Abstract
Introduction
Osteoporosis and osteopenia are progressive disorders characterized by decreased bone mass, especially in postmenopausal women. These can be associated with body pain, fractures, hearing loss and balance disorders. The present study aims to evaluate audio-vestibular function in postmenopausal patients with osteopenia or osteoporosis.
Methods
The study included 48 postmenopausal women (new subjects) diagnosed with osteoporosis (n = 23) or osteopenia (n = 25) in the age range of 50–66 years, as well as 28 normal women as controls. Audiological testing included pure tone audiometry (conventional and extended high-frequency audiometry), speech audiometry, impedance audiometry and otoacoustic emissions, including both transient evoked otoacoustic emissions (TEOAEs) and distortion product otoacoustic emissions (DPOAEs). All subjects also underwent vestibular evoked myogenic potentials testing (both ocular and cervical VEMPs).
Results
In the present study, hearing was worse at all frequencies in the osteoporosis group in comparison with the osteopenia and control groups, with worse speech recognition and discrimination scores and OAEs. Vestibular function was affected in 95.65% of women with osteoporosis and 76% of those with osteopenia.
Conclusion
Osteoporosis and osteopenia are risk factors for vestibular dysfunction and hearing deficits in postmenopausal women. Thus, hearing and vestibular function should be monitored by audiological and vestibular testing periodically in these individuals.
Keywords: Hearing loss, Audio-vestibular testing, Pure tone audiometry (PTA), OAE’S, Speech audiometry, VEMP, Sensorineural hearing
1. Introduction
Osteoporosis and osteopenia are progressive disorders characterized by decreased bone mass and increased bone brittleness that can potentially result in bone fractures (Riis, 1993). Osteoporosis, also called “a silent disease,” remains asymptomatic until it reaches the advanced stage (Kanis et al., 2013). Data from a World Health Organization (WHO) survey shows that this disorder is the second most critical health problem worldwide, following cardiovascular diseases (World Health Organization, 1994). A recent study in 2017 indicated that 29% of the Indian population has osteoporosis per consensus data (Ramalingaiah, 2017).
Osteoporosis and osteopenia can occur at any age, especially in postmenopausal women (Nilas and Christiansen, 1987), and may lead to body pain, fractures, hearing loss and balance disorders (Bigelow et al., 2016; Zuniga et al., 2012; Burke-Doe et al., 2008). A meta-analysis of five studies from different countries found that a decrease in bone mineral density (BMD) or osteoporosis was significantly associated with hearing loss (Upala et al., 2017). A nationwide cohort study of the Korean population concluded that osteoporosis increased the risk of sudden sensorineural hearing loss in patients aged ≥50 years (Kim et al., 2018). Hearing loss in individuals with osteoporosis or osteopenia can be conductive, sensorineural or mixed, potentially due to otosclerosis, ossicular fracture and neural degeneration (Matsuo et al., 2005; Doherty and Linthicum, 2004). A few studies have demonstrated that patients with osteoporosis may suffer from balance disorders as well as hearing loss (Gargeshwari et al., 2018; Mendy et al., 2014).
Most existing studies so far have focused on the association between BMD and cochlear function, and research on the impact of osteoporosis and osteopenia on vestibular function in postmenopausal women is sparse. Also, results in studies showing hearing loss in osteoporosis and osteopenia, especially in postmenopausal females, are variable especially regarding the type of hearing loss. There also lack data on extended high frequency hearing in postmenopausal women with osteoporosis and osteopenia. It was against this background that the current study was planned.
1.1. Aim of the study
The study aims to prove the hypothesis that osteoporosis and osteopenia increase the risk of hearing loss and vestibular dysfunction in postmenopausal women.
1.2. Objectives
To study the prevalence of hearing loss in postmenopausal patients with osteoporosis or osteopenia as compared to the control group.
To compare hearing in postmenopausal patients with osteoporosis or osteopenia with a control group.
To assess and compare vestibular function in postmenopausal patients with osteoporosis or osteopenia and in a control group.
2. Materials and methods
The present study quantitatively analyzed audiological and vestibular test data, using a deductive analytical research approach with a cross-sectional study design in postmenopausal females with osteoporosis or osteopenia.
2.1. The subjects were divided into three groups according to BMD measurements by dual-energy X-ray absorptiometry (DEXA)
DEXA value T-scores for osteoporosis and osteopenia were calculated by comparing to specific normative range for patients of matched gender and ethnicity (Table 1).
Table 1.
