Abstract
Solid residues obtained after essential oil extraction from Cymbopogon winterianus Jowitt (Java citronella) was explored as a potential source of phenolics/antioxidant. Both the non-distilled plant materials and their solid residues were extracted with Soxhlet extraction method using solvents of various polarity viz. petroleum ether, chloroform, ethyl acetate, acetone, ethanol, methanol, water and various combination of (50% and 75%) of methanol, ethanol, and acetone in water. Different antioxidant assays like 2,2-diphenyl-1- picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), superoxide anion (SO) radical scavenging assay, ferric-reducing antioxidant power (FRAP) and iron chelating ability along with total phenol (TPC) and flavonoid content (TFC) was measured to evaluate the extract. Compared to distilled materials, the non-distilled plant materials had significantly higher TPC/TFC content and also exhibited higher antioxidant activities. 50% aqueous methanol showed the highest extractive yield, whereas 75% aqueous methanol exhibited the highest TPC and TFC content. The 50% or 75% aqueous methanolic extract also exhibited the highest DPPH, ABTS and SO scavenging activity and ferric-reducing antioxidant power activity. However, ethyl acetate and 75% aqueous acetone extract of non-distilled and distilled plant materials, respectively showed the highest iron chelating activity. The half maximal effective concentration (IC50 = µg/mL) for DPPH, ABTS, SO and metal chelating ability in non-distilled plant extract ranged from 64–387, 92–761, 285–870, and 164–924, respectively, and corresponding value of distilled materials ranged from 144–865, 239–792, 361–833 and 374–867, respectively. The EC50 (µg/mL) for FRAP assay ranged from 118–840 and 151–952 for non-distilled and distilled materials, respectively. The findings of this study indicate the potential of these by-products as a natural antioxidants source.
Keywords: Natural antioxidant, Java citronella, Antioxidant activity, Essential oil industry by-product
Introduction
In India, the cultivation of the economically important aromatic plants like Cymbopogon winterianus Jowitt (Java Citronella) is gaining importance. Java Citronella yields essential oil known as citronella oil and hydro-distillation or steam distillation is normally used to extract essential oils. But the average percent essential oil yield obtained from this plant gives an account only up to 1% of the whole plant (Saha et al. 2019). World production of citronella oil is about 1600 tons/year and India produces almost 500 tons of oils/year with the acreage of 9000 ha (Nandapure et al. 2016). So, a substantial amount of waste (liquid/solid) is generated every year during essential oil extraction that is of concern if they are not managed properly.
Use of this biomass for generating energy (Alfa et al. 2014) or compost (Deka et al. 2011) requires huge investment, and composting is not always preferred as some of the aromatic plants have anti-germinative properties (Martino et al. 2010). Recently there is an increasing trend of use of agricultural waste as an inexpensive and sustainable resource for phenolic compounds and their possible use as natural/safe antioxidants (Santana-Méridas et al. 2012). Aromatic plants contain numerous antioxidant compounds like phenols, terpenes, nitrogen compounds, ascorbic acid, etc. During the extraction of essential oil, most of these phenolic compounds remain in the solid spent, as they are non-volatile and non-degradable with thermal treatments. Reports are also available about the presence of valuable polyphenolic dietary antioxidants in hydro-distillation residues of aromatic plants (Parejo et al. 2002; Tsimogiannis et al. 2017).
Nevertheless, antioxidant properties are not only depends on method of extraction, but also the type of extraction solvent used due to the polarity differences of the bioactive compounds (Gavarić et al. 2015). Thus, solvent selection is a key step for efficient recovery of bioactive compounds. US Food and Drug Administration (FDA 2012) define ethanol, acetone, and ethyl acetate as Class 3 solvent due to their less toxicity as far as the human health is concerned. Chloroform, petroleum ether and methanol are categorized as Class 2 solvents and should have limited use due to their inherent toxicity. Sometime combination of water with polar solvents like acetone, ethanol and methanol shows maximum antioxidant activity of extract as compared to sole solvent. Here, we intended to determine the extraction potential of different Class 2 (methanol, chloforom and pethroleum ether), Class 3 (ethanol, ethyl acetate and acetone) solvents, water and the different combination of polar solvents (acetone, methanol and ethanol)/water @ 50:50 (v/v) or 75:25 (v/v) on the total phenolic/flavonoids yield from distillation waste of Java citronella and evaluation of their antioxidant potential for possible use in food sectors.
Although, extensive studies have been carried out for essential oils extraction from Java citronella, but limited information is available regarding the antioxidant activity of this aromatic plant. In order to exploit Java citronella as a potential source of natural antioxidants, extract of both non-distilled and distilled plant residues were evaluated in this study. This work aimed to study the content of phenols/flavonoids variability amongst the different solvent extract obtained from Java citronella fresh and hydro-distilled residue, and also to evaluate their in vitro antioxidant and chelating activities. Thus, this strategy will possibly revalorize the Java citronella waste obtained from essential oil distillation industry, and also helps in understanding the prospective usage of aromatic plant waste biomass as a potential source of natural antioxidant.
Materials and methods
Plant materials
Java citronella plant materials were obtained from the experimental farm of ICAR-Directorate of Medicinal and Aromatic Plants Research (DMAPR), Anand, Gujarat, India. The freshly harvested plant materials were cut into small pieces and dried for 1 week at room temperature under dark condition. When the drying process completed, part of biomass was used for extraction of essential oil and another part of the dried biomass was grounded and preserved for further analysis.
Chemical and reagents
Gallic acid, quercetin, Folin and Ciocalteu’s reagent, aluminium nitrate, sodium carbonate, sodium nitrite, sodium hydroxide, DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS [2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammoinium salt], ferrozine [3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4,4-disulfonic acid sodium salt], NBT (nitroblue tetrazolium), PMS (phenazine methosulphate), NADH (nicotinamide adenine dinucleotide reduced), potassium persulfate, potassium ferricyanide (K3Fe(CN)6], TCA (trichloro acetic acid), ferric chloride, ferrous ammonium sulfate, Tris–HCl buffer, monosodium phosphate and disodium phosphate were procured from Sigma-Aldrich, Germany, Hi-Media, Mumbai, India, or Sisco Research Laboratories, India. Different solvents were obtained from Merck, India.
Distillation
The essential oils were extracted from dried Java citronella (200 g) plant materials by hydro-distillation in 2 L of water for 180 min using a Clevenger-type apparatus. The by-product i.e., distillation solid waste obtained after essential oil extraction was dried at room temperature for 48 h, grounded and preserved for further use. The waste liquid materials, remaining after essential oil extraction, was filtered and obtained as de-odorized extract was also used as a liquid waste.
