBST were added and incubated for 5 min at room temperature. The test strip was immersed vertically in the well and incubated for 15 min. The cross-reactivities were calculated as IC50NEO/IC50antibiotic × 100%, where IC50 is the antibiotic concentration causing 50% inhibition of the analytical signal. Totally, three replicates were performed.
NEO recoveries from spiked foodstuffs
The recovery values were estimated as the ratio between the calculated concentration of the analyte detected in the sample using the assay data and a calibration curve and the known concentration of the added analyte, expressed as a percentage.
Results and discussion
Obtaining of the specific reagents
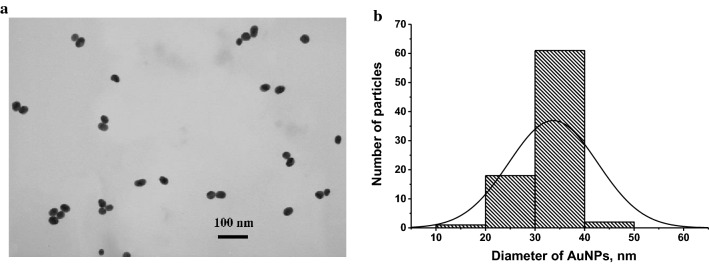
The first step of this study was the preparation and characterization of the specific reagents. AuNPs were used as a nano-dispersed label. Advantages of colloidal gold as a marker are its stability, chemical inertness, and simple synthesis of unified particles with similar size and sorption capacity. AuNPs were synthesized by the reduction of chloroauric acid with sodium citrate. Dimensional characteristics were determined by transmission electron microscopy. The average diameter of spherical non-aggregated AuNPs was 33.7 ± 8.9 nm with a mean ellipticity of 1.1 ± 0.4 (Fig. 1).
Fig. 1.
Microphotograph of AuNPs (a) and histogram of their size distribution (b)
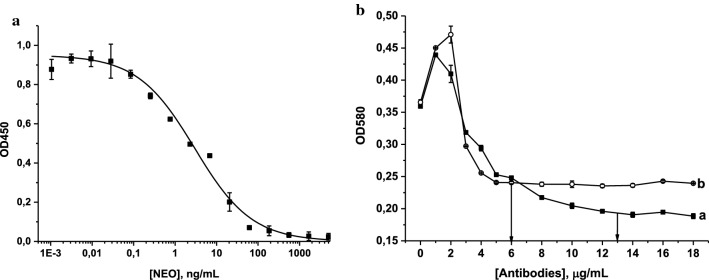
NEO-specific antibodies were first characterized by the indirect competitive ELISA and then conjugated with AuNPs. The LOD (0.2 ng/mL) and the working range of detectable concentrations (0.3–86.7 ng/mL) were calculated on the base of competitive curve of NEO obtained in the optimized conditions (Fig. 2a) of the analysis as described above. The reproducibility and the linear coefficient in the working range of the assay were 8–15% and 0.976, respectively.
Fig. 2.
Calibration curve of NEO in the ELISA (n = 3) (a) and flocculation curves for (a) anti-NEO antibodies and (b) GAMI (b). Concentrations of antibodies selected for the conjugation are marked by arrows
The high sensitivity of NEO determination in the ELISA indicated the antibodies’ suitability for the development of LFIA. The concentration of antibodies required for conjugation with a label was determined based on a flocculation curve, which illustrates the dependence of the OD of the AuNP solution on antibody concentrations in a medium with a high ionic strength (Fig. 2b).
The immobilized immunoglobulins make nanoparticles steady and prevent aggregation caused by the addition of a coagulating agent (10% NaCl). Upon an increase of antibody concentration, OD also grows, reaches a maximum, then starts declining, and reaches a plateau (flocculation point). The plateau corresponding to the stabilization of AuNPs by the immobilized protein was achieved at concentrations of ≥ 5 μg/mL for GAMI and ≥ 13 μg/mL for anti-NEO antibodies. To ensure complete coverage of the particles’ surface by immunoglobulins, antibody concentrations of ~ 20% higher than those corresponding to the flocculation points were selected for conjugates’ synthesis (e.g., 6 and 15 μg/mL for GAMI and NEO-specific antibodies, respectively) (Byzova et al. 2017).
