Abstract
Objectives
To evaluate hearing outcome of salvage treatment with intratympanic steroids (ITS) in idiopathic sudden sensorineural hearing loss (ISSNHL) refractory to initial systemic steroid (SS) therapy.
Material and methods
A retrospective medical chart review was conducted on 54 consecutive patients with ISSNHL refractory to SS. Salvage treatment with a low dose intratympanic dexamethasone (4 mg/ml) was offered after one week of primary treatment. Patients were divided into two groups: 25 patients accepted ITS (treatment group) and 29 patients did not undergo additional treatment (control group). A pure tone average (PTA) gain of at least 10 dB was considered hearing improvement.
Results
Hearing improvement rate was higher in ITS group compared to control group (40% vs. 13.8%, p = 0.035). A mean PTA improvement of 8.6 ± 9.8 dB was observed in the ITS group and, whereas the control group had an average hearing gain of 0.7 ± 2 dB (p < 0.001). Audiometric analysis revealed a significant hearing gain in ITS group at all tested frequencies compared to control group (p < 0.05). Analysis of the selected variables, identified intratympanic steroid treatment as the only independent prognostic factor for hearing improvement (OR = 4.2, 95% CI: 1.1–15.7; p = 0.04).
Conclusion
Intratympanic low dose dexamethasone is effective in patients with incomplete hearing recovery after primary systemic steroid treatment.
Keywords: Intratympanic, Steroids, Salvage, Sudden sensorineural hearing loss, Idiopathic
1. Introduction
Idiopathic sudden sensorineural hearing loss (ISSNHL) is an acute sensorineural hearing loss of at least 30 dB over three contiguous frequencies, occurring in a period up to 3 days, with no definite cause(Covelli et al., 2018; Wilson et al., 1980). ISSNHL affects between 5 and 20 per 100000 patients, annually(Alexander and Harris, 2013; Chandrasekhar et al., 2019). Its incidence increases with age, peaks at fifth and sixth decade, and presents no gender predominance(Alexander and Harris, 2013; Covelli et al., 2018). The etiology of ISSNHL remains unclear(Jiang et al., 2018). The most widely accepted etiologies are viral infection, microvascular compromise and immunologic causes(Barreto et al., 2016). Spontaneous recovery is more common within the first two weeks of symptoms onset and it is reported to range from 32 to 65%(Barreto et al., 2016; Byl, 1984; Mattox and Simmons, 1977). Patients with spontaneous recover may not present for hospital care, which can underestimate the true incidence of this condition(Byl, 1984; Covelli et al., 2018).
ISSNHL treatment remains controversial, without a standard accepted protocol(H. Y. Lee et al., 2017). Systemic steroids (SS) are the mainstay of treatment and the standard initial option(Schreiber et al., 2010; Wilson et al., 1980). Recovery rate increases to 49–89% after treatment with SS(Moskowitz et al., 1984). Nevertheless, a significant number of patients (30–50%) fails to recover after SS(Jiang et al., 2018) and recent published guidelines consider it optional based on studies that do not evidence better results compared to placebo(Chandrasekhar et al., 2019). Moreover, systemic steroids cause a range of side effects that limit their use(Chandrasekhar et al., 2019).
Intratympanic steroids (ITS) are being increasingly used in patients with ISSNHL. Due to round window semipermeable properties, higher inner ear steroid concentration and reduced systemic toxicity are achieved, compared to SS(Parnes et al., 1999). Although no advantage over SS was found as initial treatment, salvage treatment with ITS is recommended(Ng et al., 2015). ITS provide additional hearing recovery in the absence of spontaneous recovery or in patients refractory to systemic therapy(Chandrasekhar et al., 2019). On the other hand, some authors reported no significant hearing gain after salvage ITS(Plontke et al., 2009). The present study aimed to evaluate the outcome of salvage treatment with intratympanic steroids in patients with ISSNHL who failed to recover after first line systemic steroids.
2. Material and methods
2.1. Study design
A retrospective medical chart review was conducted on patients treated for ISSNHL at our center, from January 2009 to December 2018. The study protocol was approved by the Ethics Committee of our center (approval ID 33/2020) and it was performed in accordance with the principles of Helsinki declaration.
2.2. Study population
Of the 77 patients diagnosed with ISSNHL, 54 were refractory to first line systemic steroids and were enrolled in this study. Intratympanic dexamethasone was offered as salvage therapy. ISSNHL was defined as a hearing loss ≥30 dB in three contiguous frequencies, within 72 h. Patients who did not achieved a complete recovery after systemic steroid therapy (pure tone average ≤ 10 dB compared to contralateral ear or to the initial hearing level in patients with a previous audiometric evaluation before ISSNHL episode) were defined as refractory to initial treatment. Patients with incomplete audiometric data were excluded. Furthermore, patients treated initially with intratympanic steroids and those who did not underwent primary treatment with systemic steroids were excluded from the study.
