Abstract
The use of blends to produce hydrogels allows modulating their characteristics as mechanical properties and microstructure. This work aimed to study the properties of pectin and starch hydrogel blends. Pectin gel was homogeneous and porous, while pectin/starch blends containing 50% or more pectin exhibited denser and closer network, indicating that starch reduced the porosity of pectin network. Such characteristic was associated with higher gel hardness, cohesiveness, firmness, and water holding capacity. The influence of total biopolymer concentration and type of process (extrusion and atomization) on particle formation were also evaluated indicating that among the tested formulations, pectin 1% and starch 1% blend was the only sample able to form particles under extrusion and atomization. The addition of 5% (w/v) microparticles to the grape nectar presented no influence on rheological parameters, maintaining the pseudoplastic behavior. Both the starch addition and the amount of polymers used impacted the micro and macrostructure of pectin gels.
Keywords: Hydrogel, Mechanical properties, Microparticle, Gelification, Biopolymer
Introduction
To protect sensible compounds, ensure stability and promote controlled delivery, encapsulation has been a successful technique. A number of methods such as ionotropic gelation, self-assembly, complex coacervation, among others may be employed to encapsulate compounds (Burey et al. 2008; Farjami and Madadlou 2017; Okuro et al. 2015); however, the most appropriate should be chosen mainly based on the application, bioactive properties, available process scale and associated costs (Fujiwara et al. 2013; Okuro et al. 2015). In addition, the modulation of microstructure using biopolymer blends is an alternative to achieve different gel properties for application in encapsulation systems.
Ionotropic gelation is a technique used to produce soft hydrogel particles that are desirable for addition on products with high moisture content, generally due to their ability to protect bioactive compounds and to act as texture modifiers (Stokes 2011). This technique is based on the crosslinking of charged hydrocolloids with counter ions (Burey et al. 2008; Shewan and Stokes 2013). Biopolymeric solutions are generally dripped into counter ions bath, in which salts diffuse inside the polymeric chain, promoting the crosslinking of the structure that creates a three-dimensional network (Patil et al. 2012).
Low metoxilation pectin can be used to produce such structures (De Moura et al. 2018; Ferreira et al. 2009; Oidtmann et al. 2012); however, it presents high solubility, which is a disadvantage considering the early release of bioactive compounds from delivery structures (Cury et al. 2014; Liu et al. 2003). Therefore, the use of blends can be interesting to modulate its properties, achieving suitable features such as reduction of pore size and solubility, besides the strengthening or weakening of the gel network according to the application. In this context, the combination of pectin with corn starch to produce hydrogels can result in self-sustaining structures with better retention and protection of bioactive compounds. The association of hydrocolloids with starch may promote the formation of macromolecular interactions, which has shown improved functional characteristics. This association presents advantages of being effective and safe by employing native starch, therefore, free from the application of questionable chemicals (Zhang et al. 2018). Some papers have investigated the incorporation of starch into the pectin network and observed higher encapsulation efficiency of Lactobacillus plantarum and greater resistance to digestive fluids when compared to pure pectin gel (Dafe et al. 2017). Also, high amylose starch and pectin hydrogels have been developed for controlled delivery systems, with higher amounts of pectin related to the formation of stronger networks (Soares et al. 2013). These results indicate the potential to investigate further properties of these systems for their application in encapsulation systems to achieve bioactive protection and controlled delivery.
This work aimed to produce pectin-starch gels through different process preparation (dialysis, dripping and atomization) as encapsulating structures of bioactive compounds and investigate how starch concentration and polymer content impact hydrogels macro and microstructure. Anthocyanin was added to the particles as a model compound and microparticles was added to grape nectar in order to study the effects of microparticle addition on rheological properties.
Materials and methods
Materials
Low methoxyl pectin (DE 35%) was gently supplied by CPKelco (São Paulo, Brazil); Corn starch (21.33 ± 0.05% amylose) was kindly donated by Ingredion (Mogi Guaçu, Brazil). Calcium chloride was purchased from Synth (Diadema, Brazil); Blackcurrant anthocyanin extract (purity of 34.32%) was donated by Chr-Hansen (Valinhos, Brazil) and grape nectar (Dia, Brazil) was purchased from a local market. The other chemicals were of analytical grade and used without further purification.
Preparation of biopolymeric solutions and hydrogels
Pectin/starch gels were prepared in four different formulations containing pectin (P) and starch (S) [F1: 1% P and 1% S; F2: 0.5% P and 0.5% S; F3: 0.25% P and 0.75% S; and F4: 0.75% P (w/w) and 0.25% S (w/w)]. To understand the effect of starch addition into the pectin network, a control gel containing only 1.0% (w/w) pectin (F0) was produced. The solutions were prepared by heating water up to 90 ºC, followed by the addition of pectin and starch. The mixtures were kept in this temperature for 30 min, at natural pH (~ 7), for corn starch gelatinization. Solutions were submitted to external gelation in 150 mM CaCl2 solution, in order to produce macrogels, macrobeads, and microparticles.