Development of the direct LFIA of NEO
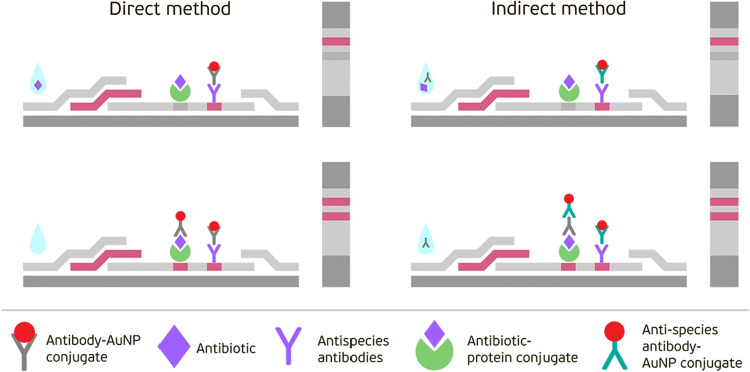
In this study, two formats of the immunochromatographic analysis of NEO were developed, which differed in the technique of a gold label introduction. A standard direct method is based on the application of specific antibodies – AuNPs conjugates and an indirect method uses anti-species immunoglobulins labeled with AuNPs. The direct competitive assay is known to be the traditional format of the immunochromatography widely used for the detection of different low-molecular-weight compounds. Here, the NEO–BSA conjugate absorbed in the test zone of the working membrane and the free NEO contained in the sample competitively interact with AuNPs-immobilized specific anti-NEO antibodies that are applied on a fiberglass membrane (see the scheme in Fig. 3).
Fig. 3.
Schemes of the direct and indirect LFIAs
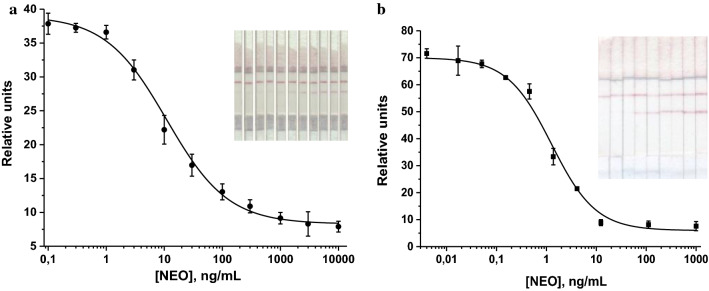
The LFIA conditions were optimized to achieve an intensive coloration of the analytical and control bands, a low LOD of NEO, and satisfactory reproducibility. For this purpose, concentrations of specific reagents and the duration of a test strip incubation with a sample were varied during the analysis. Several variants of test strips were produced and tested. The NEO–BSA conjugate was immobilized in the analytical zone in the concentration range of 0.125–1.0 mg/mL, the anti-NEO antibody–AuNPs conjugate was adsorbed onto a fiberglass membrane in the range of concentrations corresponding to OD520 = 0.5–2.5, and GAMI were applied in the control zone at a concentration range of 0.05–1.0 mg/mL. As a result of optimization, it was demonstrated that the lowest NEO LOD was achieved at concentrations of NEO–BSA and GAMI of 0.25 mg/mL, and the concentration of antibody–AuNPs conjugate corresponding to OD520 = 2. The optimal time for incubation of the test strip with the sample was 10 min. The calibration curve of the direct LFIA of NEO obtained under optimized conditions and the images of the corresponding test strips are shown in Fig. 4a.
Fig. 4.
Calibration curve of NEO in the direct (a) and indirect (b) LFIA (n = 3)
The instrumental LOD in PBST was 0.8 ng/mL (the working range of the detected concentrations was 2.1–59.3 ng/mL). The cutoff level of the direct LFIA was 500 ng/mL. The obtained sensitivity did not satisfy the requirements for maximum residue limits of veterinary medicinal products in foodstuffs of animal origin established in EU countries (https://ec.europa.eu/health/sites/health/files/files/mrl/regpdf/2001_04_25-0807_en.pdf). A further decrease in the concentration of specific antibodies, which ideally should lead to an increase in the sensitivity of the analysis, was ineffective, because this led to a decrease in the intensity of the color band formed, thus making it difficult to detect. Therefore, we proceeded to the development of the indirect LFIA, which does not have this flaw, and according to our previous studies, it allows increasing the assay sensitivity from several times to several orders of magnitude in comparison to the direct LFIA (Urusov et al. 2016, 2014; Hendrickson et al. 2018; Berlina et al. 2018).
