Abstract
The alteration of thyroxine (T4) cellular uptake by an environmental chemical can serve as a contributing factor in thyroid hormone (TH) disruption. This study describes a non-radiolabeled (LC-MS/MS) oil-filtration technique designed to characterize the mechanism(s) responsible for T4 cellular uptake in cryopreserved rat hepatocyte suspensions. The environmental chemicals perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) that are known thyroid-disrupting agents were evaluated for their potential effect on T4 cellular uptake. At 37°C, hepatic T4 uptake demonstrated saturable kinetics with increasing T4 concentrations (0.1–15 μM), while a linear uptake rate was detected at 4°C that is consistent with passive diffusion. Michaelis-Menten analysis of carrier-mediated (enzymatic) T4 uptake (37–4°C) displayed a Vmax value of 3.06 pmoles/106 cells/sec and a KM value of 2.93 μM and was determined to be the predominant uptake process (>70%) versus passive diffusion. Cyclosporin A (CsA) chemically inhibited T4 uptake rates with no discernable effect on passive diffusion, whereas PFOA/PFOS showed no inhibitory effect on T4 uptake. However, T4 uptake assays conducted with PFOA/PFOS and the T4 serum carrier protein transthyretin (TTR) displayed a dose-response increase in hepatic T4 uptake rates correlating with increased T4 free fraction values. Our findings indicate the kinetic determinant of T4 hepatic uptake was predominantly carrier-mediated as demonstrated by an enhanced first-order rate of T4 uptake compared to passive diffusion. These in vitro findings provide new mechanistic and physiological insight regarding decreased T4 serum concentrations (hypothyroxinemia) previously observed within in vivo rodent studies following perfluorinated chemical exposure.
INTRODUCTION
Thyroid hormones (THs) play a crucial role in a vast array of physiological processes including regulation of cellular metabolism, growth and neural development (Dong and Wade, 2017). The thyroid gland predominantly produces and secretes the prohormone thyroxine (T4) into circulation, where the majority (>99%) is bound to serum proteins and only a small fraction of free T4 is dissociated (Richardson et al., 2015). The free T4 fraction in blood is then available for cellular uptake by the target tissue and metabolized via an intracellular deiodination reaction to the biologically active hormone, triiodo-L-thyronine (T3) (Mendel, 1989). Thus, the process of T4translocation across the plasma membrane is required for cellular action (Visser et al., 2011).
Characterizing the mechanism(s) responsible for T4 uptake across the plasma membrane is critical for pharmacokinetic assessment of T4 serum levels during environmental chemical exposures. Historically,THs were generally believed to enter target cells solely by cellular diffusion due to their highly lipophilic nature and this assumption was highly accepted and persisted for decades without further investigation (for a historical overview, see Hennemann et al., 2001). Under this premise, environmental chemical assessment on T4 cellular uptake poses little concern as passive transcellular transport is a concentration-gradient dependent process not subject to chemical interaction (Sugano et al., 2010). However, it is now understood through results from a multitude of in vitro primary and transfected cell line studies that carrier-mediated (enzymatic) transport influences T4 cellular uptake (Jayarama-Naidu et al., 2015; Friesema et al., 2003; Visser et al., 2011). Unlike passive diffusion, T4 carrier-mediated uptake can undergo substrate saturation due to a finite number of transporter-enzyme binding sites. Importantly, T4 carrier-mediated uptake processes are also susceptible to environmental chemical interaction(s), which presents a viable mode of action for potential TH disruption that receives little attention in human health risk assessment (Marchesini et al., 2008; Connors et al., 2010).
Under physiological conditions, the rate of TH uptake from blood serum is influenced by the dissociation of free TH from the fraction bound to serum carrier proteins (Mendel, 1989). In humans, there are three main TH serum carrier proteins that include albumin, transthyretin (TTR) and thyroxine-binding globulin(TBG), whereas albumin and TTR are primarily found in adult rodent (Richardson et al., 2015). Among these proteins, TBG has the highest affinity for T4, followed by the intermediate TTR, and the low binding affinity of albumin. TTR is of particular importance due to its prominent role in T4 tissue delivery and its highly conserved nature between both human and rodent species (Richardson et al., 2015; Robbins, 2000). TTR is also found as the primary TH serum protein secreted in cerebrospinal fluid, which plays a critical role in fetal neurodevelopment and function (Palha, 2002). Thus, environmental chemical alteration of free T4 dissociation from the TTR protein-binding complex serves as a potential mode for TH disruption regarding both cellular diffusion and carrier-mediated transport.
The liver plays a major role in the metabolism and excretion of TH and strongly influences systemic blood TH concentrations (Richardson et al., 2013). Primary hepatocytes serve as an in vitro system of choice for assessing hepatocellular uptake as they maintain a multitude of endogenous transport proteins including: organic anion transporting polypeptide (OATP), organic anion transporter (OAT), monocarboxylate transporter (MCT) and sodium taurocholate co-transporting polypeptide (NTCP) families that have been previously characterized for TH uptake (Soars et al., 2007). Rodent hepatocytes provide advantages over human cells due to minimal inter-donor variability, standardized tissue storage protocols and accessibility to in vivo testing for optimization of physiologically-based pharmacokinetic models (Yabe et al., 2011). The establishment of rodent models provides valuable mechanistic information needed for further species extrapolation and variability assessment of TH hepatic uptake within advanced human systems. However, the application of diverse hepatocyte methodologies, as well as the complexity of investigating the simultaneous contributions of passive diffusion and carrier-mediated transport, has led to variable results in characterizing TH uptake processes (Hennemann et al., 2001; Rao and Rao, 1983). Furthermore, the historical radioactive method for evaluation of TH uptake is compromised by safety and handling issues and the availability of a limited number of TH substrates as well as the inability to monitor for metabolites of interest (Jayarama-Naidu et al., 2015).
The environmental chemicals PFOA and PFOS are potential thyroid disrupting agents, which have received special attention regarding chemical interaction with T4-TTR binding affinities and overall impact on TH homeostasis (Lee and Choi, 2017). PFOA and PFOS are members of a broad chemical class of per-fluorinated alkyl substances (PFASs) characterized by fully or partially fluorinated alkyl chains containing an anionic charged functional group. PFASs unique physical and chemical surfactant properties that allows for oil and water repellency has led to widespread industrial and consumer applications. Human exposure to PFOA and PFOS from general household products and their persistence in the environment has led to significant detection levels in the general population (Coperchini et al., 2017; Washington et al., 2015).
Previous in vitro studies have examined the displacement of free T4 from TTR upon PFAS exposure, which then supports PFAS in vivo rodent findings of the transient increase of free T4 available for liver metabolism and excretion (Ramhoj et al., 2018; Chang et al., 2008; Weiss et al., 2009). However, delineation of the transport mechanism(s) responsible for T4 hepatic cellular uptake in the presence of PFAS chemicals are not well defined. The objective of this study is to characterize T4hepatic uptake using cryopreserved rat hepatocyte suspensions to provide mechanistic insight during PFOA and PFOS chemical exposures.
MATERIALS AND METHODS
Chemicals and Reagents
L-thyroxine (T4; (2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5diiodo-phenyo] propanoic acid), (T3; (2S)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl] propanoic acid), cyclosporin A (CsA), PFOS, octanoic acid, silicone oil, and mineral oil were purchased from Sigma Aldrich (St. Louis, MO). PFOA was purchased from Oakwood chemicals (Estill, SC) (Fig. 1).
FIG. 1.
Chemical structure and speciation of octanoic acid, PFOA, PFOS and L-thyroxine (T4).
13C6-labeled T4 (13C6-T4) was purchased as an internal standard from Cambridge Isotope Laboratories (Tewksbury, MA). Williams’ media, hepatocyte thaw media (Invitrogen; CM7500) and hepatocyte cell maintenance supplement (CM4000) were purchased from Invitrogen (Waltham, MA). The hepatocytes were maintained in Williams’ media supplemented by the following (final concentration): Dexamethasone: 0.1 μM, Penicillin/Streptomycin: 0.5%, human recombinant insulin: 6.25 μg/mL, human transferrin: 6.25 μg/mL, selenous acid: 6.25 ng/mL, bovine serum albumin (BSA): 1.25 mg/mL, linoleic acid: 5.35 μg/mL, GlutaMAX: 2 mM, HEPES, pH 7.4: 15 mM). The serum protein transthyretin (TTR) derived from human plasma was purchased from Athens Research & Technologies (Athens, GA). Evolute Express (CX, 10 mg) 96-well solid-phase extraction (SPE) plates were purchased from Biotage (Charlotte, NC). Microcon® centrifugal filter devices with Ultracel YM-10 membrane (10,000 Da nominal molecular weight limit) were purchased from Millipore (Bedford, MA). All solvents and acids used in extraction and elution were of Optima-Grade performance and purchased from Thermo Fisher (Pittsburgh, PA).
T4 Hepatocyte Uptake Assay
Oil-filtration tube preparation.
Fisherbrand 0.4 mL polyethylene micro centrifuge tubes set in a 96-well format were used for all oil-filtration experiments. A 100 μL aliquot of 0.5 M cesium chloride(CsCl) prepared in deionized water was placed in the bottom of the 0.4 mL tubes. Next, a 100 μL aliquot of mineral oil and silicon oil (1:5 v/v, respectively) was carefully layered on top of the CsCl layer (Li et al., 2013). The tubes were then centrifuged at room temperature at 14,000 rpm for 30s to even the oil layer and disperse all air bubbles.
Cryopreserved hepatocyte thawing.