For macrogels production, the biopolymeric solutions were put into dialyses membranes (SnakeSkin Dialysis Tubing, 3500 molecular weight cut-off, Pierce, Rockford, IL, USA) in contact with a calcium chloride solution (150 mM) for 7 days for posterior mechanical properties, microstructure, and water holding capacity (WHC) evaluation.
For the macrobeads, anthocyanin 0.05% (w/w) was added in the biopolymeric solutions as a model bioactive compound. In this case, solutions were dropped (~ 1 cm height), using a 2-mm inner diameter tube coupled with a peristaltic pump (Masterflex, model 7518-00, USA), into calcium chloride (CaCl2) solution (150 mM) and maintained in contact with CaCl2 for 5 min (curing time). The amount of gelling agent used was ten times the quantity of dripped solution, and after curing, the particles were washed with deionized water to remove salt excess. Such macrobeads were tested regarding their encapsulation efficiency and process yield.
Finally, to produce microparticles all formulations also containing anthocyanin 0.05% (w/w) were passed through an atomizer nozzle with 0.7 mm diameter (Labmaq, Brazil) under compressed air (p = 1.0 bar) using a peristaltic pump (Masterflex, model 7518-00, USA). The distance between the atomizer and the gelling bath (CaCl2) was set at 30 cm. The microparticles were maintained during 5 min in CaCl2 solution for gelling. A sieve of 0.053 mm mesh diameter was used to collect the microparticles, which were submitted to particle size distribution and optical microscopy analysis. Rheological measurements of microparticles suspensions were also carried out to verify possible modifications in the grape nectar with microparticles addition. All systems were produced at least twice.
Characterization of macrogels
Mechanical properties
The macrogel samples were evaluated according to the uniaxial compression test using a TA-XT Plus Texture Analyzer (Stable Micro Systems, UK). Macrogel cylinders with 2 cm diameter and height were subjected to uniaxial compression measurements, up to 80% of their original height using a cylindrical acrylic plate (80 mm of diameter). A test velocity of 1 mm s−1 was set. The Hencky stress (Eq. 1) and strain (Eq. 2) at the fracture were obtained from the first peak of the stress–strain curve. The Young’s modulus was obtained from the slope in the linear range (up to 5% strain) (Eq. 3). At least five pieces of each gel was measured.
| 1 |
| 2 |
| 3 |
F(t) (N) and H(t) (m) correspond to the force and height at respectively time t (s); while A0 (m2) and H0 (m) are the initial area and height of the sample, respectively.
Water holding capacity (WHC)
Water holding capacity was measured according to the method described by Braga and co-authors (Braga et al. 2006) with some modifications. A cylindrical macrogel (~ 3 g) was put in Whatman # 1 filter paper (Whatman, UK) and centrifuged at 4000 g for 10 min at 22 ºC. The samples were weighed before and after centrifugation to determine WHC. Three replicates were performed for each macrogel sample.
Scanning electron microscopy
To evaluate the internal microstructure of the hydrogels, scanning electron microscopy was performed. Macrogels samples were fixed in glutaraldehyde (2.5%) prepared in cacodylate buffer 0.1 M (pH 7.2), for 24 h. Subsequently, the samples were washed in cacodylate buffer 0.1 M and crio-fractured under liquid nitrogen. Dehydration of samples in ethanol series (30%, 50%, 70%, 90%) was conducted, with the final step performed three times with 100% ethanol. A critical point drying (Balzers Critical Point Dryer CPD03) was carried out and the samples were adhered to aluminum stubs and coated with gold in a Sputter Coater (Sputter Coater POLARON, SC7620, VG Microtech, Uckfield, England). Images were captured by a scanning electron microscope (LEO440i EDS6070 Cambridge, England), using acceleration of 10 kV.
Characterization of macrobeads
Encapsulation efficiency
The encapsulation efficiency (EE) was determined by the quantification of anthocyanin losalong the whole process of the particle formation. Thus, the amount of anthocyanin present in calcium chloride solution and washing water was quantified by the pH differential method with some modifications (Lee et al. 2005). This method consists in measuring the absorbance of samples (Spectrophotometer UV–Vis; Bioespectro; Model SP 220; BRAZIL) in two pH’s (1.0 and 4.5) and two wavelengths (515 and 700 nm). The anthocyanin amount was determined based on cyanidin-3-rutinoside, the component present in higher amount in anthocyanin extract (Eq. 4).
| 4 |
where is the difference on absorbance (A) at different wavelengths and pH:
where MM = molar mass of Cyanidin-3-Rutinoside (595,53 g mol−1) (National Center for Biotechnology Information, 2017), DF = dilution factor, 1000 = Conversion factor for from g to mg, l = Pathlengh (1 cm), ε = Molar Extinction Coefficient (28,840 L x mol−1 × cm−1) (Gouvêa et al. 2012).