Development of the indirect LFIA of NEO
In this assay format, the test strip is incubated with a tested sample containing pre-added nonconjugated specific antibodies. This approach was tested in our previous studies for the detection of other contaminants (Urusov et al. 2014, 2016; Hendrickson et al. 2018; Berlina et al. 2018). It was demonstrated that thanks to the exclusion of the so-called nonproductive interactions (when the binding of the most specific antibodies conjugated to AuNPs with a free antigen does not prevent the interaction of the conjugate with the immobilized antigen), the assay sensitivity sufficiently increased (up to two orders of magnitude). Because antibodies’ interactions with the analyte in the sample results in a decrease of their binding with a label, the detection limit and the working range of an analyte’s detectable concentration shifted to lower concentrations (Urusov et al. 2014, 2016).
An indirect LFIA included two stages: pre-incubation of the tested sample with specific anti-NEO antibodies and then incubation of the test strip with this mixture. Here, instead of specific antibody–AuNPs conjugate commonly used in the standard LFIA, a combination of non-labeled anti-NEO antibodies pre-added to the sample and a conjugate of anti-species antibodies with AuNPs applied onto a fiberglass membrane was used (Fig. 4). Because antigen–antibody interactions occur in the sample and, consequently, the total amount of the bound label is reduced, the indirect labeling results in a shift of the LOD and the working range to lower concentrations while keeping its rapidity and simplicity of implementation (Urusov et al. 2014, 2016).
As in the previous format, the protocols of the indirect LFIA were also optimized to achieve the minimum LOD of NEO. This requirement was fulfilled if NEO–BSA concentration was 0.5 mg/mL (we tested concentrations of 0.1; 0.25; 0.5; 0.75; 1 mg/mL), anti-NEO antibodies were added at a concentration of 0.73 µg/mL (we tested concentrations of 0.1; 0.5; 1; 5; 10 µg/mL), and the DAGI concentration was 0.2 mg/mL (we tested concentrations of 0.05; 0.1; 0.15; 0.2; 0.5; 1.0 mg/mL). The concentration of the GAMI–AuNPs conjugate corresponded to OD520 = 1.5 (we tested concentrations corresponding to OD520 of 1; 1.5; 2; 2.5; 3). For the indirect LFIA, the optimal assay duration (a time of a test strip incubation with a sample) was 15 min. The NEO calibration curve in the indirect LFIA is presented in Fig. 4b.
The instrumental detection limit of NEO in PBST was 0.1 ng/mL; the working range of the detectable concentrations was 0.28–5.18 ng/mL, and the cutoff level was 10 ng/mL.
Therefore, it was discovered that the indirect assay demonstrated an essential gain in the assay sensitivity in comparison with the direct analysis without increasing its duration (the cutoff level of the indirect LFIA was 50 times lower than that assessed for the direct one). Therefore, the indirect assay format was selected for further experiments concerning food testing.
Specificity of the developed LFIA
In the next step, we studied the specificity of the test system. Aminoglycosides AMI, STR, and TOB as structural analogues of NEO as well as a number of antibiotics belonging to other classes (namely, lincosamide CLIN, amphenicol CAP, macrolideTYL, β-lactams AMP and CEPH, fluoroquinolones OFL and CLI, penicillins AMO, PEN G, and PEN V, polypeptide antibiotic BAC) were detected by the developed analysis. It was found that the test system was characterized by specificity only toward NEO (100%); the cross-reactivities with all other antibiotics tested were negligible (< 0.1%). Therefore, the developed LFIA allows for highly specific screening of food products for only aminoglycoside NEO.
