Abstract
Objective: Chemotherapy-induced peripheral neuropathy (CIPN) is a common adverse event experienced by cancer patients. In general, CIPN is evaluated subjectively based on patient self-assessment or clinician-reported scales; evidence supporting the utility and validity of quantitative sensory tests (QST) is lacking in this patient population. The aim of this study was to objectively assess CIPN of lower extremities by QSTs, and to evaluate the concordance between QSTs and subjective assessments. Methods: In this prospective cohort study, outpatients with cancer receiving chemotherapy were recruited at a single university hospital. We assessed CIPN at the lower extremities at baseline and three months after baseline. The QSTs were performed by applying a monofilament and a tuning fork to determine touch and vibration thresholds, respectively, at the affected site. Subjective assessments were performed based on the visual analog scale (VAS) and the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) toxicity grade. Kappa coefficients were calculated to evaluate the concordance between QSTs and subjective assessments. Results: After exclusion and drop-outs during follow-up, nineteen patients were included in the analysis. The prevalence of patients with abnormal sensation was 37% based on QSTs, 32% based on the VAS, and 14% based on CTCAE grading, respectively. Kappa coefficients were 0.32 between QSTs and VAS, and 0.28 between QSTs and CTCAE. Conclusions: The concordance rates between quantitative and subjective assessments were low. CIPN should be assessed using both quantitative and subjective assessments.
Keywords: chemotherapy-induced peripheral neuropathy, quantitative sensory testing, monofilament, tuning fork, neoplasms
Chemotherapy-induced peripheral neuropathy (CIPN) is a common adverse event1) in cancer patients undergoing treatment; the associated toxicity may be dose-limiting. CIPN manifests as sensory and motor neuropathy in the hands and feet2). Notably, CIPN of the lower extremities results in mobility impairments such as gait abnormality and sedentary behavior3,4). CIPN is also a risk factor for falls5), and necessitates dose-reduction or treatment interruption in patients receiving chemotherapy6). Therefore, accurately assessing CIPN at patients' lower extremities is crucial.
In the past, CIPN has been evaluated subjectively based on patient self-reports or National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) toxicity grades determined by clinicians3,5,7), though there may be a discrepancy between patient-reported and clinician-assessed CIPN8,9). Quantitative sensory tests (QSTs) are objective tools to assess neuropathies10), though few studies have evaluated their validity and utility in measuring CIPN at patients' lower extremities11). Some studies involving patients with painful symptoms after the completion of chemotherapy have examined the CIPN of lower extremities by QSTs12,13). However, no study has prospectively assessed the CIPN of lower extremities by QSTs in asymptomatic patients undergoing chemotherapy. Moreover, the concordance between quantitative and subjective assessments for CIPN remains unclear.
The aim of this study was to quantitatively assess CIPN of the lower extremities by QSTs in asymptomatic patients undergoing chemotherapy and to determine the degree of concordance between QSTs and subjective assessments.
Methods
Study design and patients
This prospective cohort study was performed at Kobe University Hospital. Forty-seven outpatients with cancer receiving chemotherapy, including neurotoxic agents, were recruited from February to November 2015. Inclusion criteria were: (1) receiving bortezomib, vincristine, taxane-based, or platinum-based chemotherapy; (2) over 18 years of age; and (3) able to provide informed consent. Exclusion criteria were: (1) history of central nervous system disorder, diabetes mellitus, or spine disease; (2) bone or brain metastasis; and (3) having abnormal threshold to detect touch or vibration at baseline. All patients were informed of the study protocol and provided written informed consent before enrollment. The study was performed in accordance with the tenets of the Declaration of Helsinki. The study protocol was approved by the ethics committee of the Kobe University Graduate School of Health Sciences (approval number: 323-1).
Assessment
Patient characteristics
Demographic and clinical data were collected on age, sex, body mass index (BMI), cancer stage and diagnosis, Eastern Cooperative Oncology Group performance status, recurrence, and past cancer treatment based on medical records.
