Abstract
Multicellular organisms have evolved sophisticated mechanisms to recover and maintain original tissue functions following injury. Injury responses require a robust transcriptomic response associated with cellular reprogramming involving complex gene expression programs critical for effective tissue repair following injury. Steroid receptor coactivators (SRCs) are master transcriptional regulators of cell–cell signaling that is integral for embryogenesis, reproduction, normal physiological function, and tissue repair following injury. Effective therapeutic approaches for facilitating improved tissue regeneration and repair will likely involve temporal and combinatorial manipulation of cell-intrinsic and cell-extrinsic factors. Pleiotropic actions of SRCs that are critical for wound healing range from immune regulation and angiogenesis to maintenance of metabolic regulation in diverse organ systems. Recent evidence derived from studies of model organisms during different developmental stages indicates the importance of the interplay of immune cells and stromal cells to wound healing. With SRCs being the master regulators of cell–cell signaling integral to physiologic changes necessary for wound repair, it is becoming clear that therapeutic targeting of SRCs provides a unique opportunity for drug development in wound healing. This review will provide an overview of wound healing–related functions of SRCs with a special focus on cellular and molecular interactions important for limiting tissue damage after injury. Finally, we review recent findings showing stimulation of SRCs following cardiac injury with the SRC small molecule stimulator MCB-613 can promote cardiac protection and inhibit pathologic remodeling after myocardial infarction.
Keywords: p160 steroid receptor co-activator, wound healing, myocardial infarction
Transient changes in the dynamic interplay between cells triggered by an injury are highly coordinated events required for tissue repair and recovery. We are at an exciting era regarding the identification of the cellular and molecular processes necessary for tissue regeneration and repair. In contrast to currently used systemic therapeutics, pharmacologic interventions designed to target endogenous reparative and regenerative cellular responses are expected to allow for more specific molecular targeting to promote more effective repair. As master regulators of cell type functions necessary for wound repair, understanding the selective functions of steroid receptor coactivators (SRCs) in these cell types will be critical for resolving their functional role at a cellular level.
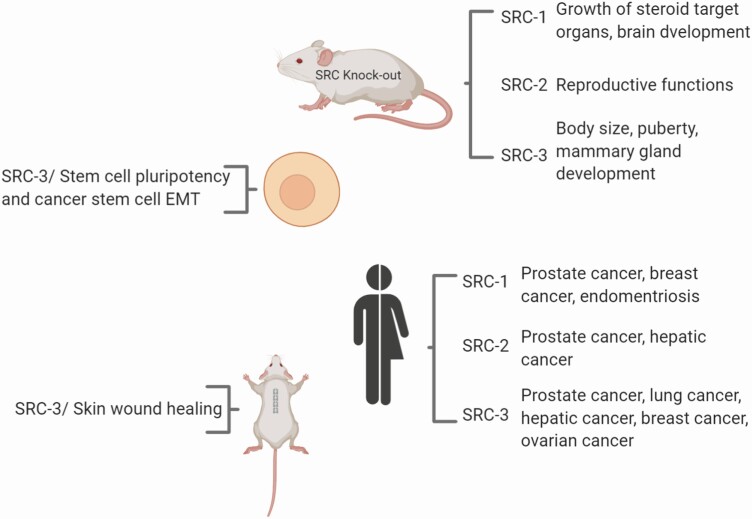
Members of the p160 SRC family, SRC-1 (NCOA1), SRC-2 (NCOA2/TIF2/GRIP1), and SRC-3 (NCOA3/AIB1/ACTR), interact with nuclear receptors (NRs) and other transcription factors to drive target gene expression (1) by assembling transcriptional coactivator complexes to increase the transcription of growth-related genes. Since the discovery of SRC-1 25 years ago (2), the pleiotropic functions of SRCs in the physiology of multiple organs critical for development and disease responses (3) continue to be uncovered. SRC family members have both overlapping and unique molecular properties and physiological roles. All 3 SRCs are frequently overexpressed or amplified in different cancers and act as primary transcriptional regulators of transcription factor activity necessary for cell proliferation, survival, cell motility, invasion, metastasis, and cell metabolism (3, 4) (Fig. 1). Because SRCs act as integrators of oncogenic steroid and tumor growth factor signaling pathways, efforts are underway to develop therapeutic targeting agents to block tumor growth and cell resistance to chemotherapy by inhibiting these proteins (5). We have recently shown that inhibiting SRC-3 with a small molecule SRC inhibitor blocks tumor growth of pancreatic ductal adenocarcinoma (6) and the tumor-initiating capacity of cancer stem-like cells (7). In addition to SRC inhibitors designed to inhibit tumor growth, we have identified small molecule activators of SRCs (MCB-613 and its derivatives) that selectively and reversibly bind to SRCs that can greatly enhance SRC transcriptional activity with no apparent toxicity in mice (8). Similar to oncogenic signaling, in conjunction with SRCs, transcription factors orchestrate the dynamic and complex gene expression programs critical for wound healing that have been implicated in tumor cell migration, proliferation, and tumor survival–promoting paracrine interactions. Accordingly, in addition to facilitating tumorigenesis, SRCs are widely expressed in different cell types and function as master regulators of cell growth and proliferation, both during normal and abnormal growth and during development (7, 9-12) (Fig. 1). A better understanding of the complex functions that SRCs perform in homeostatic and pathological states will be critical for understanding tissue damage injury responses and this should reveal new ways to promote repair following injury. Examples include roles for SRCs in key molecular pathways critical for wound healing including nuclear factor kappa B (NF-κB; NFKB1) (13), sterol regulatory element-binding protein 1 (SREBP1) (14), and Activator protein 1 (AP-1) (JUN) (15, 16), pointing to the potential for SRC targeting drugs in promoting paracrine interactions responsible for effective tissue repair after injury. Furthermore, results from animal model studies indicate that SRC-1, -2, and -3 function to promote angiogenesis via crosstalk with the vascular endothelial growth factor (VEGF) signaling pathway (17-20). SRC-3 has also been shown to function in stromal injury responses, including angiogenesis in vitro and wound healing in the skin (21) (Fig. 1).
Figure 1.