Pooled male cryopreserved Sprague-Dawley rat hepatocytes were purchased from Bioreclamation IVT (Baltimore, MD) and stored in liquid nitrogen until use. Cryopreserved rat hepatocytes were removed from liquid nitrogen and immediately thawed in a 37 °C water bath until a small ice pellet remained in the bottom of the vial. Contents of the vial were then directly transferred into 37 °C hepatocyte thaw media in a 50-mL conical tube, the cells were then gently inverted and placed in a centrifuge at room temperature to be spun at 86 × g for 6 min. The supernatant was then aspirated off not to disturb the pellet, and the cells were gently re-suspended in 3 mL of freshly prepared (37 °C) Williams’ medium containing the hepatocyte maintenance supplement, henceforth referred to as running buffer. Cell counts and viability were then performed using trypan blue exclusion and disposable cell counting chamber (Nexcelom Bioscience) and were brought to a cell concentration of 1.0 × 106 live cells/mL (− TTR) and 2.0 × 106 live cells/mL (+TTR) with viability criteria >80%. The hepatocyte suspension was then placed in a 37 °C orbital shaking water bath at 50 RPM for 10 min or placed on ice for 4 °C assays.
Chemical preparation.
All T4 (5 mM) and chemical stock solutions (20 mM) were prepared in DMSO. For experiments containing TTR, lyophilized TTR powder was first solubilized in freshly prepared running buffer (37 °C) on the day of experimentation at a concentration of 1 mg/mL. T4 solutions were then prepared in running buffer (±TTR) in a glass vial and then dispensed into low-binding 1.5 mL microcentrifuge tubes (Midsci™, MAXYmum Recovery), where upon addition of individual test chemicals, both T4 and the test chemical resulted in 4(x) the final desired concentration. DMSO was used for all vehicle control experiments and maintained <0.5% of the final concentration in all chemical mixtures. All T4 chemical solutions with TTR present were gently mixed to not disturb protein integrity and then placed on a heating block at 37 °C for a 30 min equilibration timeframe.
Kinetic assessment of T4 uptake.
Following pre-incubation of the hepatocytes at 37 °C for 10 min, 150 μL of cell suspension was added to 300 μL of running buffer in a low binding microcentrifuge tube and gently inverted to mix. The hepatic uptake assay was then initiated by the addition of 150 μL of T4 (± test chemical; ±TTR) with a gentle pipetting motion up and down to ensure proper mixing. Single aliquots (100 μL) were then subsampled from the microcentrifuge tube (25 K cells/aliquot; −TTR and 50 K cells/aliquot; +TTR), and layered on top of the previously prepared oil-filtration tubes and immediately centrifuged (14,000 rpm for 30s) at each of the designated time points (15, 30, 60, and 90s) to separate the cells from the media and cease the reaction. The experimental procedure was conducted in the sequence of vehicle control (DMSO) timeline followed by the chemical test article timeline(s), which represents one replicate. This sequence was then repeated in triplicate allowing for control replicates to account for any variability in the hepatocyte suspension over the time required to complete the experimentation.
For passive diffusion (4 °C) assays, hepatocytes and chemical reagents were placed on ice for a minimum of 15 min prior to conducting the assay. TTR chemical solutions were initially incubated at 37 °C for 30 min to ensure the same T4-TTR equilibration timeframe before being placed on ice for 15 min for the 4 °C experiments. Designated time points used for 4 °C samples were (30, 60, 90, and 120 s) due to slower uptake rates. All oil filtration tubes were then placed in a − 80 °C ultra-freezer overnight before extraction.
Free T4 concentrations
To determine free T4 concentration levels present within the running buffer solutions without hepatocytes, triple aliquots (100 μL) of the running buffer (diluted to 1×) were transferred into individual Microcon® centrifugal ultrafiltration devices and centrifuged at 12,000 rpm for 5 min. The filtrate was then prepared and analyzed for T4 following the protocol applied for hepatocyte suspensions.
T4 Quantification
Sample preparation.
Frozen oil-filtration tubes (−80 °C) were clipped through the middle of the oil layer using a plastic tubing cutter (Fisher Scientific; 22–088245). The lower CsCl layer containing the pelleted hepatocytes was then collected into a microcentrifuge tube, while carefully discarding the upper frozen level to avoid sample contamination of the test article. The CsCl layer was thawed at room temperature and digested using 400 μL of 80:20% (acetonitrile:water) containing 4% formic acid (4% FA). The samples were vortexed and placed on a mini orbital shaker (Versa-ORB) at 150 rpm for 10 min at room temperature, then centrifuged at 14,000 rpm for 10 min to pellet any residual oil or cell debris. A 200 μL aliquot of supernatant was removed and placed into a clean microcentrifuge tube, spiked with 100 μL internal standard (13C6-T4,100 ng/mL, 0.1 M NaOH), and diluted to a 2 mL final volume with water (4% FA) for solid phase extraction.
T4 solid phase extraction.
Samples were processed through solid phase extraction (SPE) using a 96-well plate (Evolute Express CX, 10 mg, 1 mL, Biotage, Charlotte, North Carolina) and a vacuum filtration manifold (Multiscreen HTS, Millipore). The SPE 96-well plate was conditioned with 400 μL of methanol followed by 400 μL of water (2% FA). Sample extracts (2 mL) were then loaded onto the SPE and vacuumed to dryness, each well was then washed with 400 μL of water (2% FA) followed by 400 μL methanol. T4 was then eluted from the SPE sorbent using a triplicate rinse (100 μL) of 50:50% (methanol:acetonitrile) containing 5% NH4OH for a 300 μL final volume collection into a 96 well plate. The 96 well plate contents were then evaporated to dryness using Microvap nitrogen dryer (Organomation, Berlin, MA), reconstituted in 100 μL of 60:40% (methanol:water) and sonicated for 5 min before LC/MS/MS analysis.
LC/MS/MS analysis.
The reconstituted samples were analyzed for T3 and T4 using an Agilent 1200 Ultra-performance liquid chromatograph (UPLC) coupled to a 6420 triple quad mass spectrometer (Agilent, Santa Clara, CA). Injections (5 uL) at a 1.0 ml/min flow rate were made into an Agilent Zorbax XDB-C18 column (4.6 mm × 50 mm, 1.8 um particle diameter; Santa Clara, CA) maintained at 40°C. Gradient elution with methanol (solvent A) and water (solvent B) with 0.2% FA was applied under the following conditions: 60% A for 0.5 min, followed by a linear gradient to 70% A at 3.0 min, increasing to 100% A at 3.2 min, and held for a 6.5 min stop time. The column was then allowed to re-equilibrate under the original conditions for a 3 min post-time. MS/MS detection was conducted using ESI+ in multiple reaction mode under the following conditions: T4 quantifying ion transition m/z 777.7→731.5, with qualifying ion transitions m/z 777.7→633.5 (collision energies 25V, respectively), with the fragmenting voltage set to 160 V and the cell accelerator at 7 V. T3 quantifying ion transition m/z 651.8→605.9, with qualifying ion transition m/z 651.8→478.7 (collision energies 30 V and 35 V, respectively), with the fragmenting voltage set at 120 V and the cell accelerator at 7 V. The internal standard, L-Thyroxine (13C6 -T4) was quantified based on the transition m/z 783.8→737.8 with the fragmenting voltage, collision energy, and cell accelerator set to 160 V, 25 V, and 7 V, respectively (Wang and Stapleton, 2010). ESI source parameters were applied according to the following: source gas temperature 350°C, gas flow 12 L/min, nebulizer 55 psi, capillary 4000 V. T3 and T4 standard curves (0.1–125 ng/mL) were prepared in (60:40, methanol:water) using L-thyroxine (13C6 -T4) as an internal standard and verified during analysis with a check standard every 12 samples, followed by a blank sample for carryover assessment. Data processing was performed using Agilent MassHunter software (version B.04.01) and sample concentrations were determined using an internal-standard response factor.
Statistical analyses.
Total (37°C) and passive diffusion (4°C) rates of T4 uptake (pmol/(106 cells*s)) were determined from the slope of the regression line (r2 > 0.7, quality criteria for 37°C control) of least squared error in plots of sample concentration (pmol/106 cells) vs time (s). Differences in uptake rates between treatment and control were determined using a Student’s t-test for differences in regression slopes having a common variance (Steel & Torrie, 1980):
| (1) |
where Rr and Rc designates uptake rate for treatment and control, respectively, is the pooled estimate of the common variance, Xj is the time at which sample j was collected and * is the mean time of sample collection. The pooled estimate of variance is defined as (Steel & Torrie, 1980):
| (2) |
where Yj is the concentration of sample j, is the regression estimate for the time at which Yj was drawn and n is the number of samples included in the regression. Significant differences in treatment vs control were evaluated by comparing the t statistic to two-tailed critical values of t at p=0.05 and 0.01 for nr+nc−4 degrees of freedom (df).
The presence of differences between treatments and controls in free T4 was determined with Students t-test with unequal variances (Steel & Torrie, 1980).
The Michaelis-Menten constants Vmax and KM were determined based on nonlinear regression of T4 uptake velocities (pmoles/sec) versus concentration using Sigma Plot (version 13.0, Systat Software,San Jose, CA) using a one-site saturation component.
RESULTS
A linear rate of T4 uptake was determined using oil filtration of cryopreserved male rat hepatocytes in suspension at both 37 °C and 4 °C. A representative kinetic profile of T4 hepatic uptake (37 °C) versus time (15, 30, 60, and 90 s) determined from individual timelines conducted in triplicate is depicted in Fig. 2. The rate of T4hepatic uptake remained linear up to approximately 120 s at physiological temperature (37 °C) before beginning to plateau. A positive y-intercept was observed from the linear regression of the measured T4 uptake time course, indicating a rapid phase of T4 cellular partitioning during the initial lag period (<15 s) of the cell separation procedure. All T4 hepatic uptake rates reported were determined from individual time lines conducted in triplicate.
FIG. 2.