Audiological evaluation included pure-tone audiometry, which was performed at admission, seven days after systemic steroid therapy and two months after the treatment was completed. Patients who underwent salvage treatment had weekly audiometric evaluation to determine the response to ITS. PTA was determined using four frequencies (0.5, 1, 2 and 4 kHz). Hearing outcome was assessed according to Wilson’s criteria (Table 1). Audiometric evaluation included the configuration of hearing loss. Audiogram shape was classified according to Sheehy’s classification in ascending, descending, flat and mid-frequency subtypes(Sheehy, 1960). Hearing loss was further classified into four groups according to its severity: mild, PTA 26–40 dB; moderate, PTA 41–70 dB; severe, PTA 71–90 dB; and profound, PTA > 90 dB. Data collected included age, gender and duration from onset of symptoms to treatment. Past medical history was evaluated, including smoking status, hypertension, hyperlipidemia, diabetes mellitus and vein thrombosis.
Table 1.
Hearing outcome (Wilson’s criteria) (Wilson et al., 1980).
| Complete Recovery (CR) | PTA within 10 dB of the initial or contralateral unaffected hearing level |
| Partial Recovery (PR) | PTA within 50% of initial hearing level or ≥ 10 dB improvement in hearing level |
| No Recovery (NR) | <10 dB improvement relative to the initial hearing level |
All patients underwent initial systemic steroid therapy (oral prednisolone, 1 mg/kg, with a maximum daily dose of 60 mg) for 7 days tapered over the next week. Profound hearing loss and an only hearing ear were criteria to admission. These patients received an equivalent dose of intravenous dexamethasone (10 mg). Salvage treatment with ITS was proposed to patients with incomplete recovery which included partial recovery (PR) and no recovery (NR) (Table 1). To assess the efficacy of salvage treatment, study population was divided according to treatment option. Treatment group (TG) included 25 patients who underwent ITS and the control group (CG) 29 patients who rejected salvage treatment.
2.3. Salvage treatment
Patients who underwent salvage treatment received 0.5–1 mL of intratympanic dexamethasone (4 mg/mL), through needle injection. Patients were placed in supine position with 45° head rotation to the contralateral ear. After injection, patients remained immobile and avoided swallowing for 30 min. Salvage treatment protocol included one ITS injection per week until a completely hearing recovery was achieved or after a fourth injection. Patients had audiometric evaluation before each injection.
2.4. Outcome
Hearing gain (HG) was calculated as the difference between the pos- and pre-treatment PTA. To assess the effectives of salvage therapy, improvement was defined as an hearing gain >10 dB.
2.5. Statistical analysis
Categorical variables were described as counts or proportions and continuous variables were expressed as means (standard deviation). Kolmogorov-Smirnov test was used to evaluate whether the data had a normal distribution. Chi-square test or Fisher’s Exact test and McNemar tests were used to compare categorical variables. Student t-test and Wilcoxon non parametric test were performed to evaluate differences continuous variables, according to whether or not their distribution was normal, respectively. A multivariate logistic regression model was developed, and included parameters statistically significative in the univariate analysis, in order to evaluate which variables were independently related to treatment outcome. Statistical analysis was performed with Statistical Package for the Social Sciences® (version 24.0 for Mac, SPSS®). A p value < 0.05 was considered statistically significant.
3. Results
3.1. Demographics
Among the 54 patients, 31 (57.4%) females and 23 (42.6%) males were enrolled in this study. Overall, the study population had a mean age of 53.8 ± 14.2 years and an average symptom duration of 6.6 ± 6.8 days. Intratympanic steroids were administered to 25 patients (treatment group), and the remaining 29 patients did not receive additional treatment (control group). The groups did not differ in demographic and clinic parameters (p > 0.05) (Table 2). A mean age of 52.5 ± 13.9 and 52.97 ± 15.4 years was observed in the ITS and control groups, respectively (p = 0.910). Patients who underwent salvage treatment presented earlier compared to the control group (4.7 vs. 8.2 days, p = 0.06) but the difference was not statistically significative.
Table 2.
