Summary
Chaperonins play an important role in folding newly synthesized or translocated proteins in all organisms. The bacterial chaperonin GroEL has served as a model system for the understanding of these proteins. In comparison, its human homolog, known as mitochondrial heat shock protein family member D1 (HSPD1) is poorly understood. Here, we present the structure of HSPD1 in the apo state determined by cryo-electron microscopy (cryo-EM). Unlike GroEL, HSPD1 forms mostly single ring assemblies in the absence of co-chaperonin (HSPE1). Comparison with GroEL shows a rotation and increased flexibility of the apical domain. Together with published structures of the HSPD1/HSPE1 co-chaperonin complex, this work gives insight into the structural changes that occur during the catalytic cycle. This new understanding of HSPD1 structure and its rearrangements upon complex formation may provide new insights for the development of HSPD1-targeting treatments against a diverse range of diseases including glioblastoma.
Subject areas: Molecular Biology, Molecular Structure
Graphical abstract
Highlights
-
•
First cryo-EM structure of the apo HSPD1 chaperone complex
-
•
Mass spectrometry has shown the presence of 7, 8, 15, and 16-mers of HSPD-1
-
•
In addition to a single ring, HSPD-1 can form an unusual inverted ring architecture
-
•
Describe grid preparation conditions that alleviate preferred orientation
Molecular Biology; Molecular Structure
Introduction
Chaperonins are molecular chaperones forming ring-shaped complexes that catalyze protein folding through the capture of unfolded polypeptides and confinement within a central cavity driven by ATP hydrolysis (Horwich et al., 2007). Bacterial GroEL, a type I chaperonin, has served as a model system to understand the structural characteristics and mechanism of chaperonins (Levy-Rimler et al., 2002). GroEL is essential in bacteria and involved in folding about 10%–15% of newly synthesized proteins (Hartl and Hayer-Hartl, 2002). The GroEL complex has been extensively studied using structural and biochemical methods over the last decades, leading to a good mechanistic understanding of its structure and function (Horwich and Fenton, 2020). GroEL forms a homo-tetradecameric structure, in which two heptameric rings are stacked back to back (Braig et al., 1994). During its catalytic cycle, GroEL binds substrate protein, ATP, and co-chaperonin GroES to form a nanoreactor in which substrate proteins can fold. Upon ATP hydrolysis, GroES, nucleotides, and substrate proteins are released (Bukau and Horwich, 1998). ATP and GroES binding between the two rings is coupled by negative cooperativity, whereas ATP binding within one ring is positively cooperative (Saibil et al., 2013; Dyachenko et al., 2013). This cooperativity is thought to be important for function: a mutant GroEL, forming single rings and lacking the negative inter-ring cooperativity, gives rise to dead-end complexes (Weissman et al., 1995).
The human homolog of GroEL is mitochondrial HSPD1, which is essential and responsible for (re)-folding of nuclear-encoded proteins that are imported into the mitochondria as well as polypeptides encoded by mtDNA (Kampinga et al., 2009). Aberrant substrate folding can lead to a variety of neurodegenerative disorders including Alzheimer, Parkinson, and Creutzfeldt-Jacob disease (Satoh et al., 2005; Chiti and Dobson 2006) that may correlate with an age-dependent decline in protein quality control (van der Putten and Lotz 2013). Mutations in HSPD1 have been associated with the hereditary brain diseases spastic paraplegia (Hansen et al. 2002, 2007) and autosomal-recessive neurodegenerative disorder (Magen et al., 2008). In the aggressive brain cancer glioblastoma, elevated HSPD1 expression confers poor prognosis and is linked with mitochondrial energy metabolism (Polson et al., 2018). Moreover, HSPD1 has been implicated in cancer proliferation and apoptosis (Cappello et al., 2014) as well as inflammatory and autoreactive conditions (Pockley et al., 2014).
Compared with GroEL, less structural and biochemical insight exists for HSPD1 (Levy-Rimler et al., 2002). Complexes of HSPD1 are unstable in vitro and dissociate in a time- and temperature-dependent manner (Viitanen et al., 1998). Upon reconstitution, HSPD1 forms mainly single ring assemblies instead of double rings (Viitanen et al., 1992). In complex with the co-chaperonin HSPE1 (the human GroES homolog), HSPD1 single and double ring assemblies have been shown to coexist. Specifically, there is evidence for single ring HSPD17/HSPE17 (half-football) and double ring HSPD114/HSPE114 (football) complexes; however, the precise order in the catalytic cycle has not been elucidated (Levy-Rimler et al., 2001). In contrast to GroEL, HSPD1 shows less requirement for inter-ring cooperativity, and HSPD1 mutants with impaired double ring formation can remain fully functional. The recently published structures of the ADP-BeF3-bound football complex and the ADP-bound football and half-football complexes represent a large step toward understanding the differences and similarities between GroEL- and HSPD1-catalyzed protein folding (Gomez-Llorente et al., 2020). Increasing evidence indicates distinct structure and enzymatic mechanism of bacterial and human chaperonins; however, an atomic model of apo HSPD1 has not been reported to date.
