Abstract
In older adults, muscle weakness contributes greatly to functional restrictions on daily living activities, increased risk of falls, and adverse physiological changes. It has been suggested that not only muscle mass but also muscular infiltration of noncontractile elements may influence muscular performance such as strength and rapid force production. It is proved that resistance training may provoke substantial increases in muscle size even if it is performed at low intensities in older individuals. Also, recent studies have demonstrated the effectiveness of resistance training on muscle quality such as muscular infiltration of noncontractile elements for older people. This review shows the age-related changes in muscle mass and muscle quality, which were measured by muscle echo intensity on ultrasound images, and low-intensity resistance training effects on muscle volume and muscle quality.
Keywords: muscle mass, muscle quality, age-related change, resistance training, older adults
To develop an evidence-based approach for improving muscle performance, it is important to understand the age-related changes in muscle characteristics such as muscle mass and muscle quality. Skeletal muscle mass decreases with aging, and it also decreases by 30%-50% between the ages of 40 and 80 years1-4). It is well established that muscle mass loss contributes to muscle weakness in older people. Furthermore, muscle weakness easily causes gait disorders, functional restrictions on daily living activities, and an increase in the risk of falls in frail older people5-11). Therefore, resistance training is essential for maintaining the ability to perform activities of daily living and preventing falls among older individuals.
Age-Related Change in Muscle Mass
In literature, there have been many studies on age-related muscle atrophy using an ultrasonographic measurement of muscle thickness. It has been reported that there have been strong correlations between muscle thickness measured by B-mode ultrasound and site-matched skeletal muscle mass measured by magnetic resonance imaging12-15). Therefore, measurements of muscle thickness using ultrasound can be used to noninvasively estimate the degree of muscle atrophy16).
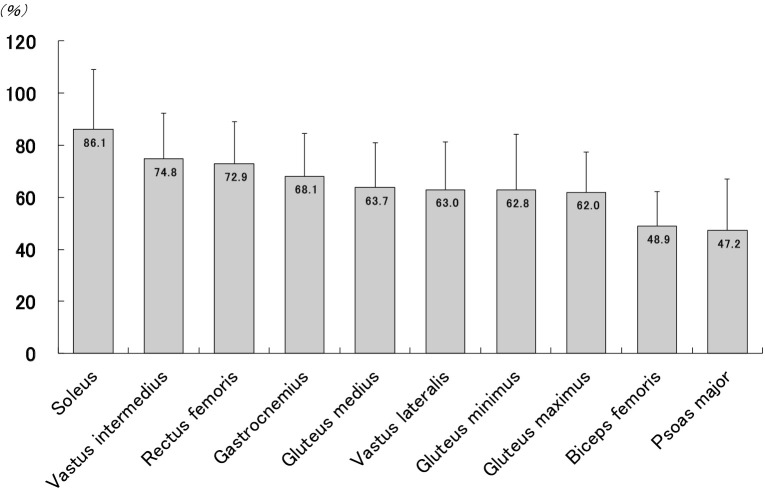
Our previous study using ultrasound showed relatively little atrophy of the soleus muscle among lower-limb muscles in older women who were able to walk without assistance17,18). Furthermore, the relatively small atrophy of soleus muscle may be as a result of the different postural role and fiber-type characteristics of muscles. It has been reported that the mean percentage of type I fibers is 86.4% in soleus, 43.5% in gastrocnemius, 42.8% in rectus femoris, 37.8% in vastus lateralis, and 52.4% in gluteus maximus19). Generally, among type II fibers, greater rates of age-related loss occur, while among type I fibers, only moderate losses occur3,20,21). Therefore, in older adults who were able to do ambulatory activity, the postural role of the soleus muscle may protect this muscle from marked reductions in thickness with aging, given its predominant type I composition. Besides, in our previous study, we demonstrated that age-related muscle atrophy was greatest for psoas major muscle among 10 muscles of the lower-limb muscles (Figure 1). The psoas major muscle is considered to be closely related to the locomotory capacity such as running and stair climbing22,23). This may be due to marked decreases in muscle mass of psoas major muscles because of decreased opportunities to run and stair climb in older adults.