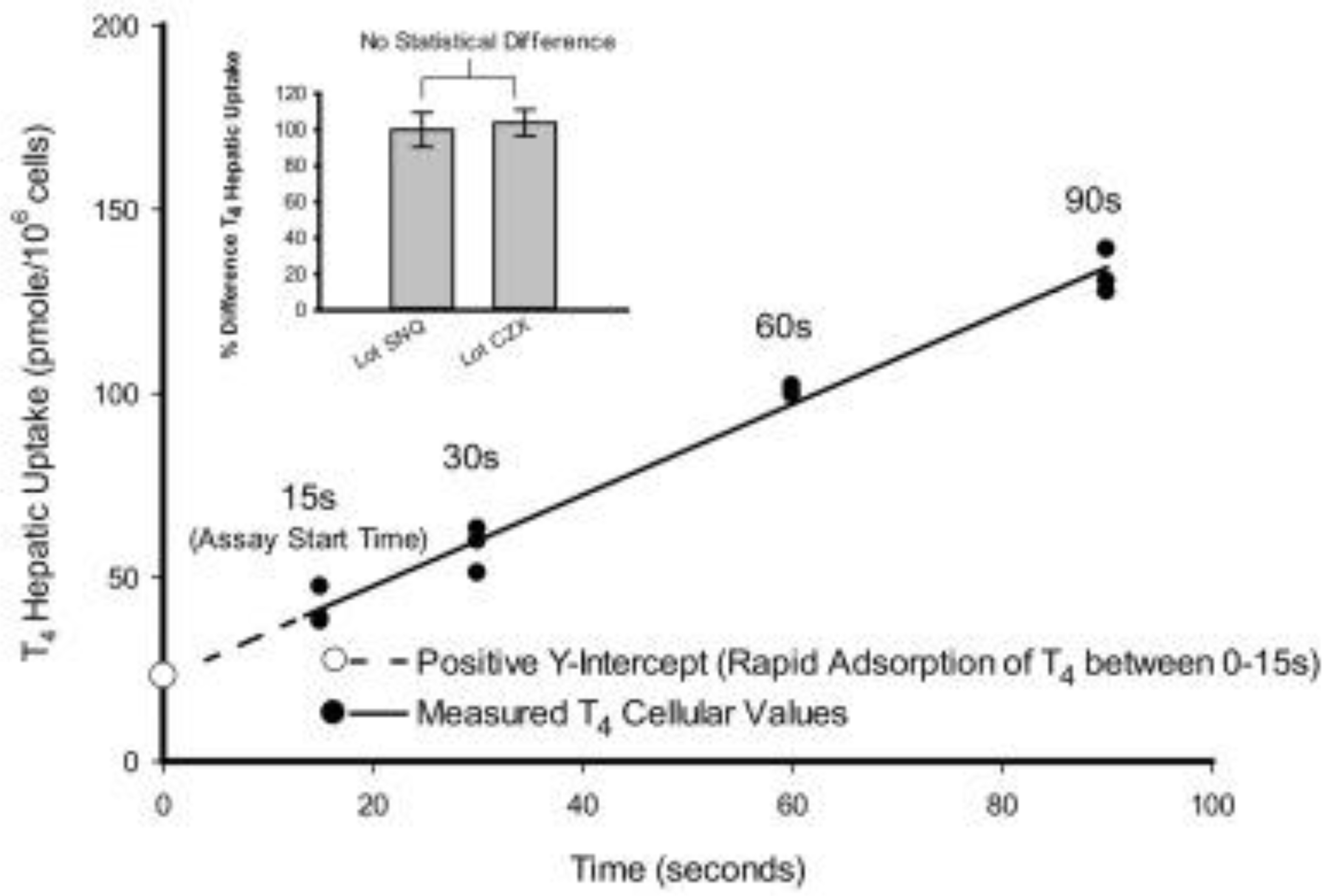
Representative kinetic profile of T4 (1.0 μM) uptake at 37 °C in cryopreserved rathepatocytes (Lot CZX) using oil filtration. A linear rate of T4 uptake (pmoles/106 cells/s) was determined from individual timelines conducted in triplicate within a single experiment. Inset depiction displays no statistical difference between duplicate pooled cryopreserved hepatocyte Lots (SNQ: 24 donors; CZX: 6 donors) used as a T4 (1.0 μM) positive control for all experimentation conducted without the serum carrier proteinTTR.
The potential deiodination of T4 resulting in T3 was not observed as T3 levels remained below the level of detection. The coefficient of variation (CV) for all 37 °C control hepatic uptake rates with and without TTR, representing daily and batch LOT variability (Table 1), remained below 30% for all experimentation. All T4hepatic uptake activity ceased via disruption of cellular function and cell membrane integrity following a freeze thaw cycle (−80 °C for 30 min) of hepatocytes.
Table 1.
Summary of experimental design with pooled cryopreserved rat hepatocyte lots.
| Experimenta | Number of individual experiments by lot | Number of individual experiments | Number of samples for timeline analysis | TTR treatment % control | |||
|---|---|---|---|---|---|---|---|
| SNQ (donors = 24) | CZX (donors = 6) | ZHN (donors = 45) | |||||
| Control | No TTR | 2 | 2 | 4 | 48 | ||
| TTR | 1 | 1 | 1 | 3 | 36 | ||
| PFOA (10 μM) | No TTR | 1 | 1 | 12 | |||
| TTR | 1 | 1 | 2 | 24 | 206–298 | ||
| PFOS (10 μM) | No TTR | 1 | 1 | 12 | |||
| TTR | 1 | 1 | 12 | 226 | |||
| Octanoic Acid (10 μM) | No TTR | ||||||
| TTR | 1 | 1 | 12 | 154 | |||
| Cyclosporin A (10 μM) | No TTR | 1 | 1 | 12 | |||
| TTR | 1 | 1 | 12 | 53 | |||
| Number of experiments | 7 | 3 | 5 | 15 | |||
| Number of samples for timeline analysis | 84 | 36 | 60 | 180 | |||
Hepatic T4 uptake velocities conducted at 37 °C demonstrated saturable enzymatic (carrier-mediated + passive diffusion) kinetics with respect to increasing T4substrate concentrations (0.1–15 μM), whereas assays conducted at 4 °C displayed a nonsaturable linear relationship to increasing T4 concentrations representative of passive diffusion (Fig. 3).
FIG. 3.
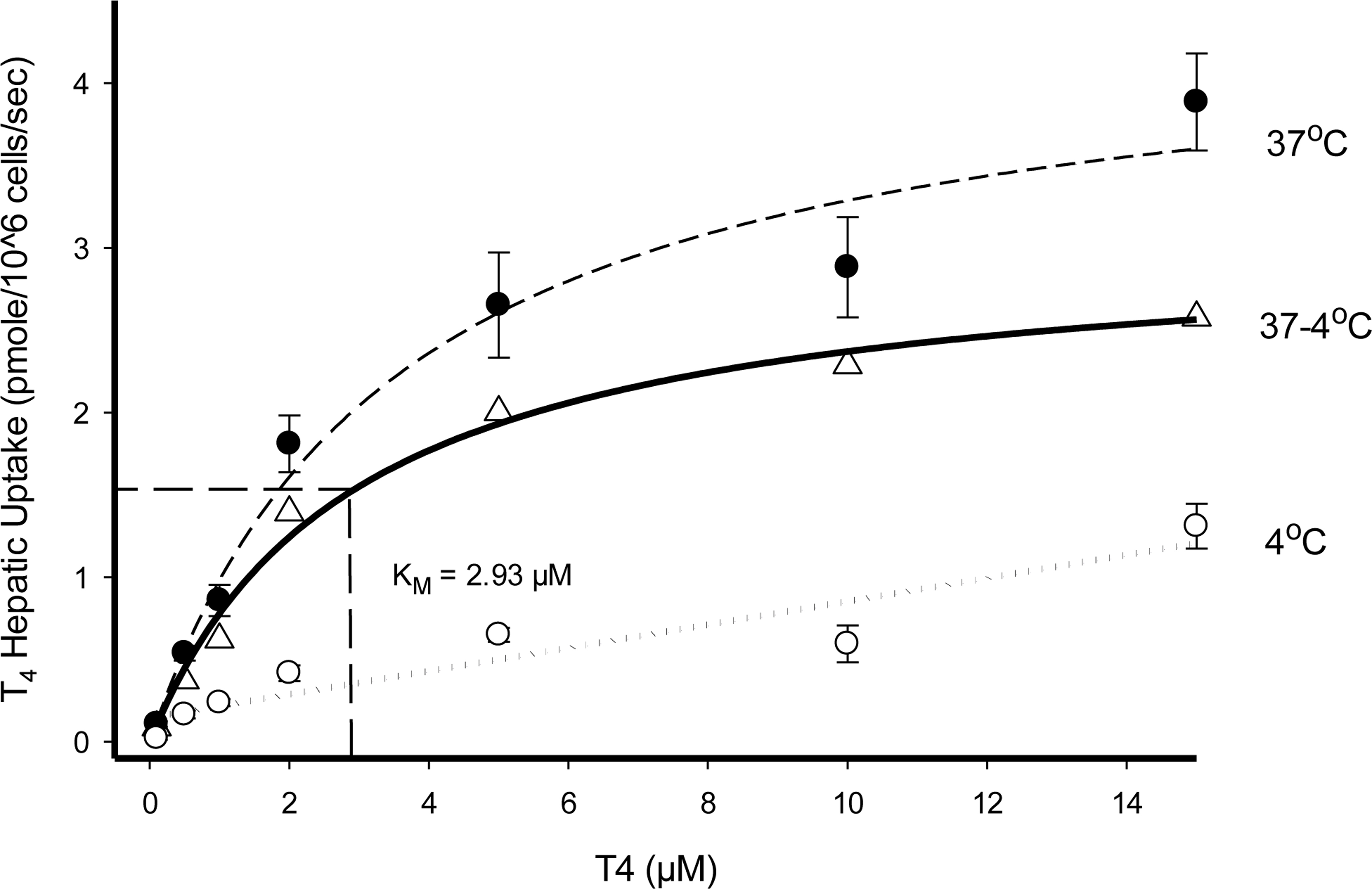
Substrate saturation kinetic profiles of T4 uptake in cryopreserved rat hepatocyte suspensions ● 37°C (carrier-mediated + passive diffusion), Δ 37°C - 4°C (carrier mediated), and ○ 4°C (passive diffusion). A linear rate of T4 hepatic uptake (pmoles/106 cells/sec) was determined from individual timelines conducted in triplicate for each substrate concentration tested. (Error bars represent ± SD.).