Demographic and clinical characteristics.
| Intratympanic Steroid Group (N = 25) | Control Group (N = 29) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 52.5 ± 13.1 | 52.9 ± 15.4 | 0.910 |
| Duration from onset to treatment, days | 4.7 ± 4.6 | 8.2 ± 8.1 | 0.075 |
| Gender, n (%) | |||
| Male | 10 (40) | 13 (44.8) | 0.787 |
| Female | 15 (60) | 16 (55.2) | |
| Symptom, n (%) | |||
| Vertigo |
5 (20) |
8 (27.6) |
0.545 |
| Comorbidities, n (%) | |||
| Smoker | 5 (20) | 11 (37.9) | 0.232 |
| Hypertension | 9 (36) | 17 (58.6) | 0.097 |
| Diabetes Mellitus | 5 (20) | 7 (24.1) | 0.755 |
| Hyperlipidemia |
11 (44) |
19 (65.5) |
0.770 |
| Audiometry at Admission | |||
| PTA, dB | 78.4 ± 21.9 | 67.2 ± 15.9 | 0.035 |
| Degree of Hearing Loss, n (%) | |||
| Moderate | 10 (40) | 20 (60) | 0.052 |
| Severe | 8 (32) | 7 (24.1) | |
| Profound | 7 (28) | 2 (6.9) | |
| Type of Hearing Loss, n (%) | |||
| Plain | 19 (76) | 20 (69) | 0.831 |
| Upward | 1 (4) | 3 (10.3) | |
| Downward | 3 (12) | 4 (13.8) | |
| Medium Frequencies | 2 (8) | 2 (6.9) | |
| Contralateral SNHL, n (%) |
6 (24) |
12 (41.4) |
0.392 |
| Audiometry after Primary Treatment | |||
| PTA, dB | 66.7 ± 26.9 | 51.5 ± 19.1 | 0.019 |
| Hearing Gain | 11.7 ± 16.9 | 15.7 ± 12.8 | 0.330 |
| Hearing Outcome | |||
| Partial Recovery | 12 (48.0) | 20 (69.0) | 0.118 |
| No Improvement | 13 (52.0) | 9 (31.0) | |
Abbreviations: PTA, Pure tone average. Continuous variables are expressed as means ± standard deviation.
Statistically significant parameters (P < 0.05) are highlighted on bold.
After primary treatment the study population achieved a mean PTA of 72.4 ± 19.6 dB (range 38.9–120 dB). Patients who received salvage treatment presented a poorer PTA compared to the control group (66.7 vs. 51.5 dB, p = 0.02). No statistically significative differences were observed between the two groups regarding the pattern of hearing loss (p = 0.831) and contralateral sensorineural hearing loss (p = 0.392). Treatment group underwent on average 2 ± 1 intratympanic dexamethasone administration, mostly one (40%) or two (32%) injections. An average time of 5.9 ± 3.2 (5–9) days between injections was noted.
3.2. Comparison of hearing improvement between ITS and control group
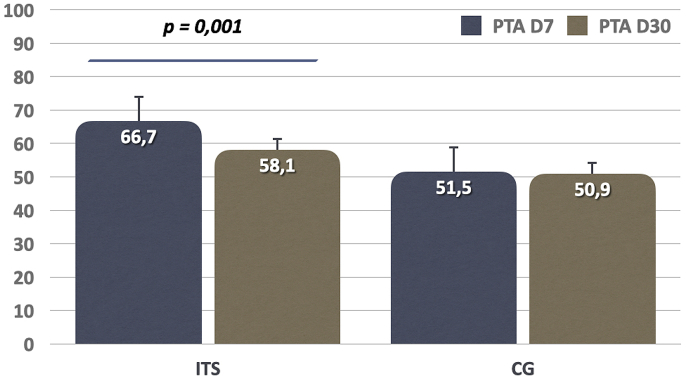
Audiometric evaluation after salvage treatment, revealed a significant difference in hearing improvement between the groups (Table 3). Patients who received intratympanic steroids had an average hearing improvement of 8.7 ± 9.8 dB, whereas it was of 0.7 ± 2 dB in the control group. This difference favoring the ITS group was statistically significative (p < 0.001). Hearing improvement rate (≥10 dB) was superior in ITS group compared to control group (40% vs. 13.8%, p = 0.035). A comparable absolute PTA thresholds in the two groups was obtained at the end of the treatment (58.1 vs. 50.9 dB, p = 0.304) (Fig. 1).
Table 3.
Hearing outcome.
| Intratympanic Steroid Group (N = 25) | Control Group (N = 29) | P Value | |
|---|---|---|---|
| Audiometry after Salvage Treatment | |||
| PTA | 58.1 ± 30.1 | 50.9 ± 19.9 | 0.304 |
| Hearing Gain | 8.7 ± 9.8 | 0.7 ± 2.0 | <0.001 |
| Hearing Improvement > 10 dB | 10 (40) | 4 (13.8) | 0.035 |
| Hearing Outcome (Wilson) | |||
| Complete Recovery | 5 (83.3) | 1 (16.7) | 0.104 |
| Partial Recovery | 11 (36.7) | 19 (63.3) | |
| No Recovery | 9 (50) | 9 (50) | |
Abbreviations: PTA, Pure tone average. Continuous variables are expressed as means ± standard deviation.
