Abstract
Obesity and related metabolic disorders have become epidemic diseases. Intermittent fasting has been shown to promote adipose tissue angiogenesis and have an anti-obesity feature; however, the mechanisms of how intermittent fasting modulates adipose tissues angiogenesis are poorly understood. We investigated the effect of fasting on vascular endothelial growth factor (VEGF) levels in white adipose tissues (WAT) and the function of fibroblast growth factor 21 (FGF21) in 1-time fasting and long-term intermittent fasting-induced VEGF expression. In the current study, fasting induced a selective and drastic elevation of VEGF levels in WAT, which did not occur in interscapular brown adipose tissue and liver. The fasting-induced Vegfa expression occurred predominantly in mature adipocytes, but not in the stromal vascular fraction in epididymal WAT and inguinal WAT (iWAT). Furthermore, a single bolus of recombinant mouse FGF21 injection increased VEGF levels in WAT. Long-term intermittent fasting for 16 weeks increased WAT angiogenesis, iWAT browning, and improved insulin resistance and inflammation, but the effect was blunted in FGF21 liver-specific knockout mice. In summary, these data suggest that FGF21 is a potent regulator of VEGF levels in WAT. The interorgan FGF21 signaling-induced WAT angiogenesis by VEGF could be a potential new therapeutic target in combination with obesity-related metabolic disorders.
Keywords: fasting, FGF21, VEGF, angiogenesis, intermittent fasting
For centuries, food acquisition has become easier for humans, while obesity and related metabolic disorders, including type II diabetes, nonalcoholic fatty liver disease, cardiovascular disease, and cancer are becoming growing health challenges in modern society, which mainly result from a dysregulated balance between energy intake and energy expenditure (1). White adipose tissue (WAT), as the main organ storing excess energy, is essential for energy homeostasis and metabolic health. To support the metabolic change, the plasticity of the adipose tissues is crucial to maintain appropriate energy balance and secretion of endocrine factors (2,3). Caloric excess or high-fat diet (HFD) have been shown to suppress adipose angiogenesis, expand the lipid droplet size, and induce the infiltration of immune cells (4), which caused inflammation and insulin resistance. In contrast, fasting regimens or hypocaloric diets are associated with improved the metabolic and inflammatory diseases in humans (5). However, the exact mechanisms by which endocrine factor modulate adipose plasticity has not been well established, particularly the onset of adipose angiogenesis.
In contrast to WAT, brown adipose tissue (BAT) is a highly vascularized organ generating heat by metabolizing glucose and fatty acids (6,7). Emerging evidence has suggested that functional brown/beige fat may enhance energy expenditure via uncoupling protein 1 (UCP-1)–mediated thermogenesis (8). However, to support the supply of oxygen and nutrients to adipose tissue, the plasticity of the embedded vasculature in adipose tissues is crucial (2). Vascular endothelial growth factor (VEGF) is classically known to be essential in vascular development, which are activated upon stimuli such as exercise (9,10) and cold exposure (11,12). Recent studies have highlighted the critical role of adipose tissue VEGF in the control of adipose tissue metabolism and systemic energy homeostasis (13). Overexpression of VEGF in adipose tissue facilitates angiogenesis, increases inguinal WAT (iWAT) UCP-1 levels and resists HFD induced obesity (14,15). Furthermore, adipose-specific VEGF knockout or pharmacological VEGF blockade by antibodies abolished the browning of WAT (13). VEGF has also been reported to increased insulin sensitivity in genetic and HFD-fed obese mouse models (16). Taken together, these findings support the WAT VEGF as the critical factor of regulating angiogenesis and metabolic homeostasis.
Fibroblast growth factor 21 (FGF21) is an endocrine factor produced by several tissues, including the liver, adipose tissue, skeletal muscle, and heart, but the liver is the major source of FGF21 and contributes to approximately 90% of circulating FGF21 levels (17-21). The expression and secretion of hepatic FGF21 were reported to be affected by several nutritional factors (17,22-24), and fasting is one of the most important factors (25-27). Recent work has demonstrated that FGF21 stimulates thermogenic gene expression BAT and induces iWAT browning (20,28-30). In addition, FGF21 indirectly regulates BAT thermogenesis by acting on the central nervous system (31). However, the effect of FGF21 on the adipose angiogenesis remains to be investigated.
In the current study, we found that fasting induced a selective and drastic elevation in VEGF levels in WAT, but this did not occur in BAT and liver. Furthermore, the fasting-induced Vegfa expression occurred predominantly in mature adipocytes, but not in the stromal vascular fraction in epididymal WAT (eWAT) and iWAT. Long-term intermittent fasting (IF) increased WAT angiogenesis and iWAT browning and improved insulin resistance and inflammation, but was blunted in FGF21 liver-specific knockout (FGF21 LKO) mice. In summary, these data suggest that FGF21 is a potent regulator of VEGF levels in WAT and emphasize the link between the dietary patterns and adipose angiogenesis.
