Abstract
BACKGROUND
Type 2 diabetes (T2D) and resistant hypertension often coexist, greatly increasing risk of target-organ damage and death. We explored the effects of empagliflozin in patients with and without presumed resistant hypertension (prHT) in a post hoc analysis of EMPA-REG OUTCOME (NCT01131676).
METHODS
Overall, 7,020 patients received empagliflozin 10, 25 mg, or placebo with median follow-up of 3.1 years. We defined baseline prHT as ≥3 classes of antihypertensive drugs including a diuretic and uncontrolled blood pressure (BP; systolic blood pressure (SBP) ≥140 and/or diastolic blood pressure ≥90 mm Hg) or ≥4 classes of antihypertensive, including a diuretic, and controlled BP. We explored the effect of empagliflozin on cardiovascular (CV) death, heart failure (HF) hospitalization, 3-point major adverse cardiac events, all-cause death, and incident/worsening nephropathy by Cox regression and BP over time by a mixed-repeated-measures-model analysis.
RESULTS
1,579 (22.5%) patients had prHT. The mean difference in change in SBP from baseline to week 12 vs. placebo was −4.5 (95% confidence interval, −5.9 to −3.1) mm Hg (P < 0.001) in prHT and −3.7 (−4.5, −2.9) mm Hg (P < 0.001) in patients without prHT. SBP was more frequently controlled (<130/80 mm Hg) with empagliflozin than with placebo. Patients with prHT had 1.5- to 2-fold greater risk of HF hospitalization, incident/worsening nephropathy, and CV death compared with those without prHT. Empagliflozin improved all outcomes in patients with and without prHT (interaction P > 0.1 for all outcomes).
CONCLUSIONS
Empagliflozin induced a clinically relevant reduction in SBP and consistently improved all outcomes regardless of prHT status. Due to these dual effects, empagliflozin should be considered for patients with hypertension and T2D.
Keywords: blood pressure, empagliflozin, hypertension, resistant hypertension, type 2 diabetes
Cardiovascular (CV) risk in patients with diabetes is graded and continuous across the entire range of blood pressure (BP). International recommendations support the start of antihypertensive treatment in persons with diabetes who have high BP with a treatment goal of reducing systolic blood pressure (SBP) to <130 mm Hg.1–3 A subset (5–30%) of patients with high BP may have resistant hypertension (rHT) defined (by European guidelines) as a seated office SBP ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg on maximally tolerated doses of ≥3 antihypertensive agents (including a diuretic) or when BP control is achieved but requires ≥4 medications.3 Diabetes increases the risk of developing rHT.4 Furthermore, the concomitant existence of diabetes and rHT is associated with a major increase in the risk of target-organ damage (including heart failure (HF), atrial fibrillation, myocardial infarction, stroke, and chronic kidney disease) and ultimately death.3–8
Spironolactone is recommended as 4th line therapy for rHT; notwithstanding, a large proportion of patients with rHT remain uncontrolled and/or may have a formal contraindication or intolerance (e.g., advanced chronic kidney disease and hyperkalemia) to spironolactone, therefore additional strategies are urgently needed.5,9
Empagliflozin is a sodium-glucose cotransporter-2 (SGLT2) inhibitor, which, compared with placebo, reduced time to first HF hospitalization, incident or worsening nephropathy, and death (CV and all-cause) in patients with type 2 diabetes (T2D) mellitus and CV disease in the EMPA-REG OUTCOME trial.10 SGLT2 inhibitors also reduce BP,11 and in the EMPA-REG BP trial empagliflozin (compared with placebo) reduced mean SBP by 3–5 mm Hg and DBP by 1–2 mm Hg at week 12.12,13 However, the effect of empagliflozin in patients with rHT is yet to be studied.
The aim of the present study is to assess the BP-lowering effects of empagliflozin in patients with and without rHT (rHT vs. no-rHT), and whether the treatment effect of empagliflozin on CV, renal and HF outcomes are consistent in those with and without rHT; as rHT was a presumptive post hoc diagnosis in this population, we refer to it as presumed resistant hypertension (prHT) throughout the manuscript (see also the Methods section).
METHODS
The methods of the EMPA-REG OUTCOME trial have been previously described.10 In short, 7,020 patients with T2D and established CV disease were assigned to receive either 10 or 25 mg of empagliflozin or placebo once daily. The primary composite outcome was death from CV causes, nonfatal myocardial infarction, or nonfatal stroke (3-point major adverse cardiac event (3P-MACE)), as analyzed in the pooled empagliflozin group vs. the placebo group. Other outcomes were CV death, hospitalization for HF, the composite of CV death or hospitalization for HF, incident/worsening nephropathy, and all-cause death. These outcomes were also assessed in the present analysis. The median follow-up time was 3.1 years.