Statistically significant parameters (P < 0.05) are highlighted on bold.
Fig. 1.
Comparison of pure tone average (PTA) hearing gain between intratympanic steroid group (ITS) and control group (CG) before and after salvage treatment.
Hearing gain was analyzed according to specific frequencies (Table 4). Higher hearing improvements were achieved in the ITS group compared to the control group for all tested frequencies (p < 0.05). Although improvement in ITS group was greater at lower frequencies, there were no significant differences in the comparison between frequencies (p > 0.05).
Table 4.
Hearing Improvement after Salvage Treatment at different frequencies.
| Frequency, kHz |
|||||
|---|---|---|---|---|---|
| 0.5 kHz | 1 kHz | 2 kHz | 4 kHz | PTA | |
| Intratympanic Steroid | 10.1 ± 13.2 | 9.2 ± 8.8 | 8.7 ± 10.9 | 7.1 ± 10.3 | 8.7 ± 9.8 |
| Control Group | 0.5 ± 2.1 | 0.3 ± 2.3 | 0.7 ± 4.4 | 1.4 ± 3.8 | 0.7 ± 2.0 |
| P Value | <0.001 | <0.001 | 0.001 | 0.001 | <0.001 |
Abbreviations: PTA, Pure tone average. Continuous variables are expressed as means ± standard deviation.
Statistically significant parameters (P < 0.05) are highlighted on bold.
3.3. Subgroup analysis: comparison of responsive and non-responsive groups in ITS patients
A ITS group restricted analysis was performed. A stratification into two groups was performed, according to the presence a hearing improvement of 10 dB or more after intratympanic dexamethasone administration. There were 10 patients who responded to ITS and 15 patients who did not respond to salvage treatment. Characteristics of both groups are presented in Table 5. Patients with partial response to initial treatment presented higher improvement rate following ITS compared to patients with no response to systemic steroids (66.7% vs. 15.4%, p = 0.015). Moreover, patients who presented response to ITS had a lower PTA before salvage treatment compared to patients who did not respond (52.8 ± 15.6 vs. 72.9 ± 29.2, p = 0.03). No association to hearing improvement was observed between age of patients (p = 0.361), duration of symptoms (p = 0.867), PTA at admission (p = 0.264) and audiogram configuration (p = 0.154).
Table 5.
Analysis of clinical factors related to hearing improvement >10 dB in the intratympanic steroid group.
|
Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| Response (N = 10) | No Response (N = 15) | P Value | OR | CI (95%) | P value | |
| Demographics | ||||||
| Age | 50.5 ± 12.4 | 55.5 ± 14 | 0.361 | |||
| Duration from onset to ITS | 10.1 ± 3.2 | 9.9 ± 3.5 | 0.867 | |||
| Gender (female) | 8 (80) | 7 (46.7) | 0.211 | |||
| Vertigo |
3 (30) |
2 (13.3) |
0.358 |
|||
| Comorbidities, n (%) | ||||||
| Smoker | 1 (10) | 4 (26.7) | 0.615 | |||
| Hypertension | 4 (40) | 5 (33.3) | 0.530 | |||
| Diabetes Mellitus | 2 (20) | 3 (20) | 0.699 | |||
| Dyslipidemia |
1 (10) |
3 (20) |
0.626 |
|||
| Audiometry at Admission | ||||||
| PTA, dB | 72.3 ± 17.2 | 82.4 ± 24.3 | 0.264 | |||
| Type of Hearing Loss, n (%) | ||||||
| Plain | 9 (90) | 10 (66.7) | 0.154 | |||
| Upward | 0 (0) | 3 (20) | ||||
| Downward | 1 (10) | 0 (0) | ||||
| Medium Frequencies | 0 (0) | 2 (13.3) | ||||
| Contralateral SNHL, n (%) |
3 (30) |
3 (20) |
0.653 |
|||
| Audiometry After Primary Treatment | ||||||
| PTA, dB | 52.8 ± 15.6 | 72.9 ± 29.2 | 0.032 | |||
| Hearing Gain | 19.5 ± 14.8 | 6.5 ± 16.7 | 0.057 | |||
| Hearing Outcome | ||||||
| Partial Recovery | 8 (80) | 4 (26.7) | 0.015 | 11.0 | 1.6–75.5 | 0.015 |
| No Improvement | 2 (20) | 11 (73.3) | ||||
Abbreviations: PTA, Pure tone average. Continuous variables are expressed as means ± standard deviation.