A potential reason for the lack of a high-resolution apo HSPD1 structure is the instability of its reconstituted oligomers. Although compositional heterogeneity can be accounted for by particle classification in cryoelectron microscopy (cryo-EM), our previous efforts to obtain a high-resolution structure were complicated by strong preferred particle orientation on cryo-EM grids. In this work, by optimization of particle concentration and buffer conditions, the structure of apo HSPD1 single ring was determined at 3.5 Å. The structure shows high similarity to GroEL and reveals high flexibility of the apical domains. Side-by-side comparison with a HSPD1/E1 complex structure shows conformational changes during the catalytic cycle. We also show that two apo HSPD1 single rings can dimerize in an unusual manner, forming an inverted double ring. The structure of apo HSPD1 represents a further important step in understanding the conformational landscape of the HSPD1 chaperonin.
Results
Reconstitution of HSPD1 into oligomers
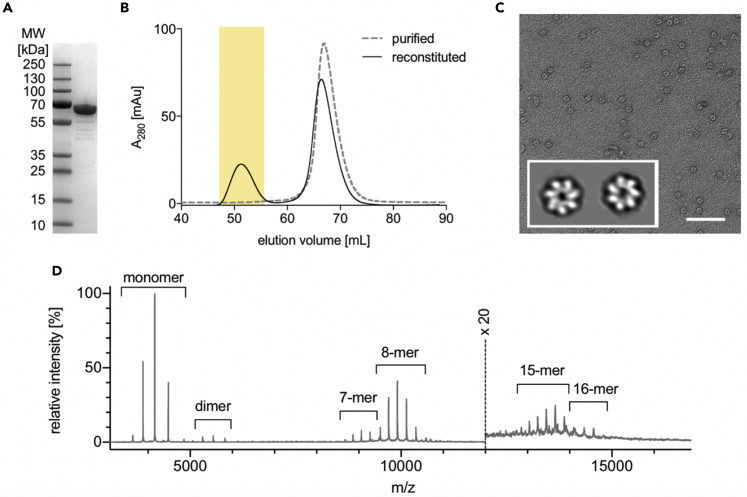
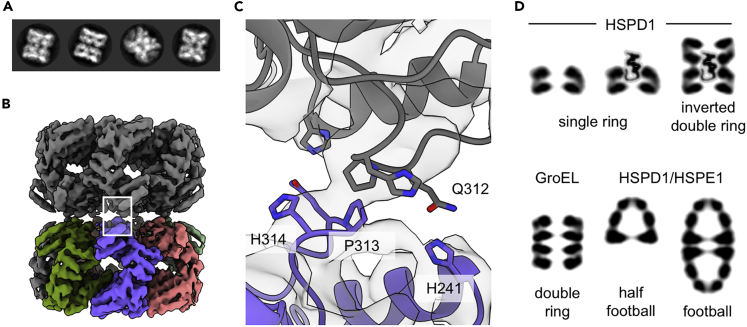
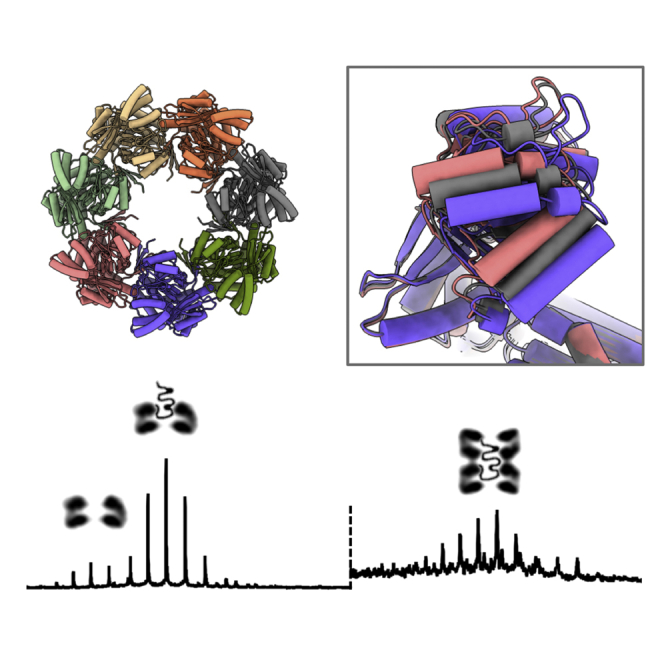
For the structural studies, HSPD1 was expressed without the mitochondrial target peptide. Overexpression of mature human mitochondrial HSPD1 in Escherichia coli resulted in a high yield of protein (amount: ~ 30 mg per 1 L of culture), purified in monomeric form. The HSPD1 oligomer was then reconstituted in the presence of ATP followed by size exclusion chromatographic purification (Figures 1A and 1B). Characterization of the oligomeric fraction by negative stain EM revealed the characteristic 7-fold symmetrical shape for chaperonins as previously described for the HSPD1 single ring (Figure 1C).
Figure 1.
Reconstitution and characterization of HSPD1 oligomers
(A) SDS PAGE gel for purified HSPD1 monomer.
(B) Size exclusion chromatography of purified (gray dashed line) and reconstituted (black solid line) HSPD1 with the peak for oligomeric HSPD1 highlighted in yellow.
(C) Negative stain electron micrograph of the oligomeric HSPD1 (scale bar, 100 nm). The inset shows two representative 2D classes.
