Abstract
The current study was employed to investigate the organochlorine pesticides (OCP) concentrations in milk, as the milk we consume, has residues of these notorious pesticides. The residual concentrations of OCP in milk have numerous harmful effects on health especially the children. Therefore, milk was analyzed using gas chromatography equipped with µECD for seven OCP residues, namely α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γ-HCH, Dieldrin, and DDT. Three hundred and sixty raw milk samples were collected from urban areas (10 areas of Lahore N = 300) and Dairy Farms (10 farms in Lahore N = 60) from September 2012 to September 2013. Samples were collected after an interval of 2 months, for 12 months. Mean values of OCPs in milk samples from urban areas were reported as α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γ-HCH, and Dieldrin with concentration of 17.44 ± 3.99, 35.74 ± 7.50, 20.28 ± 3.95, 2.51 ± 0.55, 0.93 ± 0.16 and 1.12 ± 0.18 µg kg−1, respectively, while the milk samples from dairy farms with concentration of 26.94 ± 4.63, 59.88 ± 6.76, 32.07 ± 4.51, 4.64 ± 0.48, 1.20 ± 0.17 and 1.93 ± 0.18 µg kg−1, respectively. None of the samples analyzed were found positive for the presence of DDT, just as none of the samples from area milk shops exceeded the Maximum Residual Limits (MRLs). γ-HCH and β-endosulfan were found higher in dairy farm milk samples than the MRLs. Conclusively, these pesticide residues are present in milk available in Lahore in enough quantity (some exceeding the MRLs) to threaten human health, particularly the infant and children.
Keywords: Organochlorine pesticides, Milk samples, Health hazardous effects
Introduction
Milk is a liquid of immense importance due to the nutritional value it possess for human health, particularly the infants but it has been at high risk of being contaminate and adulterated by a wide variety of chemical, water sources, metals, pesticide residues. These dangerous chemicals originate through cow’s treatments, feeding, milking atmosphere, processing plants and deliberate dilution or fake milk formation for profit purposes. These chemicals include antibiotics, hormones, disinfectants, nitrites, polychlorinated Biphenyls (PCBs), mycotoxins, toxic metals, dioxins and pesticides (Heeschen and Harding 1995). Among these, organochlorine pesticides (OCP) are the most stable and persistent ones in the environment, with some sure hazardous effects on health, with peculiar mechanism of action is human. Most of the OCP employ their hazardous effects by injuring the insect nervous system. These dangerous chemicals follow the same mechanism for human by attacking the nervous system, but the lethal doses may differ for insects and human. Due to the dependence of the organism on the dose for strong toxic effects, care should always be taken when these pesticides come in contact with human which require the knowledge of residual concentration in food chain (Joy 1982). The OCPs are categorized into three universal groups namely dichlorodiphenylethane (include DDT, DDD and dicofol), chlorinated cyclodienes (Includes Aldrin, Dieldrin and heptachlor) and hexachlorocyclohexanes (include lindane only). These compounds exits in the form of residues in all food chains from soil to animals but these may vary in toxic doses, skin absorption levels, metabolism and even elimination from the body (Hayes and Laws 1982).
OCPs have been widely used to manage both agrarian and animal pest infestation in the past, resulting in contaminated soil and environment to life threatening levels. As these compounds are highly persistent in nature, continue contaminating the food and fodder crops for a pretty longer period of time (Robertson et al. 2002). Animals reared on this feed and fodder especially the dairy animals mostly fetch these residues from the contaminated feed. Once these residues have been metabolized, they reach into fat deposits and blood flow from which these are partly removed in milk, putting human health at risk (Martínez et al. 1997). These OCPs are extremely harmful to man especially children, owing to bioaccumulation in the human body. Hazardous effects of OCPs are multiple which may lead to birth defects in infants, neurological disorders, behavior changes and reproductive defects, mild to severe gastrointestinal problems and even cancer in severe cases (Windham 2002). Currently, there are pretty few reports available on pesticide residues in different food, fruit and vegetables especially the raw milk in Pakistan. Therefore, it is necessary to assess OCP residues levels in marketed milk available in Pakistan especially the metropolitan cities like Lahore. To asses the the OCP residues present study was employed to determine the OCP residues’ concentrations in raw milk from urban areas and dairy farms in Lahore.
Materials and methods
Milk samples
Three hundred sixty raw milk samples (500 ml each) were collected from urban areas (10 areas of Lahore) and dairy farms (10 Dairy Farms in the vicinity of Lahore) from September 2012 to September 2013. Samples were collected after a 2-months interval for the whole year. Samples were collected in clean and sterilized polyethylene bottles. Milk samples were kept in an ice box during collection and transportation to National Institute of Food Science and Technology laboratory. The samples were extracted for pesticide residues and then frozen at − 40 °C.
Extraction of residues
Pesticide residue extraction was caried out using the procedural method developped by Heck et al. (2007), with some modifications. Milk samples were homogenized, mixed and afterwards, a sample of 100 ml was divided into two portions of 50 ml, each centrifuged for 15 min, at 5000 rpm. Upper layer of fat was removed for further analysis. 1 g fat was mixed with anhydrous sodium sulfate (25 g) in a flask (250 ml) with petroleum ether (100 ml) shaking vigorously for 2 min. Petroleum ether was filtered off. The final extract was evaporated and concentrated to around 1 ml, taken up in 3 ml of hexane and kept in a flask for the clean-up process (Heck et al. 2007).