Determination of NEO in foodstuffs using the developed LFIA
The testing of foodstuffs was preceded by the development of sample preparation techniques. In general, an analysis used for mass screening of food products for contamination requires an uncomplicated and rapid procedure of sample pretreatment aimed at an inhibition of biomatrix components’ influence on the immune interaction and the assay results. Unlike more sophisticated analytical methods (e.g., HPLC), immunochromatography does not require a complete disposal of the matrix. For liquid products, a simple dilution by a buffer is often practiced (Byzova et al. 2010, 2011). In our study, we tested four food samples purchased in local supermarkets: cow milk with 2.5% fat content, turkey meat, honey, and chicken eggs. The analysis of foodstuffs by the commercial ELISA kit (EuroProximaNeomycin™, r-biopharm, The Netherlands, www.europroxima.com) as the reference method revealed the absence of NEO.
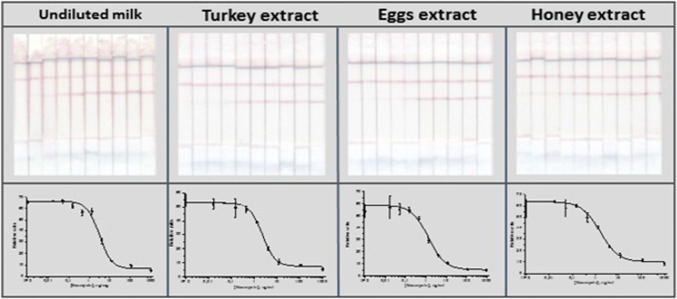
It was demonstrated that for milk, the sample preparation was not necessary at all because matrix components of undiluted milk did not interfere with the immune interactions. For turkey, eggs, and honey, a relatively quick and uncomplicated method of NEO extraction by ethyl acetate with further separation of water and organic phases by centrifugation was carried out. The images of the test strips and the corresponding competitive curves for the LFIA of NEO are presented in Fig. 5.
Fig. 5.
Images of the test strips and corresponding competitive curves for the LFIA of NEO in undiluted cow milk, turkey, chicken eggs, and honey
The cutoff levels of NEO in the tested food products coincided with that obtained in PBST: the disappearance of a colored band in the analytical zone was observed at NEO concentrations of 10 ng/g. Table 1 contains the recoveries for milk, turkey, eggs, and honey samples spiked with NEO.
Table 1.
Recoveries of NEO from milk, turkey, honey, and eggs (n = 5)
| Added amount (ng/g) | Detected amount ± SD (ng/g)/Recovery ± SD (%) | |||
|---|---|---|---|---|
| Cow milk | Turkey | Chicken eggs | Honey | |
| 0.3 |
0.22 ± 0.01/ 73.3 ± 3.3 |
0.26 ± 0.02/ 86.7 ± 6.7 |
0.25 ± 0.012/ 83.3 ± 4.0 |
0.28 ± 0.015/ 93.3 ± 5.0 |
| 0.5 |
0.35 ± 0.017/ 70.0 ± 3.4 |
0.46 ± 0.02/ 92.0 ± 4.0 |
0.44 ± 0.015/ 88.0 ± 3.0 |
0.52 ± 0.03/ 104 ± 6.0 |
| 1.0 | 0.78 ± 0.044/78.0 ± 4.4 |
1.06 ± 0.013/ 106 ± 1.3 |
0.88 ± 0.032/ 88.0 ± 3.2 |
1.06 ± 0.026/ 106 ± 2.6 |
| 2.0 |
1.72 ± 0.056/ 86.0 ± 2.8 |
2.18 ± 0.046/ 109 ± 2.3 |
1.66 ± 0.034/ 83.0 ± 1.7 |
2.27 ± 0.064/ 113.5 ± 3.2 |
| 4.0 |
3.54 ± 0.15/ 88.5 ± 3.8 |
3.73 ± 0.1/ 93.3 ± 2.5 |
3.34 ± 0.09/ 83.5 ± 2.3 |
4.76 ± 0.063/ 119 ± 1.6 |
| 5.0 |
4.56 ± 0.13/ 91.2 ± 2.6 |
4.8 ± 0.12/ 96.0 ± 2.4 |
4.66 ± 0.17/ 93.2 ± 3.4 |
5.1 ± 0.08/ 102 ± 1.6 |
*SD standard deviation, n = 5
As can be seen from the presented data, the indirect LFIA allowed for the detection of 70–119% of NEO with intra- and inter-assay coefficients of variations < 10%, which confirmed its suitability for antibiotic detection in food products. A correlation between concentrations added and detected in food samples by LFIA is presented in Fig. 2S. A high correlation coefficient (0.957, n = 10) between the amounts of NEO determined by the LFIA and the ELISA as a reference method (see above) confirmed the accuracy and reproducibility of the developed test system.