(D) Native mass spectrum of reconstituted HSPD1 showing multiple charge state distributions; different species are labeled. Above 12,000 m/z, the spectral intensity has been multiplied by 20.
Stoichiometry of the reconstituted HSPD1 complex was analyzed by native mass spectrometry (MS) (Figure 1D). Masses for the main oligomeric species (Figure 1D) were 407,635 ± 90 Da and 465,849 ± 82 Da, corresponding to assemblies of 7 and 8 subunits, respectively. Although the heptameric assembly was expected based on the negative stain EM results and previous work (Viitanen et al., 1992), the detection of 8-mer shows that an additional subunit can bind the heptameric form. The mass accuracy is sufficient to determine that, on average, the nucleotide binding site was free. In addition, the mass spectra showed species corresponding to 1, 2, 15, and 16 subunits, which are further discussed later.
Cryo-EM of oligomeric apo HSPD1
Initial attempts to determine the structure of HSPD1 by cryo-EM resulted in reconstructions at medium resolutions (5–10 Å). The major component identified by cryo-EM was the single toroidal ring with clear 7-fold symmetry. Well-distributed particles were present in the ice, and the picked particles produced well-resolved 2D classes showing distinct secondary structure. However, the resultant 3D reconstruction was significantly hindered by preferred orientation showing almost exclusively the 7-fold symmetrical top views. To alleviate problems of preferred orientation, we screened a range of conditions including carbon or graphene oxide support grids and amylamine glow discharge, none of which gave any clear improvements in angular distribution.
We used novel fast grid preparation techniques as an alternative, with a home-built device designed for time-resolved EM or a chameleon grid-making device (SPT Labtech). This could not eliminate binding to the air-water interface but gave modest improvements in angular distribution, but, in the case of the home-built device, it also increased ice thickness (Klebl et al., 2020).
Grids prepared by conventional blotting at ambient temperature (when compared with the 5°C–6°C routinely used for plunge freezing) required much higher sample concentrations to achieve the same protein density on the grids (≥1 mg/mL at 20°C versus 0.25 mg/mL at 5°C). This was counterintuitive as HSPD1 oligomers have been shown to be less stable at low temperatures (Viitanen et al., 1998). Preparing grids at ~20°C and high protein concentration (2.3 mg/mL) resulted in a small increase in angular spread, presumably due to significant crowding at the air-water interface. Yet, this approach still yielded anisotropic reconstructions, and the overall resolution was limited to ~4 Å. Using an optimized buffer, which included 1 mM DTT for grid preparation, high concentration and conventional blotting yielded the final reconstruction at a global resolution of 3.5 Å (Figure 2) with C7 symmetry. The reconstruction showed no significant anisotropy, and the resolution was sufficient to enable atomic modeling based on previous apo-GroEL and the HSPD1-HSPE1 complex crystal structures (Figure 2 and Table 1).
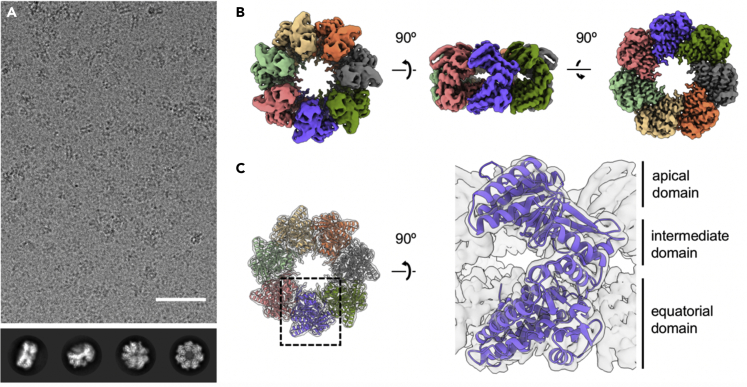
Figure 2.
Cryo-EM structure of HSPD1 single ring
(A) Raw micrograph of apo HSPD1 (scale bar, 50 nm). The bottom panel shows representative 2D classes from the final particle selection.
(B) Cryo-EM reconstruction of the apo HSPD1 single ring at 3.5 Å, colored by subunit.
(C) Atomic model for apo HSPD1 in the EM density (transparent). On the right, one subunit is shown from the side, and the three domains are labeled.
Table 1.
Summary of data collection/processing parameters and model building statistics. FSC stands for Fourier Shell Correlation.
| Data collection and processing | HSPD1 single ring |
|---|---|
| Magnification | ×75,000 |
| Voltage (kV) | 300 |
| Electron exposure (e–/Å2) | 38.5 |
| Defocus range (μm) | 1.5 to 3 |
| Pixel size (Å) | 1.065 |
| Symmetry imposed | C7 |
| Initial particle images (no.) | 355,766 |
| Final particle images (no.) | 124,784 |
| Map resolution (Å) | 3.5 |
| FSC threshold | 0.143 |
| Map resolution range (Å) | 3.2–4.8 |
| Refinement | |
| Initial models used | PDB: 4pj1, 1xck |
| Map resolution (Å) | 3.4 |
| FSC threshold | 0.5 |
| Map sharpening B factor (Å2) | −100 |
| Model composition | |
| Non-hydrogen atoms | 27,391 |
| Protein residues | 3,675 |
| B factors (Å2) | |
| Protein | 123 |
| Root mean square deviations | |
| Bond lengths (Å) | 0.004 |
| Bond angles (°) | 0.611 |
| Validation | |
| MolProbity score | 1.74 |
| Clashscore | 10.53 |
| Poor rotamers (%) | 0.71 |
| Ramachandran plot | |
| Favored (%) | 96.75 |
| Allowed (%) | 3.25 |
| Disallowed (%) | 0 |
FSC stands for Fourier Shell Correlation.