Diagnostic categories based on T-score recommended by WHO (Czerwiński et al., 2007) (Czerwiński et al., 2007).
| Normal | scores better than −1.1 |
|---|---|
| Osteopenia | scores between −1.1 and −2.5 |
| Osteoporosis | scores ≥ -2.6 |
Subjects.
Group 1: postmenopausal subjects with normal BMD values (n = 28, mean age = 59.18 ± 4.39 years), i.e. control group
Group 2: postmenopausal subjects with osteopenia (n = 25, mean age = 58.80 ± 4.35 years)
Group 3: postmenopausal subjects with osteoporosis (n = 23, mean age = 60.78 ± 4.54 years)
Age were matched across the groups (Table 2).
Table 2.
Age among osteoporosis, osteopenia and control groups.
| Groups | N | Mean Age (years)< | SD | F ratio |
|---|---|---|---|---|
| Osteoporosis | 23 | 60.78 | 4.54 | 1.35 |
| Osteopenia | 25 | 58.80 | 4.35 | |
| Control | 28 | 59.18 | 4.39 |
SD: Standard deviation.
Inclusion criteria included osteoporosis or osteopenia with no previous history of hearing loss or middle ear pathology, no apparent history of noise exposure, noise trauma or head injury. Patient evaluation was performed after obtaining ethical clearance and written consents. Ear, nose and throat examination was conducted and demographic data, including sex, age, qualification and income were obtained for all the patients as well as the control subjects.
2.2. Audiological assessment
All participants underwent pure tone audiometry, speech reception threshold (SRT), speech discrimination score (SDS) and otoacoustic emissions (OAEs) testing. Only subjects having type A tympanogram were included in this study.
2.2.1. Pure-tone audiometry was conducted at octave conventional and extended high frequencies (0.25 kHz–18 kHz) in a sound-treated room by the same audiologist with a calibrated clinical audiometer (Madsen Orbiter 922)
The TDH 39 headphones was used for conventional frequency audiometry and the HDA 2000 headphones for extended high frequency audiometry. Bone-conduction thresholds were measured at 250 Hz–4000 Hz using a B-71 bone vibrator. Three pure-tone averages (PTAs) were computed based on air conduction thresholds, i.e., PTA 1 for 500, 1000 and 2000 Hz, PTA 2 for 4000, 8000 and 10,000 10,000Hz and PTA3 for 12.5, 16 and 18 kHz. Hearing loss was graded based on the following (Clark, 1981).
| Hearing category | Audiometric threshold (dB HL) |
|---|---|
| Normal | −10–15 |
| Slight loss | 16–25 |
| Mild loss | 26–40 |
| Moderate loss | 41–55 |
| Moderately severe loss | 56–70 |
| Severe loss | 71–90 |
| Profound loss | >91 |
2.2.2. Tympanometry
A Maico (MI34) middle ear analyzer was used to assess eardrum compliance using a probe tone of 226 Hz at 85 dB SPL by varying pressure from +200 daPa to −400 daPa.
2.2.3. Otoacoustic emissions
Both distortion product and transient OAEs were measured using the Smart OAE version 1.0 (Beta Version) (Intelligent Hearing System, USA) and an ER-10D probe. Nonlinear clicks (100 μs, 85 dB peSPL) at 19.3/s were used for TEOAE testing, for 1024 sweeps of 20 ms, one ear at a time. TEOAEs were deemed present if the signal to noise ratio (SNR) at three consecutive frequencies was above 6 dB. DPOAEs were measured from 500 to 8000 Hz and considered as present if SNR was above 6 dB at three consecutive frequencies.
2.3. Vestibular testing
Vestibular-evoked myogenic potentials (VEMPs) were generated as inhibitory electrical potentials in response to sound stimulation (Neuro Audio, Russia, Version 1.0.95). Table 3 shows the parameters used in the study. Both ears of all patients and control subjects were tested.
Table 3.
Stimulus and acquisition parameters for VEMPs.
| Stimulus parameters | Acquisition parameters | ||
|---|---|---|---|
| Stimulus | 500 Hz tone burst | Filter setting | 30–1500 Hz |
| Transducer | ER3Ainsert phones | Time window | 50msec |
| Onset phase | Rarefaction | Number of sweeps | 100 |
| Intensity | 95dBnHL | Notch filter | On |
| Rate | 3.1/s | Channel (–) | Ipsilateral- cVEMP Contra- oVEMP |
2.3.1. cVEMP
cVEMPs were recorded from the sternocleidomastoid muscle (SCM) with the subject in a sitting position and the head rotated away from the stimulated side. The inverting) (–) electrode was placed at the sternoclavicular junction; non-inverting (+) electrode at the midpoint of the upper ½ of ipsilateral SCM muscle and ground electrode at the forehead. P1, N1 peak latencies and P1–N1 amplitudes were recorded.