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
Definition of rHT and BP assessment
In this post hoc analysis, we defined prHT at baseline as the use of ≥3 classes of antihypertensive drugs, including a diuretic, and uncontrolled BP (SBP ≥140 and/or DBP ≥90 mm Hg), or use of ≥4 classes of antihypertensive drugs, including a diuretic, and SBP <140 and DBP <90 mm Hg. “True” rHT definition requires a BP that remains above goal in spite of the concurrent use of 3 antihypertensive agents of different classes, with (ideally) 1 of the 3 agents being a diuretic, with all agents prescribed at maximally tolerated doses; and (ideally) also requires ambulatory confirmation of high BP.3 Typically, a clinic BP of 140/90 mm Hg corresponds to home BP values of 135/85 mm Hg and to ambulatory BP monitoring values defined as a daytime SBP/DBP of 135/85 mm Hg, a nighttime SBP/DBP of 120/70 mm Hg, and a 24-hour SBP/DBP of 130/80 mm Hg.2 For this reason, we used the 140/90 mm Hg cutoff in our rHT definition, as it may better reflect “true” rHT (i.e., be more specific). As our study is a post hoc analysis of an outcome trial, we cannot ascertain whether all of the patients herein included have “true” rHT (despite using a cutoff of office BP >140/90 mm Hg). In consequence, throughout the manuscript whenever rHT is referred to, it should be considered as prHT.
BP was measured in the seated position after at least 5 minutes of rest with a calibrated automatic sphygmomanometer and in the presence of the study physician and/or nurse at each study site, taking the average of 3 consecutive measurements with a between-measurement interval of at least 1 minute.
Statistical analysis
Patients’ baseline characteristics were derived in the prHT vs. no-prHT groups, and continuous variables are presented as mean ± SD, and categorical variables are presented as number (n) and proportion (%). Effects on BP (SBP and DBP) were evaluated using a mixed effect model repeat measurement (MMRM) model, which included subject as a random effect, baseline glycated hemoglobin (HbA1c), and baseline of the endpoint (SBP or DBP) as linear covariates and their interaction with visit in addition to baseline estimated glomerular filtration rate category, geographic region, and baseline body mass index category. Treatment, rHT at baseline, and visit were also entered as fixed effects as well as all 2- and 3-way interactions thereof. Additionally, the model included a fixed categorical effect for “time of randomization” to account for each patient’s theoretical ability to “reach” certain weeks in this study arising from the study design. A similar MMRM model was applied for the evaluation of effects on weight and HbA1c. We furthermore explored the proportion of patients that achieved SBP <130 mm Hg during the trial. We then evaluated the treatment effects (pooled empagliflozin arms vs. placebo) on time to first 3P-MACE, CV death, hospitalization for HF, the composite of CV death or HF hospitalization, incident/worsening nephropathy, and all-cause death, in those with and without prHT by Cox regression models. The Cox model included the interaction of presence of prHT at baseline by treatment to evaluate the treatment effect in patients with and without prHT at baseline separately. The model further included covariate terms for age, gender, body mass index, HbA1c, estimated glomerular filtration rate, region, rHT at baseline, and treatment. All analyses are post hoc and not adjusted for multiplicity. All statistical analyses were performed using SAS Software, Version 9.4.
RESULTS
Baseline characteristics
Overall, 1,579 (22.5%) patients had prHT (as defined in the Methods section); these patients had longer diabetes duration (>10 years: 63% vs. 55%) and more concomitant diseases, including HF (17% vs. 8%), retinopathy (27% vs. 21%) and macroalbuminuria (15% vs. 10%), as well as lower mean estimated glomerular filtration rate (68 ml/min/1.73 m2 ± 21 (SD) vs. 76 ml/min/1.73 m2 ± 21 (SD)). The mean baseline SBP/DBP ± SD was 142 ± 18/78 ± 11 mm Hg in prHT vs. 133 ± 16/76 ± 10 mm Hg in no-prHT, and patients with prHT used more antihypertensive treatments, including mineralocorticoid receptor antagonists (Table 1). More patients with prHT added antihypertensive medications including beta blockers and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers during the trial, compared with those with no-prHT. Fewer patients added concomitant medications in the empagliflozin vs. placebo arms in both prHT and no-prHT groups. Addition of mineralocorticoid receptor antagonists was balanced between treatments in patients with prHT (Table 2).
Table 1.