Quantitative assessments
CIPN baselines were assessed twice on the first day of an arbitrary cycle and on the first day of the treatment cycle three months later. QSTs were performed in a quiet, partitioned room using a series of Semmes-Weinstein monofilaments (SWM) and a tuning fork in accordance with previous studies14,15).
The SWMs (Semmes-Weinstein Monofilament SOT-DM06A, Sakai Med, Tokyo, Japan) were used to determine touch detection thresholds. This testing consisted of applying six monofilaments (whose forces were 0.07, 0.4, 2, 4, 10, and 300 g) to the surface of the skin in an up-down fashion. Starting with a bending force of 0.07 g, each monofilament was applied to the skin for approximately 1.5 seconds. We examined touch detection thresholds at three test sites, including the right plantar hallux, the right plantar-first metatarsal head, and the right dorsum-ankle as previously described2). If patients failed to detect the stimulus, the next higher force monofilament was applied to the same location. If patients detected the stimulus, the next lower force monofilament was applied. We continued the test until the same monofilament was detected thrice, and the force was defined as the touch detection threshold. If the touch detection threshold was higher than 10 g at any site, the patients were defined as having an abnormal threshold16).
In a separate session, vibration testing was conducted three times using a 128 Hz tuning fork (Luze c128 Hz 01-008, NITI-ON, Chiba, Japan) according to a previous study17). The tuning fork was placed vertically on the medial malleolus of the right foot, and held in place until the vibration ceased. We counted the seconds until the patients declared they could no longer feel the vibration. The patients were defined as having an abnormal threshold if the they did not feel any vibration. Sensation of vibration was assessed over three trials, separated by approximately ten second intervals, and averaged for each patient18). For analysis, the patients were defined as having an abnormal sensation if any of the QSTs showed an abnormal threshold.
Subjective assessments
We asked patients to report the severity of numbness at the tips of the first toe, the soles of the feet, and the ankles by drawing a line across a 100 mm visual analogue scale (VAS) as previously described2). Higher VAS scores represent more severe pain symptoms. Patients were defined as having an abnormal sensation if the VAS score was 30 mm or higher5).
The CTCAE toxicity grade version 4.0 was used to assess the severity of CIPN7). The CTCAE grade is a 5-point Likert scale; a grade of 2 is associated with impairment of activities associated with normal daily living. We collected the CTCAE grade from the medical records through the follow-up period. The patients were defined as having abnormal sensation if their CTCAE grade was greater than 1 during treatment.
Statistical Analyses
The Shapiro-Wilk test was performed to examine normality of all data. Longitudinal changes of CIPN at the lower extremities were compared by paired t-tests for normally distributed data or by the Wilcoxon signed rank test for data that violated assumptions of normality. We evaluated the convergent validity between subjective and quantitative measures by using Spearman's rank tests to estimate the correlation coefficients (Spearman's rho, ρ), and calculating kappa coefficients8). Spearman's rank tests between QST and VAS or CTCAE grade which were assessed at the follow-up period were applied to variables at each test site. Correlation coefficients were interpreted as follows: ρ = 0-0.19, very weak; 0.2-0.39, weak; 0.40-0.59, moderate; 0.60-0.79, strong; and 0.80-1.0, very strong19). The associations based on kappa values were interpreted as follows: kappa < 0.00 was poor, 0.00-0.20 was slight, 0.21-0.40 was fair, 0.41-0.60 was moderate, 0.61-0.80 was substantial, and 0.80-1.00 was almost perfect20). The 95% confidence intervals (CIs) for correlation coefficients and kappa coefficients were calculated. For all tests, a p value < 0.05 was considered statistically significant. All analyses were conducted using Stata version 13.1 (StataCorp, College Station, TX, USA).
Results
We recruited a total of 47 patients; 6 were excluded based on past medical history. Three of the remaining 41 eligible patients were excluded because of missing data from their QSTs (SWM testing, n = 1; tuning fork, n = 1; both SWM and tuning fork, n = 1). Fifteen patients were excluded from the follow-up assessment due to medical reasons, including change to supportive care, a change in drug or treatment, a change in hospital, or death. Two patients refused to participate in the assessment during follow-up and two patients were not included because we could not meet them at follow-up. Finally, 19 patients were included in the analysis. A flow chart of patient selection and exclusion is shown in Figure 1.