Soxhlet extraction
Soxhlet apparatus was used in this study for extraction. Grounded non-distilled plant materials or distilled by-product (25 g) was extracted with different polarity organic solvents (250 mL) such as petroleum ether (pet ether), chloroform, ethyl acetate, acetone, ethanol, methanol, water, and combination of water and different ratio (50% and 75%) of methanol, ethanol and acetone (Fig. 1). Extraction was carried out thrice in the same condition and then extracts were combined and evaporated under vacuum using vacuum evaporator. The combined water and organic solvent extracts were freeze-dried to remove the water and solvent. The yield of the dried extract was determined and finally the dried extract was kept in safe place.
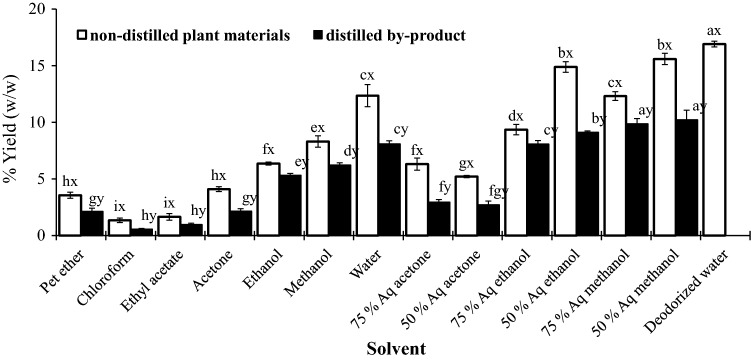
Fig. 1.
Flow chart of the processing of samples
Total polyphenols content (TPC) estimation
TPC of the different extracts were determined using Folin–Ciocalteau (FC) method (Singleton et al. 1999) followed by spectrophotometric analysis. Extract solution (0.5 mL) was mixed with FC reagent (0.5 mL) followed by the addition of distilled water (8.5 mL) and incubated at room temperature for 10 min. Then, sodium carbonate (20%; 1.5 mL) was added to this mixture and the content was boiled in a water bath for 20 min. After cooling, absorbance of the mixture was measured spectrophotometrically (at 755 nm). Gallic acid equivalent [mg GAE/g of the dry extract (DE)] was used to express the TPC content.
Estimation of total flavonoids content (TFC)
The method described by Wang et al. (2012) was used to determine TFC content with suitable adjustment. Aliquot extract (1 mL) was transferred into a 25 mL of volumetric flask and mixed with sodium nitrite (5%; 1.0 mL) for 6 min. Then aluminium nitrate (10%; 1.0 mL) was added, and the mixture was shaken (6 min) and finally sodium hydroxide (4%; 10 mL) was added to this. The mixture was then diluted with ethanol to make 25 mL solution and absorbance was measured spectrophotometrically (510 nm). Quercetin equivalent/g of dry extract (DE) was used to express the flavonoid content.
Antioxidant potential of fresh and distillation waste of Java citronella
1, 1-diphenyl-2-picryl- hydrazil (DPPH·) radical scavenging assay
A method developed by Liyana-Pathiranan and Shahidi (2005) was used for determining the DPPH radical scavenging potential of different extracts with minor modification. Methanolic solution of DPPH (0.1 mM/L; 1 mL) was mixed with 3.0 mL aliquot of (conc. 50–1000 µg/mL) methanolic solution of different extracts and then the mixture was kept in dark at room temperature (30 min). The absorbance of the mixture was measured spectrophotometrically at 517 nm. The capacity of scavenging DPPH radical was calculated as: DPPH radical scavenging activity (%) = (A517 of control–A517 of sample) × 100/A517 of control. The concentration of extract (µg/mL) which scavenge 50% of the DPPH radicals were calculated and expressed as IC50 value.
ABTS radical scavenging assay
The method described by Re et al. (1999) was used for the ABTS radical scavenging assay. Here, ABTS cation was generated by reaction between ABTS (7 mM) and potassium persulfate (2.45 mM) at room temperature for 12 h under dark condition. This ABTS stock solution was diluted with appropriate volume of methanol to get an absorbance of 0.706 ± 0.001 in spectrophotometer (at 734 nm). An 1 mL of aliquot of extracts (conc. 50–1000 µg/mL) was reacted with ABTS solution (1 mL) and after 7 min, the absorbance of the mixture was measured at 734 nm. The capacity of scavenging ABTS radical was recorded and IC50 value was calculated similar to the inhibition activity (%) of DPPH radicals.
Reducing/antioxidant power (FRAP) assay
Various concentration of extracts (conc. 50–1000 µg/mL) dissolved in methanol were mixed with phosphate buffer (0.2 M; pH 6.6; 2.5 mL) and K3[Fe(CN)6] [1% (w/v); 2.5 mL]. Then the mixture was incubated at 50 °C (20 min) and allowed to cool at room temperature. After that the TCA solution (10%; 2.5 mL) was added to this mixture. A 2.5 mL portion of this aliquot mixture was withdrawn and mixed with ferric chloride solution (0.1%; 0.5 mL). After 10 min, the absorbance reading in spectrophotometer was recorded at 700 nm. Increased absorbance of the reaction mixture indicates higher reducing power. The extract concentration giving 0.5 absorbance value (EC50: µg/mL) was calculated from the graph.
NBT superoxide (SO) radical scavenging assay
Superoxide anion radicals scavenging activity was determined according to the method described by Liu et al. (1997). 1.0 mL of different extracts solution (conc. 50–1000 µg/mL) was mixed with Tris–HCl buffer (16 mM, pH 8; 0.5 mL), NBT (0.3 mM; 0.5 mL), NADH (0.93 mM; 0.5 mL), and PMS (0.12 mM; 0.5 mL) and incubated at 25 °C (5 min) and then absorbance was measured at 560 nm. The percent NBT decolourization of the extract and IC50 values was calculated similar to the inhibition activity (%) of DPPH radicals.
Ferrous ion chelating activity
For ferrous ion chelating activity, a method developed by Lim et al. (2007) was used. Ferrous ammonium sulfate (0.125 mM/L; 1 mL) and ferrozine (0.3125 mM/L; 1.0 mL) were mixed with extracts (conc. 50–1000 µg/mL;1.0 mL) and equilibrated for 10 min before being measured spectrophotometrically (at 562 nm). Chelating capacity was calculated by comparing the control, which consists of iron and ferrozine only. The same formula for inhibition activity (%) of DPPH radicals as mentioned earlier was used to calculate the IC50 value (µg/mL).