Clean-up with florisil
Glass wool was plugged in glass column, washed with hexane and packed with Florisil followed by anhydrous sodium sulfate, rinsed with hexane. Fat extract was added in column, eluted with n-hexane to extract organochlorine pesticides and taken in rotary evaporator flask. Concentrated extract taken up into a glass vial with hexane and evaporated to dryness by nitrogen gas. Residues were dissolved in cyclohexane for GC analysis (Heck et al. 2007).
GC-µECD analysis
OCP residues were determined using an Agilent Model 6890 Gas Chromatograph equipped with a 63Ni electron capture detector (GC-ECD). Reference standards were used for individual OCP identification and quantification. 1 µl of the extract was injected into a fused silica capillary column (HP-5MS) of 30 m length and 0.25 mm i.d., using nitrogen carrier gas with a 1.0 ml/min flow rate with the following conditions: Inlet Temp. (270 °C), Detector (µECD), Detector Temp. (300 °C), Injection volume (1 µl), Carrier gas (N2), Total flow (60 ml min−1), Column (HP-5 MS), Column flow (1 ml min−1) (Fig. 1).
Fig. 1.
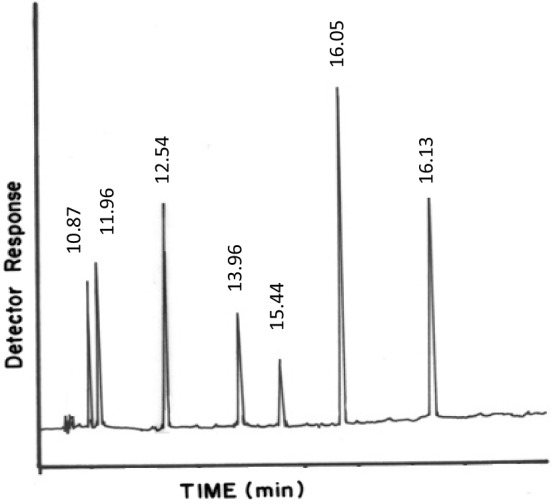
Standard chromatogram of OCP residues analyzed by GC-ECD. Detector temp. 300 °C, injection volume 1 µL, capillary column (HP-5MS). Peaks: (1) γHCH (10.87), (2) α-endosulfan (11.96), (3) β-endosulfan (12.54), (4) endosulfan-sulphate (13.96), (5) DDE (15.44), (6) DDT (16.05), (7) Dieldrin (16.13)
Oven conditions
With regards to oven conditions, these were as follows: (1) initial temp. (100 °C for 2 min); (2) temp. increase (up to 210 °C, at 6 °C min−1 and isothermal for 2 min); (3) temp. rises (260 °C, at 8 °C min−1 and isothermal for 2 min); (4) temp. increase (up to 300 °C, at 10 °C min−1 and isothermal for 10 min).
OCPs levels determination in milk samples
OCPs residues mass determination was performed using following equation:
where As and Astd corresponds to the peak height of the standard and sample, respectively; Istd of µl of the standard extract injected and Is of µl of the sample injected, Vf to the final volume of the sample extract (ml), Ms to the mass of milk fat sample in g and, finally, CRf to the concentration in ppm of the reference standard.
Data analysis
Data was analysed using the Statistical Package for Social Sciences (SPSS, IBM Corp. USA) software, version 20.0. Two-way Analysis of Variance (ANOVA) with Duncan multiple range test (DMR) were used, with an alpha set at 0.01.
Results
Table 1 shows the range and maximum residue limit (MRLs) values of seven OCP residues detected in milk samples collected from different urban areas and dairy farms in Lahore, respectively. In urban areas, mean milk samples contamination by α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γHCH, and Dieldrin were 17.44 ± 3.99, 35.74 ± 7.51, 20.28 ± 3.95, 2.51 ± 0.55, 0.92 ± 0.15 and 1.12 ± 0.17 µg kg−1, respectively. With regards to dairy farm milk samples, the mean values for α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γHCH, and Dieldrin were 26.95 ± 4.63, 59.88 ± 6.76, 32.07 ± 4.51, 4.64 ± 0.48, 1.20 ± 0.17 and 1.93 ± 0.18 µg kg−1, respectively. In both types of samples, DDT contamination was not detected and none of the milk samples from urban areas exceeded the MRLs vales. Maximum values for α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γHCH, and Dieldrin were, respectively, 48.19, 74.29, 41.77, 7.14, 2.61, 4.21 µg kg−1. The mean values for γHCH and β-endosulfan in dairy farm milk samples were higher in magnitude than the MRLs indorsed for these pesticides.
Table 1.