Figure 1.
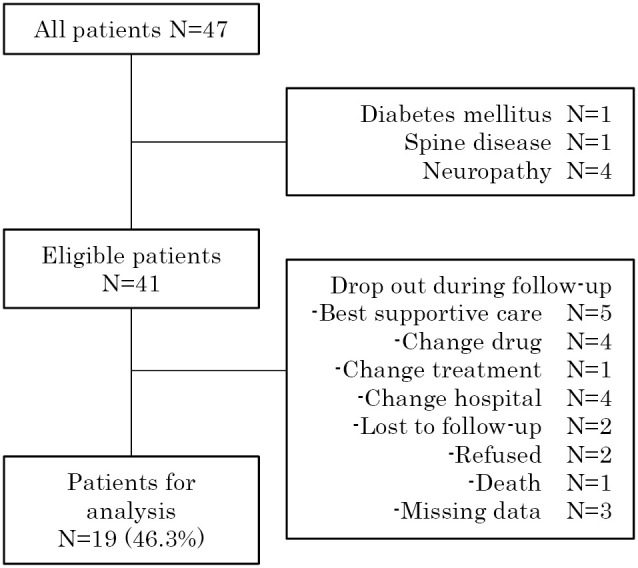
Flow chart of patient selection
Six patients were excluded before the baseline assessment, and 22 patients were excluded between the baseline and follow-up period for various reasons indicated in the flow chart. Subjects excluded for selecting the “best supportive care” chose to forego aggressive treatment in favor of supportive care to reduce their pain or other symptoms.
Patient characteristics
Demographic and clinical characteristics are shown in Table 1. The mean age was 61.5 years and almost half of patients were male (42%). The mean period from baseline to the second assessment was 103.3 days. Two patients (11%) had received chemotherapy inducing neurotoxicity in the past, and 12 patients (63%) received their first cycle of chemotherapy at baseline.
Table 1.
Characteristics of patients and treatments (n = 19)
| SD, standard deviation; BMI, body mass index; PS, performance status; PTX, paclitaxel; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone; XELOX, capecitabine and oxaliplatin; FOLFOX, leucovorin, 5-flourouracil and oxaliplatin; BD, bortezomib and dexamethasone; CIPN, chemotherapy-induced peripheral neuropathy. | |
| Age, years [mean ± SD] | 61.5 ± 13.3 |
| Sex, n (%) | |
| Male | 8 (42) |
| BMI, kg/m2 [mean ± SD] | 21.1 ± 2.6 |
| Stage, n (%) | |
| I | 1 (7) |
| II | 4 (28) |
| III | 7 (50) |
| IV | 2 (14) |
| Unknown | 5 |
| Diagnosis, n (%) | |
| Colorectal cancer | 6 (32) |
| Esophageal cancer | 1 (5) |
| Head & neck cancer | 3 (16) |
| Breast cancer | 3 (16) |
| Lymphoma | 4 (21) |
| Multiple myeloma | 2 (10) |
| Performance status, n (%) | |
| 0 | 7 (37) |
| 1 | 9 (47) |
| 2 | 2 (11) |
| 3 | 1 (5) |
| Recurrence, n (%) | 4 (21) |
| History of treatment, n (%) | |
| Surgery | 10 (53) |
| Radiation | 7 (32) |
| Chemotherapy inducing neurotoxicity | 2 (11) |
| Regimen of chemotherapy, n (%) | |
| PTX | 7 (37) |
| R-CHOP | 4 (21) |
| XELOX or FOLFOX | 6 (32) |
| BD | 2 (10) |
| Cycles of chemotherapy at baseline, n (%) | |
| 1st cycle | 12 (63) |
| 2nd cycle | 4 (21) |
| 3rd cycle | 2 (11) |
| 4th cycle | 1 (5) |
| Period from baseline to the second assessment, days [mean ± SD] | 103.3 ± 26.7 |
Changes of neuropathy and the prevalence of patients with abnormal sensation
Longitudinal changes of neuropathy are shown in Table 2. All QSTs showed significant deterioration manifesting as progressive loss of sensation through the follow-up period. The touch detection thresholds assessed by SWM testing increased significantly at all three test sites, and the vibration perception time at follow-up was significantly shorter than at baseline. VAS scores became significantly worse at the tips of the toe and the soles of the feet, while there was no significant change at the ankles. Seven of 19 patients (37%) were defined as having abnormal sensation by QSTs. The SWM testing detected 3 of 19 patients (16%) with abnormal sensation, and the tuning fork detected 6 of 19 patients (32%) with abnormal sensation. Seven (37%) and three (16%) of nineteen patients were classified as having abnormal sensation based on VAS and CTCAE assessments, respectively. All 7 patients defined as having abnormal sensation by QSTs were regarded as normal by CTCAE, while 3 patients (42.9%) were regarded as having normal sensation based on the VAS.