Statistically significant parameters (P < 0.05) are highlighted on bold.
A binary logistic regression analysis was performed, to assess independent determinants of achieving hearing improvement (≥10 dB) after salvage therapy, within the ITS group, and its relative importance. According to this analysis, patients who presented partial recovery following initial systemic steroids had 11 times the odds of improvement compared to patients with no recovery to initial treatment (OR = 11, p = 0.015, CI: 1.6–75.5). No other independent predictors of improvement were found.
4. Discussion
This study aimed to evaluate the effectiveness of salvage treatment with intratympanic low-dose dexamethasone in patients with ISSNHL refractory to systemic steroids. We found a favorable hearing outcome in patients who underwent ITS compared to control group.
The most common theories for SSNHL include viral infections, vascular compromise and immune mediated causes(Covelli et al., 2018; Erdur et al., 2014). Wilson et al. first reported the efficacy of steroids on ISSNHL management. The authors reported a recovery rate of 61% in the systemic steroid group and 32% in the placebo group(Wilson et al., 1980). Steroid administration decreases inner ear inflammation, improves cochlear blood flow, protects against cochlear ischemia and improves stria vascularis function(Lai et al., 2017). Glucocorticoid and mineralocorticoid receptors can be found in inner ear(Raymundo et al., 2010). Steroid receptors have been described in hair cells and stria vascularis, and hearing improvement occurs by modulation of ion homeostasis and immunosuppressive properties(Dallan et al., 2006). Glucocorticoid receptors modulate gene expression and control the immunologically mediated vasculitis by inhibiting cytokine secretion(Gloddek et al., 2002). Furthermore, steroids acts on mineralocorticoid receptors, restoring endocochlear potential and ion balance by increasing Na+/K+ exchange in stria vascularis, which contributes to recover from ISSHNL(Gross et al., 2002; Raymundo et al., 2010).
However, 30–50% of patients present no response to steroid therapy(Jiang et al., 2018). Some authors argue that the blood-labyrinthine barrier limits the efficacy of systemic steroids(Jiang et al., 2018). For patients with a poor response, long term administration of steroids may not be possible, due to systemic side effects(Chandrasekhar et al., 2019). Round window semipermeable properties allows intratympanic steroids to access the inner ear(Lavigne et al., 2016). Thus, a higher concentration in inner ear fluids is achieved(Chandrasekhar et al., 2019; Parnes et al., 1999). This minimizes side effects due a decreased systemic absorption (Chandrasekhar et al., 2019; Covelli et al., 2018; Parnes et al., 1999).
In literature, studies evaluating hearing outcome after salvage treatment with intratympanic steroids reported successful outcomes ranging from 8% to 95%(Chandrasekhar et al., 2019; J. B. Lee et al., 2011). Erdur et al. showed 47.6% of improvement in ITS group and 10% in control group (p = 0.002). Haynes et al. obtained a hearing improvement of 27.6% in salvage intratympanic treatment group compared to 9.1% in the control group(Haynes et al., 2007).
In the present study, there was a significantly better improvement rate in the ITS group compared to the CG (40% and 13.8%, respectively; p < 0.001). The effectiveness of ITS as salvage treatment is well described in literature(Chandrasekhar et al., 2019; Lavigne et al., 2016). Intratympanic steroids provide additional hearing improvement in 38–53% of patients with incomplete response to primary treatment(Choung et al., 2006; Xenellis et al., 2006).
Our study revealed a significative difference in hearing improvement after salvage therapy, favoring the group who underwent intratympanic steroids compared to control group (8.7 vs. 0.7 dB, respectively, p < 0.05). A metanalysis reported comparable results, with an average hearing improvement of 10 dB with salvage treatment compared to control group(Ng et al., 2015). Final hearing evaluation revealed a comparable PTA between both groups, which reflects the therapeutic effect of intratympanic steroids in the present study.
Etiology and mechanisms of ISSHNL are unknown(Chen et al., 2019). Irreversible damage can result from vascular compromise, which might be responsible for a large proportion of patients who did not recover despite medical treatment(Jiang et al., 2018; Kang et al., 2017). Several prognostic factors have been reported in literature including advanced age, presence of vertigo, profound hearing loss at presentation, descending audiometric configuration, time to onset of symptoms and cardiovascular risk factors (diabetes, hypertension)(Chen et al., 2019; Lionello et al., 2015; Nosrati-Zarenoe and Hultcrantz, 2012). Demographic and clinical characteristics of both groups in our study were comparable, except for PTA after initial systemic steroids (Table 2).