2.3.2. oVEMP
Ocular vestibular evoked myogenic potentials were also recorded with the subject in a sitting position and instructed to look 30° superomedially at a fixed point to facilitate responses. The non-inverting electrode was placed approximately 1 cm below the center of the lower eyelid, the inverting electrode about 1 cm below the non-inverting electrode on the cheek and the ground electrode on the forehead. N1, P1 peak latencies and N1–P1 amplitudes were recorded.
3. Results
3.1. Pure tone audiometry results in patients with osteoporosis or osteopenia
3.1.1. Patients with osteoporosis
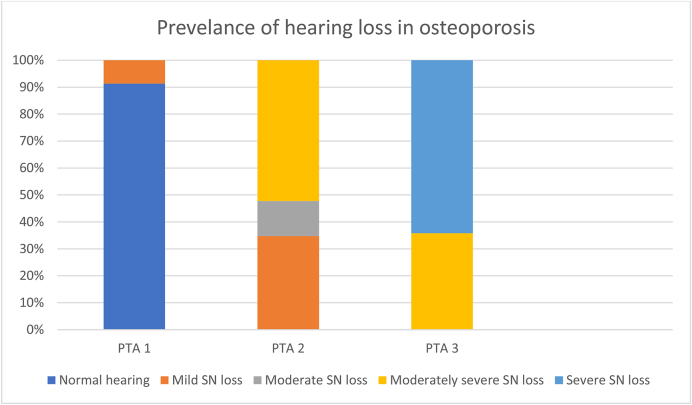
PTA 1 showed normal hearing in 21 patients (91.3%) and mild SN hearing loss in 2 (8.69%). The result of PTA2 indicated mild SN hearing loss in 8 patients (34.7%), moderate SN hearing loss in 3 (13.04%), and moderately severe SN hearing loss in 12 (52.17%). PTA3 revealed moderately severe SN hearing loss in 8 patients (36.36%) and severe SN hearing loss in 15 (65.21%) (Fig. 1).
Fig. 1.
Hearing results in patients with osteoporosis.
3.1.2. Patients with osteopenia
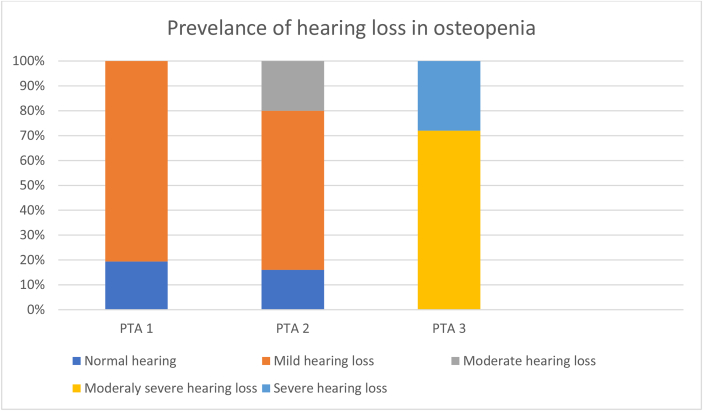
PTA 1 showed normal hearing in 24 patients (96%) and mild SN hearing loss in 1 patient (4%). PTA2 indicated normal hearing in 4 patients (16%), mild SN hearing loss in 16 (64%) and moderate SN hearing loss in 5 (20%). PTA3 revealed moderately severe SN hearing loss in 18 patients (72%) and severe SN hearing loss in 7 (28%) (see Fig. 2).
Fig. 2.
Prevalence of hearing loss in patients with osteopenia.
