Abstract
The influences of spray-drying and freeze-drying processes on functional properties of ginkgo seed proteins (GSP) were systematically investigated. It was revealed that GSP dried by spray (SGSP) displays an significantly improved water holding capacity and superior emulsifying properties than the freezing-drying GSP (FGSP), whereas, the oil binding capacity is higher in FGSP. The difference in properties of SGSP and FGSP can be attributed to their different structural characteristics. Comparing with FGSP, SGSP was demonstrated having more disulfide bonds, more amorphous and less ordered structure, accounted for big differences in functional properties. With the outstanding functional characteristics, GSP could be potentially applied in oil-in-water type food system, such as milk and mayonnaise. Finally, it is important to choose the suitable drying method according to the requirements of the specific food system.
Keywords: Ginkgo seed protein, Spray-drying, Freeze-drying, Water-holding capacity, Emulsifying properties
Introduction
Developing new food protein resources to alleviate the rapid growth of population has become an urgent subject in both scientific and industrial fields. Compared with the animal proteins, the plant proteins are much more accessible and thus are potentially a promising alternative attacking numerous attentions (Lin et al. 2017).
Recent interests in leguminous proteins as new sources of protein in foods have brought to light the potentials of many legumes that were hitherto underutilized, including faba, lentil, pea, soybean and peanut protein (Grela et al. 2017). Compared with legumin, little is known about the Ginkgo seed protein (GSP). Ginkgo biloba is the first gymnosperm for which existence of a seed protein with legumin-like properties has been demonstrated (Jensen and Berthold 1989). Ginkgo (Ginkgo biloba L.) in China, which accounted for 90% of the world’s cultivation, produced more than 12,000 tons of ginkgo seeds each year (Miao et al. 2012). As a traditional food and medicine source, the seeds of Ginkgo were used in China for several thousand years. Ginkgo seeds have a relatively high content (10–15%) of proteins, which possess rich and reasonable composition of essential amino acids, belonging to high-quality protein. Besides, ginkgo seed proteins exhibit several biological activities, including anti-oxidation and antimicrobial activity (Huang et al. 2010; Zhou et al. 2012).
To make the GSP utilization extensively, effects of different processing methods on GSP properties are systematically studied. First of all, GSP needs to be converted into dry powder form in order to extend its shelf-life and to preserve its bio-functional properties. Spray drying and freezing drying are the two most commonly used drying methods for drying protein (Wray and Ramaswamy 2015). Spray drying is industrially preferred method to produce food protein powders, because it requires lower capital cost (< 8 times) as well as operational cost (< 5 times) in comparison to freeze drying (Ahmed and Rahman 2012). However, freezing drying could better retain the native structure of protein, thus to keep the antioxidant, nutritional and biological activities of protein (Gharsallaoui et al. 2007). In addition, drying methods have been proved to affect the functional properties of protein significantly (Gong et al. 2016; Zhao et al. 2013). However, the effects of drying methods on the functional properties of GSP have not been studied. The revealing of properties of dried GSP will also contribute to the application of GSP in new food formulations.
Herein, the influences derived from the spray and freezing drying processes imposed on the key properties of GSP, such as the water holding and oil binding capacities, protein dispensability index, foaming and emulsifying properties, were systematically examined. In addition, the structure changes resulted from the different drying methods were also evaluated. Therefore, this work is proposed to provide deep insights into the rational selection of proper drying techniques for GSP production.
Materials and methods
Materials
The Ginkgo (Ginkgo biloba L.) seeds were purchased from Tancheng of Shandong province (China). NaOH, HCl, EDTA, Coomassie blue G-250, trichloroacetic acid (TCA), 5,5’-Dithiobis-(2-nitrobenzoic acid) (DTNB), bovine serum albumin (BSA), sodium dodecyl sulfate (SDS) and glycine used in the experiments were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Extraction of GSP
Fresh ginkgo nuts were shelled, clothing and cored, then freeze-dried (Free Zone 2.5 L, Labconco Instruments, USA). Freeze-dried ginkgo was ground into fine powder by grinder for 1 min, and then sieving through a fine screen. GSP was extracted using an alkaline dissolving and acid precipitating method, according to Fan et al. (2012) with a little modification. One hundred grams of ginkgo flour sample was dispersed in 0.1 mol/L NaCl solution (25%, w/v) and the pH adjusted to 9.0 with 0.5 M NaOH at 50 °C for 90 min. After centrifugation at 5900 ×g for 20 min, the pH of the supernatant was adjusted to 4.4 with 0.5 N HCl to precipitate GSP, then centrifuged at 5900 ×g for 20 min. Precipitates were dissolved in distilled water (8.0%, w/v) at pH 7, and separated into two portions, which treated either by freeze or spray drying, as follows.
Preparation of spray-dried and freeze-dried GSP
GSP dispersions (8.0%, w/v) were dried by spray- or freeze-drying methods respectively, one portion was pre-frozen at − 40 °C for 12 h, and then put into a lyophilizer at − 80 °C, 1 Pa for 24 h to obtain the freeze-dried ginkgo seed protein products (FGSP). The other portion was treated by spray-drying using a bench-top spray dryer (Büchi B-290, Büchi Labortechnik AGt, Switzerland), and the spray-dried ginkgo seed protein (SGSP) was obtained. The pump capacity was 15%, and the inlet temperature was 180 °C. The samples were stored at − 40 °C for further analysis.
Determination of functional properties of FGSP and SGSP
Water-holding capacity (WHC)
The WHC of the samples was determined according to the method of Tan et al. (2014). FGSP or SGSP (0.05 g) was mixed with 3.0 mL distilled water in a tube and vortexed for 1 min. The mixture was incubated at room temperature (25 °C) for 30 min, and then centrifuged at 22,000 ×g for 30 min. The supernatant was poured into a constant weight desiccator and the dissolved material was dried to a constant weight in an oven at 105 °C to calculate the mass. Weigh the tube containing precipitate. The WHC was calculated as given by the equation.
| 1 |
where W0 is initial weight of sample (g), W1 is the initial weight of sample and tube (g), W2 is the weight of the tube that containing the precipitation (g).
