Abstract
In order to have a better insight into the quality of minor cereals, the aim of this research was to evaluate the nutritional, biochemical, physical and rheological properties of barley, rye, triticale, oat, sorghum and millet flours. Generally, all flours could be divided into two groups according to mineral content, ω-6/ω-3 fatty acids ratio and amino acid composition. Sorghum flour was characterized by the highest total phenolic content and was the only flour which contained detectable amounts of tannins. Sorghum and millet flours differed from other flours by lower water absorption index and higher temperature of starch gelatinization. Additionally, sorghum and millet flours could be analysed by Mixolab only using constant hydration and require more time to obtain complete hydration than other flours. All flours would require modification of standard breadmaking process in order to obtain quality of product similar to those already present at the market.
Keywords: Minor cereals, Nutritional profile, Flour gelling properties, Differential scanning calorimetry, Mixolab
Introduction
Altered climatic conditions in the last few decades have influenced change in the basic biosynthetic pathways in crops, resulting in the occurrence of altered properties of protein, carbohydrate and enzyme complex of grain (DuPont and Altenbach 2003). These changes are particularly reflected in the processing quality of wheat. Consequently, this imposes the need for the use of other cereals which are cultivated in South-Eastern Europe and which, despite a satisfactory nutritional composition, are less common present in the daily diet. Due to the global heating, climate in Europe is becoming more suitable for the cultivation of millet and sorghum, cereals of poor baking properties which are highly adapted to cultivation in warm climate conditions and therefore represent a suitable future alternative for wheat in human diet.
Increasing demands for the consumption of healthier and tastier foods along with the increasing number of persons who are intolerant or allergic to gluten, causes the emergence of a new market of bakery products based on alternative cereals. One of the strategies for the development of healthy bakery products is the fortification of wheat based products with cereals which are rich in different nutritional components (mineral, vitamin, protein or dietary fibre).
Barley is an excellent source of other bioactive constituents, such as vitamin E, B-complex vitamins, minerals, phenolic compounds, and proteins which have been recognized as a rich source of some essential amino acids (Collar and Angioloni 2014). Compared to the other cereals, a considerable amount of β-glucan have been found in barley (2.5–11.3%) (Rieder et al. 2012), which is associated with reducing cholesterol and blood glucose levels. In breadmaking, wheat flour is usually substituted by barley flour at levels of 15–20% because higher levels caused the acceleration of starch retrogradation and thus more rapid bread staling during storage (Baik and Ullrich 2008).
Rye is a good source of fibre and contains many bioactive compounds such as phenolic acids, alkylresorcinols, lignans, phytosterols, minerals, tocotrienols and other vitamins (Andersson et al. 2014). Its bread making performance is mainly based on the swelling properties of endogenous pentosans contributing to the formation of viscous dough. Addition of rye pentosans to the wheat flour has beneficial effects on baking performance of obtained blends in terms of bread volume, textural properties of crumb and storage life (Denli and Ercan 2001). Triticale is cereal produced by cross-breeding wheat and rye. Despite a number of advantages such as yield and nutritional profile of triticale in comparison to wheat, the main shortcoming of this cereal is low content and quality of gluten and, therefore, inferior bread-making quality (Zhu 2018).
Oat flour is important from nutritional point, due to high level of proteins with very good amino acid balance, dietary fibre and the greatest percentage of fats among the major cereals with a good balance of the essential fatty acids (Biel et al. 2009). Oat flour is also rich in β-glucan (Brennan and Cleary 2005), and many studies have shown the beneficial health effects of oats including antioxidant and anti-inflammatory activities (Chu et al. 2013). The use of oats in the food industry is mainly related to production of flakes, breakfast cereals, cereal bars or cookies but recently use of oats as an ingredient for bread production has received increased attention (Hüttner et al. 2010).
Sorghum is recognized as important source of antioxidants because of a wide range of phenolic compounds in the grain (Dlamini et al. 2007). Millet also contains phenolic compounds but data on the phenolic compounds in millet are more limited. There are some reports which emphasis the beneficial influence of these cereals including anticancer and antidiabetic properties (Awika et al. 2009).
There are many studies which are focused on characterization of different cereals whether individually or in combination with other cereals. However, comparative studies of different cereals quality which comprise the estimation of their characteristics from various aspects are scarcely covered in the relevant literature. In addition, cereal crops grown in South-Eastern Europe other than wheat, maize and rice are not widely researched. Therefore, the aim of this research was to evaluate the nutritional, biochemical, physical and rheological properties of barley, rye, triticale, oat, sorghum and millet flours. Results obtained the above mentioned characteristics might be useful for bakers in the evaluation of flour quality for specific purpose, as well as in the selection of raw materials for development of nutritionally more balanced products.
It can be supposed that comparative data about nutritional and technological quality aspects of minor cereals would provide the insight into possibilities for application of different approaches, such as modification of standard technological process, or use of different additives in processing of non-wheat flours into standard quality bakery products.
Material and method
Material
Barley (Hordeum vulgare), rye (Secale cereale), triticale (x Triticosecale), oat (Avena sativa), sorghum (Sorghum bicolor) and proso millet (Panicum miliaceum) wholegrain flours were chosen for this study. Wholegrain barley, rye, oat and millet flours were purchased at market. Sorghum and triticale grains were provided by the Institute of Field and Vegetable Crops, Novi Sad (Serbia) and milled in a pilot scale stone mill (Klas, Trstenik, Serbia) into wholegrain flour at the laboratory of the Institute of Food Technology, Novi Sad (Serbia). Particle mean diameter for all flour was in the range of 95–115 μm, as determined by rotational sieving machine equipped with seven sieves (Bühler, Uzwil, Switzerland). Flour samples were stored in a refrigerator (− 18 °C) prior to analysis.
Nutritional composition
Moisture content, ash content, protein, fat, insoluble and total dietary fibre (TDF) content were determined according to Association Official of Analytical Chemists (AOAC 2000) methods 925.10, 925.51, 950.36, 935.38, and 985.29, respectively. Total sugars and β-glucan were determined following standard methods of American Association of Cereal Chemists (AACC 2017) (80–68, 32–23.01). Total starch content, amino acid composition and mineral composition were determined according to standards ISO 10520:1997, ISO 13903:2005 and ISO 6869:2008, respectively. Each analysis was done in three replicates.