All chemical inhibition assays were conducted with T4 (1.0 μM) initial concentration to ensure substrate levels were maintained well below the determined KM value. Cyclosporin A (CsA), a broad range chemical inhibitor for membrane bound transporting proteins (transporters), was examined in a dose-dependent (0.1–10 μM) manner for inhibition of T4 uptake (Karlgren et al., 2012). At 37 °C, a significant decrease (p < .05) in T4 total hepatic uptake was observed at low CsA (0.1 μM) concentration levels in comparison to controls (Fig. 4A). No statistical difference in T4 uptake rates were observed at 4 °C while varying CsA levels (0.1–10.0 μM). In contrast to the chemical inhibition displayed by CsA at 37 °C, T4 uptake assays conducted in the presence of the environmental chemicals PFOA (0.1–10 μM) and PFOS (10 μM; data not shown) displayed no statistical difference in T4 hepatic uptake rates at either 37 °C or 4 °C indicating no effect on T4 membrane transport (Fig. 4B).
FIG. 4.
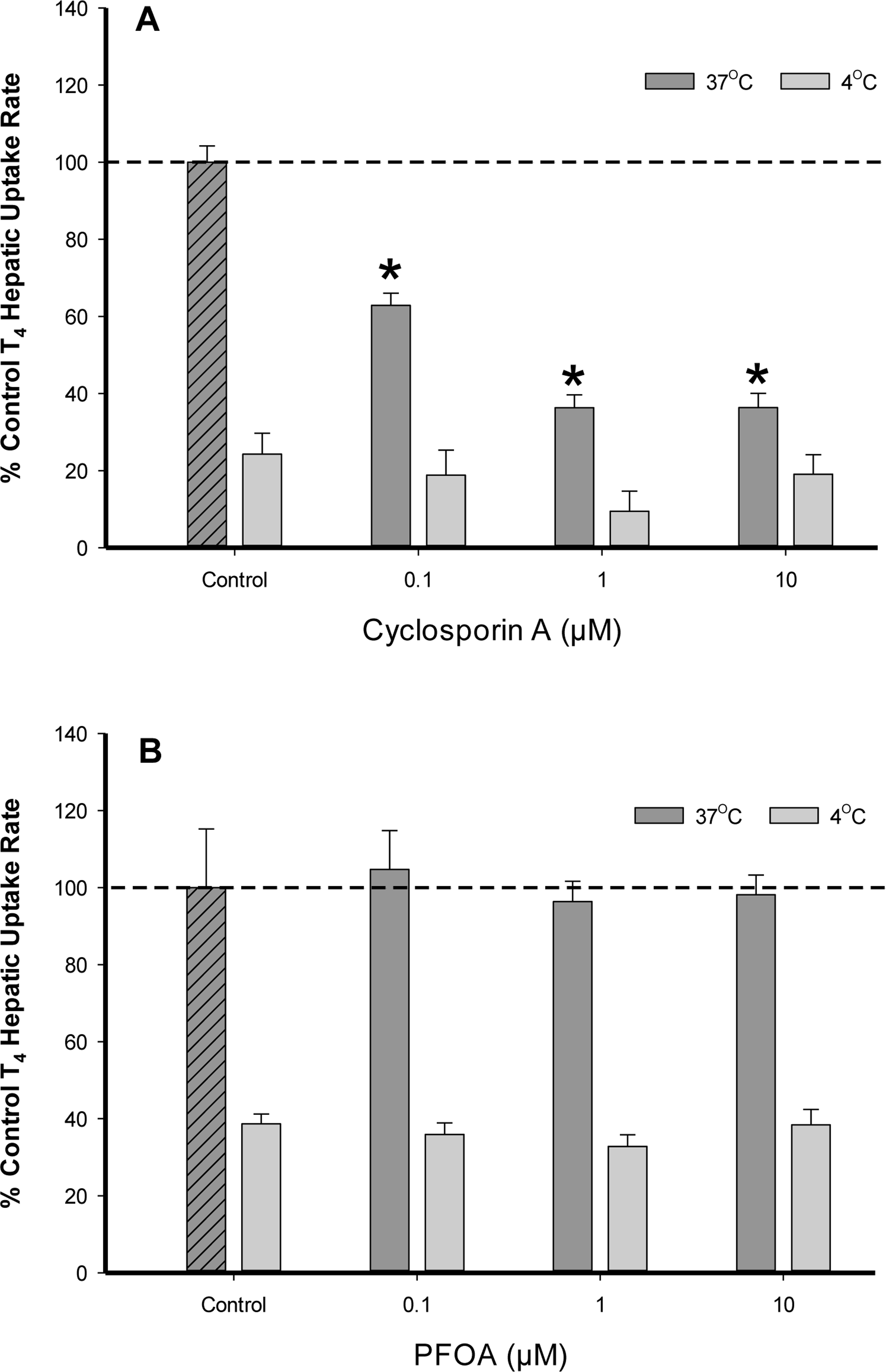
Percent difference from 37°C control of T4 (1.0 μM) uptake in cryopreserved rat hepatocyte suspensions treated with varying concentrations of the transport inhibitor (A) cyclosporin A and (B) PFOA. Data represent the linear rate of T4 hepatic uptake (pmoles/106 cells/sec) determined from individual timelines conducted in triplicate within a single experiment. Error bars represent ± SD, asterisks denote statistically significant differences versus temperature control regression slopes (*p<0.05).
PFOA and PFOS are known to competitively bind to the thyroid hormone serumcarrier protein transthyretin (TTR) resulting in the displacement of the biologically active free T4 available for uptake (Weiss et al., 2009). To assess the binding potency of PFOA to TTR and the influence on T4 hepatocyte membrane transport, we first assessed the rate of T4 uptake in the presence of varying TTR concentration levels (0–0.125 μg/mL) (Fig. 5A). Results demonstrated increasing TTR levels significantly decreased (p < .05) the rate of T4 hepatic uptake at 37 °C, which correlated with free T4 analysis determined within the initial running buffer solution as less free T4 was detected with increasing TTR concentration (Fig. 5B). T4 hepatic uptake rates at 4 °C also demonstrated a declining trend with increasing TTR levels, but no statistical significance was achieved likely due to the slower rate of passive diffusion. A temperature dependency on free T4 levels versus T4 -TTR bound was observed as less free T4 was detected in 4 °C samples compared to 37 °C. Calculations of T4-Kowvalues using the SPARC physical-chemical parameter model predicted a higher Kowvalue for T4 (log Kow 8.82) at 4 °C in comparison to 37 °C (log Kow 7.42), which corresponds with an increased T4 binding affinity for TTR at lower temperature values (Hilal and Karickhoff, 2004). In control samples containing no TTR, the decrease in free T4 at lower temperatures is likely attributed to increased T4 binding to BSA (1.25 mg/mL) and other constituents present within the running buffer.
FIG. 5.