Oil-binding capacities (OBC)
The OBC of GSP was determined according to the method of Tan et al. (2014). Fifty milligrams of samples were mixed with 3.0 mL soybean oil in a tube and vortexed for 1 min. The mixture was incubated at room temperature for 30 min and then centrifuged at 22,000 ×g for 30 min. The resulting supernatant was carefully decanted, and the tube containing the precipitation weighed. The OBC was calculated as given by the equation.
| 2 |
where W0 is initial weight of sample (g), W1 is the initial weight of sample and tube (g), W2 is the weight of the tube that containing the precipitation (g).
Protein dispensability index (PDI)
PDI, which measures the percent of water-dispersible protein over the percent of total protein, was determined following the standard method by L’hocine et al. (2006). The FGSP or SGSP (0.2 g) were dispersed in water (1%, w/v) and stirred for 30 min using a magnetic stirrer, and the pH was adjusted to 2.0–12.0, respectively using 0.5 mol/L HCl or NaOH. And then the solutions were centrifuged at 5900 ×g for 20 min. The supernatant (0.1 mL) was determined the protein content. The PDI of the sample was calculated by the equation as given.
| 3 |
where P1 is the total protein content of the supernatant, P0 is the total protein content in the sample.
Foaming properties
Foaming capacity (FC) and stability (FS) were studied according to the method of Deng et al. (2011) with slight modifications. Twenty milliliters protein samples (1.5%, w/v) in distilled water were homogenized at 10,000 rpm for 2 min. FC was expressed as the volume (%) increased due to stirring. The volume of the foam portion was recorded at 0 min for foam capacity and up to 30 min for foam stability. The foaming properties of GSP were measured at different pH values (pH 2.0–12.0). Each sample was evaluated at least in duplicate. Foaming capacity and foam stability were then calculated:
| 4 |
| 5 |
where V0 is the volume increased due to stirring (mL); V30 is the foam volume changes at 30 min of storage (mL).
Emulsifying properties
Emulsifying properties of food protein were evaluated by the emulsion activity index (EAI) and emulsion stability index (ESI) (Tirgar et al. 2017). Fifteen milliliter of 1% protein solution (pH 2.0–12.0) and 5 mL soybean oil were mixed. The mixture was homogenized at a speed of 10,000 rpm for 3 min. An aliquot of the emulsion (25 µL) was pipetted from the bottom of the container at 0 and 10 min after homogenization and mixed with 5 mL of 0.1% SDS solution. The absorbance of the emulsion at 500 nm was recorded immediately (A0) and after 10 min (A10) using a spectrophotometer (2601-UV/VIS, Beijing Beifen-Ruili Analytical Instrument Co. Ltd., China). EAI and ESI were computed using Eqs. (6) and (7), respectively.
| 6 |
| 7 |
where A0 is the initial absorbance (0 min), DF is the dilution factor (200), C is the initial concentration of protein (g/mL), ϕ is the optical path (1 cm), and θ is the proportion of the oil phase, At is the absorbance at 10 min after homogenization, Δt is 10 min.
Characterization of structure of FGSP and SGSP
Amino acid (AA)composition of FGSP and SGSP
Total amino acid compositions of the protein samples were determined by high-speed amino acid auto-analyzer (Hitachi L-8800, Japan) according to (Zhou et al. 2019). The samples (0.01 g) were hydrolyzed with 6 mol/L HCl in a sealed tube at 110 °C for 24 h and then cooled to room temperature. An equivalent volume of TCA was added to the sample to precipitate peptides or proteins. After incubation for 2 h at room temperature, the solution was filtered through Whatman No. 4 filter paper. The filtrate was centrifuged at 7000×g for 10 min, and the supernatant was stored at 4 °C before injection. Methionine and Cysteine were analyzed as methionine sulfone and cysteic acid after cold performic acid oxidation overnight before the hydrolysis. Tryptophan was determined after NaOH hydrolysis at 110 °C for 22 h. Twenty micro-liter sample was injected into Sykam Amino Acid Analyzer (Laserchrom HPLC Laboratories Ltd. Inc., Rochester, UK) to estimate the amino acid profile (except tryptophan). Amino acid composition was reported as milligrams amino acid per gram protein.
Circular dichroism (CD) spectra analysis
Protein secondary structure was determined using CD spectroscopy (Chirascan, Applied Photophysics, UK) (Zhou et al. 2016). The effect of pH on the secondary structure was performed as follows. The samples of 0.1 mg/mL at pH 7 were measured. The samples were scanned from 190 nm to 260 nm, with a scan rate of 200 nm/min, a bandwidth of 1.0 nm, and a response of 0.25 s at 20 °C. The distilled water was used as a blank. The secondary structure contents were calculated using CDNN software.
Disulphide bond (SS) and sulfhydryl group (SH) contents
SS and SH contents of samples were measured using Ellman’s reagent method with some modification (Beveridge and Nakai 1974).
Free SH content (SHF): Buffer A: Tris-Gly Buffer pH 8, Buffer B (Tris-Gly-8 mol/L urea): added 8 mol/L urea and 5 g/L SDS to buffer A. The sample (15 mg) was incubated with 50 µL Ellman’s reagent in 5 mL buffer B at room temperature for 1 h, then centrifuged at 10,000×g for 10 min. The obtained supernatant was measured at 412 nm.
Total SH content (SHT) and disulfide bonds content (SS): the sample (15 mg/mL) was incubated with 50 µL 2-ME in buffer B at room temperature for 1 h, and then added 10 mL 12% TCA and incubated for another 1 h, then centrifuged at 10,000 ×g for 10 min. The precipitate was washed 2–3 times with 5 mL 12% TCA and then dissolved in 10 mL buffer B. After the precipitate was fully dissolved, 40 µL Ellman’s reagents was added to react for 1 h, the absorbance was measured at 412 nm. The free SH content (SHF), total SH content (SHT) and disulfide bonds content (SS) was calculated as given by the equation.
| 8 |
| 9 |
Where, SH is free SH content (SHF) or total SH content (SHT) (µmol/g), A412 is the absorbance at 412 nm, D is the dilution factor, C is the protein concentration (mg/mL), SS is disulfide bonds content (µmol/g).