The encapsulation efficiency was calculated as follow (Eq. 5):
| 5 |
= Initial mass of anthocyanin added in the paste (g); = Anthocyanin mass lost in calcium chloride (g); = Anthocyanin mass lost in washing water (g).
Process yield
The yield of the dripping process was obtained by the correlation between the mass of dripped biopolymeric solution and the mass of the produced macrobeads.
Characterization of microparticles
Optical microscopy
To analyze the morphology of the produced microparticles a Scope A1 optical microscope (Carl Zeiss, Germany), with a 1000 × magnification was used. Samples were dyed with iodine to visualize the corn starch granules in the gel network.
Particle size distribution
Particle size distribution analysis was carried out for microparticles using a Mastersizer S (Malvern Instruments Ltda, UK). For the analyses, particles were dispersed in distilled water for the determination of the mean particle diameter D [3,2] and the polydispersity index (SPAN).
Rheological measurements
In order to study how the microparticles addition modify characteristics of food products, rheological properties of the grape nectar was measured with addition of different amount of microparticles (5–20% w/v). A stress-controlled rheometer (TA Instruments, AR1500ex, New Castle, DE, USA) equipped with a plate-plate geometry (d = 40 mm) was used. Gap varied depending on the sample and it was always larger than the suspended particles. Flow curves of pure nectar and nectar added of microparticles were evaluated, at 25 ºC, in a shear rate of 0 and 300 s−1. All measurements were conducted in triplicate.
Statistical analysis
The statistical treatment of the data was conducted using Sisvar software (Ferreira 2011). The significant differences between the mean values were determined by the Tukey test, with 95% confidence level.
Results and discussion
Evaluation of the macrogels
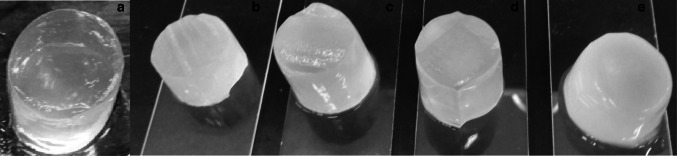
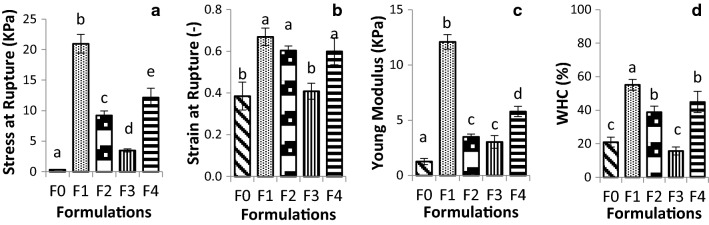
To evaluate pectin/starch hydrogels characteristics, macrogels were prepared by membrane dialysis. The physical structure of these hydrogels can be observed in Fig. 1. These hydrogels were subjected to evaluation of their mechanical properties (Fig. 2a–c), WHC (Fig. 2d), and microstructure (Fig. 3). All formulations produced self-sustained gels (Fig. 1); however, formulations F0 and F3 demonstrated strong syneresis (%WHC: 20.94 and 15.55, respectively), while the other samples exhibited lower release of water (Fig. 2d). The absence of syneresis after gel formation is related to a good stability of the gel structure (Yamamoto and Cunha 2007). For pectin-starch blends at the same polymer content (F2; F3 and F4), the lower amount of pectin caused a decrease on water uptake (F3) (Fig. 2d). Similar result was reported for pectin/starch hydrogels, in which the reduced water entrapment was associated with a reduction in the ratio of pectin (anionic biopolymer), the responsible for trapping water in the junction zones through the gelation process (Dafe et al. 2017; Da Róz et al. 2016), condition in which the hydroxyl groups of hydrocolloid structure, alow more water interaction through hydrogen bonds (Nawab et al. 2014). Zhang et al. (2018), attributed the effect of pectin on syneresis reduction to a viscosity increase.
Fig. 1.
Macrogels of pectin (P)/starch (S) produced in membranes by saline diffusion. a F0: 1% P, b F1: 1% P and 1% S, c F2: 0.5% P and 0.5% S, d F3: 0.25% P and 0.75% S and e F4: 0.75% P (w/w) and 0.25% S (w/w)
Fig. 2.
a Stress at fracture, b strain at fracture, c Young’s modulus and d water holding capacity (WHC) of pectin (P)/starch (S) macrogels (F0: 1% P; F1: 1% P and 1% S; F2: 0.5% P and 0.5% S; F3: 0.25% P and 0.75% S; F4: 0.75% P (w/w) and 0.25% S (w/w)). Lowercase letters show differences (p < 0.05) between the formulations
Fig. 3.