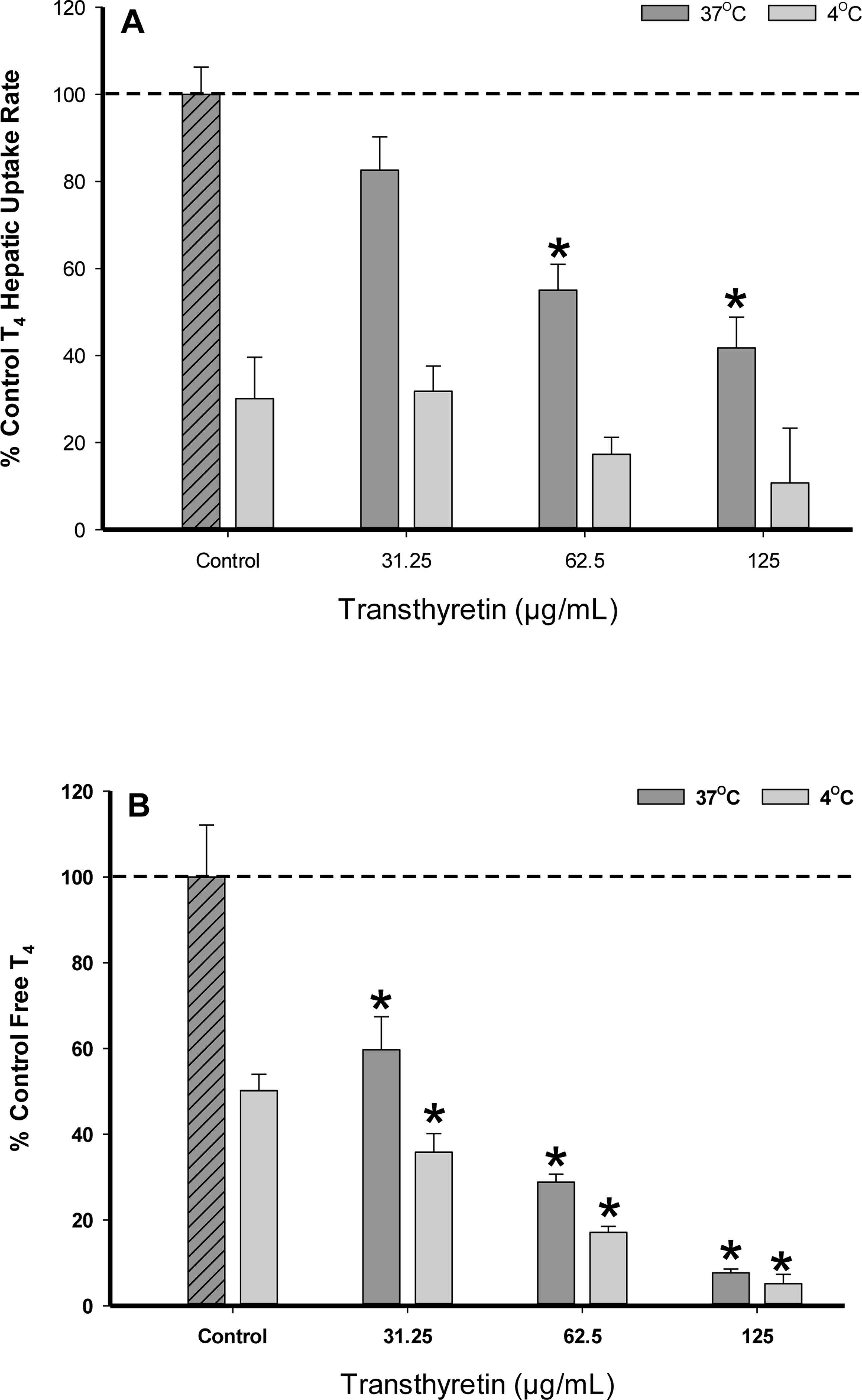
(A) Percent difference from 37°C control of T4 (1.0 μM) uptake in cryopreserved rat hepatocyte suspensions treated with varying concentrations of the serum protein transthyretin. Data represent the linear rate of T4 hepatic uptake (pmoles/106 cells/sec) determined from individual timelines conducted in triplicate within a single experiment. Error bars represent ± SD, asterisks denote statistically significant differences versus the temperature control regression slopes (*p<0.05). (B) Percent difference from 37°C control of free T4 (1.0 μM) levels determined by 10kDa filtration of running buffer solutions with varying transthyretin concentration levels (no hepatocytes). Error bars represent ± SD, asterisks denote statistically significant differences versus the temperature control (*p<0.05).
To assess PFOA influence on T4 (1.0 μM) uptake rates in the presence of TTR, PFOA concentrations were varied (0.1–10 μM) while maintaining a constant TTR (62.5 μg/mL) level (Fig. 6A). At both 37 °C and 4 °C, an upward trend in T4 uptake rates was observed in a dose-response manner with increasing PFOA concentrations. At 37 °C, a significant increase in T4 uptake rates were measured within the 8 μM (p < .05) and 10 μM (p < .01) PFOA assays, while displaying a 3 to 4 fold increase above the 4 °C T4 uptake assays. Free T4 concentrations trended upward at both 37 °C and 4 °C in the presence of increasing PFOA concentration with less free T4 being detected in 4 °C samples versus 37 °C (Fig. 6B). The evaluation of free T4levels at 4 °C containing PFOA (8–10 μM) show the free T4 levels at 4 °C exceed those within the 37 °C control; however, the T4 uptake rates at 4 °C remained well below (~50%) the 37 °C control. These results indicate an increased first-order rate associated with T4 carrier-mediated transport remained the predominant process at 37 °C.
FIG. 6.
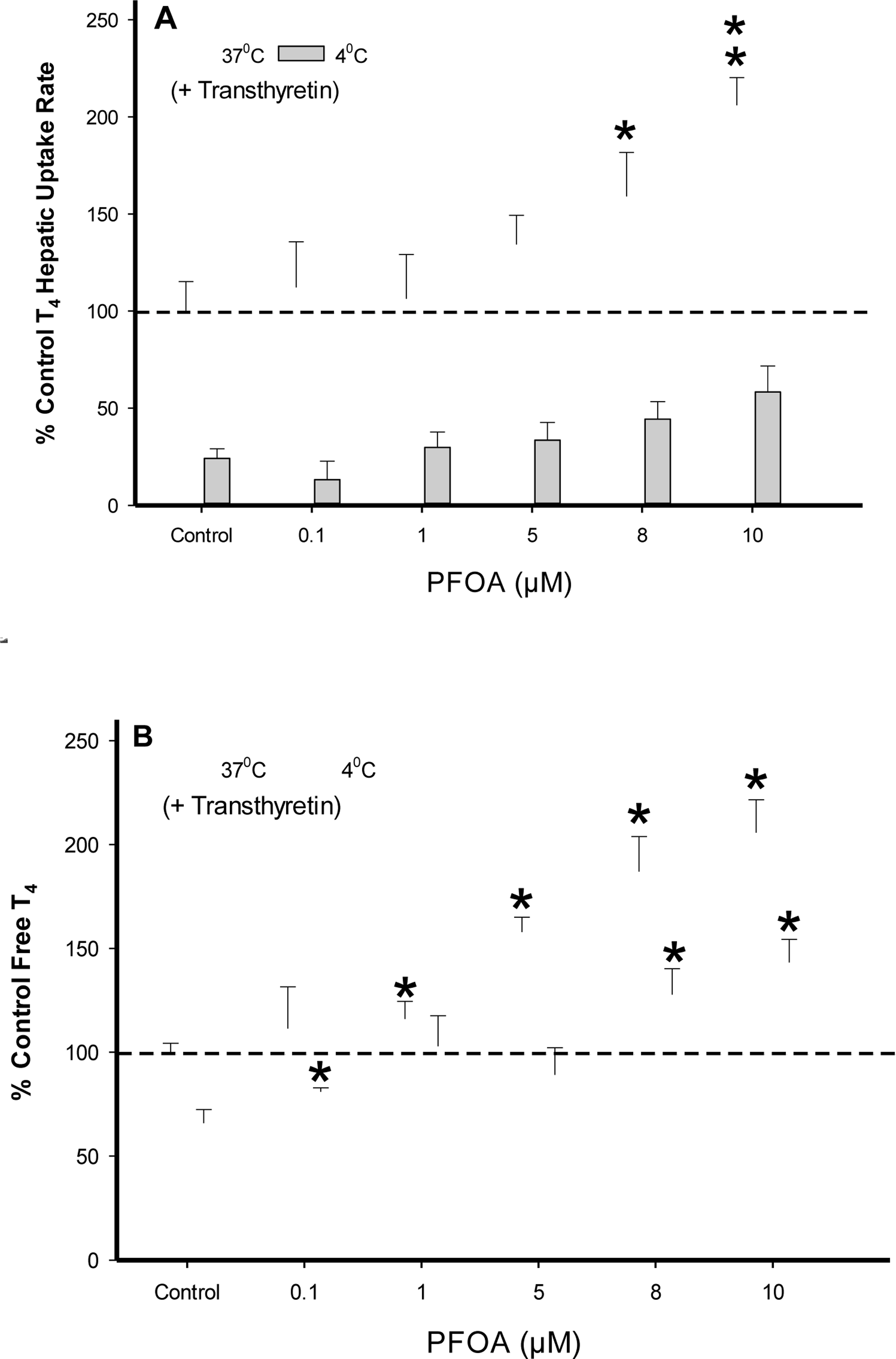
(A) Percent difference from 37°C control of T4 (1.0 μM) uptake in cryopreserved rat hepatocyte suspension treated with varying PFOA concentrations containing the serum protein transthyretin (62.5 μg/mL). Data represent the linear rate of T4 hepatic uptake (pmoles/106 cells/sec) determined from individual timelines conducted in triplicate within a single experiment. Error bars represent ± SD, asterisks denote statistically significant differences versus temperature control regression slopes (*p<0.05, **p<0.01). (B) Percent difference from 37°C control of free T4 (1.0 μM) levels in the presence of (62.5 μg/mL) transthyretin determined by 10kDa filtration of running buffer solutions (no hepatocytes). Error bars represent ± SD, asterisks denote statistically significant differences versus the temperature control (*p<0.05).
To further assess T4 membrane transport and chemical displacement of T4 in the presence of TTR, T4 (1.0 μM) hepatic assays were screened at 37 °C in the presence of (10 μM) chemical inhibitor CsA, PFOA, PFOS and the natural free fatty acid octanoic acid (Fig. 7). Perfluorinated chemical results indicate increasing T4 uptake rates correlated to free T4 levels with PFOA > PFOS, while both compounds demonstrated significantly higher T4 uptake rates (p < .01) versus control. Octanoic acid also displayed a significant increase (p < .05) in T4 hepatic uptake, however, unlike PFOA/PFOS no discernable effect on free T4 level was observed suggesting an alternative mode of action. Previous studies indicate octanoic acid can serve as a membrane-disrupting agent that may affect cell membrane fluidity and surface potential allowing for increased chemical penetration (Tan et al., 2017; Fu et al., 2015). The chemical inhibitor CsA showed no displacement of free T4 from TTR in comparison to control and significantly decreased (p < .05) T4 uptake activity, further confirming T4 carrier-mediated transport.
FIG. 7.