Figure 1.
Magnitude of age-related decline (%) in the thickness of lower-limb muscles compared to the young reference group
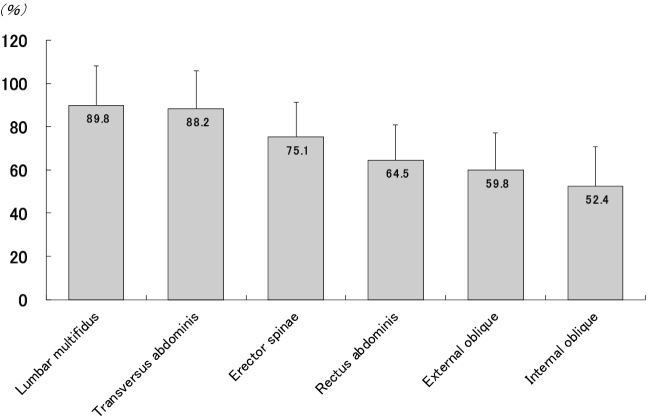
The trunk muscles have a significant role in stabilizing the body, maintaining posture, and controlling spinal and pelvic movement, so atrophy of trunk muscle may cause an increase in fall risk and disability in daily living activities in older adults24-26). In literature, there have been many studies on age-related atrophy of the trunk muscles27-31). In our previous study31), we showed that age-related muscle atrophy was smallest for the deep trunk muscles, including the transversus abdominis and lumbar multifidus muscles, in older women who were able to perform activities of daily living involving walking independently compared with young women (Figure 2). Deep trunk muscles, including the transversus abdominis and lumbar multifidus muscles, predominantly contain type I muscle fibers32). The transversus abdominis and lumbar multifidus muscles have an essential role in lumbar spine stabilization33-35). An electromyographic study showed that muscle activation levels of the trunk muscles, which are necessary to maintain stability for neutral spine posture are only 1%-3% of maximum voluntary contraction36). Therefore, the muscle mass of the transversus abdominis and lumbar multifidus muscles might be maintained by a small amount of muscle contraction during daily physical activities, regardless of the aging process. On the other hand, in older women, the magnitude of the decline in muscle mass was greater for internal and external oblique muscles than that in young women, suggesting marked age-related changes in these muscles among the trunk muscles. There may be an association between the decrease in the opportunity to perform physical activity with the movement of trunk rotation in older people and the greatest degree of atrophy for these muscles.
Figure 2.
Magnitude of age-related decline (%) in the thickness of trunk muscles compared to the young reference group
Additionally, due to the lack of specialized exercise training, decreases in muscle mass may occur in as little as 12 months and may lead to difficulty ambulating. Our longitudinal study37) showed that age-related atrophy progression may occur in the erector spinae, quadriceps femoris, and tibialis anterior muscle in as little as 12 months among trunk and lower-limb muscles (Table 1). Furthermore, our findings suggested that, among frail older women, reduced walking ability exacerbates age-related muscle atrophy in the trunk and lower-limb muscles, especially in the vastus lateralis muscle37).
Table 1.
