Abstract
Objective
Recent studies have shown that chronic inflammation contributes to the development of sudden sensorineural hearing loss (SSNHL). Some hematologic parameters have also been linked to the prognosis of SSNHL. However, the prognostic value of such hematological factors is not conclusive. This study explored the association of routine hematological parameters with SSNHL.
Methods
A systematic literature search was conducted in PubMed, Cochrane Library, Web of Science and Embase to identify eligible studies. Standardized mean deviation (SMD) and the 95% confidence interval (CI) were retried from relevant studies for analysis. Heterogeneity, subgroup, and publication bias analyses were performed.
Results
A total of 18 studies involving 1505 SSNHL patients and 1466 healthy persons were enrolled in the final analysis. The study population included 699 responders and 458 non-responders to treatment. Pooled results revealed that the neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) value in the SSNHL patient group were higher than in the healthy group (SMD = 1.05, 95% CI: 0.86,1.24, p < 0.001, SMD = 0.52, 95% CI: 0.26,0.78, p < 0.001, respectively). However, there was no significant difference in the mean platelet volumes (MPV) between the groups (SMD = 0.03, 95% CI: 0.44, 0.49, p = 0.91). Notably, NLR and PLR values were evidently higher in the unrecovered group than in the recovered group (SMD = −0.63, 95% CI: 1.02, −0.23, p = 0.002, SMD = −0.4, 95% CI: 0.76, −0.03, p = 0.03, respectively). However, the MPV value was similar in both groups (SMD = −0.35, 95% CI: 1.14,0.44, p = 0.38).
Conclusions
Our results show that NLR and PLR values can predict the onset and prognosis of SSNHL.
Keywords: Sudden sensorineural hearing loss, Prognosis, Neutrophil/ lymphocyte ratio, Platelet/lymphocyte ratio, Meta-analysis
Abbreviations
- SSNHL
sudden sensorineural hearing loss
- SMD
Standardized mean deviation
- CI
confidence interval
- NLR
neutrophil-to-lymphocyte ratio
- PLR
platelet-to-lymphocyte ratio
- MPV
mean platelet volumes
1. Introduction
SSNHL is commonly described as a quick hearing loss of not less than 30 dB at three contiguous frequencies occurring within 72 h (Leung et al., 2016). The incidence of SSNHL varies between 5 and 20 cases per 100,000 in the adult population (Stachler et al., 2012). Several factors, such as viral infections, immune mediation, microcirculatory disturbances, and vascular disturbance have been associated with the onset of SSNHL. However, other unidentified factors are thought to play a role in the pathogenesis of this condition. Recent studies indicate that chronic inflammation is a major cause of sudden deafness (Hiramatsu et al., 2012; Ulu et al., 2013). This is because it contributes to microvascular injuries, atherogenesis (Hoffman et al., 2004), and endocochlear immune responses (Masuda et al., 2012). These factors directly increase the risk of cochlear ischemia. Hematological indices, including NLR, PLR, and MPV, are typical inflammatory markers. A recent study identified NLR and PLR as the new inflammatory response biomarkers in renal illness, cerebrovascular, Alzheimer's disease, oncologic and ulcerative colitis. The study concluded that these biomarkers are more effective of inflammation than interleukin (IL)-6, IL-1b, IL-8 (K et al., 2016). Furthermore, MPV is a marker of platelet activation which can predict cardiovascular disease (Acet et al., 2016). To date, several studies have revealed a strong link between these hematologic parameters and the diagnosis/prognosis of SSNHL. Some studies reported that higher NLR, PLR, and MPV values may predict poor prognosis of SSNHL (K et al., 2016; Qiao et al., 2019; Sun et al., 2017), By contrast, other studies have challenged this association (Karli et al., 2013; Ikinciogullari et al., 2014; Lee et al., 2017). Thus, this meta-analysis was designed to clarify the association of these hematologic biomarkers with the diagnosis and prognosis of SSNHL patients.
2. Materials and methods
2.1. Search strategy
Relevant studies were searched in four databases, namely PubMed, Cochrane Library, Web of Science, and Embase from inception up to March 18, 2020. The search was performed using Medical Subject Headings (MeSH) terms combined with the following free words: “Hearing Loss, Sudden,” “Blood Cells,” “Lymphocytes,” “Neutrophils,” “Blood Platelets,” and “Leukocytes.” Additionally, we manually screened the reference lists of the retrieved papers to select other potentially relevant studies.
2.2. Inclusion and exclusion criteria
The criteria for selecting the relevant literature included: studies comparing NLR, PLR and MPV values between healthy individuals and patients diagnosed with SSNHL, and (or) between recovered SSNHL patients and unrecovered SSNHL patients; studies that measured NLR, PLR, and MPV values before any treatments; provided the mean and standard deviation data; the study provided baseline data and study was published in English. In cases where multiple studies were performed on the same study population, the most representative study was included. The following articles were excluded: letters, comments, reviews, conference abstracts, case reports, studies lacking sufficient information in English, duplicate publications, studies with factors that affect NLR and PLR, such as previous steroid treatment, acute or chronic infectious diseases, rheumatic diseases, blood or endocrine diseases, etc.
2.3. Data extraction and quality assessment
Two researchers (N.W. and S.S.P.) screened for eligible studies and performed quality assessment separately. Any disagreements were settled through discussions. The following data were retrieved from the studies: first author, year, region, study design, sample size, age, gender, period, pretreatment NLR or PLR or MPV value, follow-up time, type of steroid, and definition of recovered patients. The Newcastle-Ottawa Scale (Cook and Reed, 2015) was used to estimate the quality of the studies which has scores ranging from 0 to 9 (the higher the score, the better the quality).