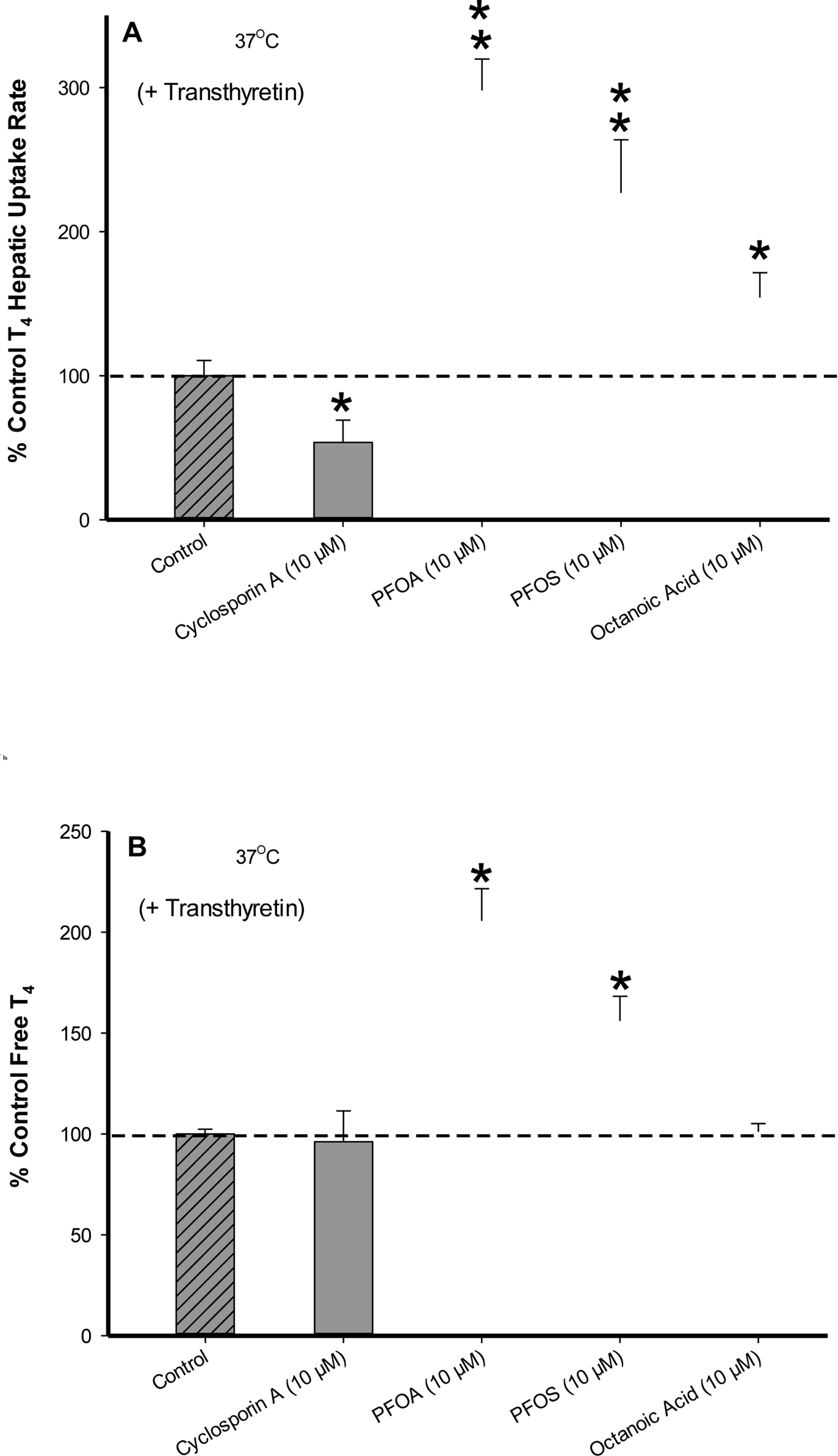
(A) Percent difference from 37°C control of T4 (1.0 μM) uptake in cryopreserved rat hepatocyte suspensions treated with CsA, PFOA, PFOS and Octanoic Acid containing the serum protein transthyretin (62.5 μg/mL). Data represent the linear rate of T4 hepatic uptake (pmoles/106 cells/sec) determined from individual timelines conducted in triplicate within a single experiment. Error bars represent ± SD, asterisks denote statistically significant differences versus temperature control regression slopes (*p<0.05, **p<0.01). (B) Percent difference from 37°C control of free T4 (1.0 μM) levels in the presence of (62.5 μg/mL) transthyretin determined by 10kDa filtration of running buffer solutions (no hepatocytes). Error bars represent ± SD, asterisks denote statistically significant differences versus the temperature control (*p<0.05).
DISCUSSION
Adequate levels of TH are necessary for proper cellular function. The alteration of T4 translocation across the cellular membrane may serve as a contributing factor in TH disruption. Due to its high lipophilicity, T4 was historically believed to enter target cells solely by passive diffusion, a process that is not susceptible to chemical perturbation (Hennemann et al., 2001). However, it is now understood from a multitude of in vitro studies that T4 cellular uptake is influenced by carrier-mediated (enzymatic) transport, which requires further evaluation for potential environmental chemical interaction (Visser et al., 2011; Jayarama-Naidu et al., 2015; Friesema et al., 2003).
In this study, we applied an oil-filtration technique using LC-MS/MS to characterize the mechanism(s) responsible for T4 cellular uptake using cryopreserved rathepatocytes in suspension. At physiological temperature (37 °C), a saturable kinetic profile for T4 cellular uptake was observed with increasing substrate concentration. These results indicate the presence of a T4 carrier-mediated uptake component as saturation occurs when the number of T4 molecules exceeds the number of enzymebinding sites present (Sugano et al., 2010). At 37 °C, both carrier-mediated transport and passive diffusion occur simultaneously, whereas under low temperatures enzymatic activity generally ceases. The coexistence of a T4 passive component was observed within our 4 °C assays as T4 uptake rates remained linear with respect to substrate concentration. Further validation of T4 passive transport was noted as the slope of the 4 °C T4 uptake rates were consistent with 37 °C rates observed at experimentally high T4 concentrations (>10 μM), where once saturation is achieved passive diffusion serves as the prevalent process (Li et al., 2013). The evaluation of the difference in T4 uptake rates at (37–4 °C) solely defines the carrier-mediated component, which fitted via nonlinear regression analysis displayed a KM value of 2.3 μM. The comparison of total T4 uptake rates (37 °C) versus carrier-mediated (37–4 °C) at substrate levels within the first-order region (<KM), demonstrated T4 carrier-mediated uptake was the predominant kinetic determinant accounting for >70% of total T4 hepatic uptake. Based on these in vitro results, the mechanistic criteria for physiologically-based modeling of TH hepatic uptake (clearance) at low physiological T4 serum levels suggests parameterization of both carrier-mediated and passive diffusion processes.
In previous studies, the application of diverse experimental protocols using primary hepatocytes has led to varying results in the quantitative assessment of T4 uptake transport (Hennemann et al., 2001). Hepatocyte uptake assays are often performed using a single incubation time point while assuming linearity. However, the linear rate of uptake can be compromised by multiple factors such as cell membrane partitioning, bidirectional passive diffusion and decrease in extracellular substrate concentration (Poirier et al., 2008). Our use of non-radiolabeled T4 with LC-MS/MS detection allows for highly quantitative analysis while simultaneously monitoring for potential TH metabolic products. The in vitro technique we applied of rapid subsampling of hepatocytes from suspension optimized at an appropriate cell concentration, allowed for multiple measured time points (full timeline) to determine T4 kinetic uptake rates based on a linear slope of regression. A positive y-intercept was observed and is consistent with a rapid membrane partitioning phaseof the highly hydrophobic T4 during the lag period in the cell separation procedure (15 s) (Hallifax and Houston, 2006). It is important to note, the incorporation of the partitioning phase using a single time point assessment may lead to variable results in characterizing T4 uptake processes (Chalmers et al., 1993; Rao and Rao, 1983; Riley and Eales, 1993).
Primary hepatocytes contain a vast array of endogenous transport proteins that can potentially mediate T4 hepatic uptake. Further characterization of the T4 carrier-mediated transport component was conducted within our hepatic assay system using the chemical inhibitor CsA. Clinical studies have shown CsA serves as a broad-based chemical inhibitor for the OATP transporter family (Takahashi et al., 2013; Karlgren et al., 2012). Previous in vitro studies indicate that approximately fifteen different OATP’s among rodent and human have been identified to facilitate thyroid hormone uptake transport; however, limited time-course data exist delineating the role of T4 carrier-mediated and passive diffusion processes within native hepatocytes (Visser et al., 2011). Our CsA results further confirmed T4 carrier-mediated transport as total T4 uptake rates (37 °C) that displayed a saturable component were chemically inhibited by CsA at low inhibitor concentration levels (0.1 μM), while showing no discernable effect on passive diffusion. These results suggest further investigation with the specific transporter rat Oatp1b2 (Human orthologs OATP1B3/1B1), which is one of the most predominantly expressed rodentOatp transporters in the liver known to mediate T4 uptake (Hagenbuch and Meier, 2004). However, it should be noted that delineation of the role of specific transport proteins in primary hepatocytes is difficult due to the lack of specific chemical inhibitors and that multiple transporters may be functioning simultaneously. Furthermore, cell line studies conducted with overexpression of individual transporters indicate that the majority of hepatic T4 uptake transporters are non-specific to TH, as they functionally accept a wide variety of other endogenous ligands essential for cellular function (Visser et al., 2011). A notable exception is the presence of monocarboxylate transporter (MCT8) in the liver, which has been identified to only selectively transport T4 and other iodothyronine related compounds (Friesema et al., 2003). To the best of our knowledge, CsA chemical inhibition of MCT8 mediated T4 uptake has not yet been investigated.
The environmental chemicals PFOA and PFOS are potential thyroid disrupting chemicals that exist as anionic surfactants at physiological pH 7.4. Research emphasis has been placed on these PFAS chemicals as they are known to reduce circulating serum levels of TH’s in vivo by increasing their metabolic clearance rate (Lee and Choi, 2017; Lau et al., 2007). In contrast to our CsA chemical inhibition results, hepatic assays conducted in the presence of increasing PFOA exposure did not alter T4 hepatic uptake. To the best our knowledge, we believe this is the first report demonstrating PFOA does not alter T4 carrier-mediated transport processeswithin primary hepatocytes. Furthermore, a distinguishable factor under physiological conditions is that TH’s are extensively bound (>99%) to blood serum proteins, which serves as a reservoir to buffer changes in the small free T4 fraction available for hepatic uptake (Palha, 2002). Therefore, a potential rate-altering step regarding T4 hepatic uptake in vivo is the dissociation of free T4 from the protein-binding complex (Mendel, 1989).