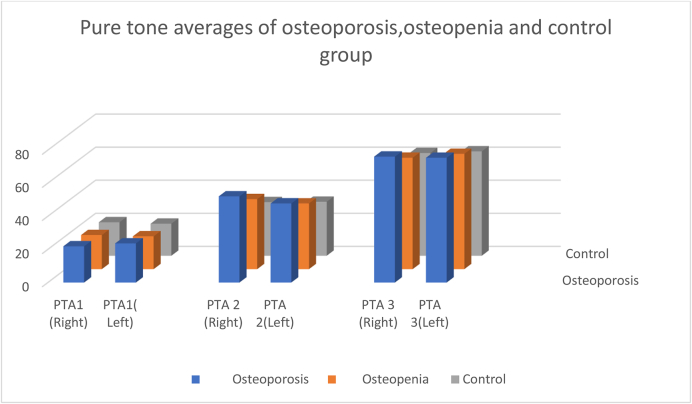
Table 4 shows better low, mid and high pure tone average thresholds in the control group than in the osteopenia and osteoporotic groups in both ears (p < 0.05). The osteoporosis group had the worst hearing sensitivity at all frequencies, followed by the osteopenia and the control group (Fig. 3).
Table 4.
Pure tone average thresholds across osteoporosis, osteopenia and control groups (dB HL).
| Osteoporosis Mean SD | Osteopenia Mean SD | Control Mean SD | F-value | |||||
|---|---|---|---|---|---|---|---|---|
| PTA1 (500 Hz,1000 Hz,2000 Hz) | Right | 21.88 | 1.690 | 20.60 | 2.45 | 20.24 | 2.26 | 3.89∗ |
| Left | 23.62 | 2.28 | 19.82 | 1.94 | 19.37 | 2.69 | 24.44∗ | |
| PTA2 (4 KHz,8 KHz,10 KHz) | Right | 52.03 | 10.27 | 42.27 | 17.97 | 32.38 | 4.83 | 16.68∗∗ |
| Left | 47.75 | 10.60 | 39.67 | 9.94 | 32.62 | 5.04 | 19.10∗∗ | |
| PTA 3 (12.5 KHz,16 KHz,18 KHz) | Right | 76.09 | 8.90 | 67.33 | 8.91 | 62.11 | 5.45 | 20.40∗∗ |
| Left | 75.38 | 5.35 | 69.67 | 8.25 | 63.10 | 4.60 | 24.76∗∗ | |
SD: Standard deviation × p < 0.05.
∗∗p < 0.01.
Fig. 3.
Pure tone averages across osteoporosis, osteopenia and control groups.
3.2. Speech audiometry
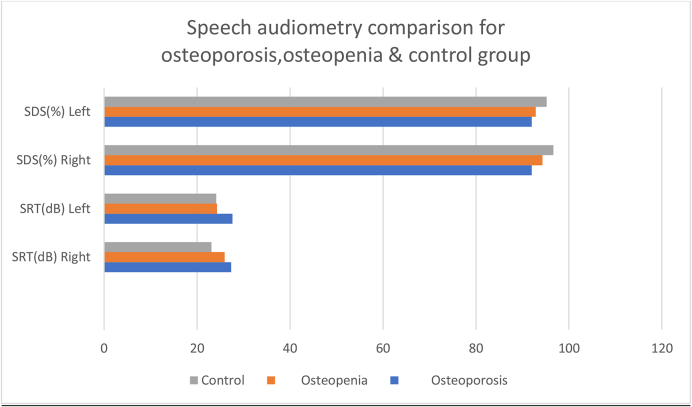
Table 5 shows the speech reception threshold (SRT) and speech discrimination scores (SDS) in all groups for both ears, with statistically significant difference among the three groups in speech reception threshold (p < 0.01) for both ears and in speech discrimination score only for the right ear (Fig. 4).
Table 5.
Speech reception thresholds (SRT) and speech discrimination scores (SDS) across osteoporosis, osteopenia and control groups.
| Osteoporosis Mean SD | Osteopenia Mean SD | Control Mean SD | F-value | |||||
|---|---|---|---|---|---|---|---|---|
| SRT(dB) | Right | 27.35 | 1.11 | 25.92 | 2.22 | 23.11 | 2.85 | 24.15∗∗ |
| Left | 27.61 | 1.92 | 24.28 | 1.95 | 24.11 | 2.14 | 23 .40∗∗ | |
| SDS (%) | Right | 92.00 | 4.82 | 94.29 | 4.95 | 96.64 | 2.98 | 6.79∗∗ |
| Left | 92.00 | 0.00 | 92.86 | 6.40 | 95.20 | 4.76 | 2.96 | |
Fig. 4.
Mean speech reception thresholds (SRT) and speech discrimination scores (SDS) across osteoporosis, osteopenia and control groups for both ears.
3.3. Otoacoustic emissions
TEOAEs were present in 7 patients with osteoporosis (30%), 8 patients with osteopenia (32%) and 10 control subjects (35%). DPOAEs were present (SNR => 6 dB at three consecutive frequencies) in 8 osteoporotic patients (34.78), 11 patients with osteopenia (44%) and 36 control subjects (46%).