Statistical analysis
Data from each solvent within the same matrix were subjected to one-way analysis of variance (ANOVA) followed by a Duncan multiple range test (DMRT) by using SPSS-20 (SPSS Inc., Chicago, USA) to determine the significant mean differences. Significant differences between non-distilled and distilled plant materials were calculated by Student’s t test using EXCEL (Microsoft Corporation, USA). Pearson’s correlation matrix between TPC, TFC and 1/IC50 or 1/EC50 value of the entire antioxidant assay was also computed.
Results and discussion
Extraction yield
Previous studies revealed that Soxhlet extraction is more efficient in terms of extraction yield as compared to the other extraction methods like ultrasonic (Annegowda et al. 2012), supercritical CO2 extraction (Plánder et al. 2012) and accelerated solvent extraction method (Shen and Shao 2005). So, in this study, we have chosen Soxhlet method as an extraction process. Previous reports also indicate that addition of water to organic solvents like acetone, methanol, and ethanol increases the polarity of the extraction medium and thus facilitates the extraction of phenolic compounds (Do et al. 2014). So, other than the sole solvent, we have also used 75 and 50% aqueous (Aq) polar organic solvent mixture for the extraction.
Polar solvents were more effective as compared to non-polar solvent for extraction yield for both fresh and distillation waste samples. Among the polar solvent, yields varied from 4.1% (acetone) to 15.6% (50% aq methanol) for fresh plant materials (Fig. 2). The decreasing trend of yields of extraction for fresh plant materials was as follows: 50% aq methanol ≈ 50% aq ethanol > water ≈ 75% aq methanol > 75% aq ethanol > methanol > ethanol ≈ 75% aq acetone > 50% aq acetone > acetone ≈ petroleum ether > ethyl acetate > chloroform (Fig. 2). The trend was almost similar to distillation waste. It can be noticed that for both fresh and distilled plant materials, the yield of pure methanol extract (6.23–8.3%) was higher as compared to that of pure ethanol (5.31–6.36%) and pure acetone (2.15–4.1%) extract, which indicates the proportional relationship of extraction yield and solvent polarity. Although the water extracts yielded (8.1–12.36%) more than that of the pure methanol extract (6.23–8.3%), but the yield of aq methanol extract (from 9.87 to 12.32% for 75% aq methanol to 10.23–15.6% for 50% aq methanol) was superior than the pure methanol and water. This exhibits that the extraction yield proportionally enhances with the increased water content in the polar solvent. With reference to the extraction yield, pure water seems to be superior solvent as compared to aqueous acetone and in few cases also superior to aq ethanol. Higher yield in water extract may be due to the extraction of compounds other than phenolics. As the proteins and carbohydrates are more soluble in methanol and water than ethanol and acetone and hence resulted to higher extraction yield. Compounds, which are soluble in both water and/or organic solvent, may get extracted in water/organic solvent combination and consequently the total yield was increased. The results corroborated the previous study of extraction yield of rice bran (Chatha et al. 2006), banana peel (González-Montelongo et al. 2010) and in some medicinal plants (Do et al. 2014).
Fig. 2.
Yield of the different solvent extract of non-distilled plant materials and distilled by-product of Java citronella [For each solvent, values in the bar between the non-distilled and distilled plant extract, followed by a different letter (x, y) are significantly different (p < 0.05); For each extraction solvent, values in the bar within the non-distilled and distilled plant extract followed by a different letter (a, b, c, d, e and f etc.) are significantly different (p < 0.05)]
The extraction yield of de-odorized water extract was found to be the highest among the different solvent. A comparison between the present study and previous reports is not feasible as the experimental condition may be totally different from the present study. However previous studies also reported the high extractive yield of de-odorized water of different aromatic essential oil bearing plant like oregano (36%), rosemary (24%), sage (25%) (Dorman et al. 2003) and for thyme (29–37%) (Dorman et al. 2003; Gavarić et al. 2015). The reason may be that water may extract a large amount of components from the original plant material. As compared to fresh plant materials, extraction yield from distillation waste was very low. Singh et al. 2014 reported 16.32 and 6.35% extractive yield, respectively for aqueous and ethanolic extract of fresh Cymbopogon winterianus leaves.
Total phenolic content (TPC)
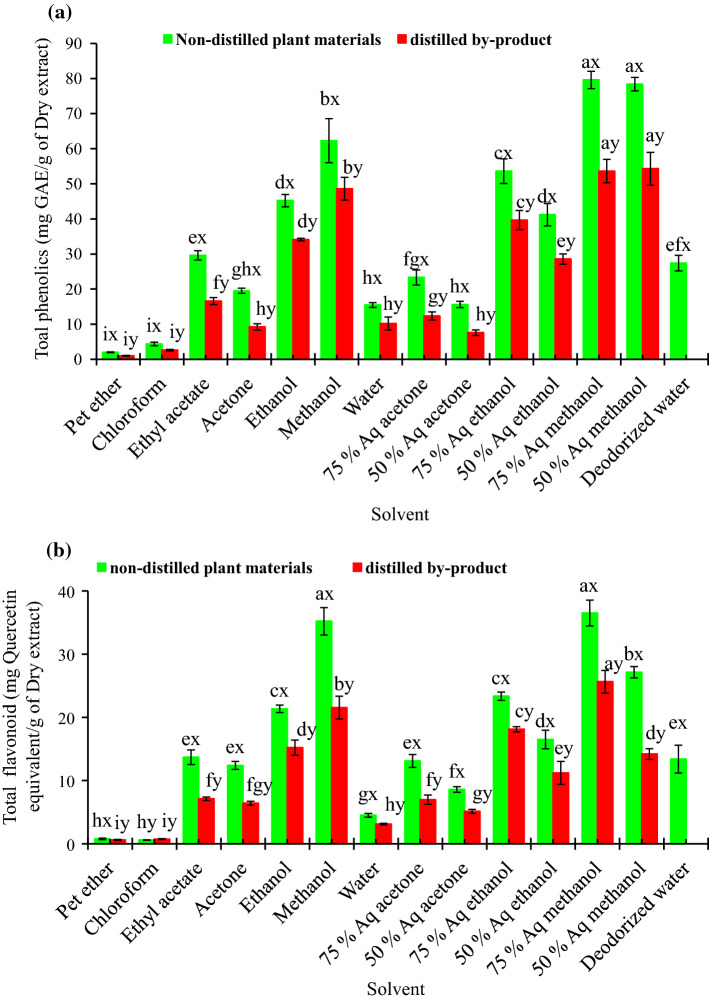
The TPC in the non-distilled plant materials was higher than the by-product (Fig. 3a). This result was expected as a fraction of the phenolic compounds may be removed along with oil extracted during hydro-distillation process. This loss ranged between 22 and 52.7%, depending on the type of solvent used for extraction. TPC (mg GAE/g of DE) value in the different extract of non-distilled materials ranged from 1.99 ± 0.11 (pet ether)-79.6 ± 2.48 (75% aq methanol) and for distilled by-product, it was ranged from 1.01 ± 0.01 (pet ether)-54.29 ± 4.68 (50% aq methanol).