Changes in muscle thicknesses between baseline and 12 months later
| Muscles | Baseline (mm) |
12 months later (mm) |
Percent change (%) |
|---|---|---|---|
| *p < 0.05 compared with baseline. **p < 0.01 compared with baseline. | |||
| Rectus abdominis | 6.2±1.9 | 6.1±1.9 | 0.4±16.4 |
| External oblique | 4.9±1.2 | 4.9±1.5 | 1.6±22.5 |
| Internal oblique | 7.1±2.0 | 6.8±2.5 | -1.1±33.1 |
| Transversus abdominis | 3.2±1.0 | 3.1±1.1 | 0.8±23.3 |
| Erector spinae | 26.6±6.4 | 21.9±5.7* | -12.0±35.6 |
| Lumbar multifidus | 26.8±5.8 | 26.3±6.3 | 1.1±25.6 |
| Psoas major | 13.7±5.2 | 11.6±3.1 | -5.4±35.7 |
| Gluteus maximus | 15.3±4.0 | 14.8±4.8 | -1.7±23.4 |
| Gluteus medius | 15.5±4.4 | 15.2±5.0 | -0.1±27.2 |
| Gluteus minimus | 12.3±3.9 | 12.3±4.0 | 1.6±30.2 |
| Rectus femoris | 16.5±4.2 | 11.5±4.2** | -28.3±25.0 |
| Vastus lateralis | 13.2±3.9 | 10.6±3.4** | -17.3±25.2 |
| Vastus intermedius | 10.8±3.1 | 8.4±2.8** | -19.8±22.9 |
| Biceps femoris | 18.3±4.6 | 17.3±5.5 | -2.6±30.8 |
| Gastrocnemius | 11.3±3.3 | 10.9±2.4 | 1.8±26.4 |
| Soleus | 29.3±6.8 | 28.3±5.5 | -0.6±20.8 |
| Tibialis anterior | 21.8±3.1 | 18.7±2.5** | -12.8±14.0 |
Age-Related Change in Muscle Quality
Several cross-sectional studies have demonstrated that the amount of intramuscular fibrous and adipose tissue (noncontractile tissue) in the muscle increases with aging38-40). Muscle echo intensity on ultrasound images using grayscale analysis has been used as an index of the amount of noncontractile tissue in the muscle. Enhanced echo intensity indicates changes in muscle quality due to increased intramuscular fibrous and adipose tissue, that is, noncontractile tissue in the muscle41,42). In our previous study43), we showed that muscle echo intensity of the quadriceps femoris muscles was significantly higher in older women than in young women, which may suggest increases in noncontractile tissue in the muscle due to aging.
In some reports, it was revealed that not only muscle mass but also muscle quality has influenced muscle strength, which were measured by muscle echo intensity on ultrasound images using grayscale analysis as an index of the amount of noncontractile tissue44-47). Fukumoto et al.44) showed that muscle echo intensity measured using computer-aided grayscale analysis of an ultrasound image independently contributes to muscle strength in middle-aged and older people, which suggests an association between increased fat and fibrous tissues within the muscle and poor muscle strength. Furthermore, several studies indicated that muscle quality, which was determined by muscle echo intensity on ultrasound images, influences muscle power, and rate of torque development, that is, the ability to produce rapid muscle contraction and the capacity to perform functional activities such as standing up from a chair and gait speed in older populations43,47-49).
Age-related changes in muscle quality may occur at an earlier age than the loss of muscle mass50,51). Ota et al.51) demonstrated that age-related decreases in muscle thickness of the rectus abdominis and external oblique muscles occur after 50 years of age; on the other hand, age-related changes in muscle quality, which were measured by muscle echo intensity occur after 30 years of age (Table 2). Thus, the proportion of noncontractile tissue such as intramuscular fat may be more susceptible to aging than muscle atrophy. Therefore, from middle age onward, interventions that are aimed at decreasing intramuscular fat and fibrous tissue may be needed.
Table 2.