3.4. Vestibular myogenic potentials (VEMPs)
cVEMP and oVEMP were affected in 95.65% and 87.33% of the patients with osteoporosis; and in 68% and 28% of the patients with osteopenia, respectively, with statistically significant differences as compared to the control group (p < 0.01).
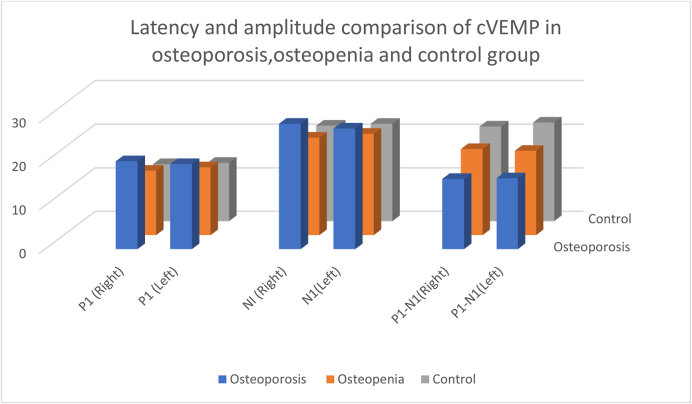
Table 6 and Fig. 5 shows Mean cVEMP latencies and amplitudes among the three groups, showing the most prolonged latencies and lowest amplitudes in the osteoporosis group, followed by the osteopenia and then the control group.
Table 6.
Mean and standard deviation of latency and amplitude of osteoporosis, osteopenia and control group for both ears in cVEMP.
| Latency (msec) | Osteoporosis Mean SD | Osteopenia Mean SD | Control | F-value | ||||
|---|---|---|---|---|---|---|---|---|
| P1 | Right | 20.20 | 0.80 | 14.82 | 1.54 | 13.10 | 0.64 | 300.14∗∗ |
| Left | 19.57 | 0.49 | 15.60 | 1.57 | 13.41 | 0.28 | 265.55∗∗ | |
| N1 | Right | 28.80 | 1.68 | 22.38 | 1.22 | 21.92 | 0.912 | 215.59∗∗ |
| Left | 27.70 | 2.02 | 23.28 | 1.06 | 22.34 | 0.67 | 113.51∗∗ | |
| Amplitude (microvolt) | ||||||||
| P1–N1 | Right | 16.15 | 1.42 | 19.78 | 1.83 | 21.72 | 1.040 | 94.45∗∗ |
| Left | 16.30 | 0.48 | 19.38 | 1.97 | 22.63 | 0.87 | 155.63∗∗ | |
Fig. 5.
Comparison of cVEMP latency and amplitude among the osteoporosis, osteopenia and control groups.
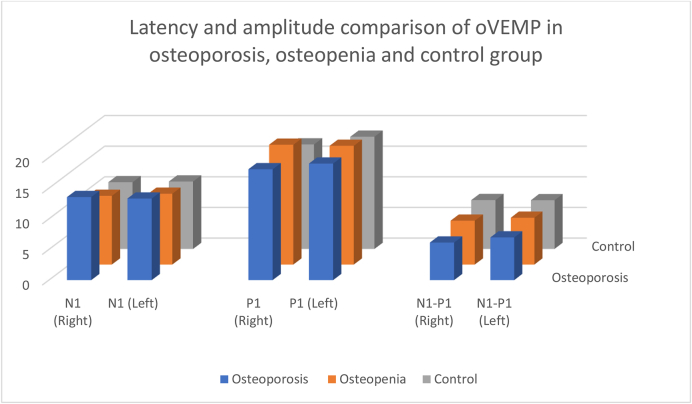
Table 7 and Fig. 6 show mean oVEMP latencies and amplitudes among the groups. The most prolonged latencies and lowest amplitudes are seen in the osteoporosis group, followed by the osteopenia group and then the control group (p < 0.01).
Table 7.