2.4. Statistical analysis
We performed statistical analyses in the Review manager 5.3 and Stata12.0 software. SMD and 95% CI values were compared between groups. A two-tailed P value < 0.05 was considered statistically significant.
Heterogeneity among the included studies was determined using the Cochran Q test (P < 0.1 implied statistically significant heterogeneity) and I2 statistic (a value below 25% indicated no heterogeneity; 25% to 50% implied moderate heterogeneity, and more than 50% signified extreme heterogeneity) (Wu et al., 2016). If heterogeneity existed among the studies, a random effect's model was used; otherwise, a fixed effect's model was employed. When extreme heterogeneity existed among studies, subgroup analyses were performed by region, hematology analyzer, age, sample size, type of steroid, follow-up, brand audiometry device, and definition of recovered to investigate the potential confounding factors.
Sensitivity analysis was implemented by sequentially omitting one study at a time to confirm the stability of the pooled effect size. Publication bias was determined using the Egger test if more than five studies were available (Sutton et al., 2000); P > 0.1 implied no publication bias.
3. Results
3.1. Literature search and study characteristics
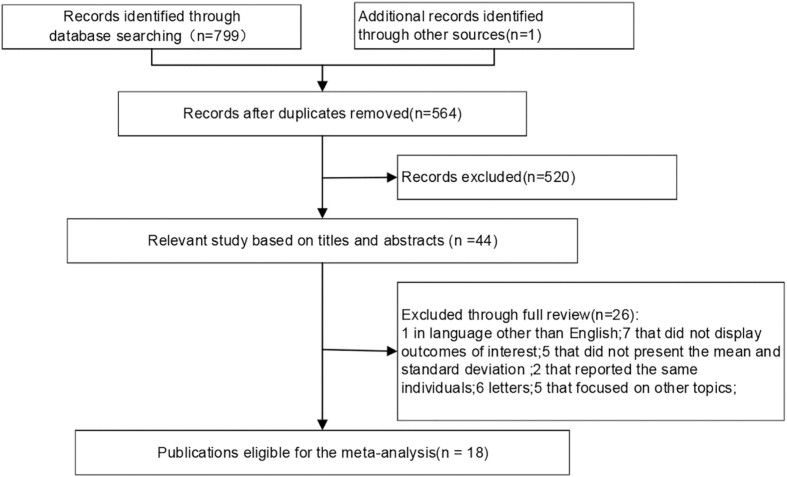
The search identified 799 papers, and one additional study was found by hand-searching the reference lists of included studies. After the removal of duplicate studies (n = 236), 564 were retained from the initial search. A further 520 articles were removed after reading the titles and abstracts of the relevant studies. From the remaining 44 articles, we excluded one study because it was not published in English, seven articles as they lacked outcomes of interest, five articles that did not give the mean and standard deviation of NLR, PLR, and MPV values, two articles that reported the same population, six letters, and five articles that focused on other topics. Finally, 18 studies (Ulu et al., 2013; K et al., 2016; Qiao et al., 2019; Karli et al., 2013; Ikinciogullari et al., 2014; Lee et al., 2017; Zheng et al., 2014; Yasan et al., 2013; Seo et al., 2014; Sagit et al., 2013; Quaranta et al., 2015; Ozler, 2014; Nonoyama et al., 2016; Mirvakili et al., 2016; Kum et al., 2015; Kocak et al., 2017; Ha et al., 2019; Bulğurcu et al., 2017) were eligible for meta-analysis. (Fig. 1).
Fig. 1.
Flow chart of the included studies.
Overall, 1505 SSNHL patients and 1466 healthy controls were involved in the included studies, of which the number varied from 21 to 348 in the patient group, and 24 to 537 in the healthy group across studies. The critical characteristics of the included literature are summarized in Table 1.
Table 1.
Summary of studies included in the meta-analysis. SD = standard deviation; M/F = male/female; NOS = Newcastle-Ottawa scale.
| First author (year) | Region | Study design | Sample size | Age (mean ± SD) | Gender (M/F) | NOS |
|---|---|---|---|---|---|---|
| Bulğurcu S (2017) | Turkey | retrospective case-control | 21 | 13.7 ± 3.2 | 13/8 | 7 |
| Ha, R (2019) | Korea | retrospective case-control | 42 | 14.5 ± 4.1 | 20/19 | 6 |
| Ikinciogullari,A (2014) | Turkey | retrospective case-control | 102 | 48.94 ± 13.86 | 54/48 | 7 |
| Karli, R. (2013) | India | retrospective case-control | 46 | 45.39 ± 15.70 | 25/21 | 7 |
| Kocak, HE (2017) | Turkey | retrospective case-control | 45 | 31.1 ± 7.4 | 25/20 | 8 |
| Kum, RO (2015) | Turkey | cross-sectional historical cohort | 59 | 46.1 ± 11.91 | 38/21 | 7 |
| Lee, JS (2017) | Korea | retrospective case-control | 46 | 14.7 ± 2.81 | 26/20 | 6 |