Steroid receptor coactivators (SRC family members) are widely expressed in different cell types and function as master regulators of normal and abnormal and cell growth and proliferation. SRCs have been shown to be overexpressed in many subsets of human cancers. More comprehensive lists of SRC expression in human cancers have been previously reviewed (3, 4). Created with BioRender.com.
SRCs Promote Pluripotency Maintenance and Stem Cell Survival
Maintenance of homeostasis and the restoration of original tissue function following injury requires the coordination of cell–cell signaling. The molecular events underlying tissue repair also are integral to physiologic changes necessary for embryonic development. In developmental angiogenesis in mouse and zebrafish embryos, endothelial tip cell sprouting requires VEGF where macrophages may be the primary source of VEGF in promoting vessel anastomosis (22). The NF-κB transcription factor plays many essential roles during embryonic development (23). Drosophila embryos deficient in NF-κB signaling display defects in muscle patterning (24), potentially as a result of control of muscle cell differentiation by myocardin and cyclin D1 in myoblasts (25). Targeting of specific repair events, when tied to the recapitulation of SRC embryonic functions after disease-related tissue damage would be expected to provide therapeutic opportunities for promoting optimal tissue reprogramming and repair. During development, stem cells give rise to all differentiated cell types present in adult animals. After maturation, stem cells continue to reside as pluripotent stem cells that retain the ability to differentiate and proliferate under certain physiologic conditions. Stem cells contribute to tissue homeostasis by replenishing cells lost to normal physiological processes and during wound repair. In response to injury, stem cells exhibit plasticity in a wide range of tissues by switching from one stem cell type to another or through terminal differentiation into cells that replace those lost to injury (26, 27). Although stem cells do not likely contribute to the limited regenerative capacity of some organs such as the heart, examples of stem cell plasticity upon wounding have been observed in skin, hair follicle, bone marrow, small intestine, and airway systems (28, 29).
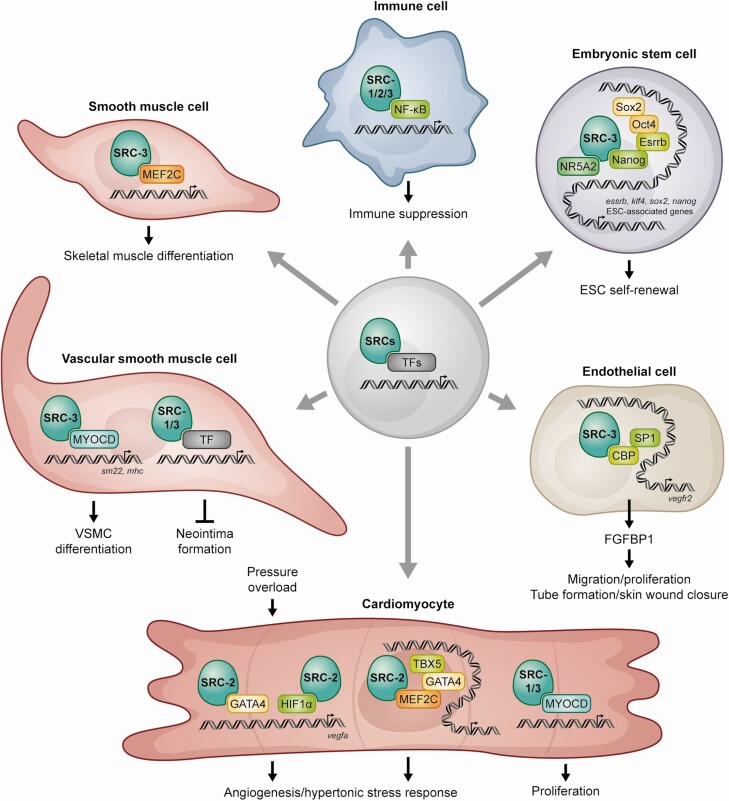
This is contrasted with reversion of differentiated cells into stem cells upon wounding through a process termed “stem cell reprogramming.” External factors involved in the maintenance of stem cells and their response to injury are diverse and tissue context dependent. In response to these factors, cell fate changes are associated with epigenetic modifications that govern access of fate-specific transcription factors. It is well established that the transcription factors Oct4, Sox2, and Nanog act as key regulators of pluripotency. In addition, NRs, including Esrrb, Dax1, and Nr5a2, have been shown to regulate the expression of pluripotency factors and are critical for pluripotency maintenance. Recent evidence indicates that, in contrast to SRC-1 and SRC-2, SRC-3 enhances stem cell reprogramming efficiency and pluripotency by cooperating with Esrrb (10) and Nanog (30) (Fig. 2). Specifically, SRC-3 supports stem cell self-renewal by binding to the Nanog promoter and recruiting the histone acetyltransferase CREB binding protein and the histone arginine methyltransferase CARM1 to activate Nanog expression (30). Also, SRC-3 supports pluripotency maintenance by binding to the Essrb2 AF-2 domain (10). In cancer cells, SRC-3 supports cancer stem cell formation and tumor growth (7). These findings establish SRC-3 as a key regulator of stem cell pluripotency maintenance. There is increasing evidence that tissue homeostasis and wound healing involve acquisition of pluripotent gene networks in tissue-dependent populations of adult stem cells (31). For example, Sox2 expressing cells contribute to regeneration of adult pituitary gland in response to injury (32-34). Taken together, SRC-3 regulation of gene programs mediating stem/progenitor cell pathways involved in both stem cell and cancer cell models highlights the underlying theme whereby tumors are able to highjack normal wound-healing responses involved at sites of tissue damage.
Figure 2.
SRCs coactivate numerous DNA-bound transcription factors in cell types critical for tissue damage responses after injury.