Baseline characteristics and baseline medication use by presumed resistant hypertension status
| Characteristic | No resistant hypertension (N = 5,441) | Resistant hypertension (N = 1,579) |
|---|---|---|
| Female sex | 1,543 (28.4) | 461 (29.2) |
| Age, years | 62.7 ± 8.7 | 64.8 ± 8.3 |
| Body mass index, kg/m2 | 30.2 ± 5.2 | 32.2 ± 5.1 |
| Time since T2D diagnosis, years | ||
| ≤1 | 143 (2.6) | 37 (2.3) |
| >1–5 | 906 (16.7) | 177 (11.2) |
| >5–10 | 1,382 (25.4) | 364 (23.1) |
| >10 | 3,010 (55.3) | 1,001 (63.4) |
| LDL-C, mg/dl | 86.5 ± 36.3 | 82.4 ± 33.7 |
| eGFR, ml/min/1.73 m2 | 75.8 ± 21.4 | 68.1 ± 20.5 |
| <60 | 1,239 (22.8) | 580 (36.7) |
| Previous stroke, n (%) | 1,256 (23.1) | 381 (24.1) |
| CADa, n (%) | 4,065 (74.7) | 1,243 (78.7) |
| Heart failure, n (%) | 433 (8.0) | 273 (17.3) |
| HbA1c, % | 8.1 ± 0.9 | 8.1 ± 0.8 |
| SBP, mm Hg | 133.4 ± 16.1 | 142.4 ± 18.2 |
| DBP, mm Hg | 76.4 ± 9.6 | 77.6 ± 10.7 |
| Retinopathy, n (%) | 1,127 (20.7) | 419 (26.5) |
| Urine albumin-to-creatinine ratio, mg/g, median (Q1, Q3) | 16.8 (6.2, 62.8) | 25.2 (7.1, 116.7) |
| Micro albuminuriab | 1,514 (27.8) | 499 (31.6) |
| Macro albuminuriab | 532 (9.8) | 237 (15.0) |
| Left ventricular hypertrophyc | 101 (2.2) | 39 (2.9) |
| Uric acid (mg/dl) | 5.8 ± 1.6 | 6.6 ± 1.8 |
| Baseline medication use | ||
| Antihypertensive drugs | 5,088 (93.5) | 1,579 (100.0) |
| Beta blockers | 3,197 (58.8) | 1,357 (85.9) |
| Diuretics | 1,456 (26.8) | 1,579 (100.0) |
| ACE inhibitors/angiotensin receptor blockers | 4,140 (76.1) | 1,526 (96.6) |
| Mineralocorticoid receptor antagonists | 170 (3.1) | 271 (17.2) |
| Statins | 4,097 (75.3) | 1,306 (82.7) |
| Antiplatelets | 4,645 (85.4) | 1,377 (87.2) |
| Metformin | 4,057 (74.6) | 1,136 (71.9) |
| Insulin | 2,447 (45.0) | 940 (59.5) |
All data are n (%) or mean ± SE unless otherwise noted. “Resistant hypertension” was a post hoc presumptive diagnosis. Abbreviations: ACE, angiotensin-converting enzyme; CAD, coronary artery disease; DBP, diastolic blood pressure; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; LDL-C, low-density lipoprotein cholesterol; LVH, left ventricular hyperthrophy; SBP, systolic blood pressure; T2D, type 2 diabetes UACR, urinary albumin-to-creatinine ratio.
aCoronary artery disease defined as any of the components of history of myocardial infarction, coronary artery bypass graft, multivessel coronary artery disease, and single vessel coronary artery disease.
bDefined as microalbuminuria UACR 30 to ≤300 mg/g; macroalbuminuria UACR >300 mg/g.
cDefined on ECG as RV5/V6 + SV1/V2 >3.5 mV or RaVL ≥1.3 mV plus ≥1 of the following: left atrial abnormality, left axis deviation, and ST- and/or T-wave changes consistent with LVH.
Table 2.
Anticoagulants, lipid-lowering, antihypertensive, and antihypertensive drugs introduced post-baseline by presumed resistant hypertension status
| No resistant hypertension (N = 5,441) | Resistant hypertension (N = 1,579) | |||
|---|---|---|---|---|
| Placebo (n = 1,817) | All empagliflozin (n = 3,624) | Placebo (n = 516) | All empagliflozin (n = 1,063) | |
| Antihypertensive | 882 (48.5) | 1,550 (42.8) | 308 (59.7) | 538 (50.6) |
| Beta blockers | 362 (19.9) | 627 (17.3) | 119 (23.1) | 231 (21.7) |
| Diuretics | 426 (23.4) | 608 (16.8) | 182 (35.3) | 291 (27.4) |
| Mineralocorticoid receptor antagonists | 89 (4.9) | 86 (2.4) | 47 (9.1) | 91 (8.6) |
| ACE inhibitors/angiotensin receptor blockers | 533 (29.3) | 922 (25.4) | 169 (32.8) | 302 (28.4) |
| Statins | 446 (24.5) | 896 (24.7) | 155 (30.0) | 249 (23.4) |
| Antiplatelets | 395 (21.7) | 732 (20.2) | 123 (23.8) | 243 (22.9) |
All data are n (%). “Resistant hypertension” was a post hoc presumptive diagnosis. Abbreviation: ACE, angiotensin-converting enzyme.