Table 2.
Longitudinal changes of neuropathy (n = 19)
| Baseline | Follow-up | P value | |
|---|---|---|---|
| IQR, inter quartile rage; SWM, Semmes-Weinstein monofilament; VAS, visual analog scale | |||
| SWM test, g [median (IQR) ] | |||
| plantar hallux | 2 (0.4 - 2) | 2 (2 - 10) | 0.01 |
| plantar-first metatarsal head | 2 (0.4 - 2) | 2 (0.4 - 4) | 0.04 |
| dorsum-ankle | 2 (2 - 2) | 2 (2 - 4) | 0.03 |
| Tuning fork, seconds [median (IQR) ] | 7.6 (6.0 -12.3) | 4.7 (0 - 7.8) | < 0.01 |
| VAS, mm [median (IQR) ] | |||
| toe | 0 (0 - 0) | 9.0 (0 - 35) | < 0.01 |
| sole | 0 (0 - 0) | 0 (0 - 18) | 0.01 |
| Ankle | 0 (0 - 0) | 0 (0 - 0) | - |
| CTCAE grade ≥ 2, n (%) | 0 (0) | 3 (16) | 0.7 |
Convergent validity of QST and subjective assessments
Correlations between QST and subjective assessments are shown in Table 3. All correlation coefficients were weak or very weak. The Kappa coefficients were calculated for the 19 patients who had no missing data. The concordance between QSTs and VAS was fair (kappa coefficient = 0.32, 95%CIs −0.11 - 0.76) and that between QSTs and CTCAE was poor (kappa coefficient = −0.28, 95%CIs −0.51 - −0.05).
Table 3.
Spearman's correlation coefficient (ρ) between quantitative sensory tests and subjective assessments.
| VAS-toe | VAS-sole | VAS-ankle | CTCAE grade | |
|---|---|---|---|---|
| Data show the ρ and (95% confidence intervals). NA: ρ could not be calculated because all data were zero in VAS-ankle. NA, not available; SWM, Semmes-Weinstein monofilament; PH, plantar hallux; PFMH, plantar-first metatarsal head; DA, dorsum-ankle; VAS, visual analog scale | ||||
| SWM test | ||||
| PH | 0.36 | −0.12 | ||
| (−0.13-0.85) | (−0.68-0.44) | |||
| PFMH | 0.08 | 0.06 | ||
| (−0.43-0.61) | (−0.39-0.52) | |||
| DA | NA | −0.36 | ||
| (−0.71-0.01) | ||||
| Tuning fork | NA | 0.06 | ||
| (−0.38-0.51) | ||||
Discussion
In this prospective study, we assessed CIPN at patients' lower extremities by QSTs, VAS and CTCAE to determine the degree of concordance between objective, quantitative measures and subjective assessments. The prevalence of patients with abnormal sensation was 37% for QSTs, 37% for VAS, and 16% for CTCAE. The correlation coefficients and concordance rates between QSTs and the subjective assessments were weak and low, respectively.