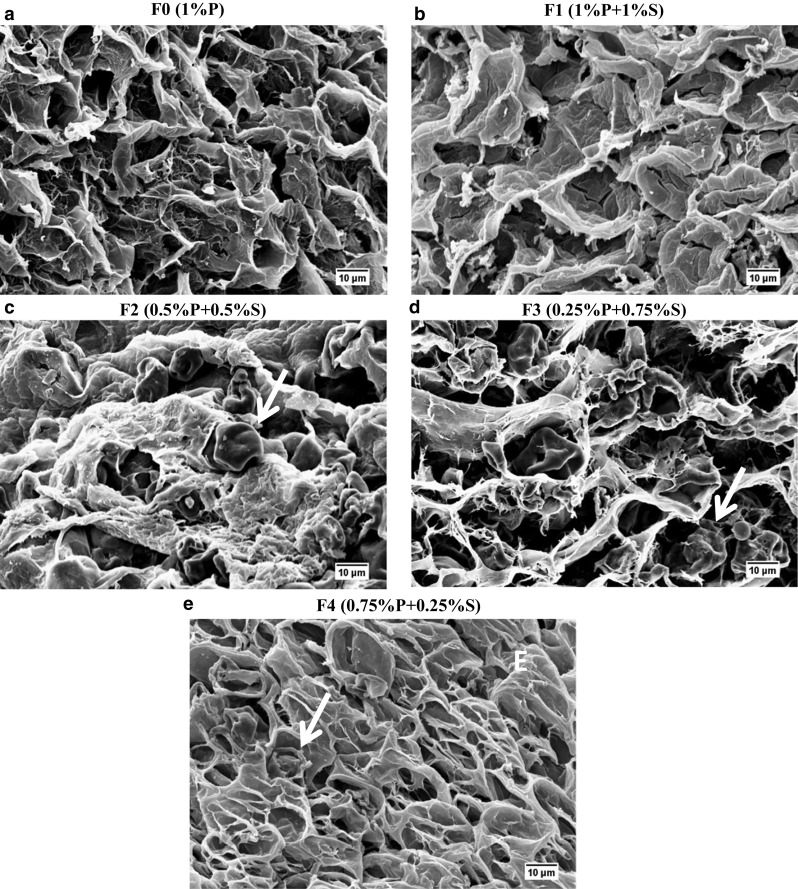
Scanning electron microscopies for macrogels formulations. a F0: 1% P; b F1: 1% P and 1% S; c F2: 0.5% P and 0.5% S; d F3: 0.25% P and 0.75% S and e F4: 0.75% P and 0.25% S, at 2500 × of magnification. The red arrows in the pictures indicate remaining starch granules
The lower water retention could be associated with a structure containing larger pores, represented by pure pectin gel (F0) and F3 (0.25% P and 0.75% S) (Fig. 3a, d). Such larger pores may also have affected the strain values (Fig. 2b), considering a less cohesive structure formed brittle gels (Fig. 3a, d), that fractured at lower strain values (0.39 and 0.41 for F0 and F3 respectively). In this samples, the maximum deformability of the network was easily achieved resulting in the rupture of the material (Zhang et al. 2005). The findings for pectin gels were the opposite of observed for gellan gels, in which the more porous structure presented greater deformability (Yamamoto and Cunha 2007). Such differences could be explained not only by the porosity and pore size but also by the different gelling mechanisms used in the present work (ionotropic gelation) and in the literature (acid gelation).
Moreover, the F0 and F3 exhibited lower stress at fracture (0.5 and 3.4 kPa respectively) (Fig. 2a) and Young Modulus (1.26 and 3.03 kPa respectively) (Fig. 2c), which was associated with the formation of a non-dense and non-resistant gel network (Pires Vilela et al. 2011; Yamamoto and Cunha 2007).
In general, results indicated that starch addition improved the pectin gel network decreasing the size of pores (Fig. 3), and increasing WHC (Fig. 2d). Amylose and amylopectin are the major starch components and they are both composed by glucose residues (Alcázar-Alay et al. 2015; Miles et al. 1985; Takeo et al. 1973), resulting on a net charge close to neutrality, which minimizes the possibility of electrostatic interactions. In addition, it was possible to observe starch remaining granules filling the pores of pectin structure (red arrows) (Fig. 3). The increase in the WHC values (Fig. 2d) could be explained not only by the larger pores but also by the structuring of pectin network. When pectin is in higher amounts (> 0.25% P w/w), it may uptake the water previously released by the starch during retrogradation to structure its own network, resulting in a denser gel (Fig. 3b, c and e). In addition, it has been reported that the presence of pectin associated with starch involves the starch granules and cause interference in gelatinization, reducing swelling and syneresis (Zhang et al. 2018). Higher water absorption capacity of pectin-starch blends compared to starch formulations were also observed for film production (Da Róz et al. 2016). The authors attributed this behavior to the presence of pectin’s galacturonic acid hydroxyl, amide and carboxyl groups in the pectin structure that interact with water.