Comparison of muscle thickness (mm) and echo intensity (0-255) among the age groups
| age group of 20’s | age group of 30’s | age group of 40’s | age group of 50’s | age group of 60’s | |
|---|---|---|---|---|---|
| (n = 23) | (n = 18) | (n = 22) | (n = 26) | (n = 23) | |
| Significant difference compared with 20’s group (*p < 0.05, **p < 0.01) Significant difference compared with 30’s group (†p < 0.05, ‡p < 0.01) Significant difference compared with 40’s group (∫p < 0.05, §p < 0.01) | |||||
| Muscle thickness | |||||
| Rectus abdominis | 9.59 ± 1.75 | 8.94 ± 1.86 | 8.20 ± 1.85 | 7.72 ± 2.13** | 7.27 ± 1.39**† |
| External oblique | 6.15 ± 1.45 | 5.77 ± 1.49 | 5.31 ± 1.40 | 4.39 ± 1.16**‡ | 4.35 ± 1.11**‡ |
| Transversus abdominis | 3.72 ± 0.49 | 3.92 ± 1.18 | 3.78 ± 1.13 | 3.28 ± 0.65 | 3.58 ± 0.82 |
| Lumbar multifidus | 26.25 ± 3.76 | 27.18 ± 4.45 | 26.65 ± 4.53 | 27.87 ± 4.66 | 26.85 ± 4.27 |
| Echo intensity | |||||
| Rectus abdominis | 38.93 ± 28.58 | 60.52 ± 18.68* | 73.13 ± 18.35** | 82.24 ± 14.34**‡ | 87.61 ± 20.98**‡ |
| External oblique | 56.77 ± 17.17 | 73.50 ± 10.82** | 81.74 ± 13.39** | 85.18 ± 10.56**† | 88.23 ± 13.49**‡ |
| Transversus abdominis | 19.42 ± 13.36 | 32.90 ± 12.84* | 34.07 ± 15.69**‡ | 48.68 ± 13.12**‡§ | 46.00 ± 17.61**‡ |
| Lumbar multifidus | 30.22 ± 13.21 | 34.23 ± 14.02 | 41.06 ± 14.91* | 49.52 ± 10.08**‡ | 51.36 ± 12.90**‡ |
Resistance Training for Older Adults
At the end of the 1980s, Frontera et al.52) reported that a heavy-resistance training program caused an increase in the knee extensor strength of older men, accompanied by muscle hypertrophy. Since then, an annually increasing number of studies have continued to document the benefits of resistance training for older people. It is traditionally believed that, to stimulate muscle hypertrophy, an individual must train with at least 60%-80% of their one-repetition maximum (1RM). However, high-load training is often unsuitable for older patients who have uncontrolled hypertension or cardiovascular diseases and degenerative joint diseases. Therefore, developing an effective method of low-load training is necessary. Regarding the effects of low-intensity resistance training, a similar degree of muscle hypertrophy has been reported to be achieved with low-intensity training by increasing the number of repetitions53-55). This result suggests that, by increasing the number of repetitions, low-intensity training can generate similar effects on muscle mass and characteristics similar to those of high-intensity training. High muscle fiber recruitment and type II fiber activation have been generally accepted to be necessary to induce muscle hypertrophy. Muscle hypertrophy seems to be largely dependent on elevated muscle protein synthesis, which is independent of training load, as long as the training volume is sufficient to recruit type II fibers56). Therefore, resistance training with high-repetition frequency has been assumed to increase fiber recruitment to sustain muscle tension and stimulate muscle protein synthesis, which promote muscle hypertrophy, regardless of low-intensity condition57).
Several studies indicated that quantitative and qualitative muscle properties, including muscle mass and accumulation of noncontractile elements in the muscles, are independent contributors to muscle strength and rapid force production among older people. Therefore, the effective interventions' development to prevent not only the aging-related muscle atrophy but also the intramuscular accumulation of noncontractile tissue may be worthwhile in a clinical setting. The recent studies have shown that resistance training can elicit significant decreases in echo intensity, which is considered an indication of intramuscular infiltration by noncontractile elements58-61). From our previous study, it is shown that, even at the low intensity, 8-week resistance training has an effect on muscle hypertrophy and results in muscle quality improvement regardless of exercise velocity in older adults (Table 3).
Table 3.