| Mirvakili, A (2016) | Iran | prospective case-control | 108 | 45.15 ± 14.42 | 61/47 | 7 |
| Ozler, GS (2014) | Turkey | retrospective case-control | 40 | 39.4 ± 11.2 | 15/25 | 7 |
| Qiao, XF (2019) | China | retrospective case-control | 60 | 45.62 ± 13.16 | 28/32 | 7 |
| Sagit, M (2013) | Turkey | retrospective case-control | 31 | 37.45 ± 15.7 | 17/14 | 8 |
| Seo, YJ (2014) | Korea | retrospective case-control | 348 | 48.19 ± 15.22 | 171/177 | 6 |
| Ulu, S (2013) | Turkey | retrospective case-control | 47 | 47.27 ± 16.88 | 27/20 | 8 |
| Yasan, H (2013) | Turkey | prospective/retrospective case-control | 147 | 30.81 ± 11.08 | 79/68 | 7 |
| Zheng, XS (2014) | China | retrospective case-control | 40 | 44.7 ± 14.1 | 22/18 | 7 |
| DurmuşK et al. (2016) | Turkey | retrospective case-control | 140 | 47.02 ± 15.72; 48.03 ± 16.85 | 88/52 | 6 |
| Nonoyama H (2016) | Japan | retrospective case-control | 89 | 54.2 ± 17.5 | 50/39 | 8 |
| Quaranta N (2015) | Italy | retrospective case-control | 94 | 48.4 ± 16.7 | NR | 8 |
3.2. Meta-analysis
3.2.1. NLR in the SSNHL and control groups
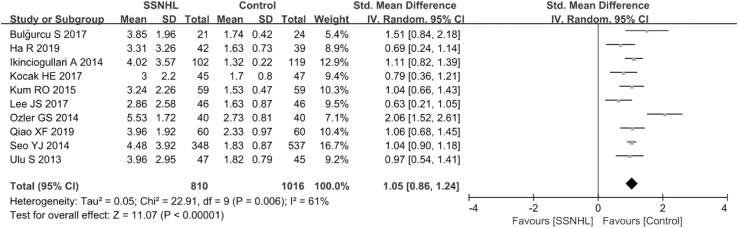
Overall, ten articles (Ulu et al., 2013; Qiao et al., 2019; Ikinciogullari et al., 2014; Lee et al., 2017; Seo et al., 2014; Ozler, 2014; Kum et al., 2015; Kocak et al., 2017; Ha et al., 2019; Bulğurcu et al., 2017) evaluated the diagnostic values of NLR in SSNHL in a total of 810 SSNHL patients and 1016 healthy controls. Owing to the extreme heterogeneity (P = 0.006, I2 = 61%)among the included studies, a random-effects model was used to assess the pooled outcome. The NLR level of the control group was remarkably lower compared with that of the SSNHL group (SMD = 1.05, 95% CI: 0.86, 1.24, P < 0.001; Fig. 2). In subgroup analysis, region, hematology analyzer, and sample size were found to be sources of heterogeneity (Table 2). However, age was not a source of heterogeneity (Table 2).
Fig. 2.
Forest plot of the differences in neutrophil-to-lymphocyte ratio (NLR) levels between SSNHL patients and healthy controls.
Table 2.
Subgroup analyses for the predictive value of NLR in SSNHL diagnosis. SMD = standard mean deviation; CI = confidence interval; NR = none reported; NLR = neutrophil-to-lymphocyte ratio; SSNHL = sudden sensorineural hearing loss.
| Categories | No. of studies | SMD (95% CI) | I2 |
|---|---|---|---|
| Total | 10 | 1.06 (0.87,1.25) | 61.7% |
| Region | |||
| Europen | 6 | 1.21 (0.90,1.53) | 69.0% |
| Asian | 4 | 0.92 (0.72,1.13) | 39.1% |
| hematology analyzer | |||
| Beckman | 2 | 1.21 (0.76,1.67) | 35.5% |
| Sysmex | 3 | 1.05 (0.93,1.71) | 0% |
| Others | 3 | 1.15 (0.34,1.97) | 89.4% |
| NR | 2 | 0.91 (0.54,1.27) | 34.5% |
| Age | |||
| Child | 3 | 0.89 (0.42,1.34) | 63.1% |
| Adult | 7 | 1.11 (0.91,1.32) | 60.7% |
| sample size | |||
| >46 | 5 | 1.05 (0.94,1.16) | 0% |
| ≤46 | 5 | 1.12 (0.60,1.65) | 82.6% |
3.2.2. PLR in SSNHL and control groups
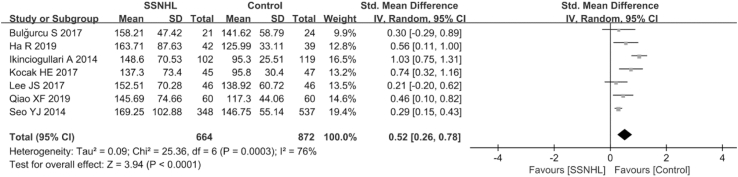
Overall, seven papers (Qiao et al., 2019; Ikinciogullari et al., 2014; Lee et al., 2017; Seo et al., 2014; Kocak et al., 2017; Ha et al., 2019; Bulğurcu et al., 2017) estimated the diagnostic value of PLR in SSNHL in a total of 664 SSNHL patients and 872 healthy individuals. Given the extreme heterogeneity among the studies (P < 0.001, I2 = 76%), a random-effects model was adopted. The PLR value of the SSNHL group was markedly higher than that of the healthy group (SMD = 0.52, 95% CI: 0.26,0.78, P < 0.001; Fig. 3). In the subgroup analyses, region, age, hematology analyzer, and sample size were sources of heterogeneity (Table 3).
Fig. 3.
Forest plot of the differences in platelet -to-lymphocyte ratio (PLR) levels between SSNHL patients and healthy controls.
Table 3.