OCP residues (µg Kg−1) in milk samples
| Parameters | Pesticides (µg Kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| α-Endosulfan | β-Endosulfan | Endosulfan-sulphate | DDE | γ-HCH | Dieldrin | DDT | |
| OCP residues (µg kg−1) in raw milk samples collected from urban areas | |||||||
| Minimum | 6.85 | 13.44 | 2.27 | 0.98 | 0.72 | 0.89 | ND |
| Maximum | 34.92 | 53.84 | 30.26 | 5.17 | 1.89 | 3.05 | ND |
| Mean ± SD | 17.44 ± 3.99 | 35.74 ± 7.51 | 20.28 ± 3.95 | 2.51 ± 0.55 | 0.92 ± 0.16 | 1.12 ± 0.17 | – |
| MRL | 50 | 50 | 50 | 40 | 1 | 6 | 40 |
| OCP residues (µg kg−1) in raw milk samples collected from dairy farms | |||||||
| Minimum | 9.45 | 18.54 | 3.13 | 1.41 | 0.99 | 1.22 | ND |
| Maximum | 48.19 | 74.30 | 41.77 | 7.14 | 2.61 | 4.21 | ND |
| Mean ± SD | 26.95 ± 4.63 | 59.88 ± 6.76 | 32.07 ± 4.51 | 4.64 ± 0.48 | 1.20 ± 0.17 | 1.93 ± 0.18 | – |
| MRL | 50 | 50 | 50 | 40 | 1 | 6 | 40 |
ND not detected, SD standard deviation
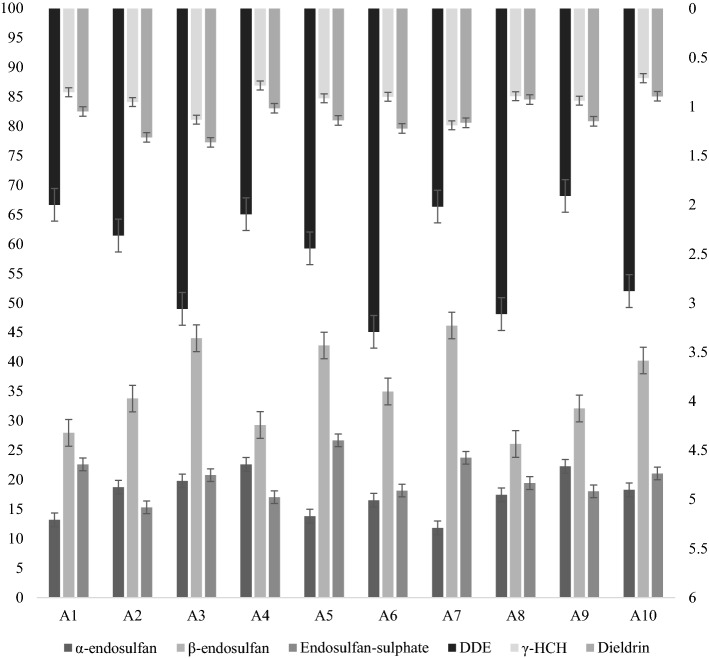
Figure 2 shows the mean values of OCP residues in milk samples collected from ten urban areas of Lahore district. The OCPs α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γ-HCH and Dieldrin achieved the highest mean values in area A9 (22.27 µg kg−1), A7 (46.18 µg kg−1), A7 (23.73 µg kg−1), A6 (3.29 µg kg−1), A3 (1.13 µg kg−1), A3 (1.36 µgkKg−1), and DDT was not detected in any sample of the ten areas studied. Concerning β-endosulfan residual concentration was highest in magnitude than all the other OCP residues. All the ten areas of milk collection were statistically significantly from each other for their residue values of pesticides detected.
Fig. 2.
Mean concentrations of OCP residues (µg kg−1) in raw milk samples collected from urban areas. A1-Iqbal Town, A2-Gulberg Town, A3-Samanabad Town, A4-Data Ganj Baksh Town, A5-Ravi Town, A6-Nishtar Town, A7-Aziz Bhatti Town, A8-Shalimar Town, A9-Wahgah Tow, A10-Cantonment, F1-Sabeel Dairy, F2-Decent Dairy, F3-Farmer's Dairy, F4-Sadaat Dairy, F5-RC Cola Dairy, F6-Zakky Dairy, F7-Star Dairy, F8-Quelts Dairy, F9-Noble Dairy, F10-Hussain Cattle and Dairy Farm
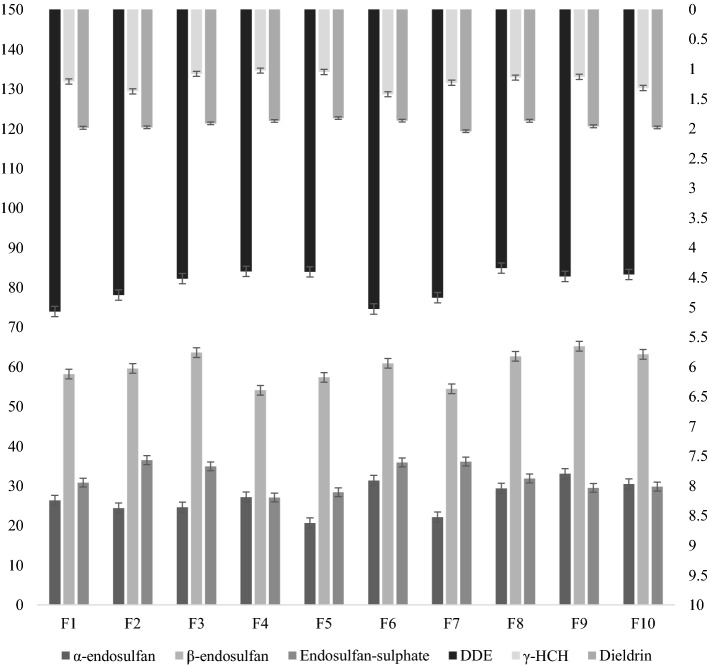
Figure 3 shows the mean contamination of OCP residues in milk samples collected from ten dairy farms of Lahore district. The OCPs α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γHCH and Dieldrin achieved the highest mean values in farm F8 (29.37 µg kg−1), F9 (65.14 µg kg−1), F2 (36.47 µg kg−1), F1 (5.07 µg kg−1), F6 (1.42 µg kg−1), F7 (2.05 µg kg−1), and DDT was not detected in any sample from the dairy farms too. Concerning to β-endosulfan residues concentration, it was highest in magnitude than all the other OCP residues. All the ten dairy farms were statistically significantly from each other for their residue values of detected pesticides.