Fig. 3.
Total phenolic (a) and flavonoid (b) content of the different solvent extract of non-distilled plant materials and distilled by-product of Java citronella [For each solvent, values in the bar between the non-distilled and distilled plant extract, followed by a different letter (x, y) are significantly different (p < 0.05); For each extraction solvent, values in the bar within the non-distilled and distilled plant extract followed by a different letter (a, b, c, d, e and f etc.) are significantly different (p < 0.05)]
Results also revealed that methanol and ethanol were found to be better solvent for extraction of TPC due to their polarity and good solubility for phenolic compounds. Low polarity solvents like pet ether and chloroform showed much lower ability in extracting TPC. Due to intermediate polarity, ethyl acetate showed better ability to extract the phenolics than the acetone. However, the addition of water to the absolute polar solvent like acetone, methanol and ethanol resulted in higher TPC yield due to increase in polarity. For instance, the extraction yield of TPC from the non-distilled plant material followed the order 75% aq methanol ≈ 50% aq methanol > methanol > 75% aq ethanol > ethanol ≈ 50% aq ethanol > ethyl acetate > 75% aq acetone > aceotone > 50% aq acetone ≈ water > chloroform > pet ether. In non-distilled plant materials, TPC content in 75% aq methanol extract and 50% aq methanol extract were statistically non-significant. It was also found that with increasing the amount of water (50%) in the aqueous solvent for ethanol and acetone, there was a decrease in TPC. 75% aq ethanol extract and 75% aq acetone extract showed higher TPC content than the absolute and 50% aqueous solvent mixture of the same solvent. For distilled by-products, the results were also following the similar trends. From the Fig. 3a it can be easily inferable that up to 75% aqueous solvent combination was best extracting solvent for extraction of phenolics than the 50% aqueous solvent combination and absolute solvent. Higher water content may result in extraction of non-phenol compounds such as carbohydrate and terpene, which may increase the yield of the extract but not the phenolic content.
The extract of de-odorized water, though highest amount of yield was obtained, but was not only poor in TPC content than the methanoloic and ethanolic extract of fresh plant but also poor than the distilled plant materials of same extract. This indicates that there was a possibility of carbohydrate richness in de-odorized extract, which attributed to higher extraction yield but not the biological activity. But still its TPC content was higher than the other low or non-polar solvent extract, indicates its moderate potential as a source of natural phenolics. Dorman et al. (2003) also reported about the antioxidant potential of de-odorized aqueous extracts of some Lamiaceae herbs. As the limited information is available about the phenol content in C. winterianus distillation waste, this study may provide useful information of the phenolic content of the distillation residue of the aromatic plants. As compared to present study, Kusmardiyani et al. 2016 reported discrepant phenol concentration (125.3 mg/g DE) in ethanolic extract of non-distilled plants of C. winterianus. These discrepancies may be due to the different factors affecting the extraction method and also the granulometry of plant materials. Sources of raw materials are also known to be another possible reason for this variability as this can affect the phenolic compound content of plant matrix.
Total flavonoid contents (TFC)
The TFC of the extracts is presented in Fig. 3b. The variability of TFC (mg quercetin equivalent/g DE) content ranged from 0.59 ± 0.12 (chloroform)-36.5 ± 2.04 (75% aq methanol) for different extract of the non-distilled plant materials. For the distillation waste, the values ranged from 0.61 ± 0.09 (pet ether) − 25.63 ± 1.79 (75% aq methanol). Compared to non-distilled plant materials, there was almost 21.8–48.3% loss of the TFC content in distilled by-product. The trend of solvent effect on extraction of TFC was similar with TPC. TFC content in 75% aq methanol extract was highest followed by the 50% and 100% methanol extract. In aromatic plant Thymus vulgaris, methanol was also found to be the best solvent for extraction of flavonoid (Hossain et al. 2013). With the increase of water content in ethanol or acetone up to 25%, the TFC content in the extract was also increased. However, further increase in water content (50%), the TFC content in the extract was decreased. In this study, flavonoid content in all the solvent extract was lower than the corresponding total phenolic content as the flavonoid is one of component of tolal phenolics in the extract. Compared to present study, Kusmardiyani et al. 2016 found little higher amount of flavonoid (almost 120 mg quercetin equivalent/g DE) in non-distilled Java citronella. The composition of flavonoid compounds is highly influenced by the location where the samples are collected and also the dominant climatic and environmental factors.
Antioxidant activity of the extract
The antioxidant activity of both fresh plant and solid residues of Java citronella was assessed by using five spectrophotometric analysis methods. These include three radical scavenging activity assays, one assay of each reduction of iron metal, and metal chelating ability. The reason for selecting these assay method was due to their simplicity, sensitivity and reproducibility.
DPPH radical scavenging activity
Solvent characteristics are the most important parameter, which influences the antioxidant capacities. To select the most appropriate solvent, the antiradical scavenging activity (DPPH·) was evaluated either using pure solvents or different combination of water/polar solvent mixture (Fig. 1). The concentrations of the extract giving a 50% DPPH free radicals inhibition i.e. IC50 values ranged from 64.2 (75% aq methanol)-386.5 µg/mL (chloroform) for fresh plant and 144.1 (75% aq methanol)-865.2 µg/mL (chloroform) for by-product (Table 1). The lower value of IC50 indicates that the extract had the highest antioxidant activity. In both the matrices, the highest DPPH scavenging potential was shown by 75% aq methanol extract. Next suitable solvent system was 50% aq methanol, followed by absolute methanol. IC50 values for extracts of acetone, ethanol and their aqueous mixture were higher than methanol and its aqueous mixture. The lowest activity was found in chloroform extract (Table 1). However, compared to the fresh plant extract, by-product showed lower DPPH scavenging potential. Depending upon the solvent system used, IC50 values of by-product extracts were 64.7–157% higher as compared to the corresponding values for non-distilled plant materials. These results also indicate that the aqueous-organic solvent mixtures are more suitable for extracting DPPH· scavenging agents. Solvent polarity, and their antioxidant extractability potential might have played an important role for this variable observation.
Table 1.