Evaluation of factors affecting hearing improvement to salvage treatment in the ITS group revealed that response to initial treatment may influence the prognosis. Our results showed that patients with an initial partial recovery to systemic steroids benefited more from intratympanic dexamethasone administration (p = 0.015). Other authors found similar results(Haynes et al., 2007). A reduced efficacy after salvage treatment has been reported in patient with profound hearing loss(Gouveris et al., 2005).
Analysis of hearing improvement showed a better hearing gain in the ITS group compared to CG at all tested frequencies (p < 0.05). Experimental studies in animals, evaluating steroid distribution through round window showed a gradient of cochlear distribution, with higher concentration achieved at the basal turn compared with the apical turn(Salt and Ma, 2001). Thus, it would be expected better results at higher frequencies. In our study, ITS group obtained better results at lower frequencies (hearing gain of 9.01 dB and 10.12 dB at 0.5 and 1 kHz) compared to higher frequencies (improvement of 7.03 and 8.66 dB at 2 kHz and 4 kHz). Other authors report similar results(Choung et al., 2006; Covelli et al., 2018; Erdur et al., 2014). Differences between animal and clinical studies are attributed to variations in the cochlear distribution of steroid receptors and a higher vulnerability of the cochlear basal turn cells to free-radical damage(Sha et al., 2001). This can explain a better recovery rate of low-frequency thresholds after ITS(Erdur et al., 2014).
Salvage treatment results are dependent on the steroid, dose, administration method, severity of hearing loss and time until treatment initiation(Chandrasekhar et al., 2019). Intratympanic dexamethasone and methylprednisolone are both effective, achieving higher inner ear concentration compared to systemic administration(Parnes et al., 1999). Animal studies have showed higher inner ear methylprednisolone concentration for a longer period of time compared to dexamethasone(Parnes et al., 1999). However other authors recommend the use of dexamethasone due to decreased discomfort during administration and a comparable hearing recovery(Chandrasekhar et al., 2019). A recent metanalysis reported better outcomes with dexamethasone(Ng et al., 2015).
IT dexamethasone dose reported in literature varies between 4 and 24 mg/mL(Chandrasekhar et al., 2019). Some studies recommend the use of higher dose based on better hearing outcome. Alexander et al. compared different concentration of IT dexamethasone as salvage treatment, and found higher improvement rate with 24 mg/mL dose compared to 10 mg/mL (53% vs. 17%, p = 0.04)(Alexander et al., 2015). Other authors suggested that a low dose of dexamethasone is inadequate as salvage treatment(Günel et al., 2015; Oue et al., 2014). Recent guidelines recommend to use an intratympanic dexamethasone dose of 16–24 mg/mL (compounded) or 10 mg/mL (stock)(Chandrasekhar et al., 2019). In our study, a lower dexamethasone dose (4 mg/mL) was used because it was the only available at our center. Our results support other studies that showed effectiveness of salvage therapy with a low dose of dexamethasone. Lee et al. used a DEX dose of 5 mg/mL and reported hearing improvement in 47.6% of ITS group compared to 16% in the control group (p = 0.027)(J. B. Lee et al., 2011). Wu et al. observed an improvement rate of 44.4% in the ITS group (8mg/2 mL of DEX) and 10.7% in the control group (p = 0.005)(Wu et al., 2011). Moreover, our effectiveness rate (improvement ≥ 10 dB) were similar to other studies that used higher DEX concentration(Taha et al., 2019).
This study has some limitations. This is a retrospective study based on medical charts with a potential information bias. Patient distribution between intratympanic steroid and control groups was not randomized, which can cause a selection bias. Moreover, our small sample size can limit some conclusions.
5. Conclusion
Our results supports salvage treatment with intratympanic low dose dexamethasone after incomplete response to systemic steroids. Patients with partial recovery after initial treatment are more likely to benefit from ITS compared to patients with no initial improvement.