Fig. 3.
Mean concentrations of OCP residues (µg kg−1) in raw milk samples collected from dairy farms. A1-Iqbal Town, A2-Gulberg Town, A3-Samanabad Town, A4-Data Ganj Baksh Town, A5-Ravi Town, A6-Nishtar Town, A7-Aziz Bhatti Town, A8-Shalimar Town, A9-Wahgah Tow, A10-Cantonment, F1-Sabeel Dairy, F2-Decent Dairy, F3-Farmer's Dairy, F4-Sadaat Dairy, F5-RC Cola Dairy, F6-Zakky Dairy, F7-Star Dairy, F8-Quelts Dairy, F9-Noble Dairy, F10-Hussain Cattle and Dairy Farm
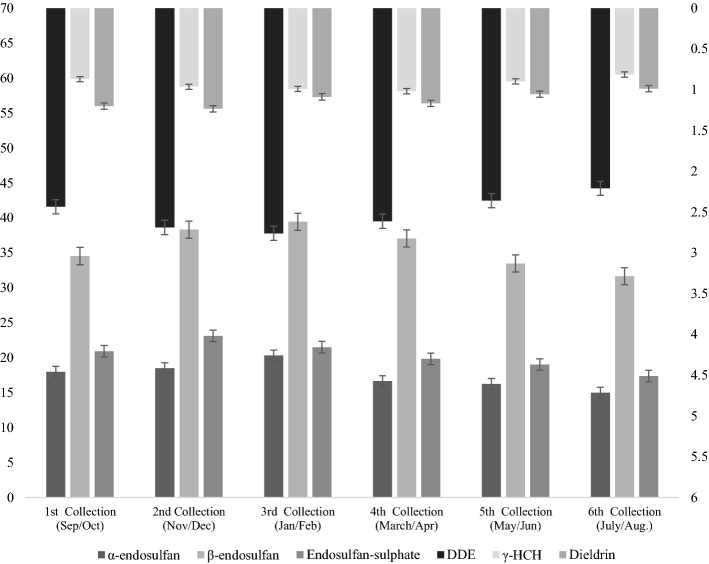
Figure 4 shows the means of OCP residues in milk samples collected at six (06) intervals from urban areas in Lahore. There were six collecting intervals, each comprising of 2 months, i.e., 1st (September/October), 2nd (November/December), 3rd (January/February), 4th (March/April), 5th (May/June) and 6th (July/August). Most of the OCPs achieved the highest mean values in winter intervals, namely 3rd (January and February) and 2nd (November and December) intervals, while the lowest values were observed during the 5th (May and June) and 6th (July and August) intervals, which corresponds to the warmer months of the year. The OCP residues α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γHCH and Dieldrin showed highest mean values during the 3rd (20.31 µg kg−1), 3rd (39.43 µg kg−1), 2nd (23.11 µg kg−1), 3rd (2.76 µg kg−1), 3rd (0.99 µg kg−1) and 2nd (1.24 µg kg−1) respectively, while DDT was not detected in any milk sample collected at any intervals from urban areas. Similarly, α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γHCH and Dieldrin showed the lowest mean values in the 6th (14.98 µg kg−1), 6th (31.64 µg kg−1), 6th (17.36 µg kg−1), 6th (2.21 µg kg−1), 6th (0.82 µg kg−1) and 6th (0.99 µg kg−1) intervals respectively, while DDT was not detected in any sample of the any area. Intervals were also statistically significantly different from each other with regards to the mean concentration of all the detected OCP residues.
Fig. 4.