Effects of low-intensity resistance training on muscle function in HV and LV groups
| HV group | LV group | |||||
|---|---|---|---|---|---|---|
| (n = 17) | (n = 15) | |||||
| Variables | Before | After | % | Before | After | % |
| Abbreviations: HV, High-velocity; LV, Low-velocity. * p < 0.05, ** p < 0.01; significant difference compared with before training Percentage indicates ratio between before and after training | ||||||
| Muscle strength (Nm) | 51.3 ± 16.6 | 64.2 ± 20.3 ** | 29.0% | 51.9 ± 11.0 | 67.8 ± 18.8 ** | 32.3% |
| Muscle thickness (cm) | 1.52 ± 0.54 | 2.10 ± 0.59 ** | 43.8% | 1.83 ± 0.52 | 2.47 ± 0.54 ** | 40.7% |
| Echo intensity (0 - 255) | 67.3 ± 14.8 | 53.8 ± 7.56 ** | 16.9% | 67.8 ± 8.95 | 53.5 ± 9.47 ** | 20.5% |
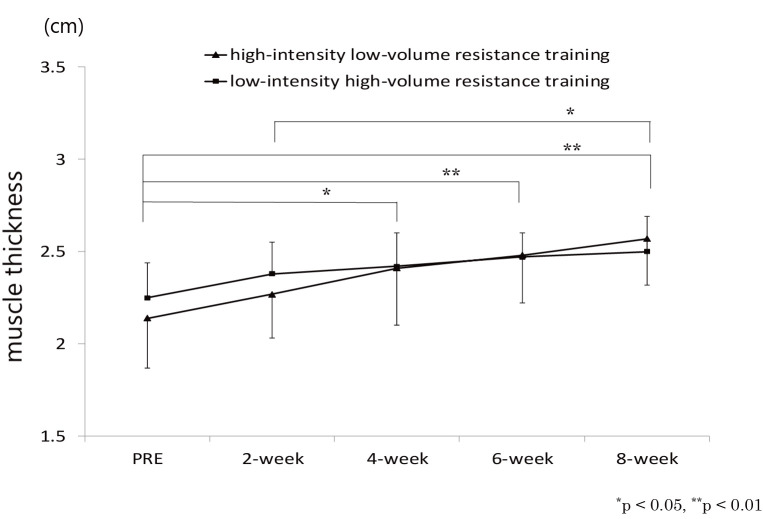
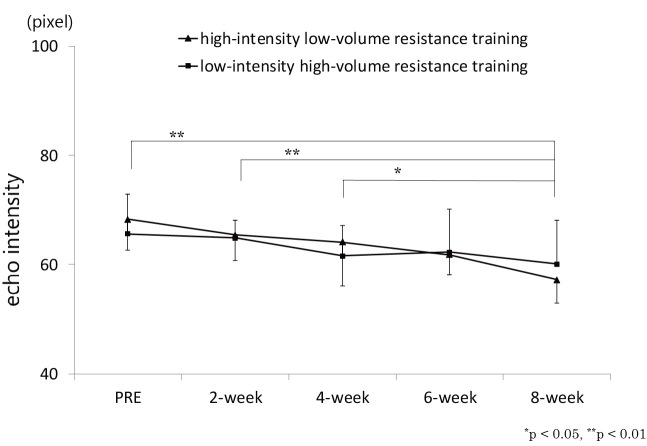
Some reports suggest that, in order to improve muscle quality than to improve muscle mass, a longer period of intervention is possibly required, which was measured by muscle echo intensity61,62). Our time-course study62) revealed that significant change in muscle thickness of rectus femoris muscle was evident after week 4 during resistance training on knee extensor muscles (Figure 3), whereas that for echo intensity of rectus femoris muscle was observed after week 8, which suggest that muscle mass changed at an earlier stage of the intervention than did muscle quality (Figure 4). Radaelli et al.61) who investigated changes in muscle thickness and echo intensity at weeks 6, 13, and 20 during resistance training in healthy older women also showed that although muscle thickness changes were observed after week 6, echo intensity changes were observed after week 13.
Figure 3.
Change in rectus femoris muscle thickness over time
Figure 4.
Change in rectus femoris echo intensity over time
Conclusion
In literature, there are numerous studies that have demonstrated that resistance strength training has an effective role in improving or maintaining muscle function of older people. Thereby, resistance training contributes to improved capacity to perform functional activities as well as enhanced quality of life among older adults. It has been pointed out that the accumulation of noncontractile elements in the muscles may be a key mechanism related to decreased capacity of generated force. Therefore, strategies that are aimed at enhancing the functional performance of older people should also focus on muscle quality improvement. Recent studies have reported the effectiveness of resistance training on muscle quality such as muscular infiltration of noncontractile elements for older people. Moreover, for examining the long-term effects of resistance training on muscle characteristics and further clarifying the most effective intervention for improving muscle function, future studies will be needed.
Conflict of Interest
There are no conflicts of interest to disclose.