BP control
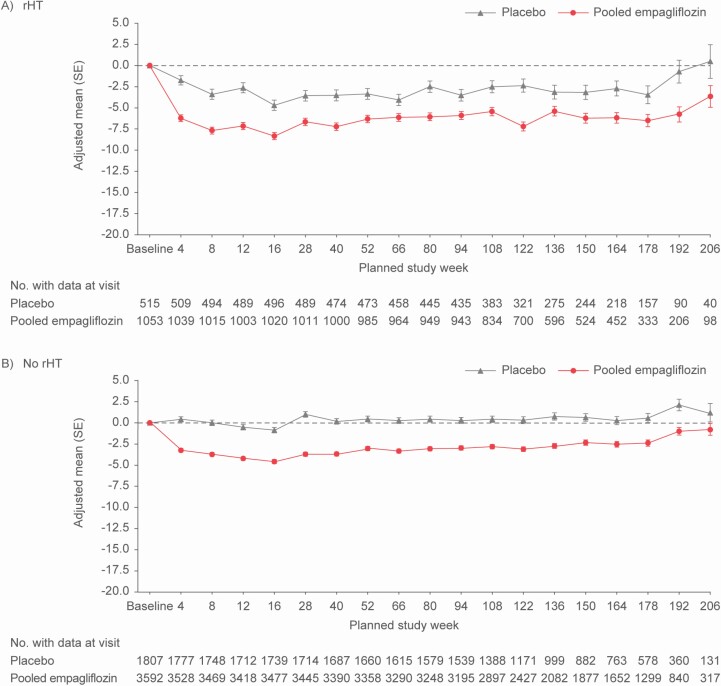
SBP and DBP were consistently reduced by empagliflozin in patients with prHT or no-prHT. Overall, the treatment effect of empagliflozin on SBP was consistent in patients with and without prHT throughout the trial (Figure 1). The mean difference (95% confidence interval) of SBP change from baseline to week 12 with empagliflozin vs. placebo was −4.5 (−5.9 to −3.1) mm Hg in prHT vs. −3.7 (−4.5 to −2.9) mm Hg in no-prHT; a difference that was sustained during the follow-up (Figure 1). These changes were generally comparable between empagliflozin doses (Supplementary Figure S1 online). The differences in change in DBP from baseline to week 12 for empagliflozin vs. placebo were smaller as compared with SBP in patients with and without prHT: −1.7 (−2.5 to −0.9) mm Hg in prHT vs. −1.2 (1.7 to −0.8) mm Hg in no-prHT; a difference that was also sustained during the follow-up (Supplementary Figure S2 online).
Figure 1.
Change from baseline in systolic blood pressure over time in patients (a) with presumed resistant hypertension and (b) no presumed resistant hypertension at baseline including all data up until individual trial termination: mixed effect repeated measurement model results. *MMRM model on the overall population including subject as random effect and, among others, treatment, visit, and presumed resistant hypertension at baseline as well as their corresponding 2- and 3-way interactions as fixed effects (for details on other fixed effects and linear covariates, see Statistical analysis section). Abbreviations: MMRM, mixed effect model repeat measurement; rHT, resistant hypertension.
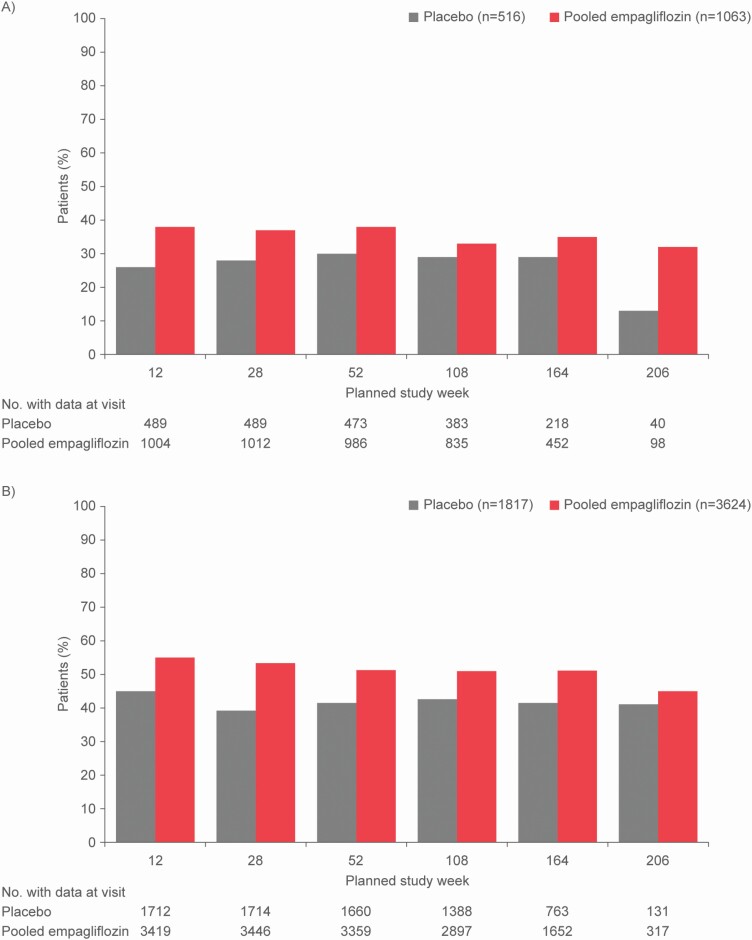
The proportion of patients with prHT that achieved SBP <130 mm Hg was higher with empagliflozin (vs. placebo) throughout the follow-up (e.g., 38% vs. 26% at week 12; Figure 2, Supplementary Figure S3 and Supplementary Table S1 online). Similarly, more patients without prHT treated with empagliflozin achieved SBP <130 mm Hg compared with placebo (Figure 2, Supplementary Table S1 online).