Fatty acid profile
Fatty acid profile of flours was determined using the procedure published in Milovanović et al. (2012). Total lipids were extracted from flours with a chloroform–methanol solution (3 × 5 mL at 2:1 ratio of chloroform to methanol) and the residue obtained after evaporation of the solvents under stream of nitrogen was weighed. Afterwards, fatty acid methyl esters were prepared from the extracted lipids by transesterification using 14% boron (III)-fluoride in methanol. The obtained samples were analysed by a GC Agilent 7890A system with flame-ionization detector (FID) equipped with fused silica capillary column (DB WAX 30 m, 0.25 mm, 0.50 μm) and auto injection module for liquid samples. Helium was used as a carrier gas (purity > 99.9997 vol %, flow rate = 1.26 mL/min). The fatty acids peaks were identified by comparison of the retention times in relation to standards from Supelco 37 Component Fatty Acid Methyl Ester Mix (Sigma-Aldrich, St. Louis, MO, USA). The results in the chromatograms were expressed as a percentage of each fatty acid in total fatty acids. All analyses were done in duplicates.
Determination of phenolic compounds
Extraction procedure
Phenolic compounds were extracted from the samples by the method of Moure et al. (2006), with slight modifications. Flour samples (2.0 g) were mixed with 16 mL of methanol containing 1% HCl for 24 h at 24 °C. The procedure was repeated twice. The methanol extracts were centrifuged at 9220 g (Sorvall® RC- 5B Refrigerated Superspeed Centrifuge, Du Pont Instruments) for 15 min and the resulting supernatants were pooled and stored at 4 °C.
Total phenolic (TP) content
TP content of prepared extracts was determined spectrophotometrically (T80 UV–Vis Spectrophotometer; PG Instruments, Lutterworth, UK). using Folin–Ciocalteu’s reagent method described by Singleton et al. (1999). The extract (0.1 mL) was diluted with distilled water (7.9 mL). Folin–Ciocalteu’s reagent (0.5 mL) and 20% sodium carbonate solution (1.5 mL) were added at the reaction mixture. The mixture was allowed to stand for 60 min and the absorbance at 750 nm was measured (T80 UV–Vis Spectrophotometer; PG Instruments, Lutterworth, UK). Gallic acid was used as a standard and results were expressed as gallic acid equivalents (GAE) (mg GAE/g of sample). Three replicates per sample were done.
Total flavonoid (TF) content
TF content of extracts was determined by a method described by Shen et al. (2009), with minor modifications. Samples (0.5 mL) of diluted extracts or standard solutions were mixed with 2 mL double distilled H2O and 0.15 mL of 5% NaNO2. After 5 min, 0.15 mL of 10% AlCl3·6H2O solution was added, and the mixture was allowed to stand for another 5 min, after which 1 mL of 1 M NaOH was added. The solution was to stand for 15 min and absorbance was measured at 415 nm. (T80 UV–Vis Spectrophotometer; PG Instruments, Lutterworth, UK). TF content was calculated from a standard rutin curve and expressed as rutin equivalents (mg RE/g sample). Three replicates per sample were done.
Tannins content
The content of tannins in extracts was determined by vanillin assay of Price et al. (1978), with some modifications. The extracts (1 mL) was added to 5 mL vanillin reagent (0.01 g/mL vanillin in methanol mixed with equal volume 8% HCl in methanol) and allowed to react for 20 min at 30 °C. Absorbance was read at 500 nm. A blank was prepared under the same reaction condition by reacting 1 mL the same aqueous sample with 5 mL 4% HCl in methanol. Absorbance was read at 500 nm in a spectrophotometer (T80 UV–Vis Spectrophotometer; PG Instruments, Lutterworth, UK). Catechin was used as a standard and results were expressed as catechin equivalents (CE) (mg CE/g of sample). Three replicates per sample were done for all analyses.
Protein fractionation
Protein fractionation was performed following the Osborne extraction procedure. Namely, flours were extracted sequentially using distilled water, 2% NaCl solution and 70% ethanol in order to obtain albumin, globulin and prolamin fractions, respectively. After the each extraction step, sample was centrifuged in order to separate supernatant (protein fraction extract) and precipitate. Supernatant was dried to obtain dry protein fraction extract, while precipitate was used for the next extraction step. The insoluble precipitate after the last extraction step represents glutelin fraction. For each investigated sample, analysis was done in two replications.
Flour gelling behavior
Water absorption index (WAI), water solubility index (WSI) and the swelling power (SP) of flours were determined to describe the gelling behaviour of flours. Flour (1 g ± 0.01 g) sample was dispersed in 20 mL of distilled water and heated at 90 °C for 15 min in an incubator (Friocell, MMM Group, Planegg, Germany). The cooked paste was cooled to room temperature, and centrifuged at 3000 g at 4 °C for 10 min (Eppendorf Centrifuge 5804 R, Hamburg, Germany). The supernatant was decanted into an evaporating dish for determination of its solid content by drying overnight at 110 °C, while the sediment was weighed. Each sample was analysed in three replicates. WSI, WAI and SP were calculated using the equations:
| 1 |
| 2 |
| 3 |
Differential scanning calorimetry (DSC)
Gelatinization temperatures and enthalpies of flour samples were studied using differential scanning calorimetry (DSC Q100, TA Instruments, New Castle, DE, USA). DSC instrument was calibrated using indium (156.6 °C). Flours were mixed with deionized water to obtain a suspension with flour/water ratio of 1:3 (w/v). Approximately 30 mg of the prepared samples were added to aluminium pans which were hermetically sealed afterwards. The sample pan was equilibrated in the instrument at 20 °C for 1 h, and then heated from 20 to 120 °C at a heating rate of 10 °C min−1, which was chosen to increase accuracy and resolution without loss of sensitivity of results. Parameters evaluated were gelatinization temperature (the onset (To), peak (Tp) and conclusion (Tc)) and the enthalpy of endothermic peak (ΔH), calculated from the area under the DSC curves and expressed as J/g.).