Subgroup analyses for the predictive value of PLR in SSNHL diagnosis. SMD = standard mean deviation; CI = confidence interval; NR = none reported; PLR = platelet -to-lymphocyte ratio; SSNHL = sudden sensorineural hearing loss.
| Categories | No. of studies | SMD (95% CI) | I2 |
|---|---|---|---|
| Total | 7 | 0.52 (0.26,0.78) | 76.6% |
| Region | |||
| Europen | 3 | 0.75 (0.38,1.15) | 61.2% |
| Asian | 4 | 0.32 (0.20,0.44) | 0% |
| hematology analyzer | |||
| Beckman | 1 | 0.31 (-0.28,0.90) | – |
| Sysmex | 2 | 0.65 (-0.08,1.38) | 95.4% |
| Others | 2 | 0.47 (-0.05,1.00) | 68.8% |
| NR | 2 | 0.50 (0.22,0.78) | 0% |
| Age | |||
| Child | 3 | 0.36 (0.09,0.63) | 0% |
| Adult | 4 | 0.62 (0.23,1.02) | 87.4% |
| sample size | |||
| >46 | 3 | 0.59 (0.11,1.07) | 90.9% |
| ≤46 | 4 | 0.47 (0.22,0.72) | 17.9% |
3.2.3. MPV in SSNHL and control groups
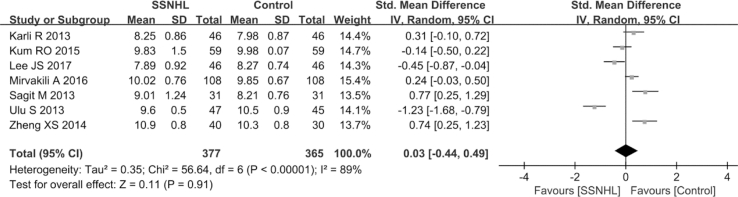
Overall, seven literature (Ulu et al., 2013; Karli et al., 2013; Lee et al., 2017; Zheng et al., 2014; Sagit et al., 2013; Mirvakili et al., 2016; Kum et al., 2015) involving 377 SSNHL patients and 365 healthy persons reported the diagnostic role of MPV in SSNHL. Extreme heterogeneity was identified among the seven articles (P < 0.1, I2 = 89%); therefore, a random-effects model was employed to inspect the pooled results. No significant difference in MPV levels was found between SSNHL patients and healthy group (SMD = 0.03, 95% CI: 0.44, 0.49, P = 0.91; Fig. 4). Subgroup analyses identified hematology analyzer as the only source of heterogeneity (Table 4).
Fig. 4.
Forest plot of the differences in mean platelet volume (MPV) levels between SSNHL patients and healthy controls.
Table 4.
Subgroup analyses for the predictive value of MPV in SSNHL diagnosis. SMD = standard mean deviation; CI = confidence interval; NR = none reported; MPV = mean platelet volumes; SSNHL = sudden sensorineural hearing loss.
| Categories | No. of studies | SMD (95% CI) | I2 |
|---|---|---|---|
| Total | 7 | 0.03 (-0.44,0.50) | 89.6% |
| Region | |||
| Europen | 4 | −0.09 (-0.81,0.62) | 92.8% |
| Asian | 3 | 0.19 (-0.49,0.87) | 86.3% |
| Hematology analyzer | |||
| Beckman | 2 | 0.30 (-0.60,1.20) | 87.7% |
| Sysmex | 1 | −1.24 (-1.69, −0.80) | – |
| Others | 2 | 0.14 (-1.04,1.32) | 92.6% |
| NR | 2 | 0.26 (0.034,0.48) | 0% |
| sample size | |||
| >46 | 3 | −0.37 (-1.17,0.44) | 93.5% |
| ≤46 | 4 | 0.33 (-0.24,0.91) | 84.4% |
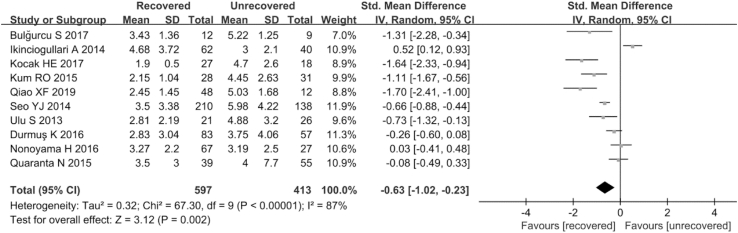
3.2.4. NLR in the recovered and unrecovered groups
Ten studies (Ulu et al., 2013; K et al., 2016; Qiao et al., 2019; Ikinciogullari et al., 2014; Seo et al., 2014; Quaranta et al., 2015; Nonoyama et al., 2016; Kum et al., 2015; Kocak et al., 2017; Bulğurcu et al., 2017) involving 597 SSNHL patients and 413 healthy individuals analyzed the association of NLR on the prognosis of SSNHL. Due to the extreme heterogeneity among the studies (P = 0.006, I2 = 61%), a random-effects model was employed. The NLR level in the unrecovered group was significantly higher than that in the recovered group (SMD = −0.63, 95% CI: 1.02, −0.23, P = 0.002; Fig. 5). Subgroup analyses performed on the clinical variables revealed that hematology analyzer and the sample size were the sources of heterogeneity, whereas region, type of steroid, brand audiometry device, the definition of recovered and follow up were not (Table 5).
Fig. 5.
Forest plot of the differences in neutrophil-to-lymphocyte ratio (NLR) levels between the recovered group and the unrecovered group.
Table 5.