Figure 2.
Proportion of patients (a) with presumed resistant hypertension and (b) without presumed resistant hypertension who achieved systolic blood pressure <130 mm Hg during the trial in empagliflozin and placebo groups.
Outcome events
In both placebo- and empagliflozin-treated patients, patients with prHT were generally at increased risk for all outcomes compared with patients with no-prHT—with the only exception of all-cause mortality in the placebo group which only showed a weak trend in this respect (heart rate 1.13, 95% confidence interval, 0.82–1.56 for prHT vs. no-prHT). For example, in placebo-treated patients, the hazard ratio for 3P-MACE was 1.31 (95% confidence interval, 1.01–1.71) (Table 3).
Table 3.
Association of outcomes with presumed resistant hypertension at baseline
| Placebo | Empagliflozin | |||||
|---|---|---|---|---|---|---|
| No resistant hypertension (N = 1,817) | Resistant hypertension (N = 516) | HRa (95% confidence interval) Resistant hypertension vs. no resistant hypertension | No resistant hypertension (N = 3,624) | Resistant hypertension (N = 1,063) | HRa (95% confidence interval) Resistant hypertension vs. no resistant hypertension | |
| No. of patients with events (%) | No. of patients with events (%) | No. of patients with events (%) | No. of patients with events (%) | |||
| CV death | 93 (5.1) | 44 (8.5) | 1.50 (1.04–2.16) | 117 (3.2) | 55 (5.2) | 1.43 (1.03–1.98) |
| HHF | 64 (3.5) | 31 (6.0) | 1.50 (0.97–2.32) | 73 (2.0) | 53 (5.0) | 2.02 (1.41–2.91) |
| CV death or HHFb | 130 (7.2) | 68 (13.2) | 1.68 (1.25–2.26) | 168 (4.6) | 97 (9.1) | 1.70 (1.32–2.19) |
| 3P-MACE | 202 (11.1) | 80 (15.5) | 1.31 (1.01–1.71) | 334 (9.2) | 156 (14.7) | 1.49 (1.23–1.81) |
| All-cause mortality | 143 (7.9) | 51 (9.9) | 1.13 (0.82–1.56) | 189 (5.2) | 80 (7.5) | 1.28 (0.98–1.67) |
| Incident/worsening nephropathy | 276 (17.0) | 112 (25.3) | 1.55 (1.24–1.93) | 356 (11.0) | 169 (18.9) | 1.68 (1.40–2.03) |
Abbreviations: 3P-MACE, 3-point major adverse cardiac event; BMI, baseline body mass index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HHF, heart failure hospitalization; rHT, resistant hypertension.
aHR by multivariable Cox regression with the following variables: age, sex, region, HbA1c (category), BMI (category), eGFR (category), treatment, rHT, and treatment by resistant hypertension interaction. “Resistant hypertension” was a post hoc presumptive diagnosis.
bExcluding fatal stroke.
Treatment effects on outcomes and HbA1c
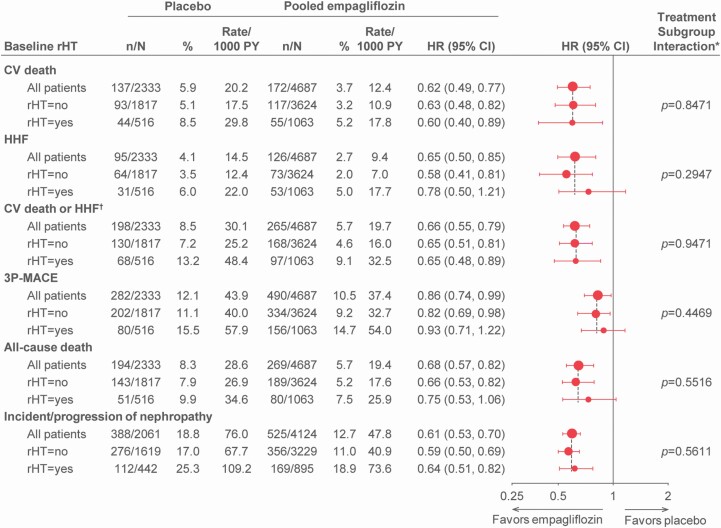
Empagliflozin (vs. placebo) consistently reduced the risk for all outcomes regardless of baseline prHT status (P value for interaction >0.1 for all outcomes; Figure 3). HbA1c levels and weight were reduced by empagliflozin treatment in patients with and without prHT (Supplementary Figures S4 and S5 online).