Mixolab properties
Mixolab measurements were done according to Chopin + protocol for rye, triticale barley and oat flours, while sorghum and millet flours were analysed at constant hydration of 55.0% (Dubat and Boinot 2012).
The Mixolab curve indicators are: WA—water absorption (%); C1—initial maximum consistence during mixing (Nm) used for determining the ability to absorb water; dough development time (min); mixing stability—elapsed time at which the torque produced is kept at 1.1 Nm (min); C2—minimum value of torque during mixing and initial heating (Nm). The Mixolab parameters which give information about starch behaviour were: C3—maximum value (peak) of torsion during heating stage (Nm); C4—stability of hot starch paste; (C3–C4)—difference between maximum and minimum of torque during heating stage (Nm); and (C5–C4)—the amount of retrogradation i.e. the difference between maximum torque after cooling period at 50 °C (C5) and torque at a point C4 (Nm).
Statistical analysis
Analysis of variance (ANOVA) and Fisher’s least significant differences test were applied at a significance level of 5% to determine differences between flour samples. Principal Component Analysis (PCA) was used to group flour samples based on the nutritional composition and technological quality. Statistical analysis was done using Statistica software (Tibco Inc., USA, 2017, https://www.tibco.com/products/data-science, version 13.3).
Results and discussion
Nutritional composition
Nutritional composition of tested flour samples is reported in Table 1. Sorghum flour had the highest protein content (12.62%) while oat flour had the lowest (8.33%). In this study, oat flour had the highest fat content but this level is not so high comparing to the literature data (Hager et al. 2012). Millet and sorghum flours had the lowest content of sugars, with millet having the highest starch content. As a result of milling process in which the bran is removed to a certain extent, millet flour had the lowest amount of dietary fibre and ash. On the other hand, triticale was characterized by the highest levels of both total and insoluble dietary fibre. However, barley, oat and rye flours had the highest β-glucan content.
Table 1.
Nutritional composition of barley, rye, triticale, oat, sorghum and millet flours (values based on fresh weight of samples)
| Barley | Rye | Triticale | Oat | Sorghum | Millet | |
|---|---|---|---|---|---|---|
| Ash (g/100 g) | 1.58e | 2.02d | 2.33a | 2.26b | 2.15c | 1.17f |
| Protein (g/100 g) | 11.60b | 11.36b | 12.30a | 8.33d | 12.62a | 9.48c |
| Fat (g/100 g) | 1.87d | 2.52b | 1.74e | 4.88a | 2.18c | 1.62f |
| Sugars (g/100 g) | 2.89b | 3.85a | 3.61a | 3.61a | 0.96c | 0.48c |
| Total starch (g/100 g) | 61.45d | 57.18e | 57.30e | 62.10c | 67.05b | 77.77a |
| TDF (g/100 g) | 15.21ab | 12.94bc | 18.13a | 11.25d | 12.13bc | 4.63e |
| IDF (g/100 g) | 8.68b | 8.72b | 12.29a | 7.52b | 9.01b | 1.77c |
| β-glucan (g/100 g) | 2.98a | 1.41b | 0.68c | 1.58b | 0.05d | 0.04d |
| TP (mg GAE/g) | 1.136b | 1.200b | 1.216b | 1.168b | 5.760a | 0.288b |
| TF (mg RE/g) | 0.321b | 0.275b | 0.196c | 0.338b | 0.875a | 0.068d |
| Tannins (mg CE/g) | n.d. | n.d. | n.d. | n.d. | 1.067 | n.d. |
| Mineral composition (mg/kg) | ||||||
| Na | 19.64cd | 105.01b | 13.73d | 127.65a | 14.34d | 23.4c |
| K | 2101c | 3691a | 3634a | 1981d | 2540b | 1165e |
| Mg | 355.5f | 927.1c | 1117b | 803.1d | 1777a | 732.5e |
| Ca | 188.45c | 147.36d | 442.15b | 531.79a | 34.12e | 42.4e |
| Mn | 6.48e | 25.36c | 43.76a | 28.12b | 16.62d | 2.94f |
| Fe | 24.42e | 31.88c | 33.74b | 29.41d | 36.81a | 23.37f |
| Cu | 5.23a | 5.22a | 4.49b | 3.81c | 3.89c | 3.47d |
| Zn | 9.82e | 15.56b | 12.39c | 18.72a | 11.87d | 18.89a |
| Fatty acid profile (% of total lipids) | ||||||
| Palmitic acid (16:0) | 24.0a | 18.9d | 20.5c | 15.3e | 21.3b | 11.0f |
| Stearic acid (18:0) | 2.1b | 2.1b | 1.8c | 1.8c | 1.5d | 2.7a |
| Oleic acid (18:1) | 20.9d | 17.2e | 16.9e | 27.8b | 38.5a | 26.8c |
| Linoleic acid (18:2) | 43.4d | 50.3b | 51.7a | 48.7c | 35.9e | 51.5a |
| Arachidic acid (20:0) | 4.6b | 3.9c | 2.6e | 3.1d | 0.3f | 5.1a |
| α-linolenic acid (18:3) | 5.0c | 7.7a | 6.6b | 3.4d | 2.5f | 2.8e |
| Saturated | 30.7a | 24.9b | 24.9b | 20.2d | 23.1c | 18.8e |
| Monounsaturated | 20.9d | 17.2e | 16.9e | 27.8b | 38.5a | 26.8c |
| Polyunsaturated | 48.4d | 58.0a | 58.3a | 52.1c | 38.4e | 54.3b |
| ω-6/ω-3 ratio | 8.7c | 6.5e | 7.8d | 14.3b | 14.4b | 18.4a |
| Amino acid composition (g/100 g) | ||||||
| Alanine (Ala) | 0.53d | 0.68c | 0.66 cd | 0.71c | 1.21b | 1.47a |
| Arginine (Arg) | 0.40c | 0.57c | 1.18a | 0.88b | 1.31a | 0.36c |
| Aspartic acid (Asp) | 0.10c | 0.78ab | 0.57b | 0.90a | 0.90a | 0.60b |
| Cysteine (Cys) | 0.30a | 0.09d | 0.22c | 0.27b | 0.07d | 0.03e |