OVEMP latency and amplitude across the osteoporosis, osteopenia and control groups.
| Latency (msec) | Osteoporosis Mean SD | Osteopenia Mean SD | Control Mean SD | F-value | ||||
|---|---|---|---|---|---|---|---|---|
| N1 | Right | 13.56 | 1.28 | 11.22 | 1.20 | 10.90 | 0.32 | 50.98∗∗ |
| Left | 13.32 | 0.87 | 11.56 | 0.41 | 11.01 | 0.46 | 99.23∗∗ | |
| P1 | Right | 18.10 | 1.42 | 19.56 | 1.17 | 17.06 | 0.19 | 38.73∗∗ |
| Left | 19.03 | 0.67 | 19.38 | 0.75 | 18.32 | 0.55 | 17.90∗∗ | |
| Amplitude (Microvolt) | ||||||||
| N1–P1 | Right | 6.13 | 0.47 | 7.16 | 0.17 | 7.98 | 0.41 | 10.25∗∗ |
| Left | 6.97 | 0.10 | 7.64 | 0.39 | 7.96 | 0.23 | 86.69∗∗ | |
Fig. 6.
Comparison of oVEMP latency and amplitude among the osteoporosis, osteopenia and control groups.
4. Discussion
4.1. Audiological results
Our findings demonstrate sensorineural hearing loss over conventional and extended high frequencies as well as affected OAEs in both the osteoporosis and osteopenia groups, suggesting hair cell damage at all frequencies, although more so for high frequencies. We have not come across a single study comparing hearing over conventional and extended high frequencies in patients with osteoporosis and osteopenia, to the best of our knowledge, although many audiological studies for frequencies up to 8 kHz in osteoporosis and osteopenia have been reported in the literature. The results in the present study are in line with studies by Henkin et al. (1972), Oghan and Coksuer (2012), Özkınrış et al. (2013) and Jung et al. (2016). Studies by Kahveci et al. (2014) and Bhavya (2016) also found OAEs affected in these patients. In the present study, extended high-frequency audiometry was conducted to help measure cochlear function and diagnose sensory injury earlier than conventional audiometry (Tweed, 2001). Furthermore, it is a sensitive tool to identify underlying causes in individuals classified by conventional audiometry as having normal hearing but reporting difficulties in speech recognition (Shaw et al., 1996). All individuals in this study showed type A tympanograms, revealing normal middle ear status, similar to studies by Özkınrış et al. (2013) and Bhavya (2016).
In the aging population, osteoporosis is a progressive bone disease that leads to reduced mineral density in all bones including the temporal bone, cochlear capsule, internal auditory canal, mandible and middle ear bone structures (Roth et al., 2011; Clark et al., 1995). Menopause also leads to a decline in estrogen levels. Estrogen induces reduction in the rate of bone loss by inhibiting osteoclastic activity. Therefore, estrogen deficiency causes further irreversible erosion in bone density (Sayegh and Stubblefield, 2002). It has been hypothesized that demineralization of the cochlear capsule may lead to sensorineural hearing loss. Upala et al. (2017), in their meta-analysis, concluded that an imbalance between bone formation and resorption might lead to dysfunctional ionic metabolism resulting in sensorineural hearing loss. Similar to our study, Kahveci et al. (2014) and Özkınrış et al. (2013) reported greater differences at higher frequencies than at lower frequencies among tested groups, although the underlying reason was not clear.
4.2. Vestibular test results
cVEMP and oVEMP were affected the most in patients with osteoporosis, followed by those with osteopenia and then the control group. Ekblad et al. (2000), Mendy et al. (2014), Li et al. (2015) and Bigelow et al. (2016) also found a positive correlation between reduced vestibular functions and low bone mineral density. In a study by Gargeshwari et al. (2018) on osteoporosis, osteopenia and control groups, both subjective and objective balance tests showed balance-related deficits in patients with osteoporosis and osteopenia.
Balance disorders are due to decreased calcium inside the vestibular system, as reported in many studies. Calcium plays a vital part in electrical signal transmission from end organs to higher centers with proper neurotransmitter support. A feature observed both in osteopenia and osteoporosis is the decrease in calcium, which may affect both sensory structures functioning in the peripheral vestibular system and neural transmission. These functional changes result in hearing loss, imbalance and other dysfunctions (Mendy et al., 2014). Vestibular function may also be affected by the role of estrogen in maintaining calcium homeostasis via coupled remodeling in postmenopausal women (Reifenstein and Albright, 1947). Research has proved that the cochlea and vestibule are functionally and anatomically associated. They share a continuous membranous labyrinth and similar receptor cell ultra-structures that are supplied by the labyrinthine artery. Along with hearing, vestibular functions can therefore also be affected (Zuniga et al., 2012; Ciaravella et al., 2007). Thus, we speculate that impaired vestibular function in these individuals may contribute to the risk of falls and, therefore, fractures.