Declaration of competing interest
The authors have no conflict of interests to declare.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Alexander T.H., Harris J.P. Incidence of sudden sensorineural hearing loss. Otol. Neurotol. 2013;34:1586–1589. doi: 10.1097/MAO.0000000000000222. [DOI] [PubMed] [Google Scholar]
- Alexander T.H., Harris J.P., Nguyen Q.T. Dose effect of intratympanic dexamethasone for idiopathic sudden sensorineural hearing loss: 24 mg/mL is superior to 10 mg/mL. Otol. Neurotol. 2015;36:1321–1327. doi: 10.1097/MAO.0000000000000834. [DOI] [PubMed] [Google Scholar]
- Barreto M.A., Ledesma A.L., de Oliveira C.A. Intratympanic corticosteroid for sudden hearing loss: does it really work? Braz. J. Otorhinolaryngol. 2016;82:353–364. doi: 10.1016/j.bjorl.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byl F.M. Sudden hearing loss: eight years’ experience and suggested prognostic table. Laryngoscope. 1984;94:647–661. [PubMed] [Google Scholar]
- Chandrasekhar S.S., Tsai Do B.S., Schwartz S.R. Clinical practice guideline: sudden hearing loss (update) Otolaryngol. Head Neck Surg. 2019;161:S1–S45. doi: 10.1177/0194599819859885. [DOI] [PubMed] [Google Scholar]
- Chen C., Shi G., He M. Characteristics and prognosis of idiopathic sudden sensorineural hearing loss in aged people: a retrospective study. Acta Otolaryngol. 2019;139:959–965. doi: 10.1080/00016489.2019.1657589. [DOI] [PubMed] [Google Scholar]
- Choung Y.H., Park K., Shin Y.R. Intratympanic dexamethasone injection for refractory sudden sensorineural hearing loss. Laryngoscope. 2006;116:747–752. doi: 10.1097/01.mlg.0000205183.29986.f6. [DOI] [PubMed] [Google Scholar]
- Covelli E., Altabaa K., Verillaud B. Intratympanic steroids as a salvage therapy for severe to profound idiopathic sudden sensorineural hearing loss. Acta Otolaryngol. 2018;138:966–971. doi: 10.1080/00016489.2018.1497805. [DOI] [PubMed] [Google Scholar]
- Dallan I., Bruschini L., Nacci A. Transtympanic steroids in refractory sudden hearing loss. Personal experience. Acta Otorhinolaryngol. Ital. 2006;26:14–19. [PMC free article] [PubMed] [Google Scholar]
- Erdur O., Kayhan F.T., Cirik A.A. Effectiveness of intratympanic dexamethasone for refractory sudden sensorineural hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2014;271:1431–1436. doi: 10.1007/s00405-013-2594-x. [DOI] [PubMed] [Google Scholar]
- Gloddek B., Lamm K., Arnold W. Pharmacological influence on inner ear endothelial cells in relation to the pathogenesis of sensorineural hearing loss. Adv. Oto-Rhino-Laryngol. 2002;59:75–83. doi: 10.1159/000059254. [DOI] [PubMed] [Google Scholar]
- Gouveris H., Selivanova O., Mann W. Intratympanic dexamethasone with hyaluronic acid in the treatment of idiopathic sudden sensorineural hearing loss after failure of intravenous steroid and vasoactive therapy. Eur. Arch. Oto-Rhino-Laryngol. 2005;262:131–134. doi: 10.1007/s00405-004-0772-6. [DOI] [PubMed] [Google Scholar]
- Gross N.D., Kempton J.B., Trune D.R. Spironolactone blocks glucocorticoid-mediated hearing preservation in autoimmune mice. Laryngoscope. 2002;112:298–303. doi: 10.1097/00005537-200202000-00018. [DOI] [PubMed] [Google Scholar]
- Günel C., Başal Y., Toka A. Efficacy of low-dose intratympanic dexamethasone for sudden hearing loss. Auris Nasus Larynx. 2015;42:284–287. doi: 10.1016/j.anl.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Haynes D.S., O’Malley M., Cohen S. Intratympanic dexamethasone for sudden sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2007;117:3–15. doi: 10.1097/01.mlg.0000245058.11866.15. [DOI] [PubMed] [Google Scholar]
- Jiang K., Li S., Cheng L. Intratympanic methylprednisolone administration promotes the recovery of idiopathic sudden sensorineural hearing loss: a retrospective case-control study. Acta Otolaryngol. 2018;138:998–1003. doi: 10.1080/00016489.2018.1504170. [DOI] [PubMed] [Google Scholar]
- Kang W.S., Yang C.J., Shim M. Prognostic factors for recovery from sudden sensorineural hearing loss: a retrospective study. J. Audiol. Otol. 2017;21:9–15. doi: 10.7874/jao.2017.21.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D., Zhao F., Jalal N. Intratympanic glucocorticosteroid therapy for idiopathic sudden hearing loss: meta-analysis of randomized controlled trials. Medicine (Baltim.) 2017;96 doi: 10.1097/MD.0000000000008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne P., Lavigne F., Saliba I. Intratympanic corticosteroids injections: a systematic review of literature. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:2271–2278. doi: 10.1007/s00405-015-3689-3. [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Kim D.K., Park Y.H. Prognostic factors for profound sudden idiopathic sensorineural hearing loss: a multicenter retrospective study. Eur. Arch. Oto-Rhino-Laryngol. 2017;274:143–149. doi: 10.1007/s00405-016-4276-y. [DOI] [PubMed] [Google Scholar]