It has been previously reported that the unique physical-chemical properties of PFOA and PFOS affect the dissociation rate constant that exists between the T4bound versus free T4 fraction with the serum protein TTR (Weiss et al., 2009). To explore this potential interaction, we first amended our hepatocyte suspensions with the serum protein TTR. Accordingly, our results showed the increased addition of TTR serum protein resulted in decreased T4 hepatic uptake rates due to detection of less available free T4. A temperature dependency of lower free T4 levels with decreasing temperature (37 °C vs 4 °C) was observed and is likely attributed to an increased T4 protein binding affinity due to increased T4-Kow values at lower temperatures (Hilal and Karickhoff, 2004). To further characterize potential PFAS interaction on T4 hepatic uptake in the presence of the serum protein TTR, a PFOA dose-response relationship was evaluated while maintaining constant TTR levels throughout experimentation. Under these conditions, our results demonstrated a significant increase in total T4 hepatic uptake rates occurred with increasing PFOA exposure levels, which correlated to an increased free T4 fraction. The rate of T4hepatic uptake remained first-order with respect to carrier-mediated transport as depicted by an enhanced response to free T4 levels under physiological temperature (37 °C) versus the passive diffusion component (4 °C). Subsequently, our screening of PFOS at high concentration levels (10 μM) with TTR displayed similar results as PFOA resulting in a significant increase in T4 uptake rates due to increased free T4values.
The identification of T4 carrier-mediated transport as the predominant kinetic determinant in the presence of TTR was reaffirmed as CsA chemically inhibited T4hepatic uptake while not affecting free T4 levels. It was previously reported in a (non-cellular) TTR-binding assay that the naturally occurring octanoic fatty acidserves as a control for assessing T4 displacement from TTR by displaying no increase in free T4 levels (Weiss et al., 2009). While our analysis confirmed no free T4displacement from TTR in the presence of octanoic acid, hepatic suspensions amended with octanoic acid displayed a significant increase in T4 hepatic uptake rate, suggesting an alternate mode of T4 translocation. Thus, previous cellular studies have shown octanoic acid can serve as a known membrane-disrupting agent that may affect cell membrane fluidity and surface potential allowing for increased chemical penetration (Tan et al., 2017; Fu et al., 2015). Our results suggest alteration of membrane fluidity was not a significant factor regarding PFOA amendments as no significant increase in T4 hepatic uptake was observed in experiments without TTR present (Fig. 4B).
Our in vitro assessment of increased T4 hepatic uptake during PFOA and PFOS exposure with TTR present provides new mechanistic insight required for in vivo physiological assessment of TH serum levels. We demonstrated that T4 hepatic uptake predominantly followed first-order carrier-mediated transport within hepatocyte suspensions and the carrier-mediated process was not altered (inhibited) by PFOA and PFOS following chemical exposure. Therefore, the increase of free T4levels from PFOA and PFOS displacement of T4 bound to TTR resulted in increased hepatic uptake rates, leading to a predicted decrease in physiological T4 serum levels. Results from our in vitro technique supports in vivo rodent toxicological studies reporting a reduction in TH serum levels related to PFOA and PFOS exposure (Martin et al., 2007; Chang et al., 2008). The decrease in total rodent T4serum levels (hypothyroxinemia) observed in vivo were attributed to increased tissue availability and hepatic metabolism due to the transient increase in free T4 levels (Lau et al., 2003). Hence, understanding the mechanistic basis for T4 hepatic uptake is critical for accurate pharmacokinetic assessment of T4 blood serum levels during PFAS chemical exposure. If physiological modeling applications of T4 hepatic uptake in the presence of PFOA/PFOS exposure are based solely on T4 passive diffusion coefficients, the predicted impact on T4 serum levels may be underestimated due to the omission of enhanced T4 carrier-mediated transport. Furthermore, our results indicate TH chemical risk assessment requires evaluating the susceptibility of T4 carrier-mediated transport to potential environmental chemical interaction.
In conclusion, an area of critical concern regarding PFAS exposure is the role maternal TH levels play during pregnancy, as a decrease in serum T4 concentrations (hypothyroidism) may lead to impaired fetal neurodevelopment (Hassan et al., 2017; Gilbert et al., 2012). Recently, perfluorohexane sulfonate was shown to decrease T4levels in rodent dams leading to developmental effects in their offspring (Ramhoj et al., 2018). Thus, further in vitro studies are needed to assess how varying PFAS chain length and functional group composition influence T4 hepatic uptake. The evaluation of TH transport processes within human hepatocytes along with enhanced analytical detection limits nearing physiological conditions would greatly improve interspecies extrapolation and health risk assessment during PFAS chemical exposure.
Footnotes
Disclaimer: The views expressed in this manuscript are those of the author’s and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
REFERENCES
- Amaraneni M, Sharma A, Pang J, Muraldhara S. Cummins BS, White CA, Bruckner JV, and Zastre J. (2016). Plasma protein binding limits the blood brain barrier permeation of the pyrethroid insecticide, deltamethrin. Toxicol Lett 250–251, 21–28. [DOI] [PubMed] [Google Scholar]
- Benvenga S. and Robbins J. (1998) Thyroid hormone efflux from monolayer cultures of human fibroblasts and hepatocytes. Effect of Lipoproteins and Other Thyroxine Transport Proteins. Endocrinology 139, 4311–4318. [DOI] [PubMed] [Google Scholar]
- Bregengard C, Kirkegaard C, Faber J, Poulsen S, Siersbaek-Nielsen K, and Friis T. (1987). The influence of free fatty acids on the free fraction of thyroid hormones in serum as estimated by ultrafiltration. Acta. Endocrinol. (Copenh.) 116(1), 102–107 [DOI] [PubMed] [Google Scholar]
- Chalmers DK, Scholz GH, Topliss DJ, Kolliniatis E, Munro SL, Craik DJ, Iskander MN, and Stockigt JR (1993). Thyroid hormone uptake by hepatocytes: structure-activity relationships of phenylanthranilic acids with inhibitory activity. J. Med. Chem. 36(9), 1272–1277. [DOI] [PubMed] [Google Scholar]
- Chang SC, Thibodeaux JR, Estavold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB, and Butenhoff JL (2008). Thyroid Hormone Status and Pituitary Function in Adult Rats Given Oral Doses of Perfluorooctanesulfonate (PFOS). Toxicology 243(3), 330–9 [DOI] [PubMed] [Google Scholar]
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, Sullivan L, Canick J, Porter TF, Luthy D, Gross S, Bianci DW, and D’Alton ME (2008). Maternal thyroid hypofunction and pregnancy outcome. Obstet. Gynecol. 112(1), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors KA, Korte JJ, Anderson GW, and Degitz SJ (2010). Characterization of thyroid hormone transporter expression during tissue-specific metamorphic events in Xenopus tropicalis. Gen. Comp. Endocrinol. 168(1), 149–59. [DOI] [PubMed] [Google Scholar]
- Coperchini F, Awwad O, Rotondi M, Santini F, Imbriani M, and Chiovato L. (2017). Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J Endocrinol. Invest. 40(2),105–121. [DOI] [PubMed] [Google Scholar]
- Dong H. and Wade MG (2017) Application of a nonradioactive assay for high throughput screening for inhibition of thyroid hormone uptake via the transmembrane transporter MCT8. Toxicol. In Vitro. 40, 234–242. [DOI] [PubMed] [Google Scholar]
- Eckel J, Rao GS, Rao ML and Breuer H. (1979). Uptake of L-tri-iodothyronine by isolated rat liver cells. A process partially inhibited by metabolic inhibitors; attempts to distinguish between uptake and binding to intracellular proteins. Biochemical Journal. 182(2), 473–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema EC, Ganguly S, Abdalla A, Fox JEM, Halestrap AP and Visser TJ (2003) Identification of Monocaroxylate Transporter 8 as a Specific Thyroid Hormone Transporter. Journal of Biological Chemistry 278(41), 40128–40135 [DOI] [PubMed] [Google Scholar]
- Fu Y, Yoon JM, Jarboe L, and Shanks JV (2015) Metabolic flux analysis of Escherichia coli MG1655 under octanoic acid (C8) stress. Appl. Microbiol. Biotechnol. 99(10), 4397–4408. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Rovet J, Chen Z. and Koibuchi N. (2012) Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology. 33(4), 842–852. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B. and Meier PJ (2004) Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 447(5), 653–665. [DOI] [PubMed] [Google Scholar]
- Hallifax D. and Houston JB (2006) Uptake and intracellular binding of lipophilic amine drugs by isolated rat hepatocytes and implications for prediction of in vivo metabolic clearance. Drug Metab Dispos. 34(11), 1829–1836. [DOI] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL and Rickard RW (2012) Renal elimination of perfluorocarboxylates (PFCAs). Chem. Res. Toxicol. 25, 35–46. [DOI] [PubMed] [Google Scholar]