Concentration, pore size, and polymers structure can also influence the water holding capacity of gels (Mohammadian and Madadlou 2016). Results indicated that different WHC was found for different total biopolymer content (F1 presented 55.2 and F2 38.8% WHC) (Fig. 2d). Lower biopolymer concentration (F2) is directly related to smaller amount of molecules available to bind water, which results in lower water retention in the gel structure (lower WHC). Moreover, comparing the mechanical properties of F1 and F2 (Fig. 2a–c), the results showed that when the pectin/starch ratio is maintained, and the total biopolymer concentration is increased (F1), an increase in the Stress at Rupture (F1: 20.95 and F2: 9.22 kPa) (Fig. 2a) and Young Modulus (F1: 12.08 and F2: 3,49 kPa) (Fig. 2c) is observed, without affecting the Strain at Fracture (Fig. 2b). According SEM images, a more packed and cohesive structure could be observed in this case (Fig. 3b). Stress at Fracture is generally related to the gel hardness which is influenced by the strength of the chains and the size of the pores (Braga et al. 2006; Zhang et al. 2005). The higher amount of biopolymer in the network results in more molecules available to interact mainly by ionic bonds between pectin and calcium ions, which resulted in a more packed network and more structured gel. The Young Modulus, on the other hand, is related to the firmness and elastic properties of the materials at low deformations, and it can be associated with the linkage of biopolymers (Braga et al. 2006). Thus, the more polymer is added, the more junction zones are formed and the higher is the firmness of the gels (F1). Among pectin-starch blend formulations, higher pectin concentrations (F1 and F4) increased the elasticity modulus (12.08 and 5.78 kPa respectively) (Fig. 2c) and stress at rupture (20.95 and 12.15 kPa respectively) (Fig. 2a), regardless polymer content. The increase of pectin content pointed to the strengthening of the structures as previously observed (Soares et al. 2013). The strain at fracture was not affected by different biopolymers concentration (F1 and F2) (Fig. 2b) indicating it was mainly influenced by how linkages were established and the organization of the network instead of the number of linkages, as observed for the Young Modulus.
Characterization of the macrobeads
Encapsulation efficiency (EE) determines the incorporated bioactive amount inside particles in comparison with the initial content added in the biopolymeric solution (Okuro et al. 2015). Depending on the process, chosen polymers, type of incorporated bioactive and interactions between the active and the network, the structure can result in higher or smaller encapsulation efficiency.
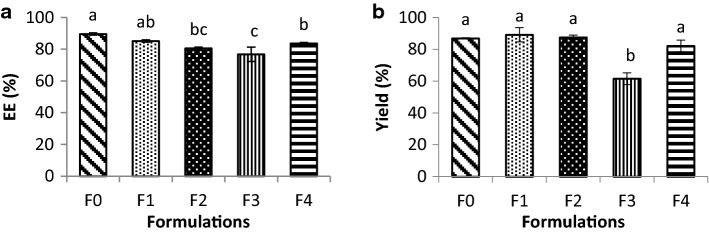
All formulations presented high EE (> 75%) and starch addition did not increase the pectin ability to entrap anthocyanin into the core of particles (Fig. 4a). Moreover, different total biopolymer content (F1 and F2) did not show significant differences (p > 0.05), entrapping the same amount of anthocyanin in their structures (Fig. 4a). Previous work has also shown that the encapsulation efficiency has not been affected by polymer ratio and drug concentration for high amylose starch/pectin blends loaded with diclofenac (Soares et al. 2013).
Fig. 4.
a Encapsulation efficiency and b yield for macrobeads of pectin (P)/starch (S) formulations (F0: 1% P; F1: 1% P and 1% S; F2: 0.5% P and 0.5% S; F3: 0.25% P and 0.75% S; F4: 0.75% P and 0.25% S). Lowercase letters show differences (p < 0.05) between the formulations
Concerning the yield, results showed that all formulations presented yield higher than 60% (Fig. 4b). The sample with the smallest amount of pectin (F3) produced fewer mass of particles (61.54%) (smallest yield), probably because pectin is the main responsible to structure the gel network. Besides, such formulation presented lower WHC (15.66% WHC) (Fig. 2d) and smallest stress at rupture (3.47 kPa) (Fig. 2a), indicating that the process yield in the production of macrobeads was directly related to water retention and strength of the gel network.
Characterization of the microparticles
The most compact and dense microstructure (Fig. 3b) found among all formulations studied (F1) was the only sample able to form particles through atomization process, showing the importance of polymer content and the process used in particle formation. In this way, the microparticles were characterized according to particle size distribution and morphology. Rheological behavior was also evaluated when the microparticles were added to grape nectar (GN), once modifications on the rheological characteristics can be related to acceptance or rejection of the product by the consumer (Herh et al. 2000).