References
- 1.Akima H, Kano Y, et al.: Muscle function in 164 men and women aged 20-84 yr. Med Sci Sports Exerc. 2001; 33: 220-226. [DOI] [PubMed] [Google Scholar]
- 2.Allen TH, Andersen EC, et al.: Total body potassium and gross body composition in relationship to age. J Gerontol. 1960; 15: 348-357. [DOI] [PubMed] [Google Scholar]
- 3.Lexell J, Taylor CC, et al.: What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988; 84: 275-294. [DOI] [PubMed] [Google Scholar]
- 4.Young A, Stokes M, et al.: The size and strength of the quadriceps muscles of old and young men. Clin Physiol. 1985; 5: 145-154. [DOI] [PubMed] [Google Scholar]
- 5.Avlund K, Schroll M, et al.: Maximal isometric muscle strength and functional ability in daily activities among 75-year-old men and women. Scand J Med Sci Sports. 1994; 4: 32-40. [Google Scholar]
- 6.Skelton DA, Greig CA, et al.: Power and related functional ability of healthy people aged 65-89 years. Age Ageing. 1994; 23: 371-377. [DOI] [PubMed] [Google Scholar]
- 7.Foldvari M, Clark M, et al.: Association of muscle power with functional status in community-dwelling olderly women. J Gerontol. 2000; 55A: M192-M199. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, et al.: Lower-extremity function in persons over age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995; 332: 556-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rantanen T, Guralnik JM, et al.: Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil. 1998; 77: 299-305. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs J, Hughes S, et al.: Predictors of change in walking velocity in older adults. J Am Geriatr Soc. 1996; 44: 126-132. [DOI] [PubMed] [Google Scholar]
- 11.Ikezoe T, Asakawa Y, et al.: Physical function screening of institutionalized elderly women to predict their risk of falling. Jpn J Phys Fit Sport. 2009; 58: 489-498. [Google Scholar]
- 12.Dupont AC, Sauerbrei EE, et al.: Real-time sonography to estimate muscle thickness: comparison with MRI and CT. J Clin Ultrasound. 2001; 29: 230-236. [DOI] [PubMed] [Google Scholar]
- 13.Fukunaga T, Miyatani M, et al.: Muscle volume is a major determinant of joint torque in humans. Acta Physiol Scand. 2001; 172: 249-255. [DOI] [PubMed] [Google Scholar]
- 14.Miyatani M, Kanehisa H, et al.: The accuracy of volume estimates using ultrasound muscle thickness measurements in different muscle groups. Eur J Appl Physiol. 2004; 91: 264-272. [DOI] [PubMed] [Google Scholar]
- 15.Sanada K, Kearns C, et al.: Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol. 2006; 96: 24-31. [DOI] [PubMed] [Google Scholar]
- 16.Heymsfield SB, Gonzalez MC, et al.: Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015; 8: 1-12. [DOI] [PubMed] [Google Scholar]
- 17.Ikezoe T, Mori N, et al.: Age-related muscle atrophy in the lower extremities and daily physical activity in elderly women. Arch Gerontol Geriatr. 2011; 53: e153-157. [DOI] [PubMed] [Google Scholar]
- 18.Ikezoe T, Mori N, et al.: Atrophy of the lower limbs in elderly women: is it related to walking ability? Eur J Appl Physiol. 2011; 111: 989-995. [DOI] [PubMed] [Google Scholar]
- 19.Johnson MA, Polgar J, et al.: Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973; 18: 111-129. [DOI] [PubMed] [Google Scholar]
- 20.Doherty TJ, Vandervoort AA, et al.: Effects of ageing on the motor unit: a brief review. Can J Appl Physiol. 1993; 18: 331-358. [DOI] [PubMed] [Google Scholar]
- 21.Roos MR, Rice CL, et al.: Age-related changes in motor unit function. Muscle Nerve. 1997; 20: 679-690. [DOI] [PubMed] [Google Scholar]
- 22.Andersson EA, Nilsson J, et al.: Intramuscular EMG from the hip flexor muscles during human locomotion. Acta Physiol Scand. 1997; 161: 361-370. [DOI] [PubMed] [Google Scholar]