IC50 (µg/mL) values of DPPH, ABTS, superoxide anion radical (SO) scavenging activities and EC50 (µg/mL) values of ferric reduction antioxidant power of the different solvent extract of non-distilled and distilled by-product of Java citronella
| Java citronella | ||||||||
|---|---|---|---|---|---|---|---|---|
| DPPH | ABTS | FRAP | SO | |||||
| Non-distilled | Distilled | Non-distilled | Distilled | Non-distilled | Distilled | Non-distilled | Distilled | |
| Pet ether | 319.8 ± 2.3 lx | 705.6 ± 10.41ky | 516.2 ± 4.4jx | > 1000 | 694.1 ± 6.0kx | 776 ± 7.8ky | > 1000 | > 1000 |
| Chloroform | 386.5 ± 3.1mx | 865.2 ± 15.48ly | 760.9 ± 5.2 lx | > 1000 | 840.3 ± 17.9 lx | 951.7 ± 21.8ly | > 1000 | > 1000 |
| Ethyl acetate | 168.5 ± 2.6fx | 316.5 ± 7.59dy | 267.4 ± 12.9ex | 421.5 ± 2.3dy | 348 ± 6.1ex | 366.3 ± 8.6ey | 870.7 ± 5.5hx | > 1000 |
| Acetone | 218.1 ± 1.7hx | 416.1 ± 7.15gy | 328.9 ± 3.3gx | 791.6 ± 9.5jy | 457. 7 ± 4.2hx | 474 ± 7.5hy | 504 ± 6.6ex | 833.7 ± 29.5gy |
| Ethanol | 133.5 ± 1.9dx | 363.2 ± 3.64ey | 201.6 ± 2.2cx | 421.9 ± 1.8dy | 290 ± 4.6dx | 400.3 ± 11.0fy | 403 ± 4.6dx | 605.7 ± 10.2dy |
| Methanol | 87.3 ± 2.3bx | 196 ± 2.31cy | 112.6 ± 3.1bx | 238.9 ± 4.5ay | 189.8 ± 7.1bx | 214.7 ± 5.1cy | 322.7 ± 9.6bx | 662.3 ± 8.5ey |
| Water | 303 ± 2.3kx | 642.7 ± 11.55jy | 569.8 ± 9.9kx | 783.5 ± 5.1iy | 658. 7 ± 2.5jx | 708 ± 17.3jy | > 1000 | > 1000 |
| 75% Aq acetone | 178.5 ± 2.1gx | 454.1 ± 7.30hy | 261.2 ± 1.8ex | 698.2 ± 3.7hy | 389.3 ± 11.7fx | 478 ± 11.0hy | 649 ± 29.5fx | 789.3 ± 21.5fy |
| 50% Aq acetone | 294.4 ± 2.9jx | 484.9 ± 3.97iy | 361.2 ± 9.9hx | 589.3 ± 1.7fy | 511.3 ± 11.2ix | 545.7 ± 13.9iy | 724 ± 8.9gx | > 1000 |
| 75% Aq ethanol | 122.6 ± 1.1cx | 315.2 ± 5.29dy | 221.2 ± 3.5dx | 456.8 ± 3.5ey | 226.3 ± 4.9cx | 331.3 ± 9.5dy | 351.7 ± 11.6cx | 452.3 ± 8.5by |
| 50% Aq ethanol | 158.3 ± 1.3ex | 399.8 ± 4.51fy | 289.6 ± 3.9fx | 687.5 ± 2.1gy | 296 ± 8.2dx | 420 ± 6.1gy | 388.7 ± 1.5dx | 525.3 ± 23.1cy |
| 75% Aq methanol | 64.2 ± 0.3ax | 144.1 ± 1.82ay | 91.8 ± 0.6ax | 261.3 ± 3.4cy | 117. 7 ± 5.1ax | 151 ± 3.6ay | 285.3 ± 6.7ax | 361.3 ± 12.7ay |
| 50% Aq methanol | 68.1 ± 1.0ax | 168.5 ± 1.04by | 111.2 ± 2.6bx | 253.8 ± 1.6by | 128 ± 6.6ax | 177.7 ± 8.1by | 307.7 ± 3.5bx | 444 ± 37.0by |
| De-odorized water | 226.21 ± 1.4i | 456.9 ± 5.1ix | 419 ± 13.5gx | 518.7 ± 6.8ex | ||||
For each solvent, values in the same column, followed by a different letter (a, b, c, d, e and f etc.) are significantly different (p < 0.05); For each solvent, values between the non-distilled and distilled plant extract, followed by a different letter (x, y) are significantly different (p < 0.05)
Analogous to the results of phenolic content, the lowest activity was exhibited by petroleum ether, ethyl acetate and chloroform and de-odorized water extracts showed moderate potential. Several reports indicate that extractability and antioxidant activity increases by the addition of low percentages of water to the solvent like methanol and ethanol (Gavarić et al.2015; Do et al. 2014). Ethanol/water (75:25) extract of Thymus vulgaris showed maximum antioxidant activity as reported by Gavarić et al. 2015. The similar trend was also established by Tohidi et al. 2017 where 80:20 methanol/water was used for extraction of phenolics and DPPH scavenging activities of fourteen Thymus accessions.
The antioxidant potential of Java citronella has not yet been well studied. The scavenging activity of Java citronella oil as tested by Leite et al. 2010 reported a much lower IC50 (12.66 ± 0.56 µg/mL) value which may be due to the rich presence of geraniol (36%) and citronellal (42.7%) in oil. Nevertheless, the DPPH scavenging potential of Java citronella leaf extract as in this study was lower than the value reported by Kusmardiyani et al. 2016 for the Java citronella leaf extract (IC50: < 50 µg/mL).
ABTS radical cation scavenging activity
ABTS assay determined the scavenging potential of ABTS radical by antioxidant compounds. Similar to DPPH assay, 75% aq methanol extract of fresh plant materials showed the highest ABTS radical scavenging potential. However, in distilled plant residues, 100% methanol showed the best activity. The water extract showed inferior ABTS scavenging potential than the aqueous organic solvent mixture and was significantly lower from that of the others polar solvents (p < 0.05). For distilled plant residues, concentration range of pet ether and chloroform extract in this study was not sufficient to inhibit the 50% ABTS free radicals. These results also re-affirmed the significant effects of extraction solvent mixtures on ABTS scavenging activity evaluation. Thus, 75% aq methanol might be the appropriate solvent for extraction of antioxidants to scavenge maximum ABTS radical (Table 1). Fresh plant materials showed lower IC50 values for ABTS assay as compared to distilled by-products. Depending upon the type of solvent used, IC50 values of by-product extracts increased from 37.5 to 184.5% as compared to the corresponding values for non-distilled plant materials (Table 1).