Comparison of the results obtained in this study with the results presented in past studies showed that having comparable cutoffs we achieved a lower instrumental LOD, namely 0.1 ng/mL (compared to 5–100 ng/mL in AuNPs-based studies with colorimetric detection). Lower LODs were achieved only in investigations based on surface-enhanced Raman scattering (SERS) detection: 0.20–0.4 pg/mL. It should be noted, however, that such low instrumental LODs were due to the use of a complex detection method based on SERS measurement, which except a marker, requires the introduction of the Raman active molecule into the test system (Shi et al. 2018a, b). The need for sophisticated equipment (Raman spectrometer) prevents the point-of-care application of the SERS-based immunochromatographic test system for mass screening of foodstuffs for contamination by antibiotics. Besides, the panel of analyzed food products was significantly expanded in our study. In addition to solely milk, which was analyzed in previous studies, we detected the antibiotic in turkey, eggs and honey.
The presented data about the developed high-sensitive LFIA of NEO demonstrates the potential in terms of its implementation to practice. The test may supplement a number of successfully commercialized developments by LFIA of veterinary drugs in food products. The assay can be applied both for qualitative visual estimation of NEO presence and quantitative measurement of its content using portable photometric devices. Milk analysis does not require sample preparation and so can be carried out directly on farms and enterprises without the involvement of any equipment. When working with solid and viscous food samples (meat, eggs, honey), standard homogenization and extraction manipulations are required, which can be completed using conventional, simple, and cheap devices. The advantages of the proposed LFIA by cost comparison with other methods depend on the features of scaled technologies and manufacturing volumes, but in general, the competitive potential of quick tests for veterinary drugs is confirmed by the active development of this segment of worldwide market (Antibiotic Residue Test Kits Market 2019).
Conclusion
Overall, a rapid and sensitive LFIA for detecting NEO based on indirect introduction of AuNPs as a label was developed. The LFIA allows detecting NEO within 15 min with an instrumental LOD/cutoff level of 0.1/10 ng/mL. The test system is able to specifically determine NEO with negligible cross-reactivity to any antibiotics from other classes tested, including other aminoglycosides. This method allows for an expansion of the applicability of immunochromatographic tests for the control of antibiotics in various food products. The LFIA enables simple screening of NEO residues in milk, turkey meat, eggs, and honey with preservation of high analytical characteristics. The sample preparation is not necessary for milk and simple enough for meat, eggs, and honey. The obtained analytical characteristics met the requirements of sanitary and hygienic control established by the Russian Federation and EU countries. Due to its availability, the proposed approach is promising for the control of various low-molecular-weight food contaminants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The work was supported by the Russian Foundation for Basic Research, project 18-58-00038, and the Belarusian Republican Foundation for Fundamental Research, project X18P-060. The authors are grateful to S. M. Pridvorova (Federal Research Center "Fundamentals of Biotechnology") for obtaining electronic microphotographs of AuNPs and D. S. Popravko for developing Fig. 3.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
The studies did not involve human participants or animals.