Thermal properties of FGSP and SGSP
The thermal properties of protein samples were determined using differential scanning calorimetry (DSC) (Diamond DSC, PerkinElmer Instruments Co., Ltd, Shelton, USA). The protein samples (1.0 mg) were dissolved in 10 µL 0.01 mol/L phosphate buffer (pH 7.4) at the aluminum pan, sealed and heated from 0 °C to 200 °C (5 °C/min). The sample was purged with 70 mL/min of nitrogen and calibrated for a baseline using an empty oven and for temperature and enthalpy using two standards. Specific heat capacity (Cp) was calibrated using a sapphire. The empty sample and reference pans were of equal mass to within ± 0.10 mg. Onset temperature (Tm), peak transition temperature or denaturation temperature (Tp), and enthalpy of denaturation (∆H) were computed from the thermograms using Thermal Analyst.
X-ray diffraction analysis
A D8 X-ray diffractometer (Bruker AXS Co., German) was used to analyze the crystalline structure of protein according to the method of with some modifications. Copper Kα was used at 40 kV and 35 mA. The 2θ range was set from 1° to 60°.
Surface morphology of FGSP and SGSP
Surface morphology of protein powder samples was observed and acquired using a scanning electron microscope (SEM, JSM-5610LV, JEOL, Japan). An accelerating potential of 10 kV was used during the test. Images were observed at magnification levels of 1000 × and 2000 ×. The protein samples (0.01 g) were deposited on aluminum stubs using double-sided adhesive carbon conductive tape and were coated with a thin gold layer with the help of gold sputter.
Statistical analysis
All experiments were performed in triplicate. Significant differences were verified by one-way analysis using SPSS 17.0 statistical software. P < 0.05 was considered statistical significance with Duncan’s test.
Results and discussion
The proximate compositions of SGSP and FGSP are shown in Table 1. It is found that the solubility of SGSP (86.12 ± 0.58%) is higher than that of FGSP (82.73 ± 0.87%), which is consistent with the effects of spray and freeze drying on the peanut protein (Gong et al. 2016). There is no significant differences in lipid between the SGSP and FGSP samples.
Table 1.
The proximate compositions and secondary structure of SGSP and FGSP
| Soluble protein(%) | Lipid (%) | Moisture (%) | Ash (%) | α-helix (%) | Anti-parallel (%) | Parallel (%) | β-turn (%) | Unordered coil (%) | |
|---|---|---|---|---|---|---|---|---|---|
| SGSP | 86.12 ± 0.58a | 2.93 ± 0.31a | 3.48 ± 0.21a | 6.19 ± 0.47a | 15.88 ± 0.07b | 24.20 ± 0.21a | 9.91 ± 0.04a | 16.17 ± 0.02b | 33.85 ± 0.14a |
| FGSP | 82.73 ± 0.87b | 2.84 ± 0.24a | 2.43 ± 0.37b | 5.43 ± 0.29b | 16.47 ± 0.07a | 23.53 ± 0.07b | 9.88 ± 0.03a | 16.28 ± 0.03a | 33.84 ± 0.10a |
Each value in the Table is the mean of three replications ± standard deviation
abc = different letters in columns indicate significant (P < 0.05) difference SGSP and FGSP
Characterization of functional properties of SGSP and FGSP
WHC and OBC
WHC of GSP affects emulsifying capacity, a highly desirable characteristic in products such as meat, bakery and beverage industries. In Fig. 1a, WHC of SGSP (541%) was significantly (P < 0.05) higher than that of FGSP (346%), which were comparable to the commercial soybean proteins (397%) and pea protein isolate (3.9–4.8 g H2O/g) (Lam et al. 2018). The spray-drying methods of GSP may be proper for the application area in the food industry which needs high WHC.
Fig. 1.
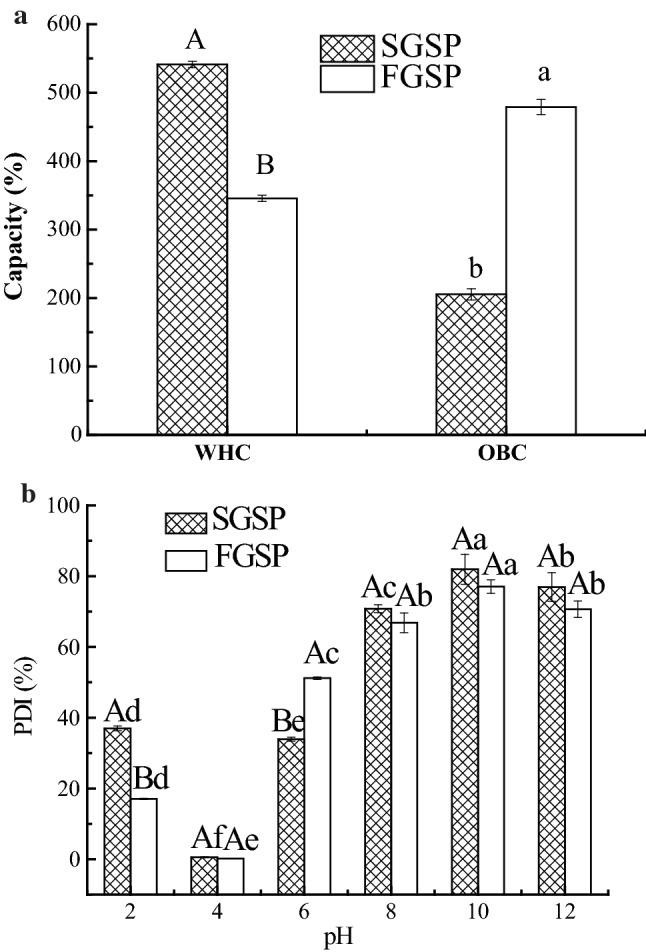
WHC, OBC (a) and PDI (b) of ginkgo seed protein dried by spray-drying and freeze-drying methods. Error bars show mean standard deviation of three determinations
The WHC of protein was affected by the greater interaction energy between protein and water, the larger average molecular weight of the peptide chain and the larger gap between the peptide chains (Hua and Gu 1999). Therefore, we could assume that WHC of SGSP might be improved by adding the hydrophilic group, increasing the chain length through disulfide cross-linking, or denaturing protein molecules into loose random structure induced by heat treatment of spray-drying. Comparing to FGSP, SGSP may be better for the application area in food industry which needs higher WHC, such as baking cake (Huang and Yang 2019).