From the IC50 values, it is easily understandable that IC50 determined by DPPH assay were comparatively lower than the value obtained in ABTS assay though for ABTS assay the reactions rate is faster than DPPH (Naik et al. 2006) and consequently lower IC50 value is expected for ABTS assay. Brown sorghum samples showed minimal difference between IC50 values of DPPH and ABTS assay as reported by Awika et al. 2003. Factors like stereo-selectivity of the radical molecules or extractability of different solvents might have affected the capacity of extract to react and quench different radicals (Yu et al. 2002). However, Dhanani et al. 2013 showed higher IC50 value for ABTS assay than DPPH assay for the Withania somnifera extract.
Ferric reducing antioxidant power (FRAP) assay
Ability of an antioxidant to reduce Fe3+ to Fe2+ in a redox-linked colourimetric reaction is measured by FRAP assay. Here, the Fe3+/ferricyanide complex is reduced to Fe2+ form in the presence of antioxidants. The extract concentration providing an absorbance of 0.5 (EC50) was calculated from the graph and EC50 values are given in the Table 1 which indicates the potential electron-donating capacity of all the extracts, as we are able to calculate the EC50 values for all the extracts in the studied concentration range. Here also, 75% and 50% aq methanol extract showed maximum reducing power and the values were significantly higher than that of the other extracts for all the concentrations. 100% methanol extract results lower reducing potential than its aqueous combination. Aq ethanol and aq acetone extracts showed intermediate value. Non-polar solvent extracts showed least activity. The extract of aqueous mixture of polar solvent showed low EC50 in-terms of FRAP value when compared with individual solvents (Table 1). The ranges of the EC50 value of different studied extract varied from 117.7 ± 0.5 (75% aq methanol)-840.3 ± 17.9 µg/mL (chloroform) for the non-distilled plant material and from 151 ± 3.6 (75% aq methanol)-951 ± 21.8 µg/mL (chloroform) for the by-product, respectively. Here also the EC50 values of by-product extract is 3.6–46.4% higher than the values related to fresh plant materials. Kusmardiyani et al. (2016) measured the FRAP in ethanolic extract of Java citronella and obtained EC50 values of approximately 12.22 µg/mL showing reducing power values were higher than those obtained in present study.
Superoxide anion radical scavenging activity (SO)
Although superoxide anion is a weak oxidant, it generates strong and dangerous hydroxyl radical and at the same time it produces singlet oxygen, which leads to oxidative stress. The IC50 values presented in the Table 1 showed that all the extracts of less polar solvent (pet ether and chloroform) and some acetone extracts did not exhibit any SO activity. Except 75% aq ethanol, other aqueous acetone/ethanol extracts showed moderate activity. Venugopalan et al. (2008) also reported the inefficiency of non-polar solvents extract to show any super oxide anion scavenging activity. For non-distilled plant materials, 50% aq methanol extract showed maximum activity (IC50 = 285.3 µg/mL) (Table 1), and the same was for distilled by-products. By-product extract showed lower SO activity and its IC50 values increases from 21.6 to 105.3% as compared to non-distilled plant materials. Surprisingly, de-odorized water extract showed good SO activity. These observations differed from the DPPH and ABTS scavenging activity. Parejo et al. (2002) also found that surprising value for superoxide scavenging activity in distilled plant of Artemisia dracunculus and Achillea millefolium. Robak and Gryglewski (1988) reported that antioxidant activity of flavonoids is mainly by scavenging superoxide anions and moderate TFC in the extract of the present study may be reason for moderate to poor SO activities.
Fe2+ ion chelating activity
Metal chelating capacity is important phenomenon as it reduced the concentration of catalyzing transition metal in lipid peroxidation (Chakraborty et al. 2015). Thus, metal chelating activity of Java citronella plant materials and distilled waste is expressed in terms of ferrous ion chelating capacity where the extract interfered with the formation of ferrous and ferrozine complex. This indicates the chelating capacity of the extract as it is expressed by capturing ferrous ion before ferrozine.
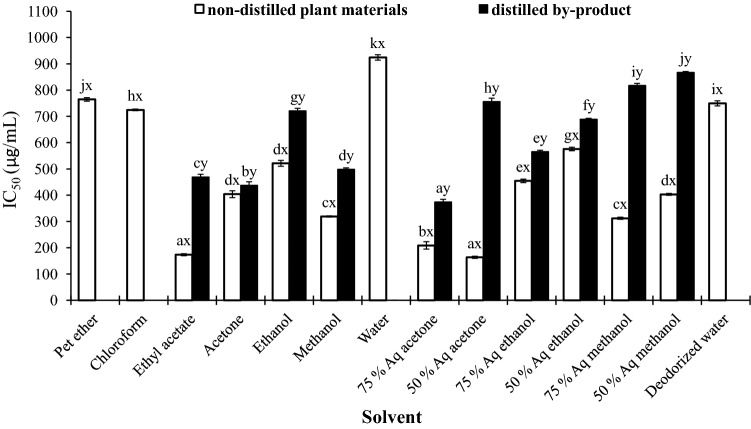
IC50 of different extract ranged from 173.4 (ethyl acetate)- 764.7 µg/mL (pet ether) and 373.6 (75% aq acetone)-867 µg/mL (50% aq methanol), for fresh plant materials and by-products, respectively (Fig. 4). Pet ether, chloroform and water extract of distilled by-products showed no IC50 values in the studied concentration range. On the contrary, Sabeena-Farvin and Jacobsen 2013 reported the superiority of water extract of seaweed over ethanolic extracts in-terms of ferrous ion chelating activity. Another discrepancy was that the ethyl acetate and aq acetone showed a better chelating capacity as compared to the methanol/ethanol or aqueous/methanol/ethanol mixture, though the former showed lower content of phenolics/flavonoides and other antioxidant activities. This indicates that chelating capacity does not solely depend on the total phenol/flavonoid content and may be associated with the presence or absence of particular chemical group of phenolics. Similarly, Ortiz-de Elguea-Culebras et al. (2017) also reported low correlation between phenols and iron chelating activity of distillation waste of four aromatic plants.
Fig. 4.