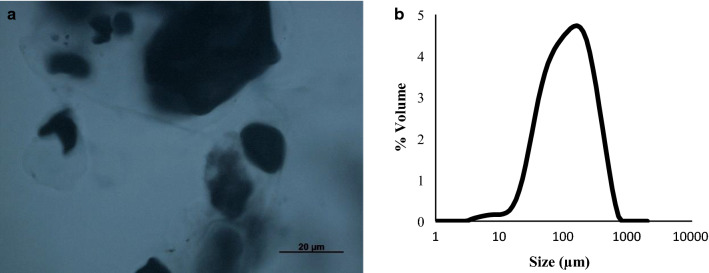
According to the optical microscopy (Fig. 5a), the obtained microparticles (1% P and 1% S) presented an irregular shape and a non-homogeneous network; with starch remaining granules (dyed with iodine) irregularly dispersed into the hydrogel particles. Similarly, the introduction of whey proteins and hydroxypropyl methylcellulose for reinforcement of alginate and pectin beads, affected negatively the morphology (Belščak-Cvitanović et al. 2016). The authors reported highest roundness for pectin particles, while the introduction of other carrier materials and the use of binary mixtures reduced the roundness of pectin microbeads. Although microparticles containing only pectin were not formed due to the low biopolymer concentration, in the present work, the role of the starch reinforcing the encapsulation system was evidenced.
Fig. 5.
Optical microscopy a and particle size distribution b of pectin (P)/starch (S) microparticles produced with 1% S (w/w) and 1% P (w/w), containing anthocyanin. The blue-black complex indicates the presence of starch granules, dyed with iodine
The particle size distribution analysis (Fig. 5b) showed a D3,2 of 65.80 µm ± µm ± 5.40 and a Span of 2.56 ± 0.09. Mean droplet diameters ranging from 77 to 275 µm were reported for pectin microparticles by atomization using varied pressure/flow rate conditions, which also showed monomodal behavior (De Moura et al. 2018). The authors reported that better protection is achieved for larger particles; however, for microparticles dispersion in food products, smaller sizes are desired.
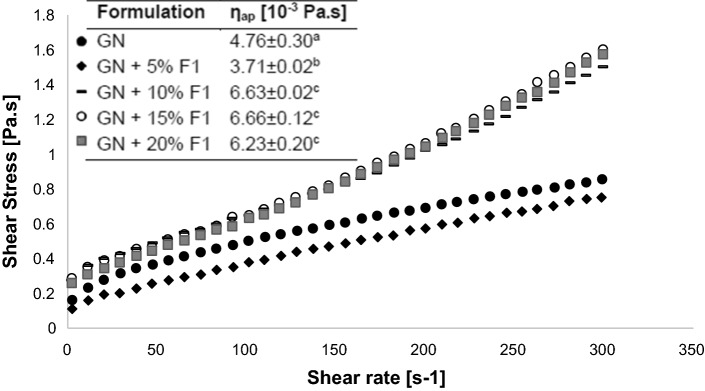
Figure 6 illustrates the rheological behavior of GN with the addition of different concentrations of pectin-starch microparticles (0–20% w/v). None of the samples presented thixotropy (data not shown). Considering that the shear rate of chewing and swallowing comprises the range of 10–100 s−1 (Steffe 1966), apparent viscosity at 100 s−1 was calculated for all suspensions (Fig. 6). Results showed that addition of 5% of microparticles resulted in a slight reduction on apparent viscosity, maintaining the pseudoplastic behavior of the grape nectar. Such behavior is the result of particles alignment in the fluid shear field. Particles are organized into layers and offer less resistance (lower viscosity) to flow (Chen et al. 2010). Furthermore, only the flow curves of the pure nectar and the nectar added with 5% of particles presented a good fit for the shear thinning model (data not shown), with correlation index (R2) higher than 0.99. Suspensions containing 10% or more particles showed an increase of apparent viscosity, which is associated with the increased particle–particle interaction (Brown and Jaeger 2014; Chen et al. 2010), due to the increased number of particles in suspension, as well as swelling of the starch contained in particles. For these samples, two distinct behaviors were noted: below 100 s−1 the suspension showed a reduction of viscosity with increasing shear rate, which is typical of a pseudoplastic fluid, while above 100 s−1 a shear thickening behavior was observed. Thus, flow model could not be adjusted in these cases.
Fig. 6.
Flow curves of microparticles (F1: 1% Pectin and 1% Starch) added in different concentrations (5–20%) in grape nectar (GN). Lowercase letters indicate significant difference (p < 0.05) in the apparent viscosity (ηap) between the formulations, at 100 s−1
Conclusion
Starch contributed to structure the pectin gel network, increasing the mechanical properties and the ability to retain larger amount of water (higher WHC). The microstructure of the gels was affected both by the starch addition and the amount of total polymers added. Pectin gel presented a homogeneous and porous structure, while pectin/starch blends containing 50% or more pectin exhibited a denser and closer network, with reduced porosity. Such characteristic was associated with higher gel hardness and firmness, besides of the greater water holding capacity. In addition, we observed the importance of total biopolymer concentration and type of process (extrusion and atomization) on particle formation, in which pectin 1% and starch 1% blend was the only sample able to form particles under atomization.
Regarding the rheological behavior of the microparticles suspensions, the addition of 5% microparticles to the grape nectar resulted in a small decrease on the apparent viscosity of the suspensions and did not influence the flow behavior. Thus, the particles will probably not affect the consumer acceptance of the grape nectar when added in this concentration.