The oil-binding capacity (OBC) of GSP is of great importance, since it affects the ability of proteins to bind fat, which is very important for applications such as meat replacement or meat extenders, mainly because it enhances flavor retention and improves mouth feel. Figure 1a shows the OBC of FGSP (479%) was found to be significantly higher than that of SGSP (205%). The differences in OBC of GSP dried by spraying and freezing were possibly due to the different conformational characteristics, surface hydrophobicity, lipophilic groups and degeneration of these proteins. The specific influencing mechanism would be discussed later by the following measurement. However, Deng et al. (2011) reported OBC of ginkgo protein isolate freeze-dried was 2.95 mL/g. The lower values reported by the authors compared to the present study might be due to differences in the the extraction conditions employed in their research (pH 9 for 30 min at room temperature) compared to our study (pH 9 for 90 min at 50 °C). The OBC of GSP is not only related to extraction methods but also processing technology (Stone et al. 2015).
In the present study, the OBC of FGSP was comparable to OBC of commercial soy protein isolate (3.29 mL/g) and winged bean protein concentrate (3.93 g/mL) (Makeri et al. 2017). The better OBC of FGSP could provide the superior fat-binding performance and apply in meat processing, such as ham and sausage. It suggested that freezing drying treated GSP could be more suitable for producing of the higher OBC of GSP, which could be used as substitutes and extenders used in cake batters, sausages or mayonnaise (Amagliani et al. 2017; Ouraji et al. 2020).
PDI
Proteins with low solubility have limited functional properties and more limited uses (Kinsella 1979). High protein dispersibility and solubility of soy flour are desirable traits for processing high-quality soy food products such as soy milk and tofu (Kumar et al. 2017). PDI is a thermodynamic representation of the balance between protein–protein and protein–water interactions. Figure 1b shows the change of PDI values over pH of SGSP and FGSP. The PDI profiles for SGSP and FGSP show the similar trends, which decrease the minimum at pH 4.0 (pI) and increase again. It is assumed that the PDI change is a result of the denaturation of GSP caused by pH variation. In addition, it is also notable that PDI of GSP was not significantly affected by the various drying methods, except at pH 2 and 6. SGSP showed lower PDI value at pH 6.0, but higher PDI at pH 2.0 than FGSP (P < 0.05). The PDI values of GSP were ranging from 60 to 90% above pH 8.0, which is similar to that of soybean protein (L’hocine et al. 2006). PDI was related to the extent of denaturation and solubility. The most severe heat treatment led to lower PDI due to more protein denaturation. However, the difference between spray and freezing drying play on the GSP was too inconspicuous to affect the PDI values at most pH values.
FC and FS
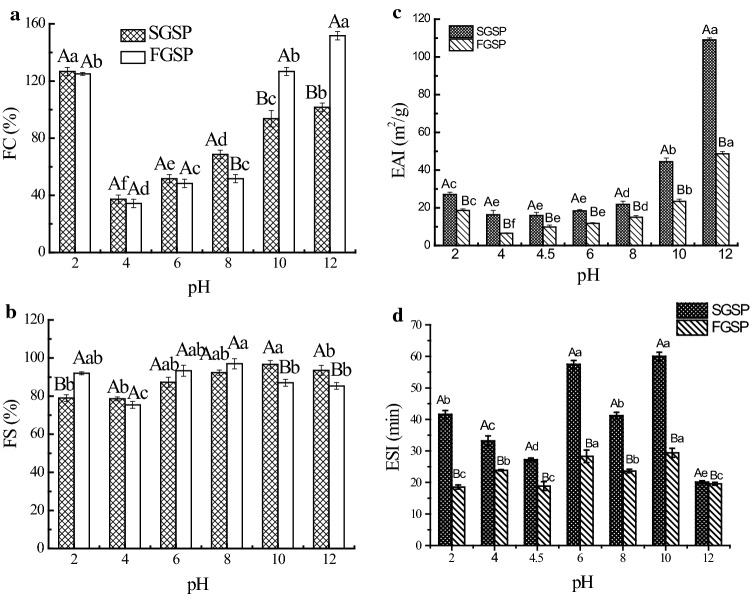
The foam formation and the respective stability generally are dependent upon the interfacial film formed made up of proteins incorporating air bubbles in the suspension and slows down the rate of coalescence (Ma et al. 2011). FC of SGSP and FGSP at various pHs are given in Fig. 2a. The FC values of SGSP and FGSP showed similar curves to that of PDI with the change of pH value. There are no significantly differences between the FC of SGSP and FGSP at pH < 8, however, there are obvious differences as pH were 8, 10 and 12. At pH 10 and 12, FGSP exhibited higher FC than that of SGSP. The similar result was also found on the rice dreg protein (Zhao et al. 2013). Drying technology could change protein conformation, affected the intensity of protein-protein repulsions and influenced FC (Ijarotimi et al. 2018). pH is another factor that could largely influence FC of protein by changing its conformation, solubility, flexibility, charge density, and hydrophobicity. In theory, high PDI of protein had high FC. However, in this study, at pH of 10 and 12, SGSP had higher PDI than that of FGSP, but lower FC. This might be because high alkaline easily make FGSP higher flexibility or hydrophobicity than those of SGSP. Ginkgo seed globulin protein (GGP) and Ginkgo seed albumin protein (GAP) are the two main storage protein in GSP. The SH and SS contents of GGP were three times lower than those of GAP, showing higher thermal sensitivities. Heat treatment will affect the structure of proteins, and make them more flexibility, especially GGP (Deng et al. 2011).
Fig. 2.
Foaming and emulsifying properties of ginkgo seed protein in different drying methods at various pHs. FC (a), FS (b), EAI (c) and ESI (d). Error bars show mean standard deviation of three determinations. Different uppercase letters (A and B) indicate significant (P < 0.05) difference between SGSP and FGSP under the same conditions; different lowercase (a and b) letters indicate significant (P < 0.05) difference between same drying methods
In Fig. 2b, the FS values of SGSP and FGSP at various pHs were more than 70%. At pH of 2, the FS of FGSP is higher than that of SGSP; however, at pH of 10 and 12, the FS of FGSP shows oppositely trends different, which is lower than that of SGSP. Different from FC, FS requires the formation of thick, cohesive and viscoelastic membranes around each bubble (Wang et al. 2016). Thus, the GSP could be used for the food required high FS, such as foaming beverage and some candies.