4.3. Limitations
The sample size was small, owing to the time-bound nature of the study. Other vestibular tests were not conducted in the study.
4.4. Future investigations may study the correlation between BMD
The degree of hearing loss and vestibular function, imaging, electrophysiological test results and the relation between abnormal tests and the onset of menopause.
5. Conclusions
Osteoporosis and osteopenia are risk factors for vestibular dysfunction and hearing deficits in postmenopausal women. Hearing loss at high frequencies is present in these women and manifests its impact as difficulty in speech discrimination. Along with hearing, vestibular functions can also be affected. Thus, postmenopausal women with osteopenia and osteoporosis should undergo audiological and vestibular testing periodically to monitor hearing and vestibular status.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
-
•
As Author Dr. Manisha K. Juneja, Dr. Sanjay Munjal, Dr. Anuradha,Dr. Ashok Gupta and Dr Sanjay Bhadada didn’t receive any research financial grant, Hence all Authors declare that they have no conflict of interest.
-
•
All procedures performed in studies involving human participants were done in Speech & Hearing Unit, Dept. of Otolaryngology, PGIMER, Chandigarh and accordance with the ethical standards of the institution
-
•
Prior Informed consent was taken from the participants as well as from the concerned institution where the participants were taking treatment.
Ethical statements
My manuscript is not submitted in any other journal. The submitted work is original.
Declaration of competing interest
The presence or absence of the conflict of interest (COI): " Dr. Manisha K. Juneja, Dr. Sanjay Munjal, Dr. Anuradha, Dr. Ashok Gupta and Dr Sanjay Bhadada declare that they have no conflict of interest."
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Bhavya M. Audiological profile in osteoporosis. Rare Dis. Orphan Drugs. 2016;3:25–32. [Google Scholar]
- Bigelow R.T., Semenov Y.R., Anson E., Du Lac S., Ferrucci L., Agrawal Y. Impaired vestibular function and low bone mineral density: data from the Baltimore Longitudinal study of aging. J. Assoc. Res. Otolaryngol. 2016;17:433–440. doi: 10.1007/s10162-016-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke-Doe A., Hudson A., Werth H., Riordan D.G. Knowledge of osteoporosis risk factors and prevalence of risk factors for osteoporosis falls and fracture in functionally independent older adults. J. Geriatr. Phys. Ther. 2008;31:11–17. doi: 10.1519/00139143-200831010-00003. [DOI] [PubMed] [Google Scholar]
- Ciaravella G., Bennequin D., Laschi C. 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2007. Biomechanical study on the sensorial epithelium of otolithic organs for creating a biomimetic sensor in 2007; pp. 4667–4670. [DOI] [PubMed] [Google Scholar]
- Clark K., Sowers M.R., Wallace R.B., Jannausch M.L., Lemke J., Anderson C.V. Age-related hearing loss and bone mass in a population of rural women aged 60 to 85 years. Ann. Epidemiol. 1995;5:8–14. doi: 10.1016/1047-2797(94)00035-r. [DOI] [PubMed] [Google Scholar]
- Czerwiński E., Badurski J.E., Marcinowska-Suchowierska E., Osieleniec J. Current understanding of osteoporosis according to the position of the World health organization (WHO) and international osteoporosis foundation. Ortop. Traumatol. Rehabil. 2007;9:337–356. [PubMed] [Google Scholar]
- Doherty J.K., Linthicum F.H., Jr. Spiral ligament and stria vascularis changes in cochlear otosclerosis: effect on the hearing level. Otol. Neurotol. 2004;25:457–464. doi: 10.1097/00129492-200407000-00010. [DOI] [PubMed] [Google Scholar]
- Ekblad S., Bergendahl A., Enler P., Ledin T., Möllen C., Hammar M. Disturbances in postural balance are common in postmenopausal women with vasomotor symptoms. Climacteric. 2000;3:192–198. doi: 10.1080/13697130008500097. [DOI] [PubMed] [Google Scholar]
- Gargeshwari A., Jha R.H., Singh N.K., Kumar P. Behavioral and objective vestibular assessment in persons with osteoporosis and osteopenia: a preliminary investigation. Braz. J. Otorhinolaryngol. 2018;84:744–753. doi: 10.1016/j.bjorl.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin R., Lifschitz M., Larson A. Hearing loss in patients with osteoporosis and Paget’s disease of bone. Am. J. Med. Sci. 1972;263:383–392. doi: 10.1097/00000441-197205000-00005. [DOI] [PubMed] [Google Scholar]