Acknowledgements
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (DEA/FEA/CAPES-AUXPE 0535/2018) for the financial support and fellowships; the companies CPKelco, Chr Hansen and Ingredion for sample donation and the access to equipment and assistance provided by the Laboratory of Process Engineering, Faculty of Food Engineering at the University of Campinas co-funded by FAPESP (2007/58017-5; EMU2009/54137-1; 2004/08517-3).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Flávia Souza Almeida, Email: flaviasouzaudi@gmail.com.
Karen Cristina Guedes Silva, Email: karen_cgs@hotmail.com.
Antônio Matias Navarrete de Toledo, Email: matiasnavarrete@yahoo.com.br.
Ana Carla Kawazoe Sato, Email: acksato@unicamp.br.
References
- Alcázar-Alay SC, Meireles MAA. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci Technol (Campinas) 2015;35(2):215–236. doi: 10.1590/1678-457X.6749. [DOI] [Google Scholar]
- Belščak-Cvitanović A, Bušić A, Barišić L, Vrsaljko D, Karlović S, Špoljarić I, Komes D. Emulsion templated microencapsulation of dandelion (Taraxacum officinale L.) polyphenols and β-carotene by ionotropic gelation of alginate and pectin. Food Hydrocoll. 2016;57:139–152. doi: 10.1016/J.FOODHYD.2016.01.020. [DOI] [Google Scholar]
- Braga ALM, Azevedo A, Julia Marques M, Menossi M, Cunha RL. Interactions between soy protein isolate and xanthan in heat-induced gels: the effect of salt addition. Food Hydrocoll. 2006;20(8):1178–1189. doi: 10.1016/J.FOODHYD.2006.01.003. [DOI] [Google Scholar]
- Brown E, Jaeger HM. Shear thickening in concentrated suspensions: phenomenology, mechanisms and relations to jamming. Rep Prog Phys. 2014;77(4):046602. doi: 10.1088/0034-4885/77/4/046602. [DOI] [PubMed] [Google Scholar]
- Burey P, Bhandari BR, Howes T, Gidley MJ. Hydrocolloid gel particles: formation, characterization, and application. Crit Rev Food Sci Nutr. 2008;48(5):361–377. doi: 10.1080/10408390701347801. [DOI] [PubMed] [Google Scholar]
- Chen DTN, Wen Q, Janmey PA, Crocker JC, Yodh AG. Rheology of soft materials. Annu Rev Condens Matter Phys. 2010;1(1):301–322. doi: 10.1146/annurev-conmatphys-070909-104120. [DOI] [Google Scholar]
- Cury B, Meneguin A, VMO C, Prezotti F (2014) Oral drug release systems based on pectin. In: Pectin: chemical properties, uses and health benefits, p 65–81. Available in: http://hdl.handle.net/11449/172436
- Da Róz AL, Veiga-Santos P, Ferreira AM, AntunesLeite TCRFDL, Yamaji FM, de Carvalho AJF. Water susceptibility and mechanical properties of thermoplastic starch–pectin blends reactively extruded with edible citric acid. Mater Res. 2016;19(1):138–142. doi: 10.1590/1980-5373-MR-2015-0215. [DOI] [Google Scholar]
- Dafe A, Etemadi H, Dilmaghani A, Mahdavinia GR. Investigation of pectin/starch hydrogel as a carrier for oral delivery of probiotic bacteria. Int J Biol Macromol. 2017;97:536–543. doi: 10.1016/J.IJBIOMAC.2017.01.060. [DOI] [PubMed] [Google Scholar]
- De Moura SCSR, Berling CL, Germer SPM, Alvim ID, Hubinger MD. Encapsulating anthocyanins from Hibiscus sabdariffa L. calyces by ionic gelation: pigment stability during storage of microparticles. Food Chem. 2018;241:317–327. doi: 10.1016/J.FOODCHEM.2017.08.095. [DOI] [PubMed] [Google Scholar]
- Farjami T, Madadlou A. Fabrication methods of biopolymeric microgels and microgel-based hydrogels. Food Hydrocoll. 2017;62:262–272. doi: 10.1016/J.FOODHYD.2016.08.017. [DOI] [Google Scholar]
- Ferreira DF. Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia (UFLA) 2011;35:1039–1042. doi: 10.1590/S1413-70542011000600001. [DOI] [Google Scholar]
- Ferreira DS, Faria AF, Grosso CRF, Mercadante AZ. Encapsulation of blackberry anthocyanins by thermal gelation of curdlan. J Braz Chem Soc. 2009;20(10):1908–1915. doi: 10.1590/S0103-50532009001000020. [DOI] [Google Scholar]
- Fujiwara GM, Campos R, Costa CK, Dias JFG, Miguel OG, Miguel MD, Zanin SMW. Production and characterization of alginate-starch-chitosan microparticles containing stigmasterol through the external ionic gelation technique. Braz J Pharm Sci. 2013;49(3):537–547. doi: 10.1590/S1984-82502013000300015. [DOI] [Google Scholar]