The seven resolved subunits show the typical three-domain chaperonin structure (Figure 2C). The equatorial domain forms the well-resolved base of the single ring (residues 1–134, 409–524), mainly composed of α-helices. The apical domain (residues 189–372) at the top of the ring contains two β-sheets and five α-helices. Among these are helices H and I (residues 232–240 and 254–266, respectively), lining the top of the central cavity. Last, the intermediate (hinge) domain (residues 135–188, 373–408) connects the equatorial and apical domains.
Conformational variability of HSPD1 subunits within the complex
Apart from the differences in complex stoichiometry (single ring) the structures of apo HSPD1 and GroEL (PDB: 1xck, Bartolucci et al., 2005) are highly similar on the subunit level, as expected with 51% sequence identity. The highest structural similarity is found around the equatorial domain, which is the most rigid and therefore best resolved region in the HSPD1 cryo-EM map. The most apparent difference in structure is an increased deviation between the apical domains (~2.6 Å average root-mean-square deviation) of HSPD1 and GroEL.
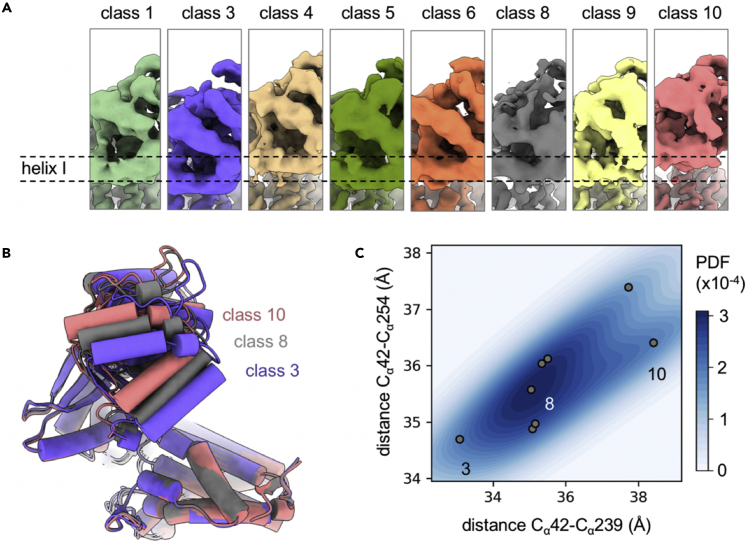
The lower local resolution in the region of the apical domain led us to examine conformational variability in the apical domain in more detail. This was done by symmetry expansion and focused classification into 10 classes (Figure S3). Only classes with clear density for helices H and I (the polypeptide binding site on the apical domain) were taken forward. These eight classes show variable positioning of helices H and I relative to the equatorial domain (Figure 3A). Atomic models for each class were generated by flexible fitting and show that the range of conformations within the data is large (Figure 3B). The distance between equatorial domain residue Trp42 and Asn239, for example, varies more than 5 Å between classes 3 and 10. Plotting the distance Trp42-Asn239 (equatorial/helix H) against the distance Trp42-Gly254 (equatorial/helix I) shows how the different conformations fall into clusters (Figure 3C). Class 3 resembles the most compact form in which apical and equatorial are closest. Most subunits adopt a conformation similar to class 8 (the central region in Figure 3C). Classes 4 and 10 represent a third class of conformation, which is more “extended” with the largest distances between apical and equatorial domains. Generally, the points in Figure 3C lie along the diagonal, which would be expected for a concerted movement of the apical domain.
Figure 3.
Conformational variability in the apical domain
(A) Eight subunit reconstructions show variable apical domain positions. The position of helix I in class 1 is indicated by dotted lines to help compare the classes.
(B) Overlay of flexibly fitted models for classes 3, 8, and 10 in purple, gray, and light red.
(C) Plot of measured equatorial/apical domain distances for all eight classes. Each class is represented by a gray point; classes 3, 8 and 10 are labeled. Points approximately lie on the diagonal, showing that the entire apical domain rotates upward. The background color is an estimate of the probability to find a subunit in a certain conformation, taking the particle number in each class into account. PDF stands for probability density function.
Class 3 is most similar to GroEL regarding the apical domain position, whereas in the more predominant conformers (65% of subunits) the apical domain is rotated upward. This shows that HSPD1 subunits can adopt a range of conformations, some of which are very similar to GroEL, but that more extended forms of HSPD1 are preferred.