Subgroup analyses for the predictive value of NLR in SSNHL prognosis. SMD = standard mean deviation; CI = confidence interval; NR = none reported; NLR = neutrophil-to-lymphocyte ratio; SSNHL = sudden sensorineural hearing loss.
| Categories | No. of studies | SMD (95% CI) | I2 |
|---|---|---|---|
| Total | 10 | −0.64 (-1.04, −0.24) | 87% |
| Region | |||
| Europen | 7 | −0.61 (-1.15, −0.07) | 87.3% |
| Asian | 3 | −0.73 (-1.47,0.01) | 88.8% |
| hematology analyzer | |||
| Beckman | 2 | −1.19 (-1.67, −0.71) | 0% |
| Sysmex | 4 | −0.21 (-0.83,0.41) | 90.1% |
| Others | 2 | −0.93 (-2.30,0.44) | 93.6% |
| NR | 2 | −0.88 (-2.49,0.73) | 87.0% |
| type of steroid | |||
| Prednisolone | 2 | −0.36 (-2.21,1.48) | 92.0% |
| Prednisone | 4 | −0.63 (-1.01, −0.24) | 70.6% |
| Others | 4 | −0.86 (-1.66, −0.05) | 89.9% |
| brand audiometry device | |||
| AC40 | 5 | −0.53 (-1.17,0.11) | 87.3% |
| Others | 1 | 0.04 (-0.41,0.48) | – |
| NR | 4 | −0.97 (-1.61, −0.32) | 88.0% |
| definition of “recovered" | |||
| ≥15 dB | 5 | −1.04 (-1.56, −0.52) | 83.2% |
| ≥10 dB | 4 | −0.31 (-1.05,043) | 89.0% |
| Others | 1 | −0.64 (-1.04, −0.24) | – |
| follow-up | |||
| 30days | 5 | −0.53 (-1.17,0.11) | 87.3% |
| Others | 4 | −0.95 (-1.64, −0.26) | 88.5% |
| NR | 1 | −0.08 (-0.49,0.33) | – |
| sample size | |||
| >60 | 5 | −0.11 (-0.53,0.32) | 86.6% |
| ≤60 | 5 | −1.28 (-1.66, −0.91) | 35.2% |
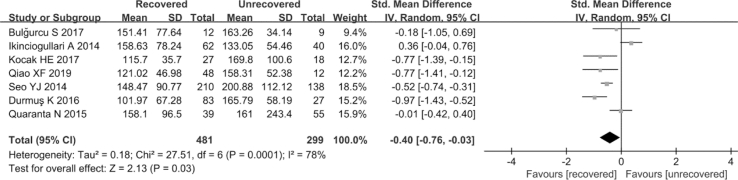
3.2.5. PLR in recovered and unrecovered group
Overall, seven studies (K et al., 2016; Qiao et al., 2019; Ikinciogullari et al., 2014; Seo et al., 2014; Quaranta et al., 2015; Kocak et al., 2017; Bulğurcu et al., 2017) consisting of 481 SSNHL patients and 299 controls estimated the impact of PLR on the prognosis of SSNHL. A random-effects model was carried out to analyze the pooled results because of the extreme heterogeneity among the seven studies (P < 0.1, I2 = 78%). The PLR level in the unrecovered group was remarkably higher than in the recovered group (SMD = −0.4, 95% CI: 0.76, −0.03, P = 0.03; Fig. 6). Subgroup analyses revealed that region, type of steroid, the definition of recovered, follow up, hematology analyzer, and sample size were sources of heterogeneity (Table 6). By contrast, the brand of audiometry device was not a source of heterogeneity (Table 6).
Fig. 6.
Forest plot of the differences in platelet -to-lymphocyte ratio (PLR) levels between the recovered group and the unrecovered group.
Table 6.
Subgroup analyses for the predictive value of PLR in SSNHL prognosis. SMD = standard mean deviation; CI = confidence interval; NR = none reported; PLR = platelet -to-lymphocyte ratio; SSNHL = sudden sensorineural hearing loss.
| Categories | No. of studies | SMD (95% CI) | I2 |
|---|---|---|---|
| Total | 7 | −0.40 (-0.77, −0.03) | 78.5% |
| Region | |||
| Europen | 5 | −0.31 (-0.86, 0.24) | 82.7% |
| Asian | 2 | −0.55 (-0.76, −0.34) | 0% |
| hematology analyzer | |||
| Sysmex | 2 | −0.10 (-0.97, 0.78) | 93.2% |
| Others | 3 | −0.77 (-1.17, −0.38) | 20.6% |
| NR | 2 | −0.35 (-1.09,0.39) | 73.6% |
| type of steroid | |||
| Prednisolone | 2 | 0.23 (-0.24, 0.70) | 22.6% |
| Prednisone | 2 | −0.30 (-0.80, 0.20) | 78.4% |
| Others | 3 | −0.88 (-1.20, −0.56) | 0% |
| brand audiometry device | |||
| AC40 | 3 | −0.27 (-1.21, 0.68) | 89.5% |
| NR | 4 | −0.47 (-0.80, −0.15) | 56.0% |
| definition of “recovered" | |||
| ≥15 dB | 5 | −0.98 (-1.43, −0.53) | 12.5% |
| Others | 2 | 0.18 (-0.20,0.55) | 40.9% |
| follow-up | |||
| 30days | 3 | −0.27 (-1.21,0.68) | 89.5% |
| Others | 3 | −0.57 (-0.77, −0.38) | 0% |
| NR | 1 | −0.02 (-0.43,0.40) | – |
| sample size | |||
| >60 | 4 | −0.29 (-0.80,0.22) | 87.80% |
| ≤60 | 3 | −0.66 (-1.05, −0.26) | 0% |
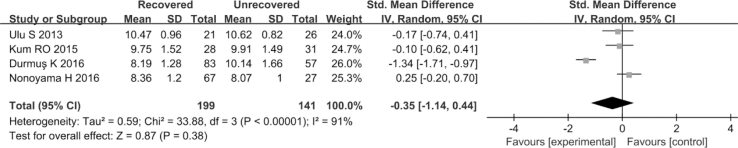
3.2.6. MPV in the recovered and unrecovered groups
Four articles (Ulu et al., 2013; K et al., 2016; Nonoyama et al., 2016; Kum et al., 2015) involving 199 SSNHL patients and 141 healthy persons examined the implications of MPV on prognosis of SSNHL. Considering the extreme heterogeneity (P < 0.1, I2 = 91%), the random-effects model was employed. There was no significant differences in MPV between the recovered and unrecovered groups (SMD = −0.35, 95% CI: 1.14–0.44, P = 0.38; Fig. 7). Results of subgroup analysis showed that the type of steroid, hematology analyzer, and sample size were the sources of heterogeneity (Table 7).