SRC-3 Inhibits Vascular Neointima Formation in Injured Vessels
Vascular smooth muscle cells (VSMCs) and endothelial cells express estrogen receptor (ER) and SRC-3 and are well known targets of estrogen action (35-37). Smooth muscle cells are found in the walls of many organs including the uterus, bladder, stomach, small and large intestines, and blood vessels. In blood vessels, smooth muscle cells are referred to as VSMCs where they act to control blood pressure and tissue oxygenation. In response to vessel damage, fibroblasts and VSMCs respond by increasing their proliferation, migration, and production of chemotactic factors and extracellular matrix proteins to heal the wound. Neointima is the formation of scar tissue within vessels in response to vascular damage. Abnormal proliferation of VSMCs contributes to atherosclerosis and neointimal hyperplasia resulting in thickening of arterial walls, which may lead to increased risk of vascular diseases. Studies using animal models clearly demonstrate the vasoprotective effects of estrogen (38). Fibroblasts and VSMCs release chemotactic factors and contribute to the cellular response to vascular injury by proliferating and migrating into the neointima. Animal models indicate that estrogen inhibits the neointimal response in acutely injured vessels (39). Similarly, in an in vitro model of cell migration, estrogen alone was shown to directly control VSMC expression of paracrine factors to inhibit the activation and migration of adventitial fibroblasts (40). The cellular mechanism by which estrogen inhibits neointima formation after vascular injury was shown to be controlled in part by rapid activation of c-myc-mediated transcription (41). SRC-3 mRNA and protein are expressed in human cardiovascular cells and tissues including aortic and coronary VSMCs (42). In the vascular smooth muscle PAC1 cell line, SRC-3 binding to the C-terminal activation domain of the major VSMC transcription factor myocardin, an essential gene for vascular development and response to injury (42) (Fig. 2), provides evidence for an additional mechanism for the vascular protective effects of SRC-3 actions via a non-NR signaling pathway. Using mouse models of vascular injury it was shown that SRC-1 and SRC-3 are also highly expressed in VSMCs and endothelial cells and are required for efficient inhibition of neointima formation by estrogen (37, 43). Unlike estrogen-mediated inhibition of adventitial fibroblasts, both fibroblast and VSMC proliferation were effected by estradiol (E2) treatment in SRC3–/– mice after injury (37). That SRC-3 was required for only 25% of neointima inhibition following injury indicates that other SRCs and/or additional signaling pathways contribute to the inhibition of neointima formation. Although the role of SRCs in cells involved in neointima formation is not well understood, the compounds targeting SRCs may reveal the roles of SRCs in neointima formation, pointing a path forward for the development of new therapeutic interventions for vascular diseases.
There is a strong link between menopause and cardiovascular disease in women (44), and in the right modality hormone-replacement therapy has been shown to reduce cardiovascular risk by about half (45). However, in the Heart Estrogen-Progestin Replacement Study, there was no therapeutic benefit of estrogen plus progestin treatment in women with established coronary disease. This estrogen resistance may be explained by results from a study showing that estrogen inhibits the early stages of neointima formation in animal models (37). Additional evidence indicates ER levels are reduced in diseased human coronary arteries. Also, reduced levels and activity of SRC-3 in diseased arteries may also contribute to estrogen resistance. These observations warrant investigation of SRC-3 and possibly other SRCs under conditions of vascular trauma and suggests that early intervention by SRC stimulation will have long-lasting effects on later events in the vascular injury response, including fibroblast migration and proliferation.
SRCs Promote Angiogenesis after Tissue Injury and During Tumor Formation
The creation of new vasculature via angiogenesis is essential for effective tissue repair. Results from our animal model studies and others indicate that SRC-1, -2, and -3 function to promote angiogenesis and tissue protection in response to injury. In the cardiovascular system of adult mice, expression of either SRC-1 or SRC-3 in VSMCs and endothelial cells is required for the protective effects of estrogen in the vascular injury response (37, 43). In mouse embryonic development, knockout of both SRC-1 and SRC-3 resulted in reduced fetal vascular development, followed by embryonic lethality at E13.5 (46). Decreased fibroblast growth factor (FGF)2 expression in placentas at E12.5 suggest that SRC-1 and SRC-3 may promote labyrinth angiogenesis by modulating FGF expression. In support of this, the FGF chaperone and angiogenic factor, FGF binding protein (FGFBP1), is regulated by SRC-3 (47, 48). In vitro, SRC-3 has been shown to integrate ERK3 signaling to promote endothelial cell migration, proliferation and tube formation by upregulating SRC-3/CREB binding protein /SP-1–mediated VEGF2 expression (20) (Fig. 2). Similar to that observed for tissue development and repair, tumors rely on neovascularization for tumorigenesis and tumor progression. In cancer models, SRC-3 may play a role in tumor maintenance by promoting tumor vascularization (49). SRCs control similar angiogenic mechanisms in response to injury in animal models. SRC-2 coordinates cardiomyocyte paracrine signaling via VEGF to promote pressure overload–induced angiogenesis (19). SRC-3 has also been shown to function in skin stromal injury responses, including angiogenesis in vitro and in wound healing in the skin via crosstalk with FGF signaling (21). SRC-1 promotes angiogenesis in breast tumors by simultaneously enhancing upregulation of both HIF-1α and AP-1-mediated VEGF-A transcription (18). Overall, SRC-1, -2, and -3 function to promote angiogenesis via crosstalk with FGF and VEGF signaling.
The formation of new vessels from pre-existing ones through sprouting angiogenesis is primarily controlled by VEGF-2A-stimulated MEF2C transcription (50). During sprouting angiogenesis, VEGFA signaling triggers the release of repressive class IIa HDACs bound to the MEF2 family of transcription factors, allowing activation of angiogenic enhancers bound to transiently activated MEF2C. MEF2C binding to the HLX-3 enhancer activates gene expression specifically during sprouting angiogenesis in the systemic vasculature (50). A recent study demonstrated a role of the VEGF-A/MEF2 sprouting angiogenesis pathway in embryonic, postnatal, and adult hearts (51). The HLX3-luc reporter was shown to be activated in embryonic and postnatal heart vessels and in vessels in response to ischemic injury at postnatal days 1 (highly regenerative stage) and 7 (an age associated with loss of regenerative potential). However, no increase in the coronary vascular development HLX-3-luc reporter was seen in injured adult hearts after myocardial infarction (MI), indicating that the VEGFA–MEF2 angiogenic pathway is specifically inactive during neovascularization post-MI in adult hearts. Although the role of SRC coactivation of MEF2C regulatory pathways governing mouse coronary vessel formation in post-MI vascular response needs to be elucidated, these findings warrant investigation of SRC activation in the heart after MI.