- 23.Masuda K, Kim J, et al.: Determinants for stair climbing by elderly from muscle morphology. Percept Mot Skills. 2002; 94: 814-816. [DOI] [PubMed] [Google Scholar]
- 24.Bogduk N, Macintosh JE, et al.: A universal model of the lumbar back muscles in the upright position. Spine. 1992; 17: 897-913. [DOI] [PubMed] [Google Scholar]
- 25.Teyhen DS, Gill NW, et al.: Rehabilitative ultrasound imaging of the abdominal muscles. J Orthop Sports Phys Ther. 2007; 37: 450-466. [DOI] [PubMed] [Google Scholar]
- 26.Mannion AF: Fibre type characteristics and function of the human paraspinal muscles: normal values and changes in association with low-back pain. J Electromyogr Kinesiol. 1999; 9: 363-377. [DOI] [PubMed] [Google Scholar]
- 27.Stokes M, Rankin G, et al.: Ultrasound imaging of lumbar multifidus muscle: normal reference ranges for measurements and practical guidance on the technique. Man Ther. 2005; 10: 116-126. [DOI] [PubMed] [Google Scholar]
- 28.Rankin G, Stokes M, et al.: Abdominal muscle size and symmetry in normal subjects. Muscle Nerve. 2006; 34: 320-326. [DOI] [PubMed] [Google Scholar]
- 29.Kanehisa H, Miyatani M, et al.: Influences of age and sex on abdominal muscle and subcutaneous fat thickness. Eur J Appl Physiol. 2004; 91: 534-537. [DOI] [PubMed] [Google Scholar]
- 30.Ota M, Ikezoe T, et al.: Age-related changes in the thickness of the deep and superficial abdominal muscles in women. Arch Gerontol Geriatr. 2012; 55: e26-30. [DOI] [PubMed] [Google Scholar]
- 31.Ikezoe T, Mori N, et al.: Effects of age and inactivity due to prolonged bed rest on atrophy of trunk muscles. Eur J Appl Physiol. 2012; 112: 43-48. [DOI] [PubMed] [Google Scholar]
- 32.Rantanen J, Rissanen A, et al.: Lumbar muscle fiber size and fiber type distribution in normal subjects. Eur Spine J. 1994; 3: 331-335. [DOI] [PubMed] [Google Scholar]
- 33.Bergmark A: Stability of the lumbar spine: a study in mechanical engineering. Acta Orthop Scand Suppl. 1989; 60: 1-54. [DOI] [PubMed] [Google Scholar]
- 34.Hodges PW: Is there a role for transversus abdominis in lumbopelvic stability? Man Ther. 1999; 4: 74-86. [DOI] [PubMed] [Google Scholar]
- 35.Kiefer A, Shirazi-Adl A, et al.: Stability of the human spine in neutral postures. Eur Spine J. 1997; 6: 45-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cholewicki J, Panjabi MM, et al.: Stabilizing function of trunk flexor-extensor muscles around a neutral spine posture. Spine. 1997; 22: 2207-2212. [DOI] [PubMed] [Google Scholar]
- 37.Ikezoe T, Nakamura M, et al.: Association between walking ability and trunk and lower-limb muscle atrophy in institutionalized elderly women: a longitudinal pilot study. J Physiol Anthropol. 2015; 34: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visser M, Kritchevsky SB, et al.: Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002; 50: 897-904. [DOI] [PubMed] [Google Scholar]
- 39.Kent-Braun JA, Ng AV, et al.: Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol. 2000; 88: 662-668. [DOI] [PubMed] [Google Scholar]
- 40.Goodpaster BH, Carlson CL, et al.: Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001; 90: 2157-2165. [DOI] [PubMed] [Google Scholar]
- 41.Pillen S, Tak RO, et al.: Skeletal muscle ultrasound: Correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009; 35: 443-446. [DOI] [PubMed] [Google Scholar]
- 42.Reimers CD, Schlotter B, et al.: Calf enlargement in neuromuscular diseases: A quantitative ultrasound study in 350 patients and review of the literature. Neurol Sci. 1996; 143: 46-56. [DOI] [PubMed] [Google Scholar]
- 43.Ikezoe T, Asakawa Y, et al.: Associations of muscle stiffness and thickness with muscle strength and muscle power in elderly women. Geriatr Gerontol Int. 2012; 12: 86-92. [DOI] [PubMed] [Google Scholar]