Emulsifying properties
EAI and ESI indicate the ability of food proteins to form and stabilize emulsions, respectively, which are critical to their role as food ingredients in a wide range of applications (Ghribi et al. 2015). Figure 2c, d show that the EAI and ESI values of SGSP and FGSP were greatly affected by the pH. At the iso-electric point (IP) of FGSP and SGSP (about pH 4.5), samples had lowest EAI values, and then increased at pH values lower or higher than the IP. The lowest EAI value of SGSP (about 16 m2/g) was obtained at pH 4–6, and EAI of FGSP was 6.5 m2/g obtained at pH 4. And at various pHs, the EAI of the SGSP was significantly (P < 0.05) higher than EAI of FGSP, especially at pH 12. The EAI of SGSP (16.32–99.05 m2/g) was higher than that of pea, chickpea and lentil protein (4–7 m2/g) (Boye et al. 2010), and comparable to peanut protein isolates.
Figure 2d shows the ESI of SGSP and FGSP solution as a function of pH. On the contrast of EAI, there was no clear pattern of changes in ESI as a function of pH. The highest ESIs of both SGSP and FGSP were obtained at pH 6 and 8. Comparing the spray and freeze dried GSP, we found that the ESI of SGSP is significantly higher (P < 0.05) than FGSP in all cases..However, these results were not consistent with the report of Gong et al. (2016), who found ESI of spray-dried peanut protein is higher than freeze-dried peanut protein only at pH 6.0. The possible reasons for the difference in effect of different drying methods on EAI and ESI of protein were related to the extraction method and protein source.
It is well established that the emulsifying property of protein is affected by many factors, including the protein structure, particle size, composition, as well as environmental conditions (Chen et al. 2019). We speculated that the high temperature of spray-drying plays effect on the GSP, make structure partial unfolding and then improve the emulsifying property of GSP. In conclusion, spray-drying produced GSP had higher EAI and ESI than those of freeze-drying dried GSP. For further application in food industry, the SGSP would be competent to stabilize oil-in-water type emulsions, such as milk and mayonnaise, and FGSP is much better use in water-in-oil type emulsion, such as butter and margarine (Lam et al. 2018).
Characterization of structural properties of SGSP and FGSP
AA composition analysis
AAs composition of SGSP and FGSP are shown in Table 2. GSP dried freeze-drying had more total content of amino acid (70.70 g/100 g) than SGSP (64.89 g/100 g), due to keep in low temperature during the freeze-drying process, which could better retain the native structure of protein. The amino acids analyzed here are divided into two categories as nutriology: Cys, His, Ile, Leu, Lys, Met, Phe, Thr, Tyr and Val as essential amino acids (EAAs), and Ala, Arg, Asp, Glu, Gly, Pro and Ser as non-essential amino acids (NEAAs). The total content of EAA of SGSP and FGSP were 23.24 g/100 g and 25.54 g/100 g protein, respectively. Both FGSP and SGSP had considerably high concentrations in the following amino acids:Glu, Leu, Val, Ile, Phe, Lys, and Tyr, whereas Thr, Cys and Met were of intermediate level. Of all the individual EAAs, Leu was the most highly available EAA in the GSP. Sulfur amino acids (SAAs), Met and Cys, are a critical group of amino acids, which could be easily lost from the body. SAAs are generally insufficient in plant source foods, and ginkgo nut was no exception in that the participation of Met and Cys in the total amino acids ranged between 1.50 and 1.65% and between 0.95 and 1.05%, respectively, which are consistent with the reports (Zhou et al. 2019).
Table 2.
Amino acid composition of SGSP and FGSP (g/100 g protein)
| SGSP | FGSP | SGSP | FGSP | ||
|---|---|---|---|---|---|
| Thr | 3.43 ± 0.01b | 3.82 ± 0.01a | Asp | 7.21 ± 0.06b | 7.93 ± 0.02a |
| Val | 3.67 ± 0.06b | 4.06 ± 0.08a | Glu | 10.23 ± 0.02b | 11.03 ± 0.02a |
| Met | 1.50 ± 0.01b | 1.65 ± 0.05a | Gly | 3.19 ± 0.09b | 3.50 ± 0.01a |
| Ile | 2.88 ± 0.01b | 3.13 ± 0.02a | Ala | 3.69 ± 0.08b | 4.02 ± 0.06a |
| Leu | 5.30 ± 0.01b | 5.76 ± 0.01a | Cys | 0.95 ± 0.02b | 1.05 ± 0.02a |
| Phe | 2.66 ± 0.01b | 2.94 ± 0.03a | Tyr | 2.55 ± 0.01b | 2.77 ± 0.02a |
| Lys | 2.51 ± 0.06b | 2.78 ± 0.02a | Pro | 2.43 ± 0.02b | 2.64 ± 0.04a |
| Trp | ND | ND | Arg | 7.75 ± 0.02b | 8.25 ± 0.07a |
| His | 1.28 ± 0.002b | 1.40 ± 0.01a | Ser | 3.58 ± 0.02b | 3.96 ± 0.02a |
| Hydrophobic AAs | 22.13 ± 0.20b | 24.2 ± 0.29a | |||
| TEAA | 23.24 ± 0.01b | 25.54 ± 0.02a | TAA | 64.89 ± 0.22b | 70.70 ± 0.23a |
Each value in the Table is the mean of three replications ± standard deviation
abc = different letters in columns indicate significant (P<0.05) difference SGSP and FGSP
It’s worth noting that the content of hydrophobic AAs of FGSP was higher than that of SGSP, which play an important effects on functional properties of protein. But the more important is effects of protein conformation on functionality, including surface hydrophobicity, disulfide bond, secondary and advanced structure, which should be continued to investigate.