SRCs Suppress the Inflammatory Response to Facilitate Wound Repair
Inflammation, although required for proper healing in the early phases of a tissue injury response, can result in tissue damage if left unabated. NRs have well-described actions in modulating inflammatory processes (52). Glucocorticoids are stress hormones that have powerful anti-inflammatory effects. It is well established that glucocorticoid receptor–mediated recruitment of coactivators including SRCs is crucial for its anti-inflammatory actions (reviewed in (53)). The evolutionary origin of glucocorticoid receptor that arose from a gene duplication and mutation of the mineralocorticoid receptor was a key event for the skeletal, pulmonary, kidney, skin, and vascular changes necessary for land adaptation which took place under conditions of increased oxygen stress and the generation of radical oxygen species (54). Thus it is not surprising that glucocorticoids are secreted in a stress-dependent manner to regulate numerous essential physiologic and developmental processes, ranging from lung maturation to immune responses. Due to glucocorticoid’s wide-ranging systemic actions on the immune system, glucocorticoids are used to treat a wide variety of inflammatory conditions. Unfortunately, glucocorticoids also increase the risk of a range of adverse systemic effects including hypertension and hyperglycemia. Furthermore, exogenous glucocorticoids are known to inhibit wound repair (55). A better understanding of local tissue repair mechanisms in homeostatic, resolving, and nonresolving disease models should point the way toward better management of tissue repair-related inflammatory responses to improve healing.
In addition to glucocorticoids, accumulating evidence suggests SRC expression and activation exhibit control over inflammatory networks and physiologic outcomes in a cell- and context-specific manner. Of particular interest for identification of local tissue injury response targets is the emerging evidence of SRC control of non-NR–mediated actions in inflammation. SRC-3 can work with a number of immune-related transcription factors such as interferon regulatory factors and NF-κB (13, 53) (Fig. 2). NF-κB is a family of signal-inducible transcription factors whose members play an essential role in the regulation of genes involved in inflammatory responses and cell survival. NF-κB signaling occurs via activation of IκB kinase (IKK), followed by phosphorylation and proteasome-mediated degradation of IκB. In breast cancer cells, SRC-3 cooperates with IKK to enhance NF-κB-mediated gene expression (13). In mice, genetic deletion of the SRC-3 gene leads to lymphoproliferation at 7 weeks and the development of B cell lymphomas at 11 to 12 months that is associated with increased numbers of B and T lymphocytes and NF-κB activation (56), indicating that SRC-3 is required to prevent lymphopoiesis as animals age. The effect of SRC-3 on the lymphoid lineage is cell autonomous as indicated by the stronger in vitro proliferative response observed after induction of T and B cells in SRC-3−/− mice and its normalization upon re-expression of SRC-3. Despite the increased proliferation in both T and B cells, it is unclear why mainly B cell lymphomas develop. In contrast to SRC-3 activation of NF-κB in breast cancer cells, IKK bound to SRC-3 (13) limits IκB phosphorylation, thus stabilizing IκB in hematopoietic tissues, inhibiting NF-κB nuclear accumulation and transcriptional activity in SRC-3 knockout mice. When challenged with infection, SRC-3 knockout mice are hypersensitive to lipopolysaccharide (LPS)-induced endotoxic shock (57, 58), indicating that SRC-3 plays a protective role against the lethal endotoxic shock triggered by an acute inflammatory response to bacterial infection. Unexpectedly, it was found that SRC-3 can suppress cytokine production at the level of mRNA translation instead of at a transcriptional level. Recent studies indicate SRC-3 cooperates with NF-κB to drive expression of CXCL2 expression in response to bacterial infection (59). In a mouse model of osteoarthritis (OA), NCOA3 loss promoted post-traumatic OA at least partially by enhancing NF-κB activation (60). Consistent with the SRC-3 knockout acute inflammation model, although SRC-3 loss is not required for initiation, SRC-3 expression protects against post-traumatic inflammatory OA. Therapeutic manipulation of SRC-3 may provide a new approach in constraining the inflammatory response mediated by the NF-κB pathway where a local inflammatory milieu in bone tissue has been established.
SRCs Promote Cardiac Tissue Development and Prevent Detrimental Tissue Remodeling after an Acute Heart Attack or Cardiac Pressure Overload
The adult mammalian heart has long been considered an essentially nonregenerative organ, with its cardiomyocytes existing as terminally differentiated cells. Remarkably, however, adult zebrafish and neonatal mice at postnatal day 1 can fully recover from surgically induced MI without the formation of scar tissue. However, the loss of regenerative capacity in the mouse fetal heart 7 days after birth has driven efforts to understand what drives this inability to reactivate intrinsic regenerative mechanisms that are fully operational only in neonatal mice. Recent findings indicate that SRC family member regulation of cardiomyocyte functions during cardiac embryonic development (46) coincides with the loss of cardiac regenerative potential at postnatal day 7. SRC-1 and SRC-3 proteins are highly expressed in proliferating cardiomyocytes at embryonic and early postnatal days and diminish rapidly after postnatal day 7 (17). Decreased ventricular wall thickness in developing embryos of SRC-1 and SRC-3 knockout mice at day E12.5 indicates that SRCs regulate ventricular wall development. In support of this, cardiomyocyte-specific knockout of SRC-1 and SRC-3 in mice resulted in a noncompaction cardiomyopathy phenotype with prominent trabeculae, deep intertrabecular recesses and a thin ventricular wall and septum. Symptoms associated with the absence of SRC-1 and SRC-3 in mice included left ventricular systolic dysfunction, blood clots, and MI. In this same study it was also shown that both SRC-1 and SRC-3 are recruited to the myocardin enhancer region in the H9C2 myoblastic cell line and in P0 mouse heart cardiomyocytes (Fig. 2).