Figure 3.
Treatment effects of empagliflozin vs. placebo on outcomes in patients with or without presumed resistant hypertension. *Based on a Cox regression model with terms for age sex, baseline BMI category, baseline HbA1c category, baseline eGFR category, geographical region, treatment, presumed resistant hypertension, and treatment by presumed resistant hypertension interaction. †Excludes fatal stroke. Abbreviations: 3P-MACE, 3-point major adverse cardiac event; BMI, baseline body mass index; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HHF, heart failure hospitalization; rHT, resistant hypertension.
Adverse events
For most adverse events, incidence rates in patients with prHT were higher compared with patients with no-prHT irrespective of treatment (Supplementary Table S2 online). Empagliflozin-treated prHT patients experienced numerically fewer serious adverse events compared with placebo-treated prHT patients (45.7% vs. 52.1%). Furthermore, rates of hyperkalemia were lower with empagliflozin than placebo, irrespective of prHT status, whereas incidence rates of genital infection were increased with empagliflozin in both subgroups.
DISCUSSION
In this analysis, treatment with empagliflozin resulted in early reductions in SBP in patients with T2D with and without prHT (−4.5 mm Hg compared with placebo in patients with prHT at week 12) with sustained BP reduction throughout the follow-up. A higher proportion of empagliflozin-treated patients achieved a SBP <130 mm Hg compared with those receiving placebo. Independent of treatment arm, patients with prHT had higher incidence of major CV (including HF) events compared with those with no-prHT after multivariable adjustment for risk factors including age and estimated glomerular filtration rate. Treatment with empagliflozin reduced major CV events irrespective of the patients’ prHT status.
Treatment with empagliflozin has consistently been shown to reduce BP in patients with T2D, thereby improving the BP control in this population.11–13 Reductions in major CV events and death with empagliflozin are likely explained by a multitude of mechanisms, including osmotic diuresis following increased urinary glucose excretion, improved glycemic control, weight loss, reduced arterial stiffness, and, importantly, a reduction in plasma volume.14–16 Although a mediation analysis identified changes in markers of plasma volume as the most important mediators of the reduction of CV death in empagliflozin-treated patients and showed that SBP and DBP had only negligible mediating effects,16 it is well established that BP control is important to improve outcomes in patients with T2D and CVD.
In the EMPA-REG OUTCOME trial, patients with prHT represented 22% of the study population, supporting the high prevalence of rHT in patients with diabetes also reported in population-based cohorts.17 Patients with rHT have high CV risk, which poses additional difficulties in the BP control, where a low BP target (SBP <130 mm Hg) is desirable.18 The Action to Control Cardiovascular Risk in Diabetes blood pressure trial (ACCORD-BP)19 tested the effect of a target SBP <120 mm Hg on major adverse cardiac effects among high-risk persons with T2D (n = 4,733). Although this trial did not show a reduction in the primary composite outcome of myocardial infarction, stroke or CV death, a reduction in stroke rate, a prespecified secondary outcome, was observed.19 Subsequent large meta-analyses strongly support intensive BP-lowering strategies over standard regimens for CV protection in persons with diabetes, with benefits seen even at SBP values <120 mm Hg.20,21 The Systolic Blood Pressure Intervention Trial (SPRINT) also supports a target of <120 mm Hg among patients at high risk for CV events but without diabetes.22 In order to achieve an adequate BP control in rHT patients, multiple drugs and drug combinations are often used. In rHT patients participating in the PATHWAY-2 trial,23 spironolactone started at 25 mg daily, and uptitrated to 50 mg, reduced home SBP at 12 weeks by a mean of −8.7 (−9.7 to −7.7) mm Hg, compared with placebo, and was more effective than alternative fourth-line drugs (bisoprolol or doxazosin) by a mean SBP margin of −4.3 (−5.1 to −3.4) mm Hg. In the present study, treatment with empagliflozin resulted in a −4.5 (−5.9 to −3.1) mm Hg greater reduction in SBP at week 12 compared with placebo in patients with prHT, suggesting that empagliflozin may have additional BP-lowering benefit on top of standard of care.24 The differences in trial design and populations preclude comparisons of BP effects between spironolactone and empagliflozin (e.g., PATHWAY-2 was a “cross-over” trial, enrolling only patients with rHT and where only 14% of the patients had T2D). Interestingly, in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, that enrolled patients with HF and preserved ejection fraction, of which 32% had diabetes, spironolactone reduced SBP by −6.1 (−8.9 to −3.3) mm Hg at 16 weeks in those with prHT.25 Together these results suggest that there may exist a potential interest in associating these drugs (spironolactone and empagliflozin) for the treatment of rHT in patients with T2D. These drugs showed morbidity and mortality reductions across different populations, and patients treated with empagliflozin had lower rates of hyperkalemia during the