- Kahveci O.K., Demirdal U.S., Yücedag F., Cerci U. Patients with osteoporosis have higher incidence of sensorineural hearing loss. Clin. Otolaryngol. 2014;39:145–149. doi: 10.1111/coa.12242. [DOI] [PubMed] [Google Scholar]
- Kanis J.A., McCloskey E.V., Johansson H., Cooper C., Rizzoli R., Reginster J.Y. Scientific advisory board of the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO) and the committee of scientific advisors of the international osteoporosis foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Layman A.J., Carey J.P., Agrawal Y. Epidemiology of vestibular evoked myogenic potentials: data from the Baltimore Longitudinal Study of Aging. Clin. Neurophysiol. 2015;126:2207–2215. doi: 10.1016/j.clinph.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Ito M., Ogawa K., Kanzaki S. Osteoporosis of ossicles and hearing loss in mice. J. Bone Miner. Res. 2005, September;20(9) 2025 M ST, NW, STE 800, WASHINGTON, DC 20036-3309 USA: AMER SOC BONE & MINERAL RES. [Google Scholar]
- Mendy A., Vieira E.R., Albatineh A.N., Nnadi A.K., Lowry D., Gasana J. Low bone mineral density is associated with balance and hearing impairments. Ann. Epidemiol. 2014;24:58–62. doi: 10.1016/j.annepidem.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Nilas L., Christiansen C. Bone mass and its relationship to age and menopause. J. Clin. Endocrinol. Metab. 1987;65:697–702. doi: 10.1210/jcem-65-4-697. [DOI] [PubMed] [Google Scholar]
- Oghan F., Coksuer H. Comparative audiometric evaluation of hearing loss between the premenopausal and postmenopausal period in young women. Am. J. Otolaryngol. 2012;33:322–325. doi: 10.1016/j.amjoto.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Organization W.H. 1994. Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group [meeting Held in Rome from 22 to 25 June 1992. [PubMed] [Google Scholar]
- Özkınrış M., Karaçavuş S., Kapusuz Z., Balbaloğlu Ö., Saydam L. Does bone mineral density have an effect on hearing loss in postmenopausal patients? Ann. Otol. Rhinol. Laryngol. 2013;122:648–652. [PubMed] [Google Scholar]
- Ramalingaiah A. Burden of osteoporosis in the urban Indian population. EC Orthop. 2017;7:74–81. [Google Scholar]
- Reifenstein E.C., Albright F. The metabolic effects of steroid hormones in osteoporosis. J. Clin. Invest. 1947;26:24–56. doi: 10.1172/JCI101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis B.J. Biochemical markers of bone turnover II: diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993;95:S17–S21. doi: 10.1016/0002-9343(93)90376-z. [DOI] [PubMed] [Google Scholar]
- Roth T.N., Hanebuth D., Probst R. Prevalence of age-related hearing loss in Europe: a review. Eur. Arch. Oto-Rhino-Laryngol. 2011;268:1101–1107. doi: 10.1007/s00405-011-1597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh R.A., Stubblefield P.G. Bone metabolism and the perimenopause overview, risk factors, screening, and osteoporosis preventive measures. Obstet. Gynecol. Clin. N. Am. 2002;29:495–510. doi: 10.1016/s0889-8545(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Shaw G.M., Jardine C.A., Fridjhon P. A pilot investigation of high-frequency audiometry in obscure auditory dysfunction (OAD) patients. Br. J. Audiol. 1996;30:233–237. doi: 10.3109/03005369609076770. [DOI] [PubMed] [Google Scholar]
- Tweed T.S. Hearing sensitivity in adults screened for selected risk factors. J. Am. Acad. Audiol. 2001;12:337–347. [PubMed] [Google Scholar]
- Upala S., Rattanawong P., Vutthikraivit W., Sanguankeo A. Associação significativa entre osteoporose e perda auditiva: uma revisão sistemática e metanálise. Braz. J. Otorhinolaryngol. 2017;83:646–652. doi: 10.1016/j.bjorl.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga M.G., Dinkes R.E., Davalos-Bichara M., Carey J.P., Schubert M.C., King W.M., Walston J., Agrawal Y. Association between hearing loss and saccular dysfunction in older individuals. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. [and] Eur. Acad. Otol. Neurotol. 2012;33:1586. doi: 10.1097/MAO.0b013e31826bedbc. [DOI] [PMC free article] [PubMed] [Google Scholar]