CD spectroscopy analysis
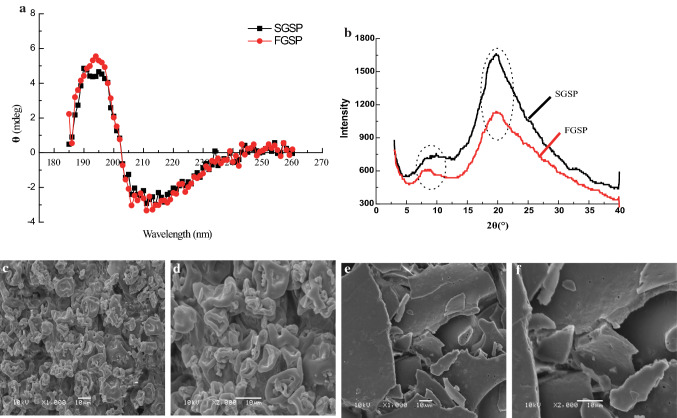
The secondary structures of SGSP and FGSP assessed by CD spectroscopy are shown in Fig. 3a. Both SGSP and FGSP had a positive peak at approximately 196 nm and a negative peak at approximately 208 nm. The proportion of the secondary structural features calculated by curve fit software of SGSP and FGSP are presented in Table 1. It can also be observed that SGSP had lower α-helix (15.88%) than FGSP (16.47%), but higher β-sheet (Anti-parallel and Parallel) (25.11%) than FGSP (24.41%). In addition, there is no significant difference in β-turn and unordered coil between SGSP and FGSP. The β-sheet structure was more stable than the α-helix structure. During spray-drying process, a little α-helix of SGSP transform into β-sheet. This may be one of the reasons that the ESI of SGSP was higher than that of freeze-drying one (Zhou et al. 2016).
Fig. 3.
Far-UVCD spectra (a), XRD (b) and ESEM micrograph of ginkgo seed protein dried by spray-drying (c, d) and freeze-drying (e, f) (1000×, 2000×, 10 kV)
SS and SH contents
Sulfhydryl-disulfide interchange (SH/SS) are important functional groups and play important roles in protein aggregation and functional properties of proteins. Table 3 shows total SH groups is higher in SGSP (60.01 μmol/g) than in FGSP (55.21 μmol/g), and there was no significant (P > 0.05) difference in the free SH content (SHF). Furthermore, it is noted that the SS content of SGSP (18.09 μmol/g) are significantly (P<0.05) higher than that of FGSP (15.22 μmol/g). SS bonds of GSP play an important role in defining its secondary and tertiary structure. Compared to freeze-drying, the spray drying could promote the formation of disulfide bonds of GSP. We speculated that the possible reason that the high temperature of spray-drying process (the air inlet temperature reached 180 °C) promote the formation of disulfide bonds. Disulfide bonds played an important role in the formation and development of three-dimensional network structures of proteins (Oak et al. 2006). Otherwise, the denatured proteins will expose hydrophobic groups, which usually leading to protein aggregation. Therefore, there is a balance between the aggregation and exposure of hydrophobic groups. Total SH and SS bonds in a protein was also positively related to EAI (Deng et al. 2011). Thus, the better emulsion stability of the SGSP compared to that of FGSP may be due to its increased ability to form covalent linkages (SS). These results were conflict with Gong et al. (2016), which showed the SS content of freeze-dried peanut protein was higher than that of spray dried one. The conflict might be accounted for the different types of protein.
Table 3.
The contents of sulfhydryl group, disulfide bonds and thermal properties of ginkgo seed protein dried by spray and freeze
| SHT (µmol/g) | SHF (µmol/g) | SS (µmol/g) | Tm (oC) | Tp (oC) | ∆H (J/g) | |
|---|---|---|---|---|---|---|
| SGSP | 60.01 ± 0.89a | 23.84 ± 1.10a | 18.09 ± 0.79a | 36.74 ± 0.06b | 68.78 ± 0.02a | 122.9141 ± 0.02b |
| FGSP | 55.21 ± 1.72b | 24.78 ± 1.22a | 15.22 ± 1.24b | 38.39 ± 0.04a | 62.70 ± 0.03b | 144.7820 ± 0.03a |
Each value in the Table is the mean of three replications ± standard deviation
abc = different letters in columns indicate significant (P<0.05) difference SGSP and FGSP
Thermal properties of SGSP and FGSP
The magnitude of changes in the thermal properties caused by the disturbance of conformational arrangements of protein was studied by DSC. The peak/denaturation temperature (Tp) and heat of transition or enthalpy (ΔH) of SGSP and FGSP are shown in Table 3. The Tp (68.78 °C) of SGSP was significantly (P<0.05) higher than that of FGSP (62.70 °C). Tp suggested that GSP can be recommended for use in the products subjected to thermal processes of lower than 70 °C.
The actual heat flow into the macromolecules during the thermal denaturation process is described by the ΔH value. Table 3 also illustrates that the ∆H of SGSP (122.91 J/g) was significantly (P<0.05) lower than that of FGSP (144.78 J/g). A lower heat flow is an indication that the protein was less native (more denatured) or lower ordered protein structure. Thus, SGSP is attributed to the lower ordered structure of the protein, which may be one of the reasons for the high WHC, EAI and ESI of SGSP. The denaturation extent of GSP dried by spray is severer than that dried by freeze, probably account for the high temperature. Although protein denaturation is beneficial to the water retention of protein products, if the protein degeneration completely, the WHC of protein will fall down. Only moderate denaturation of protein products the highest WHC.
Crystal and surface morphology of SGSP and FGSP
Food materials in solid states may be crystalline, semi-crystalline or amorphous. XRD was performed to confirm the amorphous structure of GSPs. Figure 3b shows that XRD patterns of all GSP samples exhibit a dominant amorphous haloes, a broad band with a maximum at 2θ = 20°. A dominant amorphous halo at 2θ = 20° was also reported for soy protein isolate, peanut protein, chickpea protein concentrate, fish gelatin and so on (Huang et al. 2017). It is also exhibited low intensity of typical diffraction peaks at 8.5°, which is more pronounced for FGSP. The diffraction patterns of GSP contain a combination of both amorphous and crystalline region but they presented a major amorphous structure with very low crystallinity. Since amorphous powder components are more hygroscopic than crystalline components, that is to say SGSP is much easier to absorb moisture. Thus it is important to store them in dry place.