follow-up, which may be of particular interest when used concomitantly with an mineralocorticoid receptor antagonist. Analogously, another emerging treatment option is potassium binding. For example, the AMBER study (patiromer vs. placebo to enable spironolactone use in patients with rHT and chronic kidney disease) showed that patiromer enabled more patients to continue treatment with spironolactone with less hyperkalemia26 The findings reported in the present study are also concordant with those of the EMPA-REG BP trial where, compared with placebo, empagliflozin reduced ambulatory SBP by 3.4 (−4.8 to −2.1) mm Hg and 4.2 (−5.5 to −2.8) mm Hg in the 10 and 25 mg formulations, respectively, in patients with T2D and hypertension.12 The BP-lowering effect of empagliflozin was retained in patients who were receiving 1 or multiple antihypertensive medications.13 The BP-lowering properties of empagliflozin, may likely be extended to a class effect, as dapagliflozin also reduced office BP in patients receiving background antihypertensive treatment,27 and canagliflozin also reduced BP, especially in patients with baseline SBP >140 mm Hg.28
Clinical and research implications
These findings may have major clinical and research implications as empagliflozin could be a therapeutic option for BP control in patients with hypertension and T2D, providing survival and renal protection benefits beyond its BP-lowering effects. As patients treated with empagliflozin had lower rates of hyperkalemia in our analysis as well as in previous analyses from EMPA-REG OUTCOME,29 future studies may evaluate the benefit of empagliflozin in reducing hyperkalemia risk in rHT patients treated with mineralocorticoid receptor antagonists.
Limitations
Our analysis has some limitations. First, since it is post hoc and non-prespecified, and the trial was not powered for subgroup analyses, the results must be regarded as hypothesis generating. Although BP measurement was standardized, this was not a dedicated BP trial, BP was measured in the presence of the study personnel and sphygmomanometers might have been different between study sites; therefore, the precision of the values reported may be reduced. However, this imprecision was not systematic and may even reinforce the robustness of our findings (i.e., with variability similar to a “real-world” setting). Finally, the American Heart Association (AHA) uses an inclusive definition of rHT: “rHT is defined as BP that remains above goal in spite of the concurrent use of 3 antihypertensive agents of different classes. Ideally, 1 of the 3 agents should be a diuretic and all agents should be prescribed at optimal dose amounts.” 9 The presence of “true” rHT is difficult to ascertain in non-hypertension (HT) studies, due to the difficulty in confirming adherence to therapy and performing 24-hour ambulatory BP measurements: such assessments were not performed within the framework of a CV outcome trial. Furthermore, doses of antihypertensive treatments were not captured.
Empagliflozin induced a clinically relevant reduction in SBP and consistently improved all outcomes regardless of the prHT status. Acknowledging that this is a post hoc analysis, the consistent findings suggest that due to the optimal dual effect of BP control and CV event reduction, empagliflozin should be considered a therapeutic option for patients with hypertension and T2D.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients who participated in this trial. Editorial assistance, supported financially by Boehringer Ingelheim, was provided by Jonathon Gibbs, CMPP, of Elevate Scientific Solutions.
FUNDING
The EMPA-REG OUTCOME trial was funded by the Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance.
DISCLOSURE
J.P.F. reports having received modest traveling fees from Boehringer Ingelheim. D.F. reports CME honoraria and consultation fees from Boehringer Ingelheim, Lilly, Sanofi, AstraZeneca, and Amgen, and DSMB honoraria from NovoNordisk. B.J.K. has received significant grant support from the IZKF Wuerzburg and modest honoraria from Boehringer Ingelheim. C.W. reports significant honoraria from Boehringer Ingelheim and modest honoraria from AstraZeneca, Bayer, Eli Lilly, Mitsubishi, and MSD. B.Z. has received significant speaker fees or consulting honoraria from NovoNordisk, Boehringer Ingelheim, Eli Lilly, and Merck and modest fees from Astra Zeneca, Sanofi, and Janssen. F.Z. has received modest fees for serving on the board of Boston Scientific; modest consulting fees from Novartis, Takeda, AstraZeneca, Boehringer Ingelheim, GE Healthcare, Relypsa, Servier, Boston Scientific, Bayer, Johnson & Johnson, and Resmed; modest speakers’ fees from Pfizer and AstraZeneca and significant disclosure as CardioRenal cofounder. P.R. reports modest grants and or personal fees from Ablative Solutions, AstraZeneca, Bayer, Boehringer Ingelheim, Corvidia, CVRx, Fresenius, G3P, Grunenthal, Idorsia, Novartis, NovoNordisk, Relypsa, Servier, Stealth Peptides, Vifor Fresenius Medical Care Renal Pharma, and significant disclosure as CardioRenal cofounder: A.P.O. and J.T.G. are full-time employees of Boehringer Ingelheim International GmbH, Germany. I.Z. is a full-time employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Germany. S.L. reports significant consulting fees from Hoffmann La Roche and Boehringer Ingelheim.