Informed consent
Informed consent not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Antibiotic Residue Test Kits Market . Global industry perspective, comprehensive analysis and forecast, 2018–2025. New York: Zion Market Research; 2019. p. 110. [Google Scholar]
- Bacanli M, Basaran N. Importance of antibiotic residues in animal food. Food Chem Toxicol. 2019;125:462–466. doi: 10.1016/j.fct.2019.01.033. [DOI] [PubMed] [Google Scholar]
- Berlina AN, Bartosh AV, Sotnikov DV, Zherdev AV, Xu C, Dzantiev BB. Complexes of gold nanoparticles with antibodies in immunochromatography: comparison of direct and indirect immobilization of antibodies for the detection of antibiotics. Nanotechnol Russ. 2018;13(7):430–438. doi: 10.1134/S1995078018040031. [DOI] [Google Scholar]
- Byzova NA, Safenkova IV, Slutskaya ES, Zherdev AV, Dzantiev BB. Less is more: a comparison of antibody-gold nanoparticle conjugates of different ratios. Bioconjug Chem. 2017;28(11):2737–2746. doi: 10.1021/acs.bioconjchem.7b00489. [DOI] [PubMed] [Google Scholar]
- Byzova NA, Zvereva EA, Zherdev AV, Eremin SA, Dzantiev BB. Rapid pretreatment-free immunochromatographic assay of chloramphenicol in milk. Talanta. 2010;81(3):843–848. doi: 10.1016/j.talanta.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Byzova NA, Zvereva EA, Zherdev AV, Eremin SA, Sveshnikov PG, Dzantiev BB. Pretreatment-free immunochromatographic assay for the detection of streptomycin and its application to the control of milk and dairy products. Anal Chim Acta. 2011;701(2):209–217. doi: 10.1016/j.aca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Cháfer-Pericás C, Maquieira Á, Puchades R. Fast screening methods to detect antibiotic residues in food samples. TrAC Trends Anal Chem. 2010;29(9):1038–1049. doi: 10.1016/j.trac.2010.06.004. [DOI] [Google Scholar]
- Chen J, Ying GG, Deng WJ. Antibiotic residues in food: extraction, analysis, and human health concerns. J Agric Food Chem. 2019;67(27):7569–7586. doi: 10.1021/acs.jafc.9b01334. [DOI] [PubMed] [Google Scholar]
- Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241(105):20–22. doi: 10.1038/physci241020a0. [DOI] [Google Scholar]
- Jaimee G, Halami PM. Emerging resistance to aminoglycosides in lactic acid bacteria of food origin-an impending menace. Appl Microbiol Biotechnol. 2016;100(3):1137–1151. doi: 10.1007/s00253-015-7184-y. [DOI] [PubMed] [Google Scholar]
- Jin Y, Jang JW, Lee MH, Han CH. Development of ELISA and immunochromatographic assay for the detection of neomycin. Clin Chim Acta. 2006;364(1–2):260–266. doi: 10.1016/j.cca.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Hendrickson OD, Zvereva EA, Shanin IA, Zherdev AV, Dzantiev BB. Development of a multicomponent immunochromatographic test system for the detection of fluoroquinolone and amphenicol antibiotics in dairy products. J Sci Food Agric. 2019;99(8):3834–3842. doi: 10.1002/jsfa.9605. [DOI] [PubMed] [Google Scholar]
- Hendrickson OD, Zvereva EA, Shanin IA, Zherdev AV, Tarannum N, Dzantiev BB. Highly sensitive immunochromatographic detection of antibiotic ciprofloxacin in milk. Appl Biochem Microbiol. 2018;54(6):670–676. doi: 10.1134/S000368381806008X. [DOI] [Google Scholar]
- House JR, 3rd, House LK. Ototoxicity of polymyxin B, neomycin, and hydrocortisone suspension in tympanoplasty surgery. Otolaryngol Head Neck Surg. 2014;150(2):282–284. doi: 10.1177/0194599813513007. [DOI] [PubMed] [Google Scholar]
- https://ec.europa.eu/health/sites/health/files/files/mrl/regpdf/2001_04_25-0807_en.pdf
- Lu Y, Sheng W, Liu B, Wang S. ELISA-based sensing in food safety and quality analysis. In: Lu X, editor. Sensing techniques for food safety and quality control. Cambridge: Royal Society of Chemistry; 2017. pp. 141–163. [Google Scholar]
- Parthasarathy R, Monette CE, Bracero S, S Saha M (2018) Methods for field measurement of antibiotic concentrations: limitations and outlook. FEMS Microbiol Ecol 94(8). [DOI] [PubMed]