Both touch and vibration sensory thresholds for which we objectively measured by QSTs are associated with mobility impairment and falls in healthy individuals and diabetes mellitus patients14-16),21,22). Sensations associated with both touch and vibration are conducted by A-beta sensory afferent fibers and are perceived by activation of cutaneous mechanoreceptors, such as the Merkel and the Meissner corpuscles23,24). These receptors are damaged by chemotherapeutic agents25,26); therefore, the sensory deficits observed in our cohort may be a result of chemical-induced neurotoxicity.
In our study, the correlations and concordances between QSTs and VAS were weak. About two-fifths of patients defined as having abnormal sensation based on QSTs were asymptomatic according to the VAS. This discrepancy has also been found also in a previous study27). Over three-fourths of subjects diagnosed with diabetic neuropathy based on the SWM testing are asymptomatic27), so objective quantitative assessments are recommended in patients with diabetes mellitus28). Moreover, all patients defined as having abnormal sensation based on QSTs were identified as normal by the CTCAE. Quantitative assessments are also recommended for CIPN, as subjective assessments consistently underestimate sensory impairments in these patients.
This study had several strengths and limitations. To the best of our knowledge, this is the first study to objectively assess CIPN at the lower extremities in asymptomatic patients undergoing chemotherapy and evaluated the concordance between quantitative and subjective assessments. The findings that QSTs are capable of identifying sensory impairments that were not identified by subjective VAS and CTCAE scores suggest that the results of previous studies based on subjective assessments alone should be interpreted cautiously, as they may have underestimated the prevalence. There were some limitations in this study. First, small sample size and low follow-up rate may cause selection bias. Second, we assessed only hypoesthesia. Neurotoxic agents induce not only hypoesthesia but also hyperesthesia29), so we might underestimate abnormal sensation in our cohorts. Third, we did not examine any factors related to mobility. Whether the abnormal sensation evaluated by QSTs is related to any type of motor impairment remains unclear. Further studies with a larger sample size, assessments of hyperesthesia, and functional mobility assessments are needed to validate our findings.
Clinical Implications
Present study reveals a discrepancy between subjective assessments and QSTs, suggesting that sensory abnormalities caused by CIPN precede subjective symptoms during chemotherapy. Therefore, healthcare professionals should perform a comprehensive evaluation of CIPN using QSTs and subjective assessments. Physical therapists should also assess the symptom burden and the functional limitations caused by CIPN using these assessments and tests.
Conclusion
The correlation coefficients and concordance rates between the quantitative and subjective assessments were weak and low respectively. Therefore, CIPN should be assessed using both quantitative and subjective assessments.
Conflict of Interest
The authors received no funding support and have no conflicts of interest to declare.
Acknowledgments
The authors are extremely grateful to the patients who participated in this study and all staff members of the Department of Medical Oncology and Hematology at Kobe University Hospital.
References
- 1.Argyriou AA, Bruna J, et al.: Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012; 82: 51-77. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty PM, Cata JP, et al.: Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004; 109: 132-142. [DOI] [PubMed] [Google Scholar]
- 3.Winters-Stone KM, et al.: Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017; 35: 2604-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mols F, Beijers AJ, et al.: Chemotherapy-induced peripheral neuropathy, physical activity and health-related quality of life among colorectal cancer survivors from the PROFILES registry. J Cancer Surviv. 2015; 9: 512-522. [DOI] [PubMed] [Google Scholar]
- 5.Kolb NA, Smith AG, et al.: The Association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. 2016; 73: 860-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson JK and Hurvitz EA: Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci. 1995; 50: M211-215. [DOI] [PubMed] [Google Scholar]
- 7.The National Cancer Institute: Common terminology criteria for adverse events. [Cancer Therapy Evaluation Program web site]. [cited 2019 Apr. 28]; Available from: https://evs.nci.nih.gov/ftp1/CTCAE/About.html.