- Gouvêa ACMS, Araujo MCP, Schulz DF, Pacheco S, Godoy RLO, Cabral LMC. Anthocyanins standards (cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside) isolation from freeze-dried açaí (Euterpe oleraceae Mart.) by HPLC. Food Sci and Tech. 2012;32(1):43–46. doi: 10.1590/S0101-20612012005000001. [DOI] [Google Scholar]
- Herh PKW, Colo SM, Roye N, Hedman K. Rheology of foods: new techniques, capabilities, and instruments. Am Lab. 2000;32(12):16–21. [Google Scholar]
- Lee J, Durst R, Wrolstad R. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88(5):1269–1278. doi: 10.1093/jaoac/88.5.1269. [DOI] [PubMed] [Google Scholar]
- Liu L, Fishman ML, Kost J, Hicks KB. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials. 2003;24(19):3333–3343. doi: 10.1016/S0142-9612(03)00213-8. [DOI] [PubMed] [Google Scholar]
- Miles MJ, Morris VJ, Orford PD, Ring SG. The roles of amylose and amylopectin in the gelation and retrogradation of starch. Carbohyd Res. 1985;135(2):271–281. doi: 10.1016/S0008-6215(00)90778-X. [DOI] [Google Scholar]
- Mohammadian M, Madadlou A. Cold-set hydrogels made of whey protein nanofibrils with different divalent cations. Int J Biol Macromol. 2016;89:499–506. doi: 10.1016/J.IJBIOMAC.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Nawab A, Alam F, Hasnain A. Functional properties of cowpea (Vigna unguiculata) starch as modified by guar, pectin, and xanthan gums. Starch/Stärke. 2014;66:832–840. doi: 10.1002/star.201300268. [DOI] [Google Scholar]
- Oidtmann J, Schantz M, Mäder K, Baum M, Berg S, Betz M, Richling E. Preparation and comparative release characteristics of three anthocyanin encapsulation systems. J Agric Food Chem. 2012;60(3):844–851. doi: 10.1021/jf2047515. [DOI] [PubMed] [Google Scholar]
- Okuro PK, Furtado GF, Sato ACK, Cunha RL. Structures design for protection and vehiculation of bioactives. Curr Opin Food Sci. 2015;5:67–75. doi: 10.1016/J.COFS.2015.09.003. [DOI] [Google Scholar]
- Patil P, Chavanke D, Wagh M. A review on ionotropic gelation method: novel approach for controlled gastroretentive gelispheres. Int J Pharm Pharm Sci. 2012;4:27–32. [Google Scholar]
- Pires Vilela JA, Cavallieri ÂLF, Lopes da Cunha R. The influence of gelation rate on the physical properties/structure of salt-induced gels of soy protein isolate–gellan gum. Food Hydrocoll. 2011;25(7):1710–1718. doi: 10.1016/J.FOODHYD.2011.03.012. [DOI] [Google Scholar]
- Shewan HM, Stokes JR. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J Food Eng. 2013;119(4):781–792. doi: 10.1016/J.JFOODENG.2013.06.046. [DOI] [Google Scholar]
- Soares GA, de Castro AD, Cury BSF, Evangelista RC. Blends of cross-linked high amylose starch/pectin loaded with diclofenac. Carbohyd Polym. 2013;91(1):135–142. doi: 10.1016/J.CARBPOL.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Steffe JF. Rheological methods in food process engineering. 2. USA: Freeman Press; 1996. [Google Scholar]
- Stokes JR. Rheology of industrially relevant microgels. In: Fernandez-Neives A, Wyss H, Mattson J, Weitz DA, editors. Microgel suspensions—fundamentals and applications. Weinheim: Wiley; 2011. [Google Scholar]
- Takeo K, Tokumura A, Kuge T. Complexes of starch and its related materials with organic compounds. Part X. X-Ray diffraction of amylose-fatty acid complexes. Starch Stärke. 1973;25(11):357–362. doi: 10.1002/star.19730251102. [DOI] [Google Scholar]
- Yamamoto F, Cunha RL. Acid gelation of gellan: effect of final pH and heat treatment conditions. Carbohyd Polym. 2007;68(3):517–527. doi: 10.1016/J.CARBPOL.2006.11.009. [DOI] [Google Scholar]
- Zhang J, Daubert CR, Foegeding EA. Characterization of polyacrylamide gels as an elastic model for food gels. Rheol Acta. 2005;44(6):622–630. doi: 10.1007/s00397-005-0444-5. [DOI] [Google Scholar]
- Zhang B, Bai B, Pan Y, Li XM, Cheng JS, Chen HQ. Effects of pectin with different molecular weight on gelatinization behavior, textural properties, retrogradation and in vitro digestibility of corn starch. Food Chem. 2018;264:58–63. doi: 10.1016/j.foodchem.2018.05.011. [DOI] [PubMed] [Google Scholar]