| Glutamic acid (Glu) | 0.68e | 2.13c | 2.59b | 1.67d | 3.39a | 1.48d |
| Glycine (Gly) | 0.79a | 0.76a | 0.59b | 0.62b | 0.37c | 0.27c |
| Histidine (His) | 0.39a | 0.20c | 0.33ab | 0.31abc | 0.25bc | 0.22bc |
| Isoleucine (Ile) | 1.29a | 0.34d | 0.41c | 0.59b | 0.44c | 0.46c |
| Leucine (Leu) | 0.14d | 0.92c | 1.07b | 1.10b | 1.84a | 1.83a |
| Lysine (Lys) | 0.64b | 1.16a | 0.54c | 0.30d | 0.16e | 0.17e |
| Methionine (Met) | 0.38a | 0.13c | 0.28b | 0.16c | 0.16c | 0.29ab |
| Phenylalanine (Phe) | 2.35a | 0.15d | 0.38bc | 0.28 cd | 0.49b | 0.21d |
| Proline (Pro) | 0.60d | 1.35b | 1.56a | 0.68d | 1.02c | 0.95c |
| Serine (Ser) | 1.86a | 0.64 cd | 0.76bc | 0.57d | 0.55d | 0.80b |
| Threonine (Thr) | 0.57a | 0.47bc | 0.49ab | 0.50ab | 0.39 cd | 0.35d |
| Tyrosine (Tyr) | 0.30ab | 0.10d | 0.18 cd | 0.38a | 0.24bc | 0.23bc |
| Valine (Val) | 0.65ab | 0.86a | 0.58b | 0.86a | 0.57b | 0.52b |
TDF total dietary fibre, IDF insoluble dietary fibre, TP total phenolics, GAE gallic acid equivalents, n.d. not detected
Values in the rows followed by different lowercase letters are significantly different (P < 0.05)
TP content of barley, rye, triticale, oat and sorghum flours (Table 1) were in the range of published results (0.88–10.3, 0.65–2.27, 1.3–1.6, 0.61–3.03 and 0.8–29.6 mg/g, respectively) (Hithamani and Srinivasan 2014; Chen et al. 2018; Zhu 2018; Collar and Angioloni 2014). The results obtained for millet flour were much lower than in available literature due to different milling extraction yield of grain (1.39 mg/g,) (Ragaee et al. 2006). TF content had trend similar to TP content with millet flour having much lower TF content than values obtained for pearl millet and finger millet (2.35 and 5.54 mg/g) in literature (Hithamani and Srinivasan 2014). However, TF content of rye flour was higher than values (0.067–0.075 mg/g) reported by Žilić et al. (2011). Tannins were detected only in sorghum flour, which possess significantly the highest content of TP and TF.
Mineral composition
Potassium was the most abundant macroelement in all flour samples (Table 1), followed by magnesium and calcium, which is in accordance with previous results obtained for oat and sorghum (Bagdi et al. 2011; Hager et al. 2012). Magnesium showed the highest variation among the flours, with barley having the lowest content (355.5 mg/kg f.w.) and sorghum having the highest content (1777.00 mg/kg f.w.). Both sorghum and millet flours were characterized by ten times lower calcium content than other flours.
Iron was dominant microelement in all studied flours. Results from literature also indicated that iron is the main microelement in sorghum and millet (Bagdi et al. 2011). Manganese showed wide variation among the flour samples, with values ranging from 2.94 mg/kg f.w. in millet to even 43.76 mg/kg f.w. in triticale.
According to WHO/FAO/UNU (2007), balanced sodium and potassium dietary intake should be ensured since decreased intake of potassium and increased intake of sodium can lead to hypertension. In a longer period, hypertension causes damage to small blood vessels, which can induce coronary artery disease and stroke, one of the leading causes of death in developed countries. Therefore the recommendation of World Health Organization (WHO) is to limit the intake of sodium and provide minimal prescribed amount of potassium. It is desirable that foodstuffs contain high K/Na ratio. Triticale, sorghum and barley flours had the highest K/Na ratio of all examined flours (265, 177 and 107, respectively).
Fatty acids composition
Although lipids are a minor component of cereals, they are extremely important from the nutritional point of view as a source of essential fatty acids. The ω-6/ω-3 fatty acids ratio is important factor in prevention of several chronic diseases, and its optimal value should be between 1:1 and 1:4 (Simopoulos 2002). The most abundant fatty acids in all the cereal samples were linoleic, palmitic and oleic acids (C18:2, C16:0 and C18:1, respectively) (Table 1), as expected according to literature (Hager et al. 2012). Generally rye, triticale and barley have a similar fatty acid composition.
Amino acids composition
Amino acids composition serves as the most important aspect of protein quality from nutritional point of view. The most important are the essential amino acids because they must be provided through the diet. According to the WHO/FAO/UNU (2007) suggested requirements (Table 2) it could be seen that lysine, the limiting amino acid in cereals can be found in low amounts, except in barley and rye flours. Sulphur containing (cysteine and methionine) and aromatic containing amino acids (phenylalanine and tyrosine) are present in insufficient amount in rye and sorghum flours, and rye flour respectively.
Table 2.