Comparison of apo HSPD1 with the half-football complex structure of HSPD1 and HSPE1
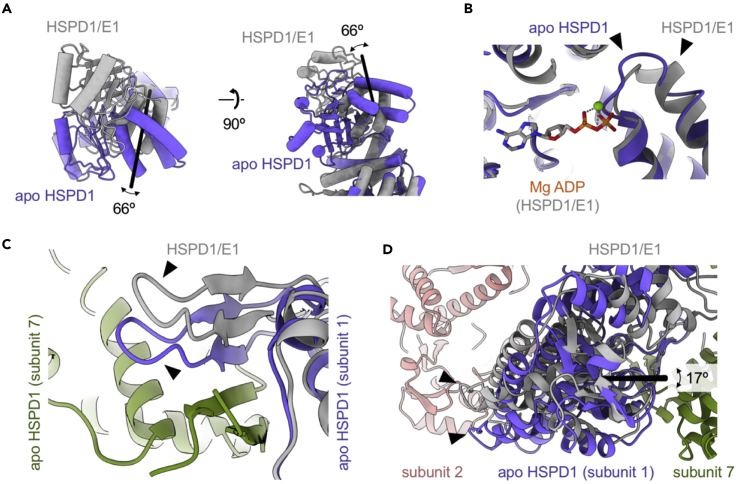
For comparison to the HSPD1/HSPE1 co-complex, we chose the ADP-bound half-football HSPD17/HSPE17 (PDB: 6mrd, Gomez-Llorente et al., 2020) as a reference as it should represent the nearest neighbor in the catalytic cycle. Relative to the half-football, the apical domains of apo HSPD1 undergo a large rigid body motion, resulting in an overall ~66° rotation (Figure 4A), which is comparable to structural changes in GroEL between ADP/GroES-bound and -unbound states and required to allow HSPE1 binding in the half-football state.
Figure 4.
Comparing structures of apo HSPD1 and the half-football complex (HSPD17/HSPE17/MgADP)
In all panels, the half-football complex is shown in gray (orange and green for neighboring subunits), and the corresponding apo HSPD1 subunit is shown in purple. Arrowheads are used to indicate the regions that show the biggest change.
(A) Rotation of the apical domain. Shown are the top (left) and side views of the domain (right). The black line is the rotation axis.
(B) Small changes near the nucleotide binding site involving Asp85.
(C) Altered position of the inter-subunit beta sheet in apo HSPD1 between subunits 1 and 7.
(D) Relative rotation of the equatorial domains in the single ring. The rotation axis is shown as black line.
Smaller differences can be found in the equatorial domain, located mostly around the nucleotide binding site. For instance, the short loop containing Asp85, which is involved in Mg-ADP coordination in the half-football structure, is located further toward the nucleotide binding site in the apo state (Figure 4B).
The position of two strands involved in forming the inter-subunit β-sheet (residues 36–38 and 46-48) is altered in apo HSPD1 relative to the half-football complex (Figure 4C). To understand the significance of this change in inter-subunit contacts, the entire single rings (HSPD1 and half-football) were aligned on their equatorial domains. This revealed a rotation of 17° for the HSPD1 equatorial domain relative to the half-football, whereas the radial distance from the ring center remained virtually unchanged (Figure 4D).
Different oligomeric states of the HSPD1 complex
Native MS showed the presence of multiple oligomeric species in the reconstituted sample (Figure 1D). The masses obtained from the six main charge state distributions are given in Table 2. They were assigned to monomer, dimeric, heptameric, octameric, 15-meric, and 16-meric assemblies, with a theoretical monomer mass of 58,235.12 Da.
Table 2.
Table of observed and calculated masses for oligomeric species by native mass spectrometry
| Observed mass (Da) | Experimental error (Da) | Main charge state | Number of subunits | Theoretical mass (Da) |
|---|---|---|---|---|
| 58,235 | 15 | 14+ | 1 | 58,235 |
| 116,470 | 32 | 21+ | 2 | 116,470 |
| 407,635 | 90 | 45+ | 7 | 407,646 |
| 465,849 | 82 | 47+ | 8 | 465,881 |
| 873,978 | 480 | 64+ | 15 | 873,527 |
| 932,402 | 640 | 64+ | 16 | 931,762 |
The monomeric and dimeric species are most likely dissociation products from larger oligomers, due to the instability of HSPD1 complexes. Heptameric and octameric species were assigned to the single ring, with or without an additional subunit bound. The larger assemblies, 15-mer and 16-mer, probably correspond to higher-order complexes composed of two single rings.
In agreement with the MS analysis, cryo-EM data under certain buffer conditions (50 mM Tris, 300 mM NaCl, 10 mM MgCl2) contained a small fraction of double ring particles (up to 8.3%, Figures 5A and 5B). We note that the relative amount of those particles varied, depending on the grid preparation and buffer conditions. Processing of the double ring species resulted in a 4.9 Å reconstruction with D7 symmetry. At this resolution the densities for the seven individual subunits per ring did not show any significant structural differences from the single ring. Unexpectedly, the overall structure is an inverted double ring, in which the two single rings contact each other face to face, with their apical domains. This arrangement is also apparent from the 2D class averages, and there was no occurrence of a different arrangement, confirming the face-to-face arrangement is either the sole or dominant species. The dimerization interface shows remarkably few interactions between the two single rings, with potential interacting residues His241, Gln312, Pro313, and His314 (Figure 5C).
Figure 5.