In addition to its well-known role as a master regulator of smooth muscle development, myocardin promotes cardiomyocyte survival. Although the transcription factor that cooperates with SRC-1 and SRC-3 at the myocardin enhancer in cardiac development is not known, SRC-3 and myocyte-specific enhancer factor 2D (MEF2D) proteins interact in a human coregulatory protein complex network in HeLa cells (61). Evidence that myocardin is a direct transcriptional target of MEF2 during cardiac development (62) suggests a role for MEF2 transcriptional activation of myocardin in cardiac development. MEF2 family members have diverse functions in a wide range of tissues in development and disease. The MEF2 family of human transcription factors consists of 4 proteins, MEF2A, -B, -C, and -D. These MEF2 transcription factors play central roles in differentiation, morphogenesis, tissue maintenance, and disease. MEF2 family members display distinct spatial and temporal expression patterns in embryonic and adult striated muscle cells, brain cells, lymphocytes, neural crest, smooth muscle, endothelium, and bone cells (63). Thus, it is reasonable to expect that SRCs are recruited to and coactivate MEF2 during stress responses and tissue remodeling, involving many other cell types including neurons, smooth muscle cells, and hematopoietic cells (63). Myocardin has also been implicated in remodeling of the adult cardiovascular system during disease. Specifically, myocardin is upregulated in the failing heart (64, 65) and overexpression of myocardin increases cardiomyocyte size (65). Overall, these findings suggest that reactivation of SRC-1 and SRC-3 and in adult cardiomyocytes in injured hearts may promote intrinsic repair responses to restore or replace damaged myocardial tissue. In contrast to SRC-1 and SRC-3, recent findings indicate that SRC-2, although not required for cardiac development, promotes angiogenesis and is required for early metabolic signaling in response to cardiac pressure overload (19, 66). SRC-2 has been shown to be critical for transcriptional control modulated by cardiac growth control factors MEF2, GATA-4, and Tbx5 (66) (Fig. 2). Unlike SRC-1 and SRC-3, SRC-2 has also been shown to coactivate MEF2C and thus affect the latter stages of skeletal muscle differentiation (67). In line with this, it was subsequently shown that activation of the cyclin D/Cdk4 cell cycle control complex promotes muscle cell proliferation by disrupting SRC-2 association with MEF2 (68). Overall, SRCs serve as master regulators of cell–cell signaling integral to physiologic changes necessary for wound repair (summarized in Fig. 2).
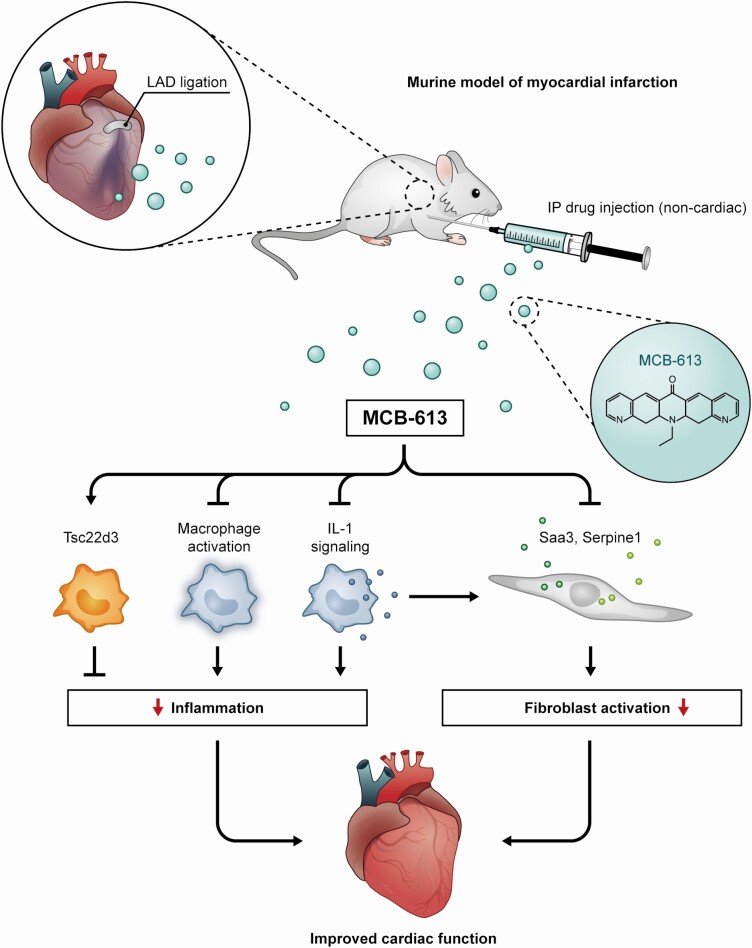
Based on the known functions of SRCs during early heart development and in cell types involved in wound healing, including myocardial injury responses as outlined above, we reasoned that our small molecule SRC stimulator MCB-613 could enable wound repair and preserve cardiac function after an acute MI (69). To test this, MCB-613 or vehicle control was administered to mice following myocardial injury induced by a permanent ligation the left anterior descending coronary artery. We showed that MCB-613, a potent small molecule stimulator of SRCs, prevents pathologic remodeling post-MI. MCB-613 decreased infarct size, apoptosis, hypertrophy, and fibrosis while maintaining significant cardiac function. In the heart, interstitial cells such as fibroblasts, immune cells and endothelial cells play an important role in the injury response. In mouse cardiac fibroblasts, although all SRC proteins were expressed, MCB-613 preferentially stimulated SRC-3 transcriptional activity. Early actions of MCB-613 included reduction of myofibroblasts and robust amelioration of reactive interstitial fibrosis responsible for myocardial stiffness and progression to heart failure. Our data indicate that early suppression of interleukin (IL)-1 signaling is part of the mechanism by which MCB-613 prevents post-MI fibroblast transdifferentiation and improves heart function post-MI. In this regard, SRC-3 is known to act as a translational repressor of IL-1β in LPS-stimulated macrophages (58) and LPS-induced IL-1β signaling is attenuated by MCB-613 (69). In vitro studies indicate that IL-1 treatment leads to a proinflammatory, proangiogenic, extracellular matrix–degrading phenotype in fibroblasts (70). The control of paracrine IL-1/fibroblast signaling post-MI has been previously shown to regulate post-MI remodeling and improve cardiac function in animal models (71, 72). Results from the CANTOS cardiovascular clinical trial indicates that therapeutic blockade of IL-1 reduces cardiovascular events in patients with atherosclerotic vascular disease (reviewed in (73, 74)). Current therapeutic approaches to prevent or reverse scar formation often can result in increased risk of cardiac rupture (75, 76). MCB-613 promotion of COMP-positive matrifibrocyte-like fibroblasts is suggestive of scar remodeling and maintenance associated with improved structural integrity that can lead to lasting protection following injury (69, 77).