Comparison of essential amino acids content of cereal flours to FAO/WHO suggested requirement (amended values from the 2007 WHO/FAO/UNU report)
| Barley | Rye | Triticale | Oat | Sorghum | Millet | FAO/WHO suggested requirements | |
|---|---|---|---|---|---|---|---|
| Amino acids | mg/g protein | Adult | |||||
| His | 33.6 | 15.5 | 26.8 | 37.2 | 18.8 | 23.2 | 15 |
| Ile | 111.2 | 26.4 | 33.3 | 70.8 | 33.1 | 48.5 | 30 |
| Leu | 12.1 | 71.3 | 87.0 | 132.1 | 138.2 | 193.0 | 59 |
| Lys | 55.2 | 89.9 | 43.9 | 36.0 | 12.0 | 17.9 | 45 |
| Met + Cys | 58.6 | 17.1 | 40.7 | 51.6 | 17.3 | 33.8 | 22 |
| Phe + Tyr | 228.4 | 19.4 | 45.5 | 79.2 | 54.8 | 46.4 | 38 |
| Thr | 49.1 | 36.4 | 39.8 | 60.0 | 29.3 | 36.9 | 23 |
| Val | 56.0 | 66.7 | 47.2 | 103.2 | 42.8 | 54.9 | 39 |
Bold italic values represent amino acid content lower than FAO/WHO suggested requirements
+ Conditionally essential amino acids
Sulphur containing amino acids are extremely important from technological quality aspects, due their role in protein network formation in dough through the S–S crosslinking. Triticale, barley and oat have the highest amount of cysteine, while triticale, barley and millet have the highest amount of methionine.
Protein fractionation
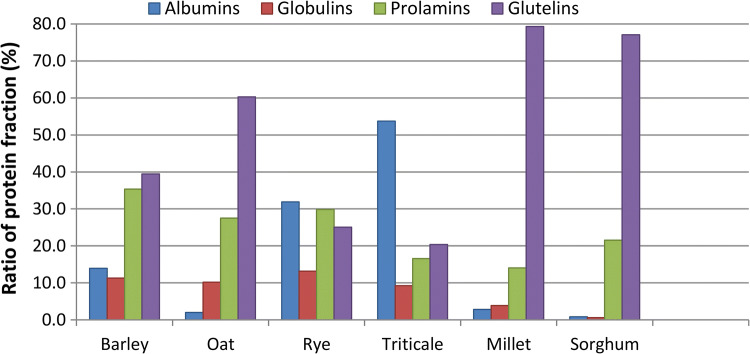
According to Osborne sequential procedure, albumin, globulin, prolamin and glutelin flour fractions were isolated and their profiles are presented in Fig. 1. According to the literature (Hager et al. 2012), prolamins are the most abundant proteins of the sorghum; however, in this study, glutelins were dominant protein fraction. On the other hand, glutelins are shown to be the main fraction of proteins in millet endosperm. This is interesting since prolamin and glutelin fractions are the most responsible for unique technological quality of wheat. Based on the similarity of proteins of sorghum, millet and oat flours (containing up to 75% of prolamin and glutelin) with wheat protein molecular weight distribution it could be concluded that these flours have the greatest potential to form a gluten-like network in the dough.
Fig. 1.
Albumin, globulin, prolamin and glutelin fractions of cereal flours
Flour gelling properties
Flour gelling properties of studied flours are presented in Table 3. It is easily observed that WAI values are related to SP values, since samples with the highest WAI value also have the highest SP values (barley and rye). Similarly, sorghum and millet are samples with the lowest WAI and SP values. These results could be interpreted as a consequence of complex interaction between starch, protein and dietary fibre components and properties of starch granules. Barley, rye and triticale flours have the highest dietary fibre content, which could be related to their high WAI values. Differences among WSI values of various cereal flours were supposed to come from several factors, such as starch and lipid content and starch crystalline structure (Robin et al. 2015). Higher lipid content could be the possible explanation of low WSI values obtained for oat flour, since lipids attached to the surface of starch granules could block the entrance of water into granule interior (Morrison et al. 1984), thus lowering WSI value.
Table 3.
Gelling properties and Mixolab parameters of barley, rye, triticale, oat, sorghum and millet flours
| Parameter | Barley | Rye | Triticale | Oat | Sorghum | Millet |
|---|---|---|---|---|---|---|
| Flour gelling properties | ||||||
| WAI (g/g) | 4.08ab | 4.27a | 3.68abc | 3.5bcd | 3.15 cd | 2.91d |
| WSI (g/g) | 0.15b | 0.11c | 0.24a | 0.04d | 0.09c | 0.03d |
| SP (g/g) | 5.22a | 5.29a | 5.18a | 4.08b | 3.91b | 3.32b |
| Mixolab parameters | ||||||
| Water absorption (%) | 70.00a | 55.00b | 68.00a | 56.40b | 55.00b | 55.00b |
| Development time (min) | 4.08c | 5.52b | 3.92d | 3.90d | 7.57a | 2.57e |
| Stability (min) | 4.10b | 6.22a | 4.05b | 4.17b | 6.55a | 2.02c |
| C2 (Nm) | 0.45b | 0.41b | 0.41b | 0.55a | 0.45b | 0.40b |
| C3 (Nm) | 1.96b | 1.80b | 1.45c | 2.27a | 1.78b | 2.25a |
| C4 (Nm) | 1.77b | 1.54b | 0.54c | 2.30a | 1.66b | 2.27a |
| C5 (Nm) | 2.35b | 2.36b | 0.29c | 2.84a | 2.26b | 2.83a |