Mean OCP residues (µg kg−1) in raw milk for collection intervals in urban areas
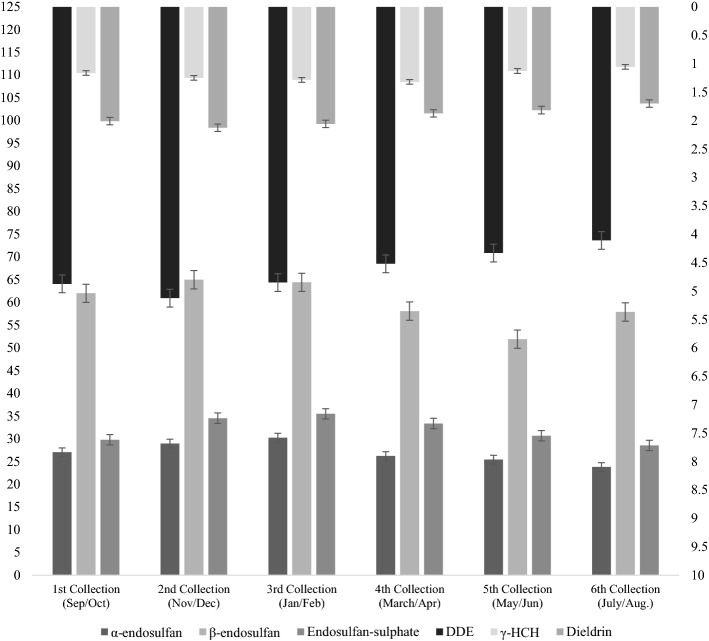
Figure 5 shows the mean contamination values of OCP residues in milk samples collected at six (06) intervals from dairy farms in Lahore. There were six collecting intervals, each one comprising 2 months, i.e., 1st (September/October), 2nd (November/December), 3rd (January/February), 4th (March/April), 5th (May/June), 6th (July/August). Most of OCPs achieved the highest mean values during winter intervals, 3rd (January and February) and 2nd (November and December), and the lowest values were observed during the 5th (May/June) and 6th (July and August) intervals, which corresponded to the warmer months of the year. The OCP residues of α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γHCH and Dieldrin showed the highest mean values for the residues in the 3rd (30.24 µg kg−1), 2nd (64.98 µg kg−1), 2nd (34.54 µg kg−1), 2nd (5.12 µg kg−1), 3rd (1.29 µg kg−1) and 2nd (2.12 µg kg−1) intervals respectively, while DDT was not detected in any of the samples from any interval of the dairy farms. Similarly, α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γHCH and Dieldrin showed the lowest mean values in the 6th (23.79 µg kg−1), 6th (57.90 µg kg−1), 6th (28.55 µg kg−1), 6th (4.11 µg kg−1), 6th (1.06 µg kg−1) and 6th (1.70 µg kg−1) intervals respectively, while DDT was not detected in any sample. Intervals were also statistically significantly different from each other with regards to the mean concentration of all detected OCP residues.
Fig. 5.
Mean OCP residues (µg kg−1) in raw milk for collection intervals in dairy farms. *Primary axis: α-endosulfan, β-endosulfan, endosulfan-sulphate. *Secondary axis: DDE, γHCH and dieldrin
Discussion
Although OCPs were banned in the early 1980s, but these pesticide chemicals continued to be used in large amounts in some tropical countries for agricultural and human pest control management. These practices polluted the environment and hence, the food chain. Specifically, organochlorines have slow decomposition rates and longer half-life, which give them the attribute of high stability in the environment (Aslam et al. 2013). Therefore, soils that were heavily treated with OCPs in the past are still a good source of OCPs for fodder and food crops (Fiedler et al. 2000). Organochlorines reach in milk and milk animals through several routs, i.e., contaminated feedstuffs, post-harvest application of pesticides, contaminated air, water, direct application to animals and application of pesticides in milk processing plants for hygienic purposes. Milk animals store pesticides in fat-rich tissues because of the lipophilic nature of OCPs and finally translocated to milk fat (Aslam et al. 2013). This study shows higher OCPs values in dairy farm milk than the area milk. This is because milk from the dairy farm is rich in fat compared to the area milk which undergoes severe water adulteration and has lower fat values due to skimming practices.
Organochlorines are lipophilic in nature due to which milk with higher fat contents have higher values of these residues, while water adulteration and other skimming practices results in a reduction of the pesticide load in area milk samples bought from vendors (John et al. 2001). In this study, samples from both sources, i.e., urban areas and dairy farms, showed the presence of OCP residues in milk. This may perhaps be explained by the widespread presence of organochlorine residues in most of the tropical zones due to its extensive use in the past. These results are in agreement with John et al. (2001), Battu et al. (2004) and Aslam et al. (2013) who reported the presence of OCPs in milk from Jaipur city, Ludhiana district and Dehli city respectively. Similarly, Ashnagar et al. (2009) showed the OCP contamination in milk samples from Ahwaz city, Iran. In 2011, Kampire et al. reported the OCP residues contamination in milk from kampla market in Uganda (Kampire et al. 2011). Mocanu et al. (2012) indicated the presence of OCPs in milk samples collected from the eastern region of Romania, while Dogheim et al. (1988) and El-Gebaly (2000) showed the existence of organochlorine residues in milk samples collected from different Governorates of Egypt. Pardio et al. (2003) also stated the residues of the OCP pollution in milk samples from a tropical region of Mexico, in North America.