As shown in Fig. 3c, e, SEM micrographs of SGSP and FGSP powders revealed that the surface morphology of GSP dried by spraying and freeze drying was quite different. Compared to the FGSP, SGSP appears to be smaller and more uniform in particle size distribution, and has collapsed particles with folded or wrinkled, and some of the particles were hollow surface morphology (Fig. 3b, c). Higher magnification (2000-fold) shows that the particles have a smooth and indented surface in Fig. 3d, which is similar to that of peanut protein by spray-drying (Gong et al. 2016). During the spray-drying process, the sample encountered with hot air, at the gas-liquid interface the interaction between rapidly decreasing diffusivity and increasing surface tension leads to the formation of collapsed particles of SGSP with uneven and folded surface morphology. Whereas the FGSP shows a plate-like morphology, a smooth and a loose porous surface morphology (Fig. 3e, f), which was caused by the increased chance that solutes in close contact with each other and solutes rapidly form ice crystals at low temperatures, thus creating opportunities for high purity ice crystals. Small and uniform particle size, hollow surface morphology and high SS content of SGSP contributed a good interpretation for the differences in high WHC and emulsifying properties of GSP dried by spray-drying.
Conclusion
Different drying methods play very important effects on the functional properties of GSP. Compared with FGSP, the GSP dried by spray-drying has higher WHC, EAI, ESI and FS, which is more suitable for application in oil-in-water type food system, such as milk and mayonnaise. FGSP possesses higher OBC, FC and more native and order. Spray drying is not only low-cost, but also can make better functional properties of GSP, which surprised us. Spray drying is industrially preferred method to produce GSP powders in most cases.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant number 31701608 and 31571783].
Abbreviations
- GSP
Ginkgo seed protein
- FGSP
Freeze-dried ginkgo seed protein
- SGSP
Spray-dried ginkgo seed protein
- AA
Amino acid
- SEM
Scanning electron microscope
- DSC
Differential scanning calorimetry
- WHC
Water-holding capacity
- OBC
Oil-binding capacity
- PDI
Protein dispersibility index
- FC
Foaming capacity
- FS
Foam stability
- EAI
Emulsion activity index
- ESI
Emulsion stability index
- CD
Circular dichroism
- XRD
X-Ray diffraction
- ESEM
Environmental field scanning electron microscopy
Compliance with ethical standards
Conflict of interest
There is no conflict of interest of all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tiesong Zheng, Email: tieszheng@sina.com.
Qiuting Zhang, Email: qiuting.zhang@njnu.edu.com.
References
- Ahmed J, Rahman MS (2012) Handbook of food process design, 2 Volume Set. Wiley, Hoboken
- Amagliani L, O’Regan J, Kelly AL, O’Mahony JA. The composition, extraction, functionality and applications of rice proteins: a review. Trends Food Sci Technol. 2017;64:1–12. doi: 10.1016/j.tifs.2017.01.008. [DOI] [Google Scholar]
- Beveridge TTSJ, Nakai S. Determination of SH-and SS-groups in some food proteins using Ellman’s reagent. J Food Sci. 1974;39:49–51. doi: 10.1111/j.1365-2621.1974.tb00984.x. [DOI] [Google Scholar]
- Boye JI, Aksay S, Roufik S, Ribéreau S, Mondor M, Farnworth E, Rajamohamed SH. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int. 2010;43:537–546. doi: 10.1016/j.foodres.2009.07.021. [DOI] [Google Scholar]
- Chen M, Lu J, Liu F, Nsor-Atindana J, Xu F, Goff HD, Ma J, Zhong F. Study on the emulsifying stability and interfacial adsorption of pea proteins. Food Hydrocolloid. 2019;88:247–255. doi: 10.1016/j.foodhyd.2018.09.003. [DOI] [Google Scholar]
- Deng Q, Wang L, Wei F, Xie B, Huang F, Huang W, Shi J, Huang Q, Tian B, Xue S. Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem. 2011;124:1458–1465. doi: 10.1016/j.foodchem.2010.07.108. [DOI] [Google Scholar]
- Fan L, Ding S, Liu Y, Ai L. Dehydration of crude protein from Ginkgo biloba L. by microwave freeze drying. Int J Biol Macromol. 2012;50:1008–1010. doi: 10.1016/j.ijbiomac.2012.02.027. [DOI] [PubMed] [Google Scholar]
- Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R. Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int. 2007;40:1107–1121. doi: 10.1016/j.foodres.2007.07.004. [DOI] [Google Scholar]
- Ghribi AM, Gafsi IM, Blecker C, Danthine S, Attia H, Besbes S. Effect of drying methods on physico-chemical and functional properties of chickpea protein concentrates. J Food Eng. 2015;165:179–188. doi: 10.1016/j.jfoodeng.2015.06.021. [DOI] [Google Scholar]
- Gong KJ, Shi AM, Liu HZ, Liu L, Hu H, Adhikari B, Wang Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J Food Eng. 2016;170:33–40. doi: 10.1016/j.jfoodeng.2015.09.011. [DOI] [Google Scholar]
- Grela ER, Kiczorowska B, Samolińska W, Matras J, Kiczorowski P, Rybiński W, Hanczakowska E. Chemical composition of leguminous seeds: part I—content of basic nutrients, amino acids, phytochemical compounds, and antioxidant activity. Eur Food Res Technol. 2017;243:1385–1395. doi: 10.1007/s00217-017-2849-7. [DOI] [Google Scholar]
- Hua Y, Gu Y. Water absorption and holding of soy protein. China Oils Fats. 1999;24:63–67. [Google Scholar]
- Huang M, Yang H. Eucheuma powder as a partial flour replacement and its effect on the properties of sponge cake. LWT Food Sci Technol. 2019;110:262–268. doi: 10.1016/j.lwt.2019.04.087. [DOI] [Google Scholar]