The HSPD1 inverted double ring
(A) Selected 2D classes of the inverted double ring.
(B) D7-symmetrical reconstruction of the inverted double ring with one ring colored by subunit and the second ring in gray. The interface between the two rings is highlighted by the white rectangle.
(C) Magnified view of the inter-ring interface of the double ring with potential interacting residues labeled.
(D) Schematic of the HSPD1 oligomeric states, apo GroEL, and HSPD1/HSPE1 complexes in comparison. Depicted are central sections of side views for the complexes. The eighth subunit is not resolved by cryo-EM, thus probably disordered, and has been placed in the substrate binding site on the apical domains. According to native MS data, the inverted double ring may contain one or two additional (unresolved) subunits.
Discussion
HSPD1 oligomers were prepared using established methods and the structure solved by single particle cryo-EM. Native MS revealed complexes with seven and eight subunits as the major oligomeric species. We propose that both heptameric and octameric HSPD1 adopt the single ring architecture observed in cryo-EM. The octameric complex must contain one additional subunit, but we see no evidence for an 8-fold symmetric complex in 2D classification suggesting that the additional subunit is disordered and bound as a substrate. HSPD1 can self-chaperone in vitro and in vivo, and the purified complex has limited stability, which explains the presence of monomers in sample (Cheng et al., 1990). Binding of these monomers by the remaining intact single rings is a plausible mechanism for formation of octameric complex (Figure 5D). This observation is consistent with previous multi-angle laser scattering measurements of apo HSPD1 oligomers, giving mass estimates of ~450 kDa, which could be explained by a mixture of heptameric and octameric complex (Levy-Rimler et al., 2001; Gomez-Llorente et al., 2020).
Compared with GroEL or double ring structures of the HSPD1/E1 complex, the inverted double ring of HSPD1 requires each ring to be rotated by 180° perpendicular to their 7-fold symmetry axes (Figure 5D). This mode of ring dimerization also occludes the HSPE1 binding site on the apical domain. In the inverted double ring structure, the apical domains containing the polypeptide binding sites are facing each other. We hypothesize that the additional one or two subunits in the double ring (14 subunits resolved by cryo-EM and 15 or 16 by native MS) are placed in the center of the complex (Figure 5D). Binding of one or two disordered subunits by two single rings would also strengthen the apparently weak interface between the two rings. The face-to-face double ring arrangement has not been described for group I chaperonins, but a similar interface has recently been reported for a group II archaeal chaperonin, proposed to be a resting state (Zeng et al., 2020). Although our data show the presence of the inverted double ring in vitro, its relevance in vivo remains unclear.
The predominant form of apo HSPD1, the single ring, was compared with apo GroEL revealing an overall difference in the position of the apical domain. In GroEL, salt bridges Arg197/Glu386 and Lys207/Glu255 between neighboring apical domains and the equatorial/apical salt bridge Asp83/Lys327 are thought to stabilize the position of the apical domain (Horwich and Fenton, 2020). Interestingly, GroEL Lys207 aligns with Thr205 in the HSPD1 sequence. In HSPD1, there is a Lys two positions downstream in the sequence, which occupies a similar position to GroEL Lys207 and may cause the upward movement of the apical domain. The presence of 27% subunits without clear density for helices H or I suggests that the true number of conformations could be larger with greater motion, than what has been captured by the analysis in this work. Conformational flexibility has also been observed in GroEL, although to a lesser extent, and is thought to be important for substrate promiscuity (Roh et al., 2017; Chen and Sigler, 1999). This conformational variability is likely limiting local resolution in the apical domain and possibly also global resolution.
A structural comparison with the nearest available neighbor in the HSPD1 catalytic cycle, namely, the ADP-bound half-football, provided insight into the conformational changes upon HSPE1/ADP release. The rearrangements between apo HSPD1 and the HSPD1/E1 half-football resemble the corresponding conformational states in GroEL closely. This suggests a similar allosteric network throughout the molecule. The complete lack of inter-ring cooperativity during the catalytic cycle of HSPD1 therefore seems surprising (Gomez-Llorente et al., 2020).
The present work provides new insights into HSPD1 complex composition and conformation, but further research is needed to understand conformational changes in HSPD1 and the order of states in the catalytic cycle. This will be essential for pharmacological targeting of the chaperonin system in HSPD1-mediated disease. For example, insight into the HSPD1 apical domain movement required for complex formation with HSPE1 may provide new opportunities for the development of inhibitors of this protein-protein interaction (PPI), a major challenge because of the relatively low affinity of the PPI (KD 7.0 μM) and the large buried surface area involved (~5,500 Å2) (Gestwicki and Shao, 2019).
This work provides the first atomic model of the HSPD1 chaperone in the apo state. It highlights the common mechanisms conserved between the well-characterized bacterial system and the less understood human mitochondrial homolog. Although significant strides in our understanding of chaperones have been achieved through studies of the bacterial GroEL complex, this work provides new insights into the human HSPD1 chaperone and highlights that direct study of this system is key if we are to design specific modulators of function that can provide potent new therapeutics.