In contrast to the conventional classification of macrophages into either M1 and M2 subtypes, the majority of cardiac macrophages in injured myocardium express prominent M2 markers that are defined by the distinct expression of C-C chemokine receptor type 2 (Ccr2) (69). It has been reported that CCR2-negative cardiac macrophages inhibit monocyte recruitment and promote healing, while CCR2-positive macrophages promote inflammation following MI (78). Overall, it was found that MCB-613 treatment contributed to improved cardiac function 24 hours post-MI by establishing a proreparative, anti-inflammatory environment by shifting the macrophage population into proreparative CCR2-negative macrophages and reduced the number of proinflammatory CCR2-positive infiltrating macrophages. Genes differentially expressed in the enriched population of CCR2-negative macrophages included TSC22 Domain Family Member 3 (Tsc22d3), an anti-inflammatory glucocorticoid-induced leucine zipper protein previously shown to provide post-MI cardioprotection (79). These studies provide evidence that therapeutic strategies designed to modulate SRC biology, especially that of SRC-3-mediated immune responses to MI, can be used to promote a broadly beneficial injury response post-MI in the adult heart in mouse models (Fig. 3).
Figure 3.
MCB-613, when given within hours post-MI, induces lasting protection from adverse remodeling concomitant with (1) inhibition of macrophage inflammatory signaling and IL-1 signaling which attenuates the acute inflammatory response, (2) prevention of fibroblast differentiation, and (3) promotion of Tsc22d3 expressing macrophages—all of which may limit inflammatory damage. SRC stimulation with MCB-613 is a potential novel therapeutic approach for inhibiting cardiac dysfunction after MI. LAD, left anterior descending artery; IP intraperitoneal.
Summary
Unlike the limited and restricted regenerative potential of adult cells, temporal manipulation of inherent plasticity in the wound response may provide a mechanism to promote tissue reprogramming and repair. Distinct molecular and cellular mechanisms related to stimulation of SRCs have been identified that pave the way for the further exploration of SRCs as drug targets that can be engaged to improve the management of tissue injury response outcomes. In this regard, we have recently shown that SRC stimulation or enhancement of reactivated SRC embryonic transcriptional programs in diverse cell types facilitates repair after cardiac damage. Our results indicate that MCB-613 can drive this process by coordinately regulating multiple key factors involved in the cardiac ischemic injury response; it occurs in part by its intrinsic and powerful transcriptional growth and repair effects on numerous nuclear genes in multiple cell types. Although timing and dosing will likely modulate the broad effects of MCB-613, our results indicate MCB-613 selectively controls the interstitial cardiac repair cellular response to ischemia and reveals that specific targeting of endogenous regenerative interstitial cell–cell communication is critical to cardiac muscle recovery.
Effective therapeutic approaches for complex diseases will likely involve temporal and combinatorial manipulation of cell-intrinsic and cell-extrinsic factors. Rational design of single targeted therapeutics for chronic disease generally has often been disappointing. Targeting of a specific cell-intrinsic or cell-extrinsic factor frequently elicits feedback or compensatory pathway mechanisms. As examples, cancer cells become resistant to single targeted therapies, blocking inflammation prevents subsequent wound healing, and fully differentiated tissues lose their stem cell-driven regenerative capacity. Coordinated regulation of multiple key factors involved in the injury response following SRC stimulation provides a mechanism by which a single stimuli can modulate a complex multifaceted repair process. Overall, new lines of investigation into modification of SRC activity represents a potential new therapeutic approach for wound healing.
Acknowledgments
Financial Support: This review was supported by Bayer/Grants4Targets Initiative (2016-08-1815), L.K.M.; and National Institutes of Health (NIH) grants HD07857 and HD08188, B.W.O.
Glossary
Abbreviations
- CCR
C-C chemokine receptor type 2
- FGF
fibroblast growth factor
- IL
interleukin
- MI
myocardial infarction
- NF-κB
nuclear factor kappa B
- NR
nuclear receptor
- OA
osteoarthritis
- SRC
steroid receptor coactivator
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cell
Additional Information
Disclosures: Authors do not have any current conflicts of interest to disclose related to coactivator “stimulating agents” or to MCB-613 employed in this study, but have equity and grant funding for other steroid receptor coactivator “inhibiting agents” with Coactigon, Inc.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Lonard DM, O’malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27(5):691-700. [DOI] [PubMed] [Google Scholar]
- 2. Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270(5240):1354-1357. [DOI] [PubMed] [Google Scholar]
- 3. Dasgupta S, Lonard DM, O’Malley BW. Nuclear receptor coactivators: master regulators of human health and disease. Annu Rev Med. 2014;65:279-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9(9):615-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lonard DM, O’Malley BW. Molecular pathways: targeting steroid receptor coactivators in cancer. Clin Cancer Res. 2016;22(22):5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Song X, Chen H, Zhang C, et al. SRC-3 inhibition blocks tumor growth of pancreatic ductal adenocarcinoma. Cancer Lett. 2019;442:310-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rohira AD, Yan F, Wang L, et al. Targeting SRC coactivators blocks the tumor-initiating capacity of cancer stem-like cells. Cancer Res. 2017;77(16):4293-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang L, Yu Y, Chow DC, et al. Characterization of a steroid receptor coactivator small molecule stimulator that overstimulates cancer cells and leads to cell stress and death. Cancer Cell. 2015;28(2):240-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lanz RB, Bulynko Y, Malovannaya A, et al. Global characterization of transcriptional impact of the SRC-3 coregulator. Mol Endocrinol. 