- Huang W, Deng Q, Xie B, Shi J, Huang FH, Tian B, Huang Q, Xue S. Purification and characterization of an antioxidant protein from Ginkgo biloba seeds. Food Res Int. 2010;43:86–94. doi: 10.1016/j.foodres.2009.08.015. [DOI] [Google Scholar]
- Huang T, Tu Z-C, Wang H, Liu W, Zhang L, Zhang Y, ShangGuan X-C. Comparison of rheological behaviors and nanostructure of bighead carp scales gelatin modified by different modification methods. J Food Sci Tech Mys. 2017;54:1256–1265. doi: 10.1007/s13197-017-2511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijarotimi OS, Malomo SA, Fagbemi TN, Osundahunsi OF, Aluko RE. Structural and functional properties of Buchholzia coriacea seed flour and protein concentrate at different pH and protein concentrations. Food Hydrocolloid. 2018;74:275–288. doi: 10.1016/j.foodhyd.2017.08.018. [DOI] [Google Scholar]
- Jensen U, Berthold H. Legumin-like proteins in gymnosperms. Phytochemistry. 1989;28:1389–1394. doi: 10.1016/S0031-9422(00)97753-7. [DOI] [Google Scholar]
- Kinsella JE. Functional properties of soy proteins. J Am Oil Chem Soc. 1979;56:242–258. doi: 10.1007/BF02671468. [DOI] [Google Scholar]
- Kumar V, Rani A, Hussain L, Yadav M, Jha P, Petwal V, Dwivedi J. Changes in physico-chemical properties of native and toasted defatted soy flour on submission to electron beam radiation. Food Bioprod Process. 2017;105:141–146. doi: 10.1016/j.fbp.2017.07.001. [DOI] [Google Scholar]
- Lam ACY, Can Karaca A, Tyler RT, Nickerson MT. Pea protein isolates: structure, extraction, and functionality. Food Rev Int. 2018;34:126–147. doi: 10.1080/87559129.2016.1242135. [DOI] [Google Scholar]
- L’hocine L, Boye JI, Arcand Y. Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. J Food Sci. 2006;71:C137–C145. doi: 10.1111/j.1365-2621.2006.tb15609.x. [DOI] [Google Scholar]
- Lin M, Tay SH, Yang H, Yang B, Li H. Development of eggless cakes suitable for lacto-vegetarians using isolated pea proteins. Food Hydrocolloid. 2017;69:440–449. doi: 10.1016/j.foodhyd.2017.03.014. [DOI] [Google Scholar]
- Ma Z, Boye JI, Simpson BK, Prasher SO, Monpetit D, Malcolmson L. Thermal processing effects on the functional properties and microstructure of lentil, chickpea, and pea flours. Food Res Int. 2011;44:2534–2544. doi: 10.1016/j.foodres.2010.12.017. [DOI] [Google Scholar]
- Makeri MU, Abdulmannan F, Ilowefah MA, Chiemela C, Shu’aibu MB, Muhammad K. Comparative physico-chemical, functional and structural characteristics of winged bean [Psophocarpus tetragonolobus DC] and Soybean [Glycine max.] Protein isolates. J Food Meas Charact. 2017;11:835–846. doi: 10.1007/s11694-016-9455-4. [DOI] [Google Scholar]
- Miao M, Jiang H, Jiang B, Cui SW, Jin Z, Zhang T. Structure and functional properties of starches from Chinese ginkgo (Ginkgo biloba L.) nuts. Food Res Int. 2012;49:303–310. doi: 10.1016/j.foodres.2012.07.038. [DOI] [Google Scholar]
- Oak MD, Sissons M, Egan N, Tamhankar SA, Rao VS, Bhosale SB. Relationship between gluten strength and pasta firmness in Indian durum wheats. Int J Food Sci Tech. 2006;41:538–544. doi: 10.1111/j.1365-2621.2005.01103.x. [DOI] [Google Scholar]
- Ouraji M, Alimi M, Motamedzadegan A, Shokoohi S. Faba bean protein in reduced fat/cholesterol mayonnaise: extraction and physico-chemical modification process. J Food Sci Tech Mys. 2020 doi: 10.1007/s13197-019-04211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AK, Karalash A, Tyler RT, Warkentin TD, Nickerson MT. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res Int. 2015;76:31–38. doi: 10.1016/j.foodres.2014.11.017. [DOI] [Google Scholar]
- Tan ES, Ying-Yuan N, Gan CY. A comparative study of physicochemical characteristics and functionalities of pinto bean protein isolate (PBPI) against the soybean protein isolate (SPI) after the extraction optimisation. Food Chem. 2014;152:447–455. doi: 10.1016/j.foodchem.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Tirgar M, Silcock P, Carne A, Birch EJ. Effect of extraction method on functional properties of flaxseed protein concentrates. Food Chem. 2017;215:417–424. doi: 10.1016/j.foodchem.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang L, Wang R, Chen Z. Effects of freeze-milling on the physicochemical properties of rice protein isolates. LWT Food Sci Technol. 2016;65:832–839. doi: 10.1016/j.lwt.2015.09.016. [DOI] [Google Scholar]
- Wray D, Ramaswamy HS. Novel concepts in microwave drying of foods. Dry Technol. 2015;33:769–783. doi: 10.1080/07373937.2014.985793. [DOI] [Google Scholar]
- Zhao Q, Xiong H, Selomulya C, Chen XD, Huang S, Xia R, Zhou Q, Sun W. Effects of spray drying and freeze drying on the properties of protein isolate from rice dreg protein. Food Bioprod Process. 2013;6:1759–1769. [Google Scholar]
- Zhou H, Chen X, Wang C, Ye J, Chen H. Purification and characterization of a novel ~18 kDa antioxidant protein from Ginkgo biloba seeds. Molecules. 2012;17:14778–14794. doi: 10.3390/molecules171214778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wang C, Ye J, Chen H, Tao R, Cao F. Effects of high hydrostatic pressure treatment on structural, allergenicity, and functional properties of proteins from ginkgo seeds. Innov Food Sci Emerg. 2016;34:187–195. doi: 10.1016/j.ifset.2016.02.001. [DOI] [Google Scholar]
- Zhou M, Hua T, Ma X, Sun H, Xu L (2019) Protein content and amino acids profile in 10 cultivars of ginkgo (Ginkgo biloba L.) nut from China. Roy Soc Open Sci 6:181571 [DOI] [PMC free article] [PubMed]