- Lee J.B., Choi S.J., Park K. The efficiency of intratympanic dexamethasone injection as a sequential treatment after initial systemic steroid therapy for sudden sensorineural hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2011;268:833–839. doi: 10.1007/s00405-010-1476-8. [DOI] [PubMed] [Google Scholar]
- Lionello M., Staffieri C., Breda S. Uni- and multivariate models for investigating potential prognostic factors in idiopathic sudden sensorineural hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2015;272:1899–1906. doi: 10.1007/s00405-014-2992-8. [DOI] [PubMed] [Google Scholar]
- Mattox D.E., Simmons F.B. Natural history of sudden sensorineural hearing loss. Ann. Otol. Rhinol. Laryngol. 1977;86:463–480. doi: 10.1177/000348947708600406. [DOI] [PubMed] [Google Scholar]
- Moskowitz D., Lee K.J., Smith H.W. Steroid use in idiopathic sudden sensorineural hearing loss. Laryngoscope. 1984;94:664–666. [PubMed] [Google Scholar]
- Ng J.H., Ho R.C., Cheong C.S. Intratympanic steroids as a salvage treatment for sudden sensorineural hearing loss? A meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2015;272:2777–2782. doi: 10.1007/s00405-014-3288-8. [DOI] [PubMed] [Google Scholar]
- Nosrati-Zarenoe R., Hultcrantz E. Corticosteroid treatment of idiopathic sudden sensorineural hearing loss: randomized triple-blind placebo-controlled trial. Otol. Neurotol. 2012;33:523–531. doi: 10.1097/MAO.0b013e31824b78da. [DOI] [PubMed] [Google Scholar]
- Oue S., Jervis-Bardy J., Stepan L. Efficacy of low-dose intratympanic dexamethasone as a salvage treatment for idiopathic sudden sensorineural hearing loss: the Modbury Hospital experience. J. Laryngol. Otol. 2014;128(Suppl. 2):S27–S30. doi: 10.1017/S0022215113003472. [DOI] [PubMed] [Google Scholar]
- Parnes L.S., Sun A.H., Freeman D.J. Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope. 1999;109:1–17. doi: 10.1097/00005537-199907001-00001. [DOI] [PubMed] [Google Scholar]
- Plontke S.K., Löwenheim H., Mertens J. Randomized, double blind, placebo controlled trial on the safety and efficacy of continuous intratympanic dexamethasone delivered via a round window catheter for severe to profound sudden idiopathic sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2009;119:359–369. doi: 10.1002/lary.20074. [DOI] [PubMed] [Google Scholar]
- Raymundo I.T., Bahmad F., Barros Filho J. Intratympanic methylprednisolone as rescue therapy in sudden sensorineural hearing loss. Braz. J. Otorhinolaryngol. 2010;76:499–509. doi: 10.1590/S1808-86942010000400015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt A.N., Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear. Res. 2001;154:88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- Schreiber B.E., Agrup C., Haskard D.O. Sudden sensorineural hearing loss. Lancet. 2010;375:1203–1211. doi: 10.1016/S0140-6736(09)62071-7. [DOI] [PubMed] [Google Scholar]
- Sha S.H., Taylor R., Forge A. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear. Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Sheehy J.L. Vasodilator therapy in sensory-neural hearing loss. Laryngoscope. 1960;70:885–914. doi: 10.1288/00005537-196007000-00002. [DOI] [PubMed] [Google Scholar]
- Taha A., Shlamkovitch N., Abu-Eta R. High dose of intratympanic steroids for sudden sensorineural hearing loss salvage. Otol. Neurotol. 2019;40:1134–1138. doi: 10.1097/MAO.0000000000002386. [DOI] [PubMed] [Google Scholar]
- Wilson W.R., Byl F.M., Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch. Otolaryngol. 1980;106:772–776. doi: 10.1001/archotol.1980.00790360050013. [DOI] [PubMed] [Google Scholar]
- Wu H.P., Chou Y.F., Yu S.H. Intratympanic steroid injections as a salvage treatment for sudden sensorineural hearing loss: a randomized, double-blind, placebo-controlled study. Otol. Neurotol. 2011;32:774–779. doi: 10.1097/MAO.0b013e31821fbdd1. [DOI] [PubMed] [Google Scholar]
- Xenellis J., Papadimitriou N., Nikolopoulos T. Intratympanic steroid treatment in idiopathic sudden sensorineural hearing loss: a control study. Otolaryngol. Head Neck Surg. 2006;134:940–945. doi: 10.1016/j.otohns.2005.03.081. [DOI] [PubMed] [Google Scholar]