Fig. 7.
Forest plot of the differences in mean platelet volume (MPV) levels between the recovered group and the unrecovered group.
Table 7.
Subgroup analyses for the predictive value of MPV in SSNHL prognosis. SMD = standard mean deviation; CI = confidence interval; NR = none reported; MPV = mean platelet volumes; SSNHL = sudden sensorineural hearing loss.
| Categories | No. of studies | SMD (95% CI) | I2 |
|---|---|---|---|
| Total | 4 | −0.35 (-1.15,0.44) | 91.2% |
| Region | |||
| Europen | 3 | −0.56 (-1.44,0.32) | 89.9% |
| Asian | 1 | 0.25 (-0.20,0.70) | – |
| hematology analyzer | |||
| Sysmex | 2 | 0.08 (-0.32,0.49) | 22.2% |
| Others | 2 | −0.74 (-1.96,0.48) | 93.2% |
| type of steroid | |||
| Prednisone | 2 | −0.13 (-0.52,0.25) | 0% |
| Others | 2 | −0.55 (-2.12,1.02) | 96.5% |
| brand audiometry device | |||
| AC40 | 3 | −0.56 (-1.44,0.32) | 89.9% |
| Others | 1 | 0.25 (-0.20,0.70) | – |
| definition of recovered | |||
| ≥15 dB | 1 | −1.35 (-1.72,-0.98) | – |
| Others | 3 | 0.03 (-0.26,0.32) | 0% |
| follow-up | |||
| 30days | 3 | −0.56 (-1.44,0.32) | 89.9% |
| Others | 1 | 0.25 (-0.20,0.70) | – |
| sample size | |||
| >60 | 2 | −0.55 (-2.12,1.06) | 96.5% |
| ≤60 | 2 | −0.13 (-0.52,0.25) | 0% |
3.2.7. Sensitivity analysis and publication bias
The leave-one-out approach was employed to assess the hematological indices. Notably, the direction and degree of pooled results did not change significantly. This implied that the meta-analysis was robust. Moreover, there was no evidence of publication bias among the hematologic parameters used for the diagnosis of SSNHL (Egger test: NLR P = 0.675; PLR P = 0.403; MPV P = 0.954), and prognosis of SSNHL (Egger test: NLR P = 0.357; PLR P = 0.974). However, we could not assess the level of publication bias of MPV in the prognosis of SSNHL because the number of studies were fewer than five.
4. Discussion
Understanding the etiopathogenesis of diseases is a prerequisite to effective treatment of patients. Although the detailed mechanisms of SSNHL are currently unclear, compelling evidence indicate that inflammation may contribute significantly to this disease (Hiramatsu et al., 2012; Ulu et al., 2013). Chronic inflammation causes microvascular injuries, atherogenesis (Hoffman et al., 2004), and endocochlear immune responses (Masuda et al., 2012), all of which promote the risk of cochlear ischemia directly.
It has been reported that NLR, PLR, and MPV play a role in chronic inflammation. NLR or PLR are the two distinct subtypes of WBC which are more stable and reliable than a single inflammatory indicator (Yang et al., 2016). This is because these ratios are less influenced by circumferential factors, such as exercise and dehydration (Demir et al., 2015). Elevated NLR is associated with increased inflammatory activity (Imtiaz et al., 2012). Similarly, PLR levels can reflect the severity of systematic inflammation (Ye et al., 2019). For instance, elevated PLR indicates damage to blood vessels and platelet adhesion (Gary et al., 2013). High platelet aggregation in the damaged vessel walls result in vascular obstruction and perfusion problems (Sertoglu et al., 2015). MPV is negatively related to platelet count (Avci et al., 2017) and is a measure of platelet function or activation (Panova-Noeva et al., 2017). High levels of MPV are an established risk factor for thrombotic disposition and indicate abnormal platelet function (Ji et al., 2019).
In this meta-analysis, we analyzed 18 publications involving 1505 SSNHL patients and 1466 healthy individuals to determine the association of NLR, PLR, and MPV values with SSNHL. Our findings suggest that NLR and PLR values of SSNHL patients are much higher than those of healthy people. However, there is no distinct difference in the MPV levels between these two groups. These results imply that the levels of NLR and PLR influence the pathogenesis of SSNHL. Hence, NLR and PLR are prospective biomarkers for predicting the pathogenesis of SSNHL. However, these results should be interpreted with caution considering the extreme heterogeneity among analyzed studies. In the subgroup analyses performed to identify the sources of heterogeneity, the region, hematology analyzer, and sample size were major factors causing the heterogeneity. Specifically, age was a source of heterogeneity in PLR levels between the two groups. Moreover, sensitivity analysis revealed no significant difference in the impact of these hematological indices on the onset of SSNHL.