| C2 (min) | 18.57f | 19.07e | 19.23d | 19.88c | 21.73a | 20.90b |
| C3 (min) | 27.33c | 26.92d | 23.77e | 26.90d | 27.82b | 32.05a |
| C4 (min) | 33.18c | 31.60d | 30.00f | 31.53e | 33.25b | 36.25a |
WAI water absorption index, WSI water solubility index, SP swelling power
Values in the rows followed by different lowercase letters are significantly different (P < 0.05)
Differential scanning calorimetry
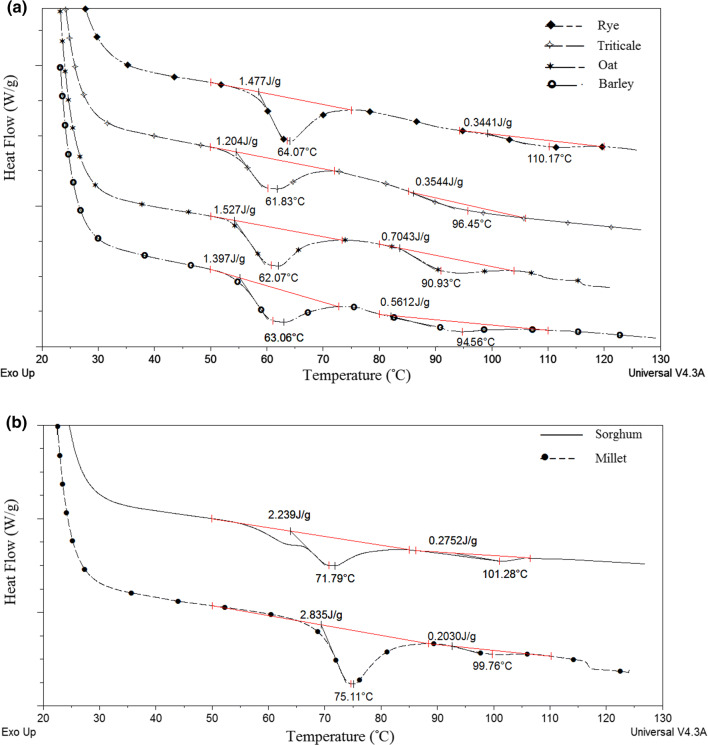
The thermal properties of rye, triticale, barley and oat flours do not differ significantly, and their gelatinization process takes place at lower temperatures (the minimum in DSC curves varied from 61.8 to 64.1 °C) (Fig. 2a) compared to those obtained for sorghum (Tp1 registered at 71.8 °C) and millet (Tp1 at 75.1 °C) (Fig. 2b). Similar results were obtained for different millet flours by Shinoj et al. (2006). High transition temperatures obtained for sorghum and millet are supposed to result from the high degree of starch crystallinity, more stable amorphous regions or a lower degree of amylopectin chain branching (Shinoj et al. 2006). The obtained values of gelatinization enthalpies (∆H1) vary in the range from 1.21 to 2.84 J/g. The highest gelatinization enthalpies (higher than 2 J/g) were obtained for sorghum and millet flours and could be also related to lower amount of starch in amorphous phase.
Fig. 2.
DSC plots showing the endothermic curves of barley, rye, triticale, oat (a) and sorghum and millet (b) flours
The second transition peak with a higher temperature, in the range from 90 to 110 °C, was observed in all tested flour samples. These peaks could be linked to dissociation of amylose–internal lipid complexes (Morrison et al. 1980).
The enthalpies of amylose–internal lipid complexes dissociation (∆H2) were much smaller in comparison with the main endothermic peaks, similarly to findings by Robin et al. (2015). Differences among the analysed flours probably originated from different ratios of monoacyl lipids (FAME = fatty acid methylesters) in total lipids, which are able to modify some of the properties of the non-waxy cereal starches, as shown in the study of Morrison et al. (1980).
Dough rheological properties assessed by Mixolab
The highest values of water absorption were recorded for barley flour (70.0%) and triticale flour (68.0%) which could be attributed to the highest level of β-glucan in barley flour and the highest content of TDF in triticale flour. Development time (C1, min) values show that the sorghum and rye flours needed the most time to hydrate and achieve the specified maximum consistency of the dough (Table 3). Barley, triticale and oat flours had the lowest stability, indicating the need for shorter mixing time. Proteins of oat, triticale and rye flours were able to resist to weakening during mixing and heating for a longest time, while proteins of oat flour expressed the least physical change (based on the highest torque values in C2, Nm). Millet and sorghum flours showed pronounced differences in the time required for the hydration of the flour and in the elasticity of the dough (Table 3).
Parameters C3–C5 indicate the properties of the starch component. The lowest value of maximum viscosity (C3, Nm) was recorded for triticale flour and this value was reached in the shortest time (C3, min) (Table 3). This could be attributed to greater presence and activity of enzymes, since albumins were the most abundant fraction in that flour (Fig. 1). This means that triticale flour perhaps requires shorter fermentation time than those commonly used in the baking industry. In the case of sorghum and millet flours, in the measurement conditions of consistent hydration, dough of sorghum flour had a lower viscosity.
Triticale dough had the lowest final torque in C5 (Nm), which might be related to the fact that triticale flour had the lowest content of starch due to the higher ratio of proteins and dietary fibre in comparison to other flours. This indicates the possibility of longer shelf-life of products prepared from this flour.
Overall, all tested flours showed certain potential to make three-dimensional network in dough or batters.