REFERENCES
- 1. American Diabetes Association. 9. Cardiovascular disease and risk management: standards of medical care in diabetes—2018. Diabetes Care 2018; 41:S86–S104. [DOI] [PubMed] [Google Scholar]
- 2. Whelton PK, Carey RM, Aronow WS, Casey DE. Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2018; 138:e484–e594. [DOI] [PubMed] [Google Scholar]
- 3. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; Authors/Task Force Members . 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 4. Viazzi F, Piscitelli P, Ceriello A, Fioretto P, Giorda C, Guida P, Russo G, De Cosmo S, Pontremoli R. Resistant hypertension, time-updated blood pressure values and renal outcome in Type 2 diabetes mellitus. J Am Heart Assoc, published online September 22 2017; 6:pii: e006745. doi: 10.1161/JAHA.117.006745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension (Dallas, Tex.: 1979) 2018; 72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2018; 138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 7. Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, O’Connor PJ, Selby JV, Ho PM. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; 125:1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mondesir FL, Brown TM, Muntner P, Durant RW, Carson AP, Safford MM, Levitan EB. Diabetes, diabetes severity, and coronary heart disease risk equivalence: REasons for Geographic and Racial Differences in Stroke (REGARDS). Am Heart J 2016; 181:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rimoldi SF, Messerli FH, Bangalore S, Scherrer U. Resistant hypertension: what the cardiologist needs to know. Eur Heart J 2015; 36:2686–2695. [DOI] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 11. Azizi M, Rossignol P, Hulot JS. Emerging drug classes and their potential use in hypertension. Hypertension 2019;74:1075–1083. [DOI] [PubMed] [Google Scholar]
- 12. Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, Woerle HJ; EMPA-REG BP Investigators . Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015; 38:420–428. [DOI] [PubMed] [Google Scholar]
- 13. Mancia G, Cannon CP, Tikkanen I, Zeller C, Ley L, Woerle HJ, Broedl UC, Johansen OE. Impact of empagliflozin on blood pressure in patients with type 2 diabetes mellitus and hypertension by background antihypertensive medication. Hypertension (Dallas, Tex.: 1979) 2016; 68:1355–1364. [DOI] [PubMed] [Google Scholar]
- 14. Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, Woerle HJ, von Eynatten M, Broedl UC. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014; 13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 2015; 17:1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2018; 41:356–363. [DOI] [PubMed] [Google Scholar]
- 17. Bayliss G, Weinrauch LA, D’Elia JA. Resistant hypertension in diabetes mellitus. Curr Diab Rep 2014; 14:516. [DOI] [PubMed] [Google Scholar]
- 18. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure ; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 19. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, Woodward M, MacMahon S, Turnbull F, Hillis GS, Chalmers J, Mant J, Salam A, Rahimi K, Perkovic V, Rodgers A. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016; 387:435–443. [DOI] [PubMed] [Google Scholar]
- 21. Aggarwal R, Steinkamp J, Chiu N, Petrie B, Mirzan H. Intensive blood pressure targets for diabetic and other high-risk populations: a pooled individual patient data analysis. Hypertension (Dallas, Tex.: 1979) 2018; 71:833–839. [DOI] [PubMed] [Google Scholar]
- 22. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society’s PATHWAY Studies Group . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015; 386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moore MN, Atkins ER, Salam A, Callisaya ML, Hare JL, Marwick TH, Nelson MR, Wright L, Sharman JE, Rodgers A. Regression to the mean of repeated ambulatory blood pressure monitoring five studies. J Hypertens 2019; 37:24–29. [DOI] [PubMed] [Google Scholar]
- 25. Rossignol P, Claggett BL, Liu J, Vardeny O, Pitt B, Zannad F, Solomon S. Spironolactone and resistant hypertension in heart failure with preserved ejection fraction. Am J Hypertens 2018; 31:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, Ma J, White WB, Williams B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019; 394:1540–1550. [DOI] [PubMed] [Google Scholar]
- 27. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin-angiotensin system blockade. Blood Press 2016; 25:93–103. [DOI] [PubMed] [Google Scholar]
- 28. Weir MR, Januszewicz A, Gilbert RE, Vijapurkar U, Kline I, Fung A, Meininger G. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich) 2014; 16:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B; EMPA-REG OUTCOME Investigators . Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018; 137:119–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.