IC50 (µg/mL) values of iron metal chelating ability [For each solvent, values in the bar between the non-distilled plant materials and distilled plant extract, followed by a different letter (x, y) are significantly different (p < 0.05); For each extraction solvent, values in the bar within the non-distilled and distilled plant extract followed by a different letter (a, b, c, d, e and f etc.) are significantly different (p < 0.05)]
Coefficients of Pearson’s correlation among total Phenols/flavonoid content and different antioxidant assay
A multiple correlation analysis was performed for both non-distilled plant and by-product extracts to evaluate the association between the TPC, TFC and corresponding antioxidant assay derived as 1/IC50 values (Table 2). Significant correlations were found between DPPH, ABTS, FRAP and SO assays and TPC. In the fresh plant, TPC showed the highest correlation with 1/IC50 value for DPPH, whereas TPC of by-product exhibit highest correlation with FRAP assays. SO, assay showed the lowest but significant correlation with the TPC in both fresh and by-product. Significant positive correlations were also found between TFC and different antioxidant activity. In fresh plant, TFC showed highest correlation with 1/IC50 value derived from ABTS assay and for by-product maximum correlation of TFC was with 1/IC50 value of DPPH and FRAP assay. The lowest but significant correlation was found between SO assay and TFC in both fresh and by-product. TPC showed higher correlation with antioxidant assay as compared to the TFC. However, for fresh plant materials, the correlation was non-significant between metal chelating activity with TPC and TFC and was negatively associated for by-products. A strong positive correlation was found between the TPC and TFC (Table 2) irrespective of type of matrix [r = 0.949 (p < 0.01) and 0.928, (p < 0.01), respectively]. A poor correlation between metal chelating activities and flavonoids/phenolics content was also reported by Ortiz-de Elguea-Culebras et al. (2017).
Table 2.
Correlation between phenolics, flavonoids and antioxidant activity
| Non-distilled plant materials | ||||||||
|---|---|---|---|---|---|---|---|---|
| TPC | TFC | 1/IC50 DPPH | 1/IC50 ABTS | 1/EC50 FRAP | 1/IC50 SO | 1/IC50 Metal chelating ability | ||
| Distilled By-product | TPC | 1 | 0.949** | 0.965** | 0.925** | 0.957** | 0.914** | 0.046 |
| TFC | 0.928** | 1 | 0.920** | 0.928** | 0.892** | 0.888** | 0.135 | |
| 1/IC50 DPPH | 0.902** | 0.856** | 1 | 0.979** | 0.989** | 0.861** | 0.078 | |
| 1/IC50 ABTS | 0.877** | 0.792** | 0.928** | 1 | 0.959** | 0.814** | 0.167 | |
| 1/EC50 FRAP | 0.907** | 0.856** | 0.997** | 0.916** | 1 | 0.854** | 0.077 | |
| 1/IC50 SO | 0.759** | 0.703** | 0.719** | 0.536** | .736** | 1 | − 0.595** | |
| 1/IC50 Metal chelating ability | − 0.536** | − 0.402* | − 0.445* | − 0.410* | − 0.458* | − 0.787** | 1 | |
*Correlation is significant at P = 0.05 level (2-tailed), **Correlation is significant at P = 0.01 level (2-tailed)
Significant correlations were also found between the various antioxidant assay, especially between DPPH and FRAP assays, [r = 0.989 (p < 0.01) and 0.997, (p < 0.01) for fresh plant and distilled by-product, respectively], (Table 2) and DPPH and ABTS assays [r = 0.979 (p < 0.01) and 0.928, (p < 0.01) for fresh plant and distilled by-product, respectively] (Table 2). A good correlation was also found between the 1/IC50 values of FRAP and ABTS assay also. The lowest correlations were found between the SO assay and others. However, the correlation was non-significant between 1/IC50 values of metal chelating activity and other antioxidant assay (except SO, where it is negative)for fresh plant of Java citronella and it was negative for by-products. The results revealed that several factors such as type of extraction solvent, the sample material and type of phenolic/flavonoid compounds which react in different ways in these assays.
Conclusion
The distillation residue of Java citronella produces a non-profitable waste, which can be valorized as a potential source of natural antioxidants. The content of total phenolics/flavonoids as well as antioxidant activity was highly dependent on type of solvent used and it was found that aqueous combination of polar solvent showed better efficiency on extraction of the compounds from both non-distilled and distilled plant materials. Aqueous 75% and 50% methanol were found to be the best solvent for extracting total phenolics/flavonoids as well as antioxidant activity of extracts in terms DPPH, ABTS, SO and FRAP assay. However, ethyl acetate and aq acetone extract showed maximum iron chelating ability. Results of this study showed that the distillation waste of Java citronella exhibits moderate antioxidant potential and it’s antioxidant activity was lower than the original plant materials. However, recovery of appreciable amounts of antioxidants from the hydro-distilled by-product indicates that this could be exploited as a natural and safe antioxidant source for dietary applications. Further extensive investigation on phyotochemical composition and characterisations of this Java citronella distillation waste are still required for better exploitation of this agro-waste.
Acknowledgements
The senior author is thankful to the Director, ICAR-DMAPR, Anand for providing necessary facilities during the period of this investigation.
Author contribution
All the authors contributed equally for this work.