DDT was not detected in raw milk samples from both sources. This is possibly due to the gradual decomposition and decline in DDT use since its legal prohibition (John et al. 2001). Researchers also found a steady drop in DDT values in foods, especially milk. Studies have proven the gradual decline in DDT like Dogheim et al. (1988) reported organochlorine pesticides in milk from Beni-Suef with mean values of DDT as 2031 ppb, and in 1996 he observed that the DDT level in dairy milk was much lower than previously estimated DDT levels.
El-Gebaly (2000) studied organochlorine compounds in Great Cairo, and found presence of DDT in 25% of milk samples with a mean value of 173 ppb, while in Spain, Martinez et al. (1997) found that only 1% of milk samples were contaminated with DDT, with a mean level of 7 ppb. Nida’M et al. (2009) reported absence of DDT in all samples at their detection limits, which support results of this study. In 2012, total DDT levels were detected below the detection limit by Mocanu et al. (2012), in agreement of the data obtained in this study.
Dieldrin residues ranged from 0.89 to 3.05 µg kg−1 (urban areas) and 1.22 to 4.21 µg kg−1 (dairy farms), with mean concentrations of 1.12 ± 0.18 µg kg−1 (urban areas) and 1.93 ± 0.18 µg kg−1 (dairy farms). The MRL for Dieldrin is 6 µg kg−1 showing that the results are well within safe limits. Mean Dieldrin values obtained in this study were found lower than the mean value found by El-Gebaly (175 µg kg−1) in Great Cairo (El-Gebaly 2000), and Martinez et al. (28 ppb) in Spain (Martínez et al. 1997). Ashnagar et al. (2009) found higher mean values (33 ppb) than those obtained in this study. On the contrary, Kampire et al. (2011) found mean values at many low concentrations in raw milk samples collected from Kampala market, Uganda. These results are in agreement with those stated in this work. Interestingly, Bulut et al. (2011) found a mean value of 0.63 ppb, which are even lower than our readings obtained in this study.
α-Endosulfan residues ranged from 6.85 to 34.92 µg kg−1 (urban areas) and 9.45 to 48.19 µg kg−1 (dairy farms) with mean concentrations of 17.44 ± 3.99 µg kg−1 (urban areas) and 26.94 ± 4.63 µg kg−1 (dairy farms). The MRL for α-Endosulfan is 50 µg kg−1, revealing the study results to be well in the safe limits. Nag and Raikwar (2008), Aslam et al. (2013) and Bulut et al. (2011) found mean values for α-Endosulfan lower than those of this research, i.e., 6.5 ppb, 2 ppb, and 2.45 ppb, respectively. However, Ciscato et al. (2002) reported levels of 140 ppb in raw milk samples collected in Brazil, which is higher than that of this work.
β-Endosulfan Residues, the samples analyzed in this study ranged from 13.44 to 53.84 µg kg−1 (urban areas) and 18.54 to 74.30 µg kg−1 (dairy farms) with mean concentration of 35.74 ± 7.50 µg kg−1 (urban areas) and 59.88 ± 6.76 µg kg−1 (dairy farms). The MRL is 50 µg kg−1, showing that the results of the area samples are slightly within safe limits, but are well above the safe limits for dairy farm samples. This work agrees with Nag and Raikwar (2008), who found mean values of 22.9 ppb. Aslam et al. (2013) detected lower mean values (4.1 ppb), while Ciscato et al. (2002) reported values of higher concentration (150 ppb) in milk samples collected from Sao Paulo city of Brazil.
Endosulfan-sulphate levels detected in this study ranged from 2.27 to 30.27 µg kg−1 (urban areas) and 3.13 to 41.77 µg kg−1 (dairy farms) with mean concentration of 20.28 ± 3.95 µg kg−1 (urban areas) and 32.07 ± 4.51 µg kg−1 (dairy farms). The MRL is 50 µg kg−1, being slightly within safe limits for both type of samples. These results resembles to that of Nag and Raikwar (2008) and Aslam et al. (2013), who found mean values of 19.8 ppb and 43 ppb, respectively, in collected raw milk samples. However, Bulut et al. (2011) found lower mean values (7.25 ppb) in milk samples than that fount in this study.
On the other hand, milk samples analyzed in this study ranged from 0.72 to 1.89 µg kg−1 (urban areas) and 0.99 to 2.61 µg kg−1 (dairy farms) with regards to γ-HCH residues, and mean concentration was of 0.93 ± 0.16 µg kg−1 (urban areas) and 1.20 ± 0.17 µg kg−1 (dairy farms). The MRL for γ-HCH is 1 µg·kg−1, showing that our results are not within safe limits. Kampire et al. (2011), Battu et al. (2004), Pandit et al. (2002), Ashnagar et al. (2009), John et al. (2001), Nag and Raikwar (2008) and Pardio et al. (2003) reported mean values of γ-HCH residues in raw milk of 26 ppb, 13.49 ppb, 52 ppb, 21 ppb,46 ppb, 10 ppb and 128 ppb, respectively, which were higher than that obtained in this study.