Further analysis showed that NLR and PLR levels in the unrecovered group were markedly higher than those of the recovered group. However, there is no correlation in the MPV level between these two groups. A possible explanation for this result might be that patients in the unrecovered group experience a higher degree of inflammation. Considering the extreme heterogeneity among the studies, subgroup analyses were conducted. Hematology analyzer and sample size were found to be the sources of heterogeneity. For PLR levels, the region, type of steroid, the definition of recovered and follow-up were the sources of heterogeneity in the two groups. In sensitivity analysis, the direction and degree of the pooled results did not vary significantly, indicating that this meta-analysis was robust. Altogether, these findings provide evidence that chronic inflammation is involved in the pathogenesis of SSNHL, and NLR/PLR are promising prognostic predictors of SSNHL.
Although some findings in this meta-analysis have been previously reported in other studies (Chen et al., 2018; Cao et al., 2018), there are some differences to be noted. Chen's study included only ten articles, and analyzed the relationship between NLR and the diagnosis and prognosis of SSNHL; Ji's research only investigated the association between platelet parameters and SSNHL. However, we included 18 studies, and comprehensively analyzed the relationship between NLR, PLR, and MPV and SSNHL. Furthermore, in the subgroup analysis, we analyzed the effect of hematology analyzer and brand audiometry device on the merged results and identified them as sources of heterogeneity.
Nevertheless, there are several limitations to be considered in this study. First, the included studies were retrospectively designed and the number of patients in many of the studies were relatively small. This could potentially introduce selection and information bias. Second, there are no universally accepted cut-off values for NLR and PLR levels This should be addressed in future research. Third, different criteria were used to define recovery among the included studies when assessing the prognostic values of NLR and PLR levels in SSNHL. This may result in heterogeneity in the results obtained across studies. Finally, we could not analyze some factors (e.g., audiogram, time to treatment, underlying diseases) due to lack of information.
5. Conclusions
Overall, we have established that chronic inflammation is involved in the onset of SSNHL. Moreover, NLR and PLR might be convenient, cheap and routinely available prognostic predictors of SSNHL. However, considering the limitations of this work, further high-quality and large-scale studies such as randomized, controlled, prospective clinical trials (RCT), and several inflammatory factors, including C-reactive protein, cytokines, interleukins in SSNHL patients, are required to validate our findings.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
We declare that we have no conflict of interest.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Acet H., Ertaş F., Akıl M.A. Relationship between hematologic indices and global registry of acute coronary events risk score in patients with ST-segment elevation myocardial infarction. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2016;22:60–68. doi: 10.1177/1076029614533145. [DOI] [PubMed] [Google Scholar]
- Avci A., Avci D., Erden F. Can we use the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume values for the diagnosis of anterior uveitis in patients with Behcet's disease? Therapeut. Clin. Risk Manag. 2017;13:881–886. doi: 10.2147/TCRM.S135260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulğurcu S., Dikilitaş B., Arslan İ.B. Neutrophil-to-Lymphocyte and platelet-to-lymphocyte ratios in pediatric patients with idiopathic sudden hearing loss. The journal of international advanced otology. 2017;13:217–220. doi: 10.5152/iao.2017.3404. [DOI] [PubMed] [Google Scholar]
- Cao Z., Li Z., Xiang H. Prognostic role of haematological indices in sudden sensorineural hearing loss: review and meta-analysis. Clin. Chim. Acta. 2018;483 doi: 10.1016/j.cca.2018.04.025. 104-1. [DOI] [PubMed] [Google Scholar]
- Chen L., Zhang G., Zhang Z. Neutrophil-to-lymphocyte ratio predicts diagnosis and prognosis of idiopathic sudden sensorineural hearing loss. Medicine. 2018;97 doi: 10.1097/MD.0000000000012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D.A., Reed D.A. Appraising the quality of medical education research methods: the medical education research study quality instrument and the newcastle-Ottawa scale-education. Acad. Med. 2015;90:1067–1076. doi: 10.1097/ACM.0000000000000786. [DOI] [PubMed] [Google Scholar]
- Demir F., Karadeniz C., Ozdemir R. Usefulness of neutrophil to lymphocyte ratio in prediction of coronary artery lesions in patients with kawasaki disease. Balkan Med. J. 2015;32:371–376. doi: 10.5152/balkanmedj.2015.151108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary T., Pichler M., Belaj K. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PloS One. 2013;8 doi: 10.1371/journal.pone.0067688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha R., Lim B.W., Kim D.H. Predictive values of neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and other prognostic factors in pediatric idiopathic sudden sensorineural hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2019;120:134–139. doi: 10.1016/j.ijporl.2019.02.023. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Teranishi M., Uchida Y. Polymorphisms in genes involved in inflammatory pathways in patients with sudden sensorineural hearing loss. J. Neurogenet. 2012;26:387–396. doi: 10.3109/01677063.2011.652266. [DOI] [PubMed] [Google Scholar]
- Hoffman M., Blum A., Baruch R. Leukocytes and coronary heart disease. Atherosclerosis. 2004;172:1–6. doi: 10.1016/s0021-9150(03)00164-3. [DOI] [PubMed] [Google Scholar]
- Ikinciogullari A., Koseoglu S., Kilic M. New inflammation parameters in sudden sensorineural hearing loss: neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio. Journal of International Advanced Otology. 2014;10:197–200. [Google Scholar]