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alfa IM, Dahunsi SO, Iorhemen OT, Okafor CC, Ajayi SA. Comparative evaluation of biogas production from poultry droppings, cow dung and lemon grass. Bioresour Technol. 2014;157:270–277. doi: 10.1016/j.biortech.2014.01.108. [DOI] [PubMed] [Google Scholar]
- Annegowda HV, Mordi MN, Ramanathan S, Hamdan MR, Mansor SM. Effect of extraction techniques on phenolic content, antioxidant and antimicrobial activity of Baulina purpurea: HPTLC determination of antioxidants. Food Anal Method. 2012;5(2):226–233. doi: 10.1007/s12161-011-9228-y. [DOI] [Google Scholar]
- Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J Agric Food Chem. 2003;51(23):6657–6662. doi: 10.1021/jf034790i. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Joseph D, Praveen NK. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J Food Sci Technol. 2015;52(4):1924–1935. doi: 10.1007/s13197-013-1189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatha SAS, Anwar F, Manzoor M. Evaluation of the antioxidant activity of rice bran extracts using different antioxidant assays. Grasas Aceites. 2006;57:328–335. [Google Scholar]
- Deka H, Deka S, Baruah CK, Das J, Hoque S, Sarma NS. Vermicomposting of distillation waste of citronella plant (Cymbopogon winterianus Jowitt.) employing Eudriluseugeniae. Bioresour Technol. 2011;102(13):6944–6950. doi: 10.1016/j.biortech.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Dhanani T, Shah S, Gajbhiye NA, Kumar S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem. 2013;10:S1193–S1199. doi: 10.1016/j.arabjc.2013.02.015. [DOI] [Google Scholar]
- Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22(3):296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman HJD, Peltoketo A, Hiltunen R, Tikkanen MJ. Characterization of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003;83(2):255–262. doi: 10.1016/S0308-8146(03)00088-8. [DOI] [Google Scholar]
- FDA (2012) Guidance for industry. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073395.pdf. Accessed 28 Sept 2015
- Gavarić N, Kladar N, Mišan A, Nikolić A, Samojlik I, Mimica-Dukić N, Božin B. Post distillation waste material of thyme (Thymus vulgaris L., Lamiaceae) as a potential source of biologically active compounds. Ind Crops Prod. 2015;74:457–464. doi: 10.1016/j.indcrop.2015.05.070. [DOI] [Google Scholar]
- González-Montelongo R, Lobo MG, González M. Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem. 2010;119(3):1030–1039. doi: 10.1016/j.foodchem.2009.08.012. [DOI] [Google Scholar]
- Hossain MA, AL-Raqmi KAS, AL-Mijizy ZH, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Biomed. 2013;3(9):705–710. doi: 10.1016/S2221-1691(13)60142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmardiyani S, Fidrianny I, Alfianti F. Antioxidant profile and phytochemical content of three kinds of lemon grass grown in West Java-Indonesia. Asian J Pharm Clin Res. 2016;9(4):381–385. [Google Scholar]
- Leite BL, Bonfim RR, Antoniolli AR, Thomazzi SM, Araújo AA, Blank AF, Estevam CS, Cambui EV, Bonjardim LR, Albuquerque Júnior RL, Quintans-Júnior LJ. Assessment of antinociceptive, anti-inflammatory and antioxidant properties of Cymbopogon winterianus leaf essential oil. Pharm Biol. 2010;48(10):1164–1169. doi: 10.3109/13880200903280000. [DOI] [PubMed] [Google Scholar]
- Lim YY, Lim TT, Tee JJ. Antioxidant properties of several tropical fruits: a comparative study. Food Chem. 2007;103:1003–1008. doi: 10.1016/j.foodchem.2006.08.038. [DOI] [Google Scholar]
- Liu F, Ooi VEC, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997;60:763–771. doi: 10.1016/S0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Liyana-Pathiranan CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L) as affected by gastric pH conditions. J Agric Food Chem. 2005;53:2433–2440. doi: 10.1021/jf049320i. [DOI] [PubMed] [Google Scholar]
- Martino LD, Mancini E, Almeida LFRD, Feo VD. The anti germinative activity of twenty-seven monoterpenes. Molecules. 2010;15(9):6630–6637. doi: 10.3390/molecules15096630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SR, Pilgaonkar VW, Panda VS. Evaluation of antioxidant activity of Ginkgo biloba phytosomes in rat brain. Phytother Res. 2006;20(11):1013–1016. doi: 10.1002/ptr.1976. [DOI] [PubMed] [Google Scholar]
- Nandapure SP, Wankhade SG, Jadhao SM, Bhoyar SM, Wanjari SS, Sarode RB. Quality parameters of Java citronella oil as influenced by nutrient management under inceptisols. Int J Trop Agric. 2016;34(3):579–584. [Google Scholar]
- Ortiz-de Elguea-Culebras G, Berruga MI, Santana-Méridas O, Herraiz-Peñalver D, Sánchez-Vioque R. Chemical composition and antioxidant capacities of four Mediterranean industrial essential oils and their resultant distilled solid by-products. Eur J Lipid Sci Technol. 2017;119(12):1700242. doi: 10.1002/ejlt.201700242. [DOI] [Google Scholar]
- Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codina C. Comparison between the radical scavenging activity and antioxidant activity of six distilled and non-distilled Mediterranean herbs and aromatic plants. J Agric Food Chem. 2002;50(23):6882–6890. doi: 10.1021/jf020540a. [DOI] [PubMed] [Google Scholar]
- Plánder S, Gontaru L, Blazics B, Veres K, Kéry A, Kareth S, Simándi B. Major antioxidant constituents from Satureja hortensis L. extracts obtained with different solvents. Eur J Lipid Sci Technol. 2012;114:772–779. doi: 10.1002/ejlt.201100273. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Robak J, Gryglewski IR. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- Sabeena-Farvin KH, Jacobsen C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013;138:1670–1681. doi: 10.1016/j.foodchem.2012.10.078. [DOI] [PubMed] [Google Scholar]
- Saha A, Basak BB, Ponnuchamy M. Performance of activated carbon derived from Cymbopogon winterianus distillation waste for scavenging of aqueous toxic anionic dye Congo red: comparison with commercial activated carbon. Sep Sci Technol. 2019 doi: 10.1080/01496395.2019.1620277. [DOI] [Google Scholar]
- Santana-Méridas O, González-Coloma A, Sánchez-Vioque R. Agricultural residues as a source of bioactive natural products. Phytochem Rev. 2012;11(4):447–466. doi: 10.1007/s11101-012-9266-0. [DOI] [Google Scholar]
- Shen J, Shao X. A comparison of accelerated solvent extraction, Soxhlet extraction, and ultrasonic-assisted extraction for analysis of terpenoids and sterols in tobacco. Anal Bioanal Chem. 2005;383:1003–1008. doi: 10.1007/s00216-005-0078-6. [DOI] [PubMed] [Google Scholar]
- Singh NK, Vemu B, Nandi A, Singh H, Kumar R, Dumka VK. Acaricidal activity of Cymbopogon winterianus, Vitex negundo and Withania somnifera against synthetic pyrethroid resistant Rhipicephalus (Boophilus) microplus. Parasitol Res. 2014;113(1):341–350. doi: 10.1007/s00436-013-3660-4. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Tohidi B, Rahimmalek M, Arzani A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017;220:153–161. doi: 10.1016/j.foodchem.2016.09.203. [DOI] [PubMed] [Google Scholar]
- Tsimogiannis D, Choulitoudi E, Bimpilas A, Mitropoulou G, Kourkoutas Y, Oreopoulou V. Exploitation of the biological potential of Satureja thymbra essential oil and distillation by-products. J Appl Res Med Aromat Plants. 2017;4:12–20. doi: 10.1016/j.jarmap.2016.07.002. [DOI] [Google Scholar]
- Venugopalan S, Sharma A, Venugopalan V, Gautam H. Comparative study on the antioxidant activities of extracts from Piper betle leaves. Biomed Pharmacol J. 2008;1(1):115–120. [Google Scholar]
- Wang J, Zhao YM, Guo CY, Zhang SM, Liu CL, Zhang DS, Bai XM. Ultrasound-assisted extraction of total flavonoids from Inula helenium. Pharmacogn Mag. 2012;8(30):166. doi: 10.4103/0973-1296.96581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Haley S, Perret J, Harris M. Antioxidant properties of hard winter wheat extracts. Food Chem. 2002;78(4):457–461. doi: 10.1016/S0308-8146(02)00156-5. [DOI] [Google Scholar]