Regarding DDE, milk samples analyzed in this study ranged from 0.98 to 5.17 µg kg−1 (urban areas) and 1.41 to 7.14 µg kg−1 (dairy farms) with mean concentration of of 2.51 ± 0.55 µg kg−1 (urban areas) and 4.64 ± 0.48 µg kg−1 (dairy farms). The MRL is 40 µg kg−1 for DDE, showing that the results of this study are well within safe limits. Data obtained in this study is similar to that of reported by Kampire et al. (2011), who stated mean values in raw milk samples of 9 ppb. Mean DDE values were observed at higher concentrations by Costabeber et al. (2001), Mallatou et al. (1997), Ashnagar et al. (2009), John et al. (2001), Nag and Raikwar (2008) and Pardioa et al. (2003) with readings of 58 ppb, 22 ppb, 17 ppb, 30 ppb, 20 ppb and 39 ppb, respectively. Barkatina et al. (1999) reported mean value of 1.41 ppb lower than the mean values of this study for both type of sample sources.
Regarding the seasonal variation, statistically significant variations were observed in this study among the different collection intervals. Organochlorine pesticide residues were found higher in magnitude during the colder months and lower during the warmer months of the year. These results are in agreement with the results obtained by Watterson (1991), Robertson et al. (2002), John et al. (2001) and Tsiplakou et al. (2010). This variation in OCP residues in raw milk during different collecting intervals is essentially due to environmental stresses and feed variation of the animal feed (Tsiplakou et al. 2010). Presence of organochlorines at higher values during intervals of collection falling in winter months and lower values during warmer months may further be explained by environmental elements, like heat, wind, and rain, interval’s characteristics falling during summer and rainy seasons. However, the influence of these factors is much lower during colder months of the year.
On the other hand, residue dissipation is not fast enough from the field and cattle feed in the winter. As the feeding of milk animals during winter have more oil seeds, which are also one of the richest sources of OCP accumulation and transport OCPs residues is maximum for animal’s body in winter. Thus animals receive more pesticide residues in winter seasons, which results in higher residual levels of pesticides in milk during the winter time intervals (John et al. 2001).
Conclusion
Despite the ban of OCP, these are still positive in foods, particularly in milk. In present study, seven OCP (α-endosulfan, β-endosulfan, Endosulfan-sulphate, DDE, γ-HCH, Dieldrin, and DDT) were studied their presence in raw milk samples from dairy farms and urban areas. Milk samples from urban areas presented lower OCPs loads than those collected in dairy farms in Lahore. During the months of winter OCP residues were higher in magnitude than warmer months of the year. None of the samples analyzed were found positive for the presence of DDT, while none of the samples from area milk shops exceeded the Maximum Residual Limits (MRLs). γ-HCH and β-endosulfan residues were found in higher magnitude than the MRLs in samples collected from dairy farms.
The study showed the presence of pesticide residues in the most vital component of human food, the Milk. Results reveals that these residues are in sufficient quantities in milk to threat the health, showing the alarming situation of milk available in Lahore. Such milk, when consumed, endangers human health, particularly infants and children. It is established fact that these pesticides have very deleterious effects on health leading to irreversible damages even at their lower levels too. Thus, it is of utmost need of the time to deepen regulatory investigations in this matter to improve the overall food security and safety of humans at the whole.
Acknowledgements
N. Martins would like to thank the Portuguese Foundation for Science and Technology (FCT-Portugal) for the Strategic project ref. UID/BIM/04293/2013 and “NORTE2020—Northern Regional Operational Program” (NORTE-01-0145-FEDER-000012). Thanks Higher Education Commission (HEC) Pakistan for support to the study.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bahare Salehi, Email: bahar.salehi007@gmail.com.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Natália Martins, Email: natalia.martins@ceb.uminho.pt.