2010;24(4):859-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Percharde M, Lavial F, Ng JH, et al. Ncoa3 functions as an essential Esrrb coactivator to sustain embryonic stem cell self-renewal and reprogramming. Genes Dev. 2012;26(20):2286-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O’Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci U S A. 2000;97(12):6379-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. York B, O’Malley BW. Steroid receptor coactivator (SRC) family: masters of systems biology. J Biol Chem. 2010;285(50):38743-38750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu RC, Qin J, Hashimoto Y, et al. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by I kappa B kinase. Mol Cell Biol. 2002;22(10):3549-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dasgupta S, Putluri N, Long W, et al. Coactivator SRC-2-dependent metabolic reprogramming mediates prostate cancer survival and metastasis. J Clin Invest. 2015;125(3):1174-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su Q, Hu S, Gao H, et al. Role of AIB1 for tamoxifen resistance in estrogen receptor-positive breast cancer cells. Oncology. 2008;75(3-4):159-168. [DOI] [PubMed] [Google Scholar]
- 16. Yan J, Erdem H, Li R, et al. Steroid receptor coactivator-3/AIB1 promotes cell migration and invasiveness through focal adhesion turnover and matrix metalloproteinase expression. Cancer Res. 2008;68(13):5460-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen X, Qin L, Liu Z, Liao L, Martin JF, Xu J. Knockout of SRC-1 and SRC-3 in mice decreases cardiomyocyte proliferation and causes a noncompaction cardiomyopathy phenotype. Int J Biol Sci. 2015;11(9):1056-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qin L, Xu Y, Xu Y, et al. NCOA1 promotes angiogenesis in breast tumors by simultaneously enhancing both HIF1α- and AP-1-mediated VEGFa transcription. Oncotarget. 2015;6(27):23890-23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suh JH, Lai L, Nam D, et al. Steroid receptor coactivator-2 (SRC-2) coordinates cardiomyocyte paracrine signaling to promote pressure overload-induced angiogenesis. J Biol Chem. 2017;292(52):21643-21652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang W, Bian K, Vallabhaneni S, et al. ERK3 promotes endothelial cell functions by upregulating SRC-3/SP1-mediated VEGFR2 expression. J Cell Physiol. 2014;229(10):1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Otaiby M, Tassi E, Schmidt MO, et al. Role of the nuclear receptor coactivator AIB1/SRC-3 in angiogenesis and wound healing. Am J Pathol. 2012;180(4):1474-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fantin A, Vieira JM, Gestri G, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Espín-Palazón R, Traver D. The NF-κB family: Key players during embryonic development and HSC emergence. Exp Hematol. 2016;44(7):519-527. [DOI] [PubMed] [Google Scholar]
- 24. Halfon MS, Hashimoto C, Keshishian H. The Drosophila toll gene functions zygotically and is necessary for proper motoneuron and muscle development. Dev Biol. 1995;169(1):151-167. [DOI] [PubMed] [Google Scholar]
- 25. Bakkar N, Wang J, Ladner KJ, et al. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol. 2008;180(4):787-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304(5675):1331-1334. [DOI] [PubMed] [Google Scholar]
- 27. Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502(7472):513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019;21(1):18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ge Y, Fuchs E. Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat Rev Genet. 2018;19(5):311-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Z, Yang M, Liu H, et al. Role of nuclear receptor coactivator 3 (Ncoa3) in pluripotency maintenance. J Biol Chem. 2012;287(45):38295-38304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wollenzien H, Voigt E, Kareta MS. Somatic pluripotent genes in tissue repair, developmental disease, and cancer. SPG Biomed. 2018;1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andoniadou CL, Matsushima D, Mousavy Gharavy SN, et al. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13(4):433-445. [DOI] [PubMed] [Google Scholar]
- 33. Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A. 2008;105(8):2907-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fu Q, Gremeaux L, Luque RM, et al. The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology. 2012;153(7):3224-3235. [DOI] [PubMed] [Google Scholar]
- 35. Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10(5):615-624. [PubMed] [Google Scholar]
- 36. Orimo A, Inoue S, Ouchi Y, Orimo H. Vascular smooth muscle cells possess estrogen receptor and respond to estrogen. Ann N Y Acad Sci. 1995;748:592-594. [DOI] [PubMed] [Google Scholar]
- 37. Yuan Y, Liao L, Tulis DA, Xu J. Steroid receptor coactivator-3 is required for inhibition of neointima formation by estrogen. Circulation. 2002;105(22):2653-2659. [DOI] [PubMed] [Google Scholar]
- 38. Foegh ML, Zhao Y, Farhat M, Ramwell PW. Oestradiol inhibition of vascular myointimal proliferation following immune, chemical and mechanical injury. Ciba Found Symp. 1995;191:139-145; discussion 145. [DOI] [PubMed] [Google Scholar]
- 39. Oparil S. Arthur C. Corcoran memorial lecture. Hormones and vasoprotection. Hypertension. 1999;33(1 Pt 2):170-176. [DOI] [PubMed] [Google Scholar]
- 40. Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29(3):289-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen SJ, Chen YF, Miller DM, Li H, Oparil S. Mithramycin inhibits myointimal proliferation after balloon injury of the rat carotid artery in vivo. Circulation. 1994;90(5):2468-2473. [DOI] [PubMed] [Google Scholar]
- 42. Li HJ, Haque Z, Lu Q, Li L, Karas R, Mendelsohn M. Steroid receptor coactivator 3 is a coactivator for myocardin, the regulator of smooth muscle transcription and differentiation. Proc Natl Acad Sci U S A. 2007;104(10):4065-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yuan Y, Xu J. Loss-of-function deletion of the steroid receptor coactivator-1 gene in mice reduces estrogen effect on the vascular injury response. Arterioscler Thromb Vasc Biol. 2007;27(7):1521-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sullivan JM, Fowlkes LP. The clinical aspects of estrogen and the cardiovascular system. Obstet Gynecol. 1996;87(2 Suppl):36S-43S. [DOI] [PubMed] [Google Scholar]
- 45. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801-1811. [DOI] [PubMed] [Google Scholar]