- 8.Shimozuma K, Ohashi Y, et al.: Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer. 2009; 17: 1483-1491. [DOI] [PubMed] [Google Scholar]
- 9.Nyrop KA, Deal AM, et al.: Patient-reported and clinician-reported chemotherapy-induced peripheral neuropathy in patients with early breast cancer: Current clinical practice. Cancer. 2019; 125: 2945-2954. [DOI] [PubMed] [Google Scholar]
- 10.Backonja MM, Walk D, et al.: Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009; 25: 641-647. [DOI] [PubMed] [Google Scholar]
- 11.Molassiotis A, Cheng HL, et al.: Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. 2019; 19: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhi WI, Chen P, et al.: Chemotherapy-induced peripheral neuropathy (CIPN) in breast cancer survivors: a comparison of patient-reported outcomes and quantitative sensory testing. Breast Cancer Res Treat. 2019; 178: 587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ventzel L, Madsen CS, et al.: Chronic Pain and Neuropathy Following Adjuvant Chemotherapy. Pain Med. 2018; 19: 1813-1824. [DOI] [PubMed] [Google Scholar]
- 14.Ward RE, Boudreau RM, et al.: Sensory and motor peripheral nerve function and incident mobility disability. J Am Geriatr Soc. 2014; 62: 2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson PG, Delforge M, et al.: Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012; 26: 595-608. [DOI] [PubMed] [Google Scholar]
- 16.Wilson SJ, Garner JC, et al.: The influence of multiple sensory impairments on functional balance and difficulty with falls among U.S. adults. Prev Med. 2016; 87: 41-46. [DOI] [PubMed] [Google Scholar]
- 17.Sawacha Z, Gabriella G, et al.: Diabetic gait and posture abnormalities: a biomechanical investigation through three dimensional gait analysis. Clin Biomech (Bristol, Avon). 2009; 24: 722-728. [DOI] [PubMed] [Google Scholar]
- 18.Jamali A, Sadeghi-Demneh E, et al.: Somatosensory impairment and its association with balance limitation in people with multiple sclerosis. Gait Posture. 2017; 57: 224-229. [DOI] [PubMed] [Google Scholar]
- 19.Cohen LH: Measurement of life events. In: Cohen LH (ed). Life Events and Psychological Functioning: Theoretical and Methodological Issues. Newbury Park, Sage, 1988, pp. 11-30. [Google Scholar]
- 20.Kundel HL and Polansky M: Measurement of observer agreement. Radiology. 2003; 228: 303-308. [DOI] [PubMed] [Google Scholar]
- 21.Deshpande N, Ferrucci L, et al.: Association of lower limb cutaneous sensitivity with gait speed in the elderly: the health ABC study. Am J Phys Med Rehabil. 2008; 87: 921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergin PS, Bronstein AM, et al.: Body sway and vibration perception thresholds in normal aging and in patients with polyneuropathy. J Neurol Neurosurg Psychiatry. 1995; 58: 335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancelliere P: A review of the pathophysiology and clinical sequelae of diabetic polyneuropathy in the feet. J Diabetes Metab Disor Control. 2016; 3: 21-24. [Google Scholar]
- 24.Garcia-Mesa Y, Garcia-Piqueras J, et al.: Merkel cells and Meissner's corpuscles in human digital skin display Piezo2 immunoreactivity. J Anat. 2017; 231: 978-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Carvalho Barbosa M, Kosturakis AK, et al.: A quantitative sensory analysis of peripheral neuropathy in colorectal cancer and its exacerbation by oxaliplatin chemotherapy. Cancer Res. 2014; 74: 5955-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosturakis AK, He Z, et al.: Subclinical peripheral neuropathy in patients with multiple myeloma before chemotherapy is correlated with decreased fingertip innervation density. J Clin Oncol. 2014; 32: 3156-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregg EW, Sorlie P, et al.: Prevalence of lower-extremity disease in the US adult population >= 40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004; 27: 1591-1597. [DOI] [PubMed] [Google Scholar]
- 28.Boulton AJ, Vinik AI, et al.: Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005; 28: 956-962. [DOI] [PubMed] [Google Scholar]
- 29.Ling B, Authier N, et al.: Behavioral and pharmacological description of oxaliplatin-induced painful neuropathy in rat. Pain. 2007; 128: 225-234. [DOI] [PubMed] [Google Scholar]