- 44.Fukumoto Y, Ikezoe T, et al.: Skeletal muscle quality assessed by echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol. 2012; 112: 1519-1525. [DOI] [PubMed] [Google Scholar]
- 45.Cadore EL, Izquierdo M, et al.: Echo intensity is associated with skeletal muscle power and cardiovascular performance in elderly men. Exp Gerontol. 2012; 47: 473-478. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe Y, Yamada Y, et al.: Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin Interv Aging. 2013; 8: 993-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilhelm EN, Rech A, et al.: Relationship between quadriceps femoris echo intensity, muscle power, and functional capacity of older men. Age (Dordr). 2014; 36: 1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rech A, Radaelli R, et al.: Echo intensity is negatively associated with functional capacity in older women. Age (Dordr). 2014; 36: 9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez P, Wilhelm EN, et al.: Echo intensity independently predicts functionality in sedentary older men. Muscle Nerve. 2017; 55: 9-15. [DOI] [PubMed] [Google Scholar]
- 50.Fukumoto Y, Ikezoe T, et al.: Age-related ultrasound changes in muscle quantity and quality in women. Ultrasound Med Biol. 2015; 41: 3013-3017. [DOI] [PubMed] [Google Scholar]
- 51.Ota M, Ikezoe T, et al.: Age-related changes in muscle thickness and echo intensity of trunk muscles in healthy women: Comparison of 20s to 60s age groups. Eur J Appl Physiol. 2020; 120: 1805-1814. [DOI] [PubMed] [Google Scholar]
- 52.Frontera WR, Meredith CN, et al.: Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988; 64: 1038-1044. [DOI] [PubMed] [Google Scholar]
- 53.Taaffe DR, Pruitt L, et al.: Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. Clin Physiol. 1996; 16: 381-392. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins ND, Housh TJ, et al.: Neuromuscular adaptations after 2 and 4 weeks of 80% versus 30% 1 repetition maximum resistance training to failure. J Strength Cond Res. 2016; 30: 2174-2185. [DOI] [PubMed] [Google Scholar]
- 55.Van Roie E, Delecluse C, et al.: Strength training at high versus low external resistance in older adults: effects on muscle volume, muscle strength, and force-velocity characteristics. Exp Gerontol. 2013; 48: 1351-1361. [DOI] [PubMed] [Google Scholar]
- 56.Loenneke JP, Fahs CA, et al.: Blood flow restriction: The metabolite/volume threshold theory. Med Hypotheses. 2011; 77: 748-752. [DOI] [PubMed] [Google Scholar]
- 57.Morton RW, McGlory C, et al.: Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front Physiol. 2015; 6: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radaelli R, Brusco CM, et al.: Muscle quality and functionality in older women improve similarly with muscle power training using one or three sets. Exp Gerontol. 2019; 128: 110745. [DOI] [PubMed] [Google Scholar]
- 59.Radaelli R, Botton CE, et al.: Low- and high-volume strength training induces similar neuromuscular improvements in muscle quality in elderly women. Exp Gerontol. 2013; 48: 710-716. [DOI] [PubMed] [Google Scholar]
- 60.Wilhelm EN, Rech A, et al.: Concurrent strength and endurance training exercise sequence does not affect neuromuscular adaptations in older men. Exp Gerontol. 2014; 60: 207-214. [DOI] [PubMed] [Google Scholar]
- 61.Radaelli R, Botton CE, et al.: Time course of low- and high-volume strength training on neuromuscular adaptations and muscle quality in older women. Age (Dordr). 2014; 36: 881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikezoe T, Kobayashi T, et al.: Effects of low-load, higher-repetition versus high-load, lower-repetition resistance training not performed to failure on muscle strength, mass, and echo intensity in healthy young men: a time-course study. J Strength Cond Res. 2017. 10.1519/JSC.0000000000002278. Online ahead of print. [DOI] [PubMed] [Google Scholar]