Limitations of the study
This work provides the first structure of the HSPD1 complex, but many questions remain unanswered. We do not know the physiological significance of the double ring that is seen in both EM and MS experiments. Although a similar interaction has been seen for a group II archaeal chaperonin (Zeng et al., 2020), only time will tell if this interaction is physiologically relevant. We provide evidence for an 8-mer where we predict that one HSPD1 subunit is acting as a substrate for the 7-mer. This additional subunit appears to be highly dynamic and disordered, and therefore we could not resolve it by cryo-EM.
Protein folding requires a multipart mechanical cycle with HSPD1 forming a complex with HSPE in either a half-football (HSPD17/HSPE17) or football (HSPD114/HSPE114). Further work will be required to determine the precise order in the catalytic cycle with techniques such as time-resolved cryo-EM.
Resource availability
Lead contact
Further requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Stephen Muench (s.p.muench@leeds.ac.uk).
Materials availability
No new reagents were generated in this study.
Data and code availability
The atomic model and map for the HSPD1 single ring have been deposited to the PDB and EMDB with accession codes PDB: 7AZP and EMD: 11950, respectively. A map for the inverted double ring has been deposited to the EMDB with accession code EMD: 11189.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
The authors would like to thank Dr. Hao Shao and Dr. Jason Gestwicki (UCSF) for providing the HSPD1 plasmid. D.P.K. is a PhD student on the Wellcome Trust 4-year PhD program in The Astbury Center funded by The University of Leeds. The Titan Krios microscopes were funded by the University of Leeds (UoL ABSL award) and Wellcome Trust (108466/Z/15/Z). The time-resolved device was developed by a Biotechnology and Biological Sciences Research Council grant BB/P026397/1. The Orbitrap UHMR was funded by Wellcome Trust multi-user equipment grant 208385/Z/17/Z.
Author contributions
Conceptualization and experimental design, D.P.K., M.C.F., E.L.H., N.A.R., H.W., F.S., R.S.B., and S.P.M.; performed experiments, D.P.K., M.C.F., and E.L.H.; analysis of data: D.P.K., M.C.F., E.L.H., N.A.R., F.S., R.S.B., and S.P.M.; Supervision, N.A.R., F.S., R.S.B., and S.P.M.; wrote the manuscript, D.P.K., M.C.F., H.W., F.S., R.S.B., and S.P.M.
Declaration of interests
The authors declare no conflicts of interest.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.102022.
Supplemental information
References
- Bartolucci C., Lamba D., Grazulis S., Manakova E., Heumann H. Crystal structure of wild-type chaperonin GroEL. J. Mol. Biol. 2005;354:940–951. doi: 10.1016/j.jmb.2005.09.096. [DOI] [PubMed] [Google Scholar]
- Braig K., Otwinowski Z., Hegde R., Boisvert D.C., Joachimiak A., Horwich A.L., Sigler P.B. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Bukau B., Horwich A.L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Cappello F., Marino Gammazza A., Palumbo Piccionello A., Campanella C., Pace A., Conway de Macario E., Macario A.J. Hsp60 chaperonopathies and chaperonotherapy: targets and agents. Expert Opin. Ther. Targets. 2014;18:185–208. doi: 10.1517/14728222.2014.856417. [DOI] [PubMed] [Google Scholar]
- Chen L., Sigler P.B. The crystal structure of a GroEL/peptide complex: plasticity as a basis for substrate diversity. Cell. 1999;99:757–768. doi: 10.1016/s0092-8674(00)81673-6. [DOI] [PubMed] [Google Scholar]
- Cheng M.Y., Hartl F.U., Horwich A.L. The mitochondrial chaperonin hsp60 is required for its own assembly. Nature. 1990;348:455–458. doi: 10.1038/348455a0. [DOI] [PubMed] [Google Scholar]
- Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Dyachenko A., Gruber R., Shimon L., Horovitz A., Sharon M. Allosteric mechanisms can be distinguished using structural mass spectrometry. Proc. Natl. Acad. Sci. USA. 2013;110:7235–7239. doi: 10.1073/pnas.1302395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestwicki J.E., Shao H. Inhibitors and chemical probes for molecular chaperone networks. J. Biol. Chem. 2019;294:2151–2161. doi: 10.1074/jbc.TM118.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Llorente Y., Jebara F., Patra M., Malik R., Nisemblat S., Chomsky-Hecht O., Parnas A., Azem A., Hirsch J.A., Ubarretxena-Belandia I. Structural basis for active single and double ring complexes in human mitochondrial Hsp60-Hsp10 chaperonin. Nat. Commun. 2020;11:1916–2014. doi: 10.1038/s41467-020-15698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J.J., Dürr A., Cournu-Rebeix I., Georgopoulos C., Ang D., Nielsen M.N., Davoine C.S., Brice A., Fontaine B., Gregersen N., Bross P. Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am. J. Hum. Genet. 2002;70:1328–1332. doi: 10.1086/339935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Svenstrup K., Ang D., Nielsen M.N., Christensen J.H., Gregersen N., Nielsen J.E., Georgopoulos C., Bross P. A novel mutation in the HSPD1 gene in a patient with hereditary spastic paraplegia. J. Neurol. 2007;254:897. doi: 10.1007/s00415-006-0470-y. [DOI] [PubMed] [Google Scholar]