- 46. Chen X, Liu Z, Xu J. The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival. Mol Endocrinol. 2010;24(10):1917-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harris VK, Coticchia CM, Kagan BL, Ahmad S, Wellstein A, Riegel AT. Induction of the angiogenic modulator fibroblast growth factor-binding protein by epidermal growth factor is mediated through both MEK/ERK and p38 signal transduction pathways. J Biol Chem. 2000;275(15):10802-10811. [DOI] [PubMed] [Google Scholar]
- 48. Lahusen T, Fereshteh M, Oh A, Wellstein A, Riegel AT. Epidermal growth factor receptor tyrosine phosphorylation and signaling controlled by a nuclear receptor coactivator, amplified in breast cancer 1. Cancer Res. 2007;67(15):7256-7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fereshteh MP, Tilli MT, Kim SE, et al. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res. 2008;68(10):3697-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sacilotto N, Chouliaras KM, Nikitenko LL, et al. MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes Dev. 2016;30(20):2297-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Payne S, Gunadasa-Rohling M, Neal A, et al. Regulatory pathways governing murine coronary vessel formation are dysregulated in the injured adult heart. Nat Commun. 2019;10(1):3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol. 2006;6(1):44-55. [DOI] [PubMed] [Google Scholar]
- 53. Rollins DA, Coppo M, Rogatsky I. Minireview: nuclear receptor coregulators of the p160 family: insights into inflammation and metabolism. Mol Endocrinol. 2015;29(4):502-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Torday JS. Homeostasis as the mechanism of evolution. Biology (Basel). 2015;4(3):573-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jozic I, Vukelic S, Stojadinovic O, et al. Stress signals, mediated by membranous glucocorticoid receptor, activate PLC/PKC/GSK-3β/β-catenin pathway to inhibit wound closure. J Invest Dermatol. 2017;137(5):1144-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coste A, Antal MC, Chan S, et al. Absence of the steroid receptor coactivator-3 induces B-cell lymphoma. EMBO J. 2006;25(11):2453-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen Q, Chen T, Xu Y, et al. Steroid receptor coactivator 3 is required for clearing bacteria and repressing inflammatory response in Escherichia coli-induced septic peritonitis. J Immunol. 2010;185(9):5444-5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yu C, York B, Wang S, Feng Q, Xu J, O’Malley BW. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell. 2007;25(5):765-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen W, Lu X, Chen Y, et al. Steroid receptor coactivator 3 contributes to host defense against enteric bacteria by recruiting neutrophils via upregulation of CXCL2 expression. J Immunol. 2017;198(4):1606-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang B, Li Z, Wang W, et al. NCOA3 loss disrupts molecular signature of chondrocytes and promotes posttraumatic osteoarthritis progression. Cell Physiol Biochem. 2018;49(6):2396-2413. [DOI] [PubMed] [Google Scholar]
- 61. Malovannaya A, Lanz RB, Jung SY, et al. Analysis of the human endogenous coregulator complexome. Cell. 2011;145(5):787-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Creemers EE, Sutherland LB, McAnally J, Richardson JA, Olson EN. Myocardin is a direct transcriptional target of Mef2, Tead and Foxo proteins during cardiovascular development. Development. 2006;133(21):4245-4256. [DOI] [PubMed] [Google Scholar]
- 63. Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134(23):4131-4140. [DOI] [PubMed] [Google Scholar]
- 64. Torrado M, López E, Centeno A, Medrano C, Castro-Beiras A, Mikhailov AT. Myocardin mRNA is augmented in the failing myocardium: expression profiling in the porcine model and human dilated cardiomyopathy. J Mol Med (Berl). 2003;81(9):566-577. [DOI] [PubMed] [Google Scholar]
- 65. Xing W, Zhang TC, Cao D, et al. Myocardin induces cardiomyocyte hypertrophy. Circ Res. 2006;98(8):1089-1097. [DOI] [PubMed] [Google Scholar]
- 66. Reineke EL, Benham A, Soibam B, et al. Steroid receptor coactivator-2 is a dual regulator of cardiac transcription factor function. J Biol Chem. 2014;289(25):17721-17731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen SL, Dowhan DH, Hosking BM, Muscat GE. The steroid receptor coactivator, GRIP-1, is necessary for MEF-2C-dependent gene expression and skeletal muscle differentiation. Genes Dev. 2000;14(10):1209-1228. [PMC free article] [PubMed] [Google Scholar]
- 68. Lazaro JB, Bailey PJ, Lassar AB. Cyclin D-cdk4 activity modulates the subnuclear localization and interaction of MEF2 with SRC-family coactivators during skeletal muscle differentiation. Genes Dev. 2002;16(14):1792-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mullany LK, Rohira AD, Leach JP, et al. A steroid receptor coactivator stimulator (MCB-613) attenuates adverse remodeling after myocardial infarction. Proc Natl Acad Sci U S A. 2020;117(49):31353-31364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Turner NA. Effects of interleukin-1 on cardiac fibroblast function: relevance to post-myocardial infarction remodelling. Vascul Pharmacol. 2014;60(1):1-7. [DOI] [PubMed] [Google Scholar]
- 71. Bageghni SA, Hemmings KE, Yuldasheva NY, et al. Fibroblast-specific deletion of interleukin-1 receptor-1 reduces adverse cardiac remodeling following myocardial infarction. JCI Insight. 2019;5(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bujak M, Dobaczewski M, Chatila K, et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173(1):57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Buckley LF, Carbone S, Trankle CR, et al. Effect of Interleukin-1 blockade on left ventricular systolic performance and work: a post hoc pooled analysis of 2 clinical trials. J Cardiovasc Pharmacol. 2018;72(1):68-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Libby P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol. 2017;70(18):2278-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol. 2014;70:74-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7(1):30-37. [DOI] [PubMed] [Google Scholar]
- 77. Fu X, Khalil H, Kanisicak O, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128(5):2127-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bajpai G, Bredemeyer A, Li W, et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. 2019;124(2):263-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baban B, Yin L, Qin X, Liu JY, Shi X, Mozaffari MS. The role of GILZ in modulation of adaptive immunity in a murine model of myocardial infarction. Exp Mol Pathol. 2017;102(3):408-414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.