- Hassan I. et al. (2017). Neurodevelopment and thyroid hormone synthesis inhibition in the rat: quantitative understanding within the adverse outcome pathway framework. Toxicol. Sci. 160(1), 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennemann G, Docter R, Fiesema EC, de Jong M, Krenning EP and Visser TJ (2001) Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr. Rev. 22, 451–476. [DOI] [PubMed] [Google Scholar]
- Henrichs J, Ghassabian A, Peeters RP and Tiemeier H. (2013). Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin. Endocrinol. (Oxf.) 79(2), 152–162. [DOI] [PubMed] [Google Scholar]
- Hilal SH and Karickhoff SW (2004) Prediction of the solubility, activity coefficient and liquid/liquid partition coefficient of organic compounds. QSAR Comb. Sci. 23, 709–720. [Google Scholar]
- Iwabuchi K, Senzaki N, Mazawa D, Sato I, Hara M, Ueda F, Liu W. and Tsuda S. (2017). Tissue toxicokinetics of perfluoro compounds with single and chronic low doses in male rats. J Toxicol. Sci. 42(3), 301–317. [DOI] [PubMed] [Google Scholar]
- Jayarama-Naidu R, Johannes J, Meyer F, Wirth EK, Schomburg L, Kohrle J. and Renko K. (2015) A Nonradioactive Uptake Assay for Rapid Analysis of Thyroid Hormone Transporter Function. Endocrinology. 156(7), 2739–2745. [DOI] [PubMed] [Google Scholar]
- Karlgren M. Vildhede A, Norinder U, Wisniewski JR, Kimoto E, Lai Y, Haglund U. and Artursson P. (2012). Classification of inhibitors of hepatic organic anion transporting polypeptides (OATPs): influence of protein expression on drug-drug interactions. J. Med. Chem. 55, 4740–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A. and Seed J. (2007). Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci. 99(2), 366–394. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Roger JM, Grey BE, Stanton ME, Butenhoff JL and Stevenson LA (2003). Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol. Sci. 74(2), 382–392. [DOI] [PubMed] [Google Scholar]
- Lee J. and Choi K. (2017). Perfluoroalkyl substances exposure and thyroid hormones in human: epidemiological observations and implications. Ann. Pediatr. Endocrinol. Metab. 22(1), 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Nouraldeen A. and Wilson AGE (2013). Evaluation of transporter-mediated hepatic uptake in a non-radioactive high-throughput assay: a study of kinetics, species differences and plasma protein effect. Xenobiotica 43(3), 253–262. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. (2010). Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin. Endocrinol. (Oxf.) 72(6), 825–829. [DOI] [PubMed] [Google Scholar]
- Liu G, Zhang S, Yang K, Zhu L. and Lin D. (2016). Toxicity of perfluorooctane sulfonate and perfluorooctanoic acid to Escherichia coli: Membrane disruption, oxidative stress, and DNA damage induced cell inactivation and/or death. Environ. Pollut. 214, 806–815. [DOI] [PubMed] [Google Scholar]
- Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, Takeuchi M, Irth H. and Murk AJ (2008) Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol. 232(1), 150–160. [DOI] [PubMed] [Google Scholar]
- Martin MT, Brennan RJ, Hu W, Aanoglu E, Lau C, Ren H, Wood CR, Corton JC, Kavlock RJ and Dix DJ (2007). Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol. Sci. 97, 595–613. [DOI] [PubMed] [Google Scholar]
- Mats L, Pettersson T, and Cralstrom A. (1984) Thyroid hormone binding in serum of 15 vertebrate species: Isolation of thyroxine-binding globulin and Preablbumin analogs. Gen. and Comp. Endocrinol. 58, 360–375. [DOI] [PubMed] [Google Scholar]
- Mendel CM. (1989). The free hormone hypothesis: a physiologically based mathematical model. Endocr. Rev. 10(3), 232–274. [DOI] [PubMed] [Google Scholar]
- Min H, Dong J, Wang Y, Teng W, Xi Q. Chen, J. (2016). Maternal Hypothyroxinemia-Induced Neurodevelopmental Impairments in the Progeny. Mol. Neurobiol. 53(3), 1613–1624. [DOI] [PubMed] [Google Scholar]
- Noctor TA, Wainer IW and Hage DS (1992) Allosteric and competitive displacement of drugs from human serum albumin by octanoic acid, as revealed by high-performance liquid affinity chromatography, on a human serum albumin-based stationary phase. J. Chromatogr. 577, 305–315. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Eresman DJ, Froehlich JW, Seacat AM, Butenhoff JL and Zobel LR (2007). Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9), 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW and Zobel LR (2007). Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int. Arch. Occup. Environ. Health 81(2), 231–246. [DOI] [PubMed] [Google Scholar]
- Palha JA (2002) Transthyretin as a thyroid hormone carrier: function revisited. Clin. Chem. Lab. Med. 40(12), 1292–1300. [DOI] [PubMed] [Google Scholar]
- Perez F, Nadal M, Navarro-Ortega A, Fabrega F, Domingo JL, Barcelo D. and Farre M. (2013). Accumulation of perfluoroalkyl substances in human tissues. Environment International 59, 354–362. [DOI] [PubMed] [Google Scholar]
- Poirier A, Lave T, Portmann R, Brun ME, Senner F, Kansy M, Grimm HP and Funk C. (2008) Design, data analysis, and simulation of in vitro drug transport kinetic experiments using a mechanistic in vitro model. Drug Metab. Dispos. 36(12), 2434–2444. [DOI] [PubMed] [Google Scholar]
- Ramhoj L, Hass U, Boberg J, Scholze M, Chritiansen S, Nielsen F. and Axelstad M. (2018) Perfluorohexane Sulfonate (PFHxS) and a Mixture of Endocrine Disrupters Reduce Thyroxine Levels and Cause Antiandrogenic Effects in Rats. Toxicol. Sci. 163(2), 579–591. [DOI] [PubMed] [Google Scholar]
- Rao GS and Rao M. (1983). L-thyroxine enters the rat liver cell by simple diffusion. J. Endocrinol. 97, 277–282. [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Wijayagunaratne RC, D’souza DG, Darras VM and Van Herck SL (2015). Transport of thyroid hormones via the choroid plexus into the brain: the roles of transthyretin and thyroid hormone transmembrane transporters. Front. Neurosci. 9, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson VM, Ferguson SS, Sey YM an Devito MJ (2013). In vitro metabolism of thyroxine by rat and human hepatocytes. Xenobiotica 44(5), 391–403. [DOI] [PubMed] [Google Scholar]
- Riley WW and Eales JG (1993). Characterization of L-thyroxine transport into hepatocytes isolated from juvenile rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 90(1), 31–42. [DOI] [PubMed] [Google Scholar]
- Robbins J. (2000) Editorial: new ideas in thyroxine-binding globulin biology. J Clin Endocrinol. Metab. 85(11), 3994–3995. [DOI] [PubMed] [Google Scholar]
- Roman GC, Gassabian A, Bongers-Schokking JJ, Jaddoe VW, Hofman A, de Rijke YB, Verhulst FC and Tiemeier H. (2013). Association of gestational maternal hypothyroxinemia and increased autism risk. Ann. Neurol. 74(5), 733–742. [DOI] [PubMed] [Google Scholar]
- Soars MG, Grime K, Sproston JL, Webborn PJ and Riley RJ (2007). Use of hepatocytes to assess the contribution of hepatic uptake to clearance in vivo. Drug Metab. Dispos. 35(6), 859–65. [DOI] [PubMed] [Google Scholar]
- Sugano K, et al. (2010). Coexistence of passive and carrier-mediated processes in drug transport. Nat. Rev. Drug Discov. 9(8), 597–614. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Ohtsuka T, Yoshikawa T, Tatekawa I, Uno Y, Utoh M, Yamazaki H. and Kume T. (2013). Pitavastatin as an in vivo probe for studying hepatic organic anion transporting polypeptide-mediated drug-drug interactions in cynomolgus monkeys. Drug Metab. Dispos. 41(10), 1875–1882. [DOI] [PubMed] [Google Scholar]
- Tan Z, Khakbaz P, Chen Y, Lombardo J, Yoon JM, Shanks JV, Klauda JB and Jarboe LR (2017). Engineering Escherichia coli membrane phospholipid head distribution improves tolerance and production of biorenewables. Metab. Eng. 44, 1–12. [DOI] [PubMed] [Google Scholar]
- Treiber A, Schneiter R, Hausler S. and Stieger B. (2007). Bosentan is a substrate of human OATP1B1 and OATP1B3: inhibition of hepatic uptake as the common mechanism of its interactions with cyclosporin A, rifampicin, and sildenafil. Drug Metab. Dispos. 35(8), 1400–1407. [DOI] [PubMed] [Google Scholar]
- Visser WE, Friesema EC and Visser TJ (2011). Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol. Endocrinol. 25, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. and Stapleton HM (2010). Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 397(5), 1831–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington JW, Jenkins TM, Rankin K, and Naile JE (2015) Decades-scale degradation of commercial, side-chain, fluorotelomer-based polymers in soils and water. Environ. Sci. Technol. 49(2), 915–923. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP and Hamers T. (2009) Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol. Sci. 109, 206–216. [DOI] [PubMed] [Google Scholar]
- Winquist A. and Steenland K. (2014). Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts. Epidemiology 25(2), 255–264. [DOI] [PubMed] [Google Scholar]
- Woody CJ, Weber SL, Lauback HE, Ingram-Wiley V, Amini-Alashti P. and Sturbaum BA (1998). The effects of chronic exercise on metabolic and reproductive functions in male rats. Life Sci 62(4), 327–332. [DOI] [PubMed] [Google Scholar]
- Yabe Y, Galetin A. and Houston JB (2011). Kinetic characterization of rat hepatic uptake of 16 actively transported drugs. Drug Metab. Dispos. 39, 1808–1814. [DOI] [PubMed] [Google Scholar]
- Yang X, Lyakurwa F, Xie H, Chen J, Li X, Qiao X. and Cai X. (2017). Different binding mechanisms of neutral and anionic poly-/perfluorinated chemicals to human transthyretin revealed by in silico models. Chemosphere 182, 574–583. [DOI] [PubMed] [Google Scholar]