- Hartl F.U., Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Horwich A.L., Fenton W.A. Chaperonin-assisted protein folding: a chronologue. Q. Rev. Biophys. 2020;53:e4. doi: 10.1017/S0033583519000143. [DOI] [PubMed] [Google Scholar]
- Horwich A.L., Fenton W.A., Chapman E., Farr G.W. Two families of chaperonin: physiology and mechanism. Annu. Rev. Cell Dev. Biol. 2007;23:115–145. doi: 10.1146/annurev.cellbio.23.090506.123555. [DOI] [PubMed] [Google Scholar]
- Kampinga H.H., Hageman J., Vos M.J., Kubota H., Tanguay R.M., Bruford E.A., Cheetham M.E., Chen B., Hightower L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebl D.P., Gravett M.S., Kontziampasis D., Wright D.J., Bon R.S., Monteiro D., Trebbin M., Sobott F., White H.D., Darrow M. Need for speed: examining protein behaviour during cryoEM grid preparation at different timescales. Structure. 2020;28:1238–1248. doi: 10.1016/j.str.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Rimler G., Bell R.E., Ben-Tal N., Azem A. Type I chaperonins: not all are created equal. FEBS Lett. 2002;529:1–5. doi: 10.1016/s0014-5793(02)03178-2. [DOI] [PubMed] [Google Scholar]
- Levy-Rimler G., Viitanen P., Weiss C., Sharkia R., Greenberg A., Niv A., Lustig A., Delarea Y., Azem A. The effect of nucleotides and mitochondrial chaperonin 10 on the structure and chaperone activity of mitochondrial chaperonin 60. Eur. J. Biochem. 2001;268:3465–3472. doi: 10.1046/j.1432-1327.2001.02243.x. [DOI] [PubMed] [Google Scholar]
- Magen D., Georgopoulos C., Bross P., Ang D., Segev Y., Goldsher D., Nemirovski A., Shahar E., Ravid S., Luder A. Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am. J. Hum. Genet. 2008;83:30–42. doi: 10.1016/j.ajhg.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley A.G., Henderson B., Multhoff G. Extracellular cell stress proteins as biomarkers of human disease. Biochem. Soc. Trans. 2014;42(6):1744–1751. doi: 10.1042/BST20140205. [DOI] [PubMed] [Google Scholar]
- Polson E.S., Kuchler V.B., Abbosh C., Ross E.M., Mathew R.K., Beard H.A., da Silva B., Holding A.N., Ballereau S., Chuntharpursat-Bon E. KHS101 disrupts energy metabolism in human glioblastoma cells and reduces tumor growth in mice. Sci. Transl Med. 2018;10:454. doi: 10.1126/scitranslmed.aar2718. [DOI] [PubMed] [Google Scholar]
- van der Putten H., Lotz G.P. Opportunities and challenges for molecular chaperone modulation to treat protein-conformational brain diseases. Neurotherapeutics. 2013;10:416–428. doi: 10.1007/s13311-013-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh S.H., Hryc C.F., Jeong H.H., Fei X., Jakana J., Lorimer G.H., Chiu W. Subunit conformational variation within individual GroEL oligomers resolved by Cryo-EM. Proc. Natl. Acad. Sci. USA. 2017;114:8259–8264. doi: 10.1073/pnas.1704725114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H.R., Fenton W.A., Clare D.K., Horwich A.L. Structure and allostery of the chaperonin GroEL. J. Mol. Biol. 2013;425:1476–1487. doi: 10.1016/j.jmb.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Satoh J., Onoue H., Arima K., Yamamura T. The 14-3-3 protein forms a molecular complex with heat shock protein Hsp60 and cellular prion protein. J. Neuropathol. Exp. Neurol. 2005;64:858–868. doi: 10.1097/01.jnen.0000182979.56612.08. [DOI] [PubMed] [Google Scholar]
- Viitanen P.V., Lorimer G.H., Seetharam R., Gupta R.S., Oppenheim J., Thomas J.O., Cowan N.J. Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J. Biol. Chem. 1992;267:695–698. [PubMed] [Google Scholar]
- Viitanen P.V., Lorimer G., Bergmeier W., Weiss C., Kessel M., Goloubinoff P. Purification of mammalian mitochondrial chaperonin 60 through in vitro reconstitution of active oligomers. Meth Enzymol. 1998;290:203–217. doi: 10.1016/s0076-6879(98)90020-9. [DOI] [PubMed] [Google Scholar]
- Weissman J.S., Hohl C.M., Kovalenko O., Kashi Y., Chen S., Braig K., Saibil H.R., Fenton W.A., Horwich A.L. Mechanism of GroEL action: productive release of polypeptide from a sequestered position under GroES. Cell. 1995;83:577–587. doi: 10.1016/0092-8674(95)90098-5. [DOI] [PubMed] [Google Scholar]
- Zeng Y.C., Sobti M., Stewart A.G. Structural Analysis of a filamentous chaperonin from Sulfolobus Solfataricus. bioRxiv. 2020 doi: 10.1101/2020.01.13.905216. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic model and map for the HSPD1 single ring have been deposited to the PDB and EMDB with accession codes PDB: 7AZP and EMD: 11950, respectively. A map for the inverted double ring has been deposited to the EMDB with accession code EMD: 11189.