Principal component analysis of flour nutritional and technological quality
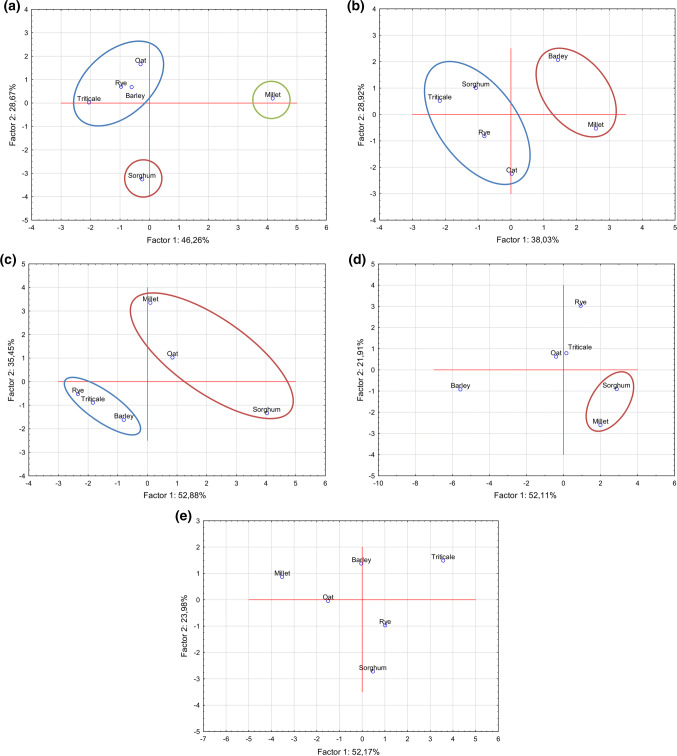
PCA (Fig. 3) was used to classify samples based on their nutritional profile (ash, protein, fat, sugars, total starch, total dietary fibre, insoluble dietary fibre, β-glucan, TP and TF) (a), mineral composition (b), fatty acid profile (c) amino acid composition (d) and Mixolab characteristics (water absorption, stability, C2–C5 in Nm and C1–C4 in min) (e). Results of PCA showed that the first two principal components (F1 and F2) explained high percentage of the total variance (66.95–88.33%) for all quality parameters. According to nutritional profile (Fig. 3a) triticale, rye, barley and oat flours were grouped on opposite side of PCA diagram from sorghum and millet flours based on their TP and TF content. On the other hand, sorghum is below the x axis contrary to all others flours according to tannin content. Samples with lower ash content (barley and millet) were grouped on the right side (Fig. 3b). Related to fatty acid composition, flour samples were separated on the basis of ω-6/ω-3 ratio (Fig. 3c). Genetically related species were grouped together according to amino acid composition (Fig. 3d). Previously described classification of flours based on their nutritional profile could not be used to predict the classification of flours based on their technological quality. Millet flour was separated from other flours by the most pronounced C3 (min) and C4 (min) values (Fig. 3e), which indicate that dough prepared from this flour requires longer thermal processing. Sorghum flour was distinguished by the longest time needed for dough formation, which imposes longer mixing phase during breadmaking. As above mentioned, triticale flour was differentiated by the properties of starch component (C3, C4, C5 in Nm) pointing out necessity for shorter thermal processing.
Fig. 3.
Classification of flour samples based on their nutritional profile (a), mineral composition (b), fatty acid composition (c), amino acid composition (d) and Mixolab characteristics (e)
Conclusion
Key nutritional difference between sorghum, millet and other flours is high TP content and presence of tannins in sorghum flour and low TP and TF content in millet flour. Barley and millet flours were characterized by lower mineral content than other flours. Ratio of ω-6/ω-3 fatty acids separated all flours into two groups; the first included those with lower ratio (rye, triticale and barley) and the second included flours with higher ratio (millet, oat and sorghum). Amino acid composition was mostly related to the climate conditions of cultivation; millet and sorghum, which are usually cultivated in tropical climate areas in Asia and Africa, were separated from the cereals cultivated in temperate climate conditions of Europe and Northern America. Sorghum and millet flours had different physico-chemical properties from other flours, namely lower water absorption index and higher temperature of starch gelatinization, which could be linked to higher degree of starch crystallinity. Regarding the technological potential of analysed flours, it was concluded that sorghum and millet flours could be analysed by Mixolab only using constant hydration and require more time to obtain complete hydration than other flours. This comparative study of different minor cereals contributes to technological knowledge of possible ways of their application, solely or in combination, with aim to achieve nutritionally and sensory balanced food.
Funding
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia within the Project of Technological Development No. TR 31007 and grant No. 451-03-68/2020-14/200222.
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC (2017) Approved methods of analysis, 11th edn. Methods 80–68.01, 76–31.01 and 32–23.01. American Association of Cereal Chemists International, St. Paul, MN, USA. https://aaccipublications.aaccnet.org. Accessed 02 Jul 2019
- Andersson AA, Dimberg L, Åman P, Landberg R. Recent findings on certain bioactive components in whole grain wheat and rye. J Cereal Sci. 2014;59(3):294–311. doi: 10.1016/j.jcs.2014.01.003. [DOI] [Google Scholar]
- AOAC (2000) Official methods of analysis, 17th edn. Methods 925.10, 925.51, 950.36, 935.38, and 985.29. The Association of Official Analytical Chemists, Gaithersburg, MD, USA
- Awika JM, Yang L, Browning JD, Faraj A. Comparative antioxidant, antiproliferative and phase II enzyme inducing potential of sorghum (Sorghum bicolor) varieties. LWT Food Sci Technol. 2009;42(6):1041–1046. doi: 10.1016/j.lwt.2009.02.003. [DOI] [Google Scholar]
- Bagdi A, Balázs G, Schmidt J, Szatmári M, Schoenlechner R, Berghofer E, Tömösközia S. Protein characterization and nutrient composition of Hungarian proso millet varieties and the effect of decortication. Acta Aliment. 2011;40(1):128–141. doi: 10.1556/AAlim.40.2011.1.15. [DOI] [Google Scholar]
- Baik BK, Ullrich SE. Barley for food: characteristics, improvement, and renewed interest. J Cereal Sci. 2008;48(2):233–242. doi: 10.1016/j.jcs.2008.02.002. [DOI] [Google Scholar]
- Biel W, Bobko K, Maciorowski R. Chemical composition and nutritive value of husked and naked oats grain. J Cereal Sci. 2009;49(3):413–418. doi: 10.1016/j.jcs.2009.01.009. [DOI] [Google Scholar]
- Brennan CS, Cleary LJ. The potential use of cereal (1 → 3, 1 → 4)-β-D-glucans as functional food ingredients. J Cereal Sci. 2005;42(1):1–13. doi: 10.1016/j.jcs.2005.01.002. [DOI] [Google Scholar]
- Chen C, Wang L, Wang R, Luo X, Li Y, Li J, Li Y, Chen Z. Phenolic contents, cellular antioxidant activity and antiproliferative capacity of different varieties of oats. Food Chem. 2018;239:260–267. doi: 10.1016/j.foodchem.2017.06.104. [DOI] [PubMed] [Google Scholar]