- Imtiaz F., Shafique K., Mirza S.S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012;5:2. doi: 10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S., Zhang B., Wang X. Effects of statin therapy on mean platelet volume in patients with risk of cardiovascular diseases: a systematic review and meta-analysis. Biosci. Rep. 2019;39 doi: 10.1042/BSR20190180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K D., H T., TD K. Assessment of hematological factors involved in development and prognosis of idiopathic sudden. Sensorineural Hearing Loss. 2016;27:e85–e91. doi: 10.1097/SCS.0000000000002241. [DOI] [PubMed] [Google Scholar]
- Karli R., Alacam H., Unal R. Mean platelet volume: is it a predictive parameter in the diagnosis of sudden sensorineural hearing loss? Indian Journal of Otolaryngology and Head & Neck Surgery. 2013;65:350–353. doi: 10.1007/s12070-013-0648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocak H.E., Elbistanli M.S., Acipayam H. Are neutrophil/lymphocyte and platelet/lymphocyte ratios related with formation of sudden hearing loss and its prognosis? European annals of otorhinolaryngology, head and neck diseases. 2017;134:383–386. doi: 10.1016/j.anorl.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Kum R.O., Ozcan M., Baklaci D. Investigation of neutrophil-to-lymphocyte ratio and mean platelet volume in sudden hearing loss. Brazilian journal of otorhinolaryngology. 2015;81:636–641. doi: 10.1016/j.bjorl.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., Hong S.K., Kim D.H. The neutrophil-to-lymphocyte ratio in children with sudden sensorineural hearing loss: a retrospective study. Acta Otolaryngol. 2017;137:35–38. doi: 10.1080/00016489.2016.1217561. [DOI] [PubMed] [Google Scholar]
- Leung M.A., Flaherty A., Zhang J.A. Sudden sensorineural hearing loss: Primary care update. Hawai‘i J. Med. Public Health. 2016;75:172–174. [PMC free article] [PubMed] [Google Scholar]
- Masuda M., Kanzaki S., Minami S. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol. Neurotol. : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012;33:1142–1150. doi: 10.1097/MAO.0b013e3182635417. [DOI] [PubMed] [Google Scholar]
- Mirvakili A., Dadgarnia M.H., Baradaranfar M.H. Role of platelet parameters on sudden sensorineural hearing loss: a case-control study in Iran. PloS One. 2016;11 doi: 10.1371/journal.pone.0148149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama H., Tanigawa T., Shibata R. Red blood cell distribution width predicts prognosis in idiopathic sudden sensorineural hearing loss. Acta Otolaryngol. 2016;136:1137–1140. doi: 10.1080/00016489.2016.1195919. [DOI] [PubMed] [Google Scholar]
- Ozler G.S. Increased neutrophil-lymphocyte ratio in patients with idiopathic sudden sensorineural hearing loss. J. Craniofac. Surg. 2014;25:e260–e263. doi: 10.1097/SCS.0000000000000565. [DOI] [PubMed] [Google Scholar]
- Panova-Noeva M., Arnold N., Hermanns M.I. Mean platelet volume and arterial stiffness - clinical relationship and common genetic variability. Sci. Rep. 2017;7:40229. doi: 10.1038/srep40229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao X.F., Li X., Wang G.P. Neutrophil-to-Lymphocyte ratio and platelet-to-lymphocyte ratio in patients with sudden sensorineural hearing loss. Medical principles and practice. international journal of the Kuwait University, Health Science Centre. 2019;28:23–27. doi: 10.1159/000494556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta N., Squeo V., Sangineto M. High total cholesterol in peripheral blood correlates with poorer hearing recovery in idiopathic sudden sensorineural hearing loss. PloS One. 2015;10 doi: 10.1371/journal.pone.0133300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagit M., Kavugudurmaz M., Guler S. Impact of mean platelet volume on the occurrence and severity of sudden sensorineural hearing loss. J. Laryngol. Otol. 2013;127:972–976. doi: 10.1017/S002221511300193X. [DOI] [PubMed] [Google Scholar]
- Seo Y.J., Jeong J.H., Choi J.Y. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio: novel markers for diagnosis and prognosis in patients with idiopathic sudden sensorineural hearing loss. Dis. Markers. 2014;2014:702807. doi: 10.1155/2014/702807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sertoglu E., Kayadibi H., Uyanik M. Comment on "Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio: novel markers for diagnosis and prognosis in patients with idiopathic sudden sensorineural hearing loss. Dis. Markers. 2015;2015:745879. doi: 10.1155/2015/745879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachler R.J., Chandrasekhar S.S., Archer S.M. Clinical practice guideline: sudden hearing loss. Otolaryngology--head and neck surgery. official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012;146:S1–S35. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- Sun Y., Guo Y., Wang H. Differences in platelet-related parameters among patients with audiographically distinct sudden sensorineural hearing loss. Medicine. 2017;96 doi: 10.1097/MD.0000000000007877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A.J., Duval S.J., Tweedie R.L. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulu S., Ulu M.S., Bucak A. Neutrophil-to-lymphocyte ratio as a new, quick, and reliable indicator for predicting diagnosis and prognosis of idiopathic sudden sensorineural hearing loss. Otol. Neurotol. : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2013;34:1400–1404. doi: 10.1097/MAO.0b013e31829b57df. [DOI] [PubMed] [Google Scholar]
- Wu J., Liang C., Chen M. Association between tumor-stroma ratio and prognosis in solid tumor patients: a systematic review and meta-analysis. Oncotarget. 2016;7:68954–68965. doi: 10.18632/oncotarget.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.B., Xing M., Ma L.N. Prognostic significance of neutrophil-lymphocyteratio/platelet-lymphocyteratioin lung cancers: a meta-analysis. Oncotarget. 2016;7:76769–76778. doi: 10.18632/oncotarget.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasan H., Tuz M., Yariktas M. The significance of routine laboratory parameters in patients with sudden sensorineural hearing loss. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2013;65:553–556. doi: 10.1007/s12070-012-0480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G.L., Chen Q., Chen X. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: a cohort study. Sci. Rep. 2019;9:10639. doi: 10.1038/s41598-019-47143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Hou N., Hao R. The relationship of mean platelet volume and sudden sensorineural hearing loss. J. Audiol. Speech Pathol. 2014;22:600–602. [Google Scholar]