- Peng J, Wang Y, Liu L, Kuang H, Li A, Xu C. Multiplex lateral flow immunoassay for five antibiotics detection based on gold nanoparticle aggregations. RSC Adv. 2016;6(10):7798–7805. doi: 10.1039/C5RA22583C. [DOI] [Google Scholar]
- Perez-Rodriguez F, Mercanoglu Taban B. A state-of-art review on multi-drug resistant pathogens in foods of animal origin: risk factors and mitigation strategies. Front Microbiol. 2019;10:2091. doi: 10.3389/fmicb.2019.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HM, Acuff G, Bergeron G, Bourassa MW, Gill J, Graham DW, Kahn LH, Morley PS, Salois MJ, Simjee S, Singer R, Smith TC, Storrs C, Wittum TE. Critically important antibiotics: criteria and approaches for measuring and reducing their use in food animal agriculture. Ann N Y Acad Sci. 2019;1441(1):8–16. doi: 10.1111/nyas.14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Huang J, Sun Y, Deng R, Teng M, Li Q, Yang Y, Hu X, Zhang Z, Zhang G. A SERS-based multiple immuno-nanoprobe for ultrasensitive detection of neomycin and quinolone antibiotics via a lateral flow assay. Microchim Acta. 2018;85(2):84. doi: 10.1007/s00604-017-2556-x. [DOI] [PubMed] [Google Scholar]
- Shi Q, Huang J, Sun Y, Yin M, Hu M, Hu X, Zhang Z, Zhang G. Utilization of a lateral flow colloidal gold immunoassay strip based on surface-enhanced Raman spectroscopy for ultrasensitive detection of antibiotics in milk. Spectrochim Acta A Mol Biomol Spectrosc. 2018;197:107–113. doi: 10.1016/j.saa.2017.11.045. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Darwish WS (2019) Environmental chemical contaminants in food: review of a global problem. J Toxicol 2345283 [DOI] [PMC free article] [PubMed]
- Uhrovcik J. Strategy for determination of LOD and LOQ values–some basic aspects. Talanta. 2014;119:178–180. doi: 10.1016/j.talanta.2013.10.061. [DOI] [PubMed] [Google Scholar]
- Urusov AE, Petrakova AV, Zherdev AV, Dzantiev BB. "Multistage in one touch" design with a universal labelling conjugate for high-sensitive lateral flow immunoassays. Biosens Bioelectr. 2016;86:575–579. doi: 10.1016/j.bios.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Urusov AE, Zherdev AV, Dzantiev BB. Use of gold nanoparticle-labeled secondary antibodies to improve the sensitivity of an immunochromatographic assay for aflatoxin B1. Microchim Acta. 2014;181(15):1939–1946. doi: 10.1007/s00604-014-1288-4. [DOI] [Google Scholar]
- van Duijkeren E, Schwarz C, Bouchard D, Catry B, Pomba C, Baptiste KE, Moreno MA, Rantala M, Ružauskas M, Sanders P, Teale C, Wester AL, Ignate K, Kunsagi Z, Jukes H. The use of aminoglycosides in animals within the EU: development of resistance in animals and possible impact on human and animal health: a review. J Antimicrob Chemother. 2019;74(9):2480–2496. doi: 10.1093/jac/dkz161. [DOI] [PubMed] [Google Scholar]
- Wu Q, Peng D, Liu Q, Shabbir MAB, Sajid A, Liu Z, Wang Y, Yuan Z. A novel microbiological method in microtiter plates for screening seven kinds of widely used antibiotics residues in milk, chicken egg and honey. Front Microbiol. 2019;10:436. doi: 10.3389/fmicb.2019.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Ren K, Yang Y-z, Guo J-p, Ma G-p, Liu Y-m, Lu Y-q, Li X-b. Immunoassay of chemical contaminants in milk: a review. J Integr Agricult. 2015;14(11):2282–2295. doi: 10.1016/S2095-3119(15)61121-2. [DOI] [Google Scholar]
- Yang B, Wang L, Luo C, Wang X, Sun C. Simultaneous determination of 11 aminoglycoside residues in honey, milk, and pork by liquid chromatography with tandem mass spectrometry and molecularly imprinted polymer solid phase extraction. J AOAC Int. 2017;100(6):1869–1878. doi: 10.5740/jaoacint.16-0399. [DOI] [PubMed] [Google Scholar]
- Zheng J, Li Y, Guan H, Zhang J, Tan H (2019) Enhancement of neomycin production by engineering the entire biosynthetic gene cluster and feeding key precursors in Streptomyces fradiae CGMCC 4.576. Appl Microbiol Biotechnol 103(5):2263–2275. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.