References
- Ashnagar A, Naseri NG, Farmad MC. Determination of organochlorine pesticide residues in cow's milk marketed in Ahwaz city of Iran. Int J PharmTech Res. 2009;1(2):247–251. [Google Scholar]
- Aslam M, Rais S, Alam M. Quantification of organochlorine pesticide residues in the buffalo milk samples of Delhi City, India. J Environ Prot. 2013;4(09):964. doi: 10.4236/jep.2013.49111. [DOI] [Google Scholar]
- Barkatina E, Pertsovsky A, Murokh V, Kolomiets N, Shulyakovskaya O, Venger O, Makarevich V. Organochlorine pesticide residues in basic food products and diets in the Republic of Belarus. Bull Environ Contam Toxicol. 1999;63(2):235–242. doi: 10.1007/s001289900971. [DOI] [PubMed] [Google Scholar]
- Battu R, Singh B, Kang BJE. Contamination of liquid milk and butter with pesticide residues in the Ludhiana district of Punjab state, India. Ecotoxicol Environ Saf. 2004;59(3):324–331. doi: 10.1016/j.ecoenv.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Bulut S, Akkaya L, Gök V, Konuk M. Organochlorine pesticide (OCP) residues in cow’s, buffalo’s, and sheep’s milk from Afyonkarahisar region, Turkey. Environ Monit Assess. 2011;181(1–4):555–562. doi: 10.1007/s10661-010-1849-x. [DOI] [PubMed] [Google Scholar]
- Costabeber I, Trindade R, Fries L. Organochlorine pesticides level in cow milk. Alimentaria. 2001;38:127–129. [Google Scholar]
- Dogheim S, Almaz M, Kostandi S, Hegazy M. Pesticide residues in milk and fish samples collected from upper Egypt. J Assoc Off Anal Chem. 1988;71(5):872–874. [PubMed] [Google Scholar]
- El-Gebaly F (2000) Detection of organochlorine pesticides residue in animal milk collected from Great Cairo Governorate. Thesis of Forensic Medicine and Toxicology (Al-Azhar University), pp 117–137
- Fiedler H, Hutzinger O, Welsch-Pausch K, Schmiedinger A, Umlauf G (2000) Evaluation of the occurrence of PCDD/PCDF and POPs in wastes and their potential to enter the foodchain. Final Report, University of Bayreuth, Bayreuth
- Hayes W, Laws E (1982) Chlorinated hydrocarbon insecticides. In: Hayes WJ (ed) Pesticides studied in man. Williams & Wilkins
- Heck M, dos Santos JS, Junior SB, Costabeber I, Emanuelli T. Estimation of children exposure to organochlorine compounds through milk in Rio Grande do Sul, Brazil. Food Chem. 2007;102(1):288–294. doi: 10.1016/j.foodchem.2006.05.019. [DOI] [Google Scholar]
- Heeschen W, Harding F (1995) Contaminants. In: Harding F (ed) Milk quality. Springer, pp 133–150
- John P, Bakore N, Bhatnagar P. Assessment of organochlorine pesticide residue levels in dairy milk and buffalo milk from Jaipur City, Rajasthan, India. Environ Int. 2001;26(4):231–236. doi: 10.1016/S0160-4120(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Joy RM. Chlorinated hydrocarbon insecticides. In: Ecobichon DJ, Joy RM, editors. Pesticides and neurological diseases. Boca Raton: CRC Press; 1982. pp. 91–150. [Google Scholar]
- Kampire E, Kiremire BT, Nyanzi SA, Kishimba M. Organochlorine pesticide in fresh and pasteurized cow’s milk from Kampala markets. Chemosphere. 2011;84(7):923–927. doi: 10.1016/j.chemosphere.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Mallatou H, Pappas C, Kondyli E, Albanis T. Pesticide residues in milk and cheeses from Greece. Sci Total Environ. 1997;196(2):111–117. doi: 10.1016/S0048-9697(96)05404-6. [DOI] [PubMed] [Google Scholar]
- Martínez MP, Angulo R, Pozo R, Jodral M. Organochlorine pesticides in pasteurized milk and associated health risks. Food Chem Toxicol. 1997;35(6):621–624. doi: 10.1016/S0278-6915(97)00028-8. [DOI] [PubMed] [Google Scholar]
- Mocanu GD, Nistor OV, Botez E, Andronoiu DG, Macovei VM. Trace elements and organochlorine pesticides in raw milk from south eastern regions of Romania. J Food Sci Eng. 2012;2(3):143. [Google Scholar]
- Nag SK, Raikwar MK. Organochlorine pesticide residues in bovine milk. Bull Environ Contam Toxicol. 2008;80(1):5–9. doi: 10.1007/s00128-007-9276-6. [DOI] [PubMed] [Google Scholar]
- Nida’M S, Ahmad R, Estaitieh HJC. Organochlorine pesticide residues in dairy products in Jordan. Chemosphere. 2009;77(5):673–678. doi: 10.1016/j.chemosphere.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Pandit G, Sharma S, Srivastava P, Sahu S. Persistent organochlorine pesticide residues in milk and dairy products in India. Food Addit Contam. 2002;19(2):153–157. doi: 10.1080/02652030110081155. [DOI] [PubMed] [Google Scholar]
- Pardio V, Waliszewski K, Landin L, Bautista R. Organochlorine pesticide residues in cow's milk from a tropical region of Mexico. Food Addit Contam. 2003;20(3):259–269. doi: 10.1080/0265203021000046207. [DOI] [PubMed] [Google Scholar]
- Pastor Ciscato CH, Gebara AB, de Souza Spinosa H. Pesticide residues in cow milk consumed in Sao Paulo City (Brazil) J Environ Sci Health Part B. 2002;37(4):323–330. doi: 10.1081/PFC-120004473. [DOI] [PubMed] [Google Scholar]
- Robertson G, Lebowitz M, Needham L, O’Rourke M, Rogan S, Petty J, Huckins J (2002) Distributions of residential organochlorine pesticide residuals along the Arizona/Mexico border. In: Paper presented at the proceedings of the 9th international conference on indoor air quality and climate. Indoor Air 2002
- Tsiplakou E, Anagnostopoulos C, Liapis K, Haroutounian S, Zervas G. Pesticides residues in milks and feedstuff of farm animals drawn from Greece. Chemosphere. 2010;80(5):504–512. doi: 10.1016/j.chemosphere.2010.04.069. [DOI] [PubMed] [Google Scholar]
- Watterson A. Pesticides and your food. London: Green Print; 1991. [Google Scholar]
- Windham B (2002) The health effects of pesticides. In: Pesticid