- Chu YF, Wise ML, Gulvady AA, Chang T, Kendra DF, Van Klinken BJW, Shi Y, O’Shea M, O’Shea M. In vitro antioxidant capacity and anti-inflammatory activity of seven common oats. Food Chem. 2013;139(1–4):426–431. doi: 10.1016/j.foodchem.2013.01.104. [DOI] [PubMed] [Google Scholar]
- Collar C, Angioloni A. Nutritional and functional performance of high β-glucan barley flours in breadmaking: mixed breads versus wheat breads. Eur Food Res Technol. 2014;238(3):459–469. doi: 10.1007/s00217-013-2128-1. [DOI] [Google Scholar]
- Denli E, Ercan R. Effect of added pentosans isolated from wheat and rye grain on some properties of bread. Eur Food Res Technol. 2001;212(3):374–376. doi: 10.1007/s002170000281. [DOI] [Google Scholar]
- Dlamini NR, Taylor JR, Rooney LW. The effect of sorghum type and processing on the antioxidant properties of African sorghum-based foods. Food Chem. 2007;105(4):1412–1419. doi: 10.1016/j.foodchem.2007.05.017. [DOI] [Google Scholar]
- Dubat A, Boinot N. Rheological and enzymes analyses. Villeneuve-la-Garenne: Chopin Technology; 2012. Mixolab applications handbook; pp. 10–15. [Google Scholar]
- DuPont FM, Altenbach SB. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J Cereal Sci. 2003;38(2):133–146. doi: 10.1016/S0733-5210(03)00030-4. [DOI] [Google Scholar]
- Hager AS, Wolter A, Jacob F, Zannini E, Arendt EK. Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J Cereal Sci. 2012;56(2):239–247. doi: 10.1016/j.jcs.2012.06.005. [DOI] [Google Scholar]
- Hithamani G, Srinivasan K. Effect of domestic processing on the polyphenol content and bioaccessibility in finger millet (Eleusine coracana) and pearl millet (Pennisetum glaucum) Food Chem. 2014;164:55–62. doi: 10.1016/j.foodchem.2014.04.107. [DOI] [PubMed] [Google Scholar]
- Hüttner EK, Dal Bello F, Arendt EK. Rheological properties and bread making performance of commercial wholegrain oat flours. J Cereal Sci. 2010;52(1):65–71. doi: 10.1016/j.jcs.2010.03.004. [DOI] [Google Scholar]
- ISO . Determination of starch content—Ewers polarimetric method. ISO:10520. Geneva: International Organization for Standardization; 1997. [Google Scholar]
- ISO . Animal feeding stuffs—determination of amino acid composition. ISO:13903. Geneva: International Organization for Standardization; 2005. [Google Scholar]
- ISO . Animal feeding stuffs—determination of the contents of calcium, copper, iron, magnesium, manganese, potassium, sodium and zinc—method using atomic absorption spectrometry. ISO:6869. Geneva: International Organization for Standardization; 2008. [Google Scholar]
- Milovanović I, Mišan A, Šarić B, Kos J, Mandić A, Simeunović J, Kovač D (2012) Evaluation of protein and lipid content and determination of fatty acid profile in selected species of cyanobacteria. In: Proceedings of the 6th central European congress on food, CEFood2012. http://fins.uns.ac.rs/uploads/zbornici/CEFood-proceedings2012.pdf
- Morrison WR, Tan SL, Hargin KD. Methods for the quantitative analysis of lipids in cereal grains and similar tissues. J Sci Food Agric. 1980;31(4):329–340. doi: 10.1002/jsfa.2740310402. [DOI] [PubMed] [Google Scholar]
- Morrison WR, Milligan TP, Azudin MN. A relationship between the amylose and lipid contents of starches from diploid cereals. J Cereal Sci. 1984;2(4):257–271. doi: 10.1016/S0733-5210(84)80014-4. [DOI] [Google Scholar]
- Moure A, Domínguez H, Parajó JC. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006;41(2):447–456. doi: 10.1016/j.procbio.2005.07.014. [DOI] [Google Scholar]
- Price ML, Van Scoyoc S, Butler LG. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J Agric Food Chem. 1978;26(5):1214–1218. doi: 10.1021/jf60219a031. [DOI] [Google Scholar]
- Ragaee S, Abdel-Aal ESM, Noaman M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006;98(1):32–38. doi: 10.1016/j.foodchem.2005.04.039. [DOI] [Google Scholar]
- Rieder A, Holtekjølen AK, Sahlstrøm S, Moldestad A. Effect of barley and oat flour types and sourdoughs on dough rheology and bread quality of composite wheat bread. J Cereal Sci. 2012;55(1):44–52. doi: 10.1016/j.jcs.2011.10.003. [DOI] [Google Scholar]
- Robin F, Théoduloz C, Srichuwong S. Properties of extruded whole grain cereals and pseudocereals flours. Int J Food Sci Technol. 2015;50(10):2152–2159. doi: 10.1111/ijfs.12893. [DOI] [Google Scholar]
- Shen Y, Jin L, Xiao P, Lu Y, Bao J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J Cereal Sci. 2009;49(1):106–111. doi: 10.1016/j.jcs.2008.07.010. [DOI] [Google Scholar]
- Shinoj S, Viswanathan R, Sajeev MS, Moorthy SN. Gelatinisation and rheological characteristics of minor millet flours. Biosyst Eng. 2006;95(1):51–59. doi: 10.1016/j.biosystemseng.2006.05.012. [DOI] [Google Scholar]
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–379. doi: 10.1016/S0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Wilchek M, Bayer EA, editors. Methods in enzymology. Cambridge: Academic Press; 1999. pp. 152–178. [Google Scholar]
- WHO/FAO/UNU (2007) Protein and amino acid requirements in human nutrition; Report of a joint WHO/FAO/UNU expert consultation, WHO Tech Rep Ser no. 935. WHO, Geneva. Dietary protein quality evaluation in human nutrition. Report of an FAO expert consultation. http://apps.who.int/iris/handle/10665/43411. Accessed 21 Aug 2019
- Zhu F. Triticale: nutritional composition and food uses. Food Chem. 2018;241:468–479. doi: 10.1016/j.foodchem.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Žilić S, Šukalović VHT, Dodig D, Maksimović V, Maksimović M, Basić Z. Antioxidant activity of small grain cereals caused by phenolics and lipid soluble antioxidants. J Cereal Sci. 2011;54(3):417–424. doi: 10.1016/j.jcs.2011.08.006. [DOI] [Google Scholar]