Materials and Methods
Animals
All animal procedures in this study were approved by the Institutional Animal Care and Research Committee of Sichuan Agricultural University (SICAU-2015–033). The mice were fed a standard diet (Figs. 1 and 2) comprising 70%, 20%, and 10% of calories from carbohydrate, protein, and fat, respectively (D12450J), or a HFD (Figs. 3 and 4) comprising 20%, 20%, and 60% of calories from carbohydrates, protein, and fat (D12492), from Research Diets (New Brunswick, NJ, USA). C57BL/6J male mice were obtained from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The FGF21 LKO mice were generated as previously described (20). FGF21Liver+/-, Alb-Cre mice were generated by mating FGF21loxp/loxp mice (022361; The Jackson Laboratory, Bar Harbor, ME, USA) with Alb-Cre mice (J003574; Model Animal Research Center, Nanjing University, Nanjing, China) transgenic mice. FGF21Liver+/+, Alb-Cre mice were generated by crossing FGF21Liver+/-, Alb-Cre mice with FGF21loxp/loxp mice. Littermates of FGF21loxp/loxp mice were used as control. Recombinant mouse FGF21 (8409; R&D Systems; Bio-Techne, Minneapolis, MN, USA) or equal volume of phosphate-buffered saline was injected from tail vein at the dose of 1 mg/kg bodyweight. Mice were kept in 24 ± 2℃ facilities with a 12-h light/dark cycle and had free access to water.
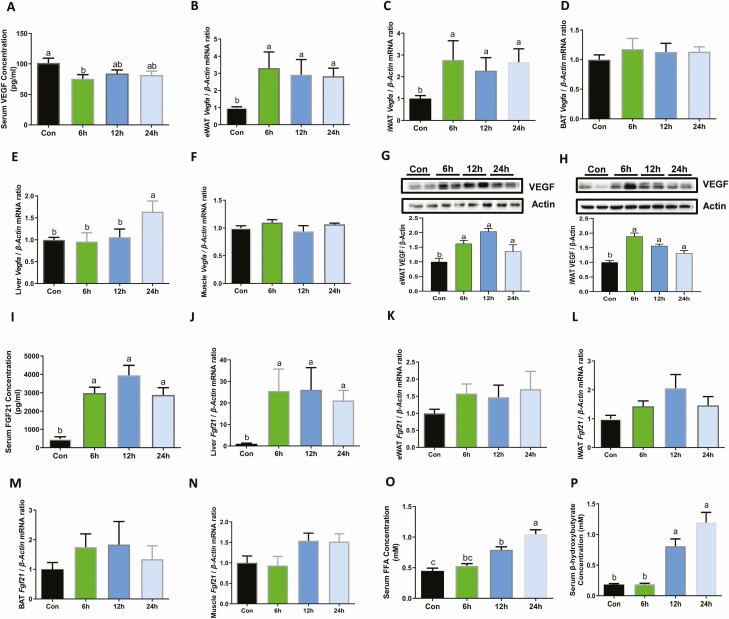
Figure 1.
Fasting induced adipose-specific VEGF expression and liver Fgf21 expression. Twelve-week-old male C57BL/6J mice were either fed with chow diets ad libitum or fasting for various time periods (6 h, 12 h, and 24 h) as indicated. (A) Serum VEGF levels and (B) the mRNA expression of Vegfa in epididymal WAT (eWAT) (B), subcutaneous iWAT (C), interscapular BAT (D), liver (E), and muscle (F) as determined by real-time PCR analysis. The protein levels of VEGF in eWAT (G) and iWAT (H) as determined by western blot; (I) serum FGF21 levels as determined by enzyme-linked immunosorbent assay; and real-time transcription PCR analysis for Fgf21 mRNA expression levels of liver (J), eWAT (K), iWAT (L), BAT (M), and muscle (N). Serum FFA (O) and ketone bodies (P) levels. Data are mean ± SEM; n = 6/group. Statistical significance was evaluated by 1-way ANOVA with Tukey’s test for multiple comparisons to determine differences between each group. Labeled means without a common letter differ, P < 0.05. Abbreviations: BAT, brown adipose tissue; eWAT, epididymal white adipose tissue; FFA, free fatty acid; FGF21, fibroblast growth factor 21; iWAT, inguinal white adipose tissue; VEGF, vascular endothelial growth factor.
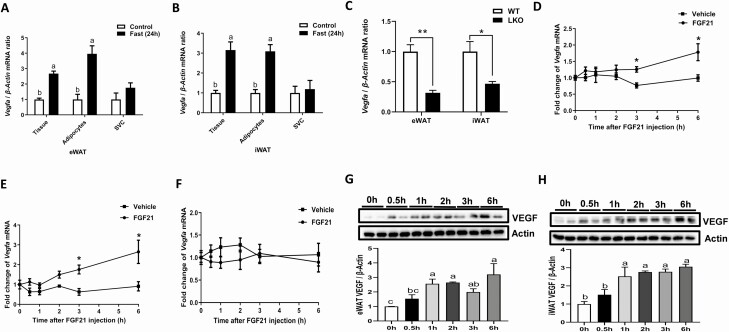
Figure 2.
FGF21 promoted expression and accumulation of VEGF in WAT. The relative mRNA abundance of Vegfa in tissue, adipocytes and stromal vascular fraction isolated from eWAT (A) and iWAT (B) at fed or 24 h of fasting. Twelve-week-old male WT (FGF21fl/fl) and FGF21 LKO mice were fasted for 24 h; the Vegfa mRNA expression in eWAT and iWAT (C). Quantitative reverse transcription PCR analysis for Vegfa mRNA expression in eWAT (D), iWAT (E), and BAT (F) at the indicated time points after tail vein injection of rmFGF21 (1 mg/kg). The protein levels of VEGF at various time points after mice receiving a delivery of rmFGF21 with tail vein injection in eWAT (G) and iWAT (H). n = 6/group. Statistical significance was evaluated by unpaired Student’s t test. *P < 0.05, **P < 0.01, versus control; labeled means without a common letter differ, P < 0.05. Abbreviation: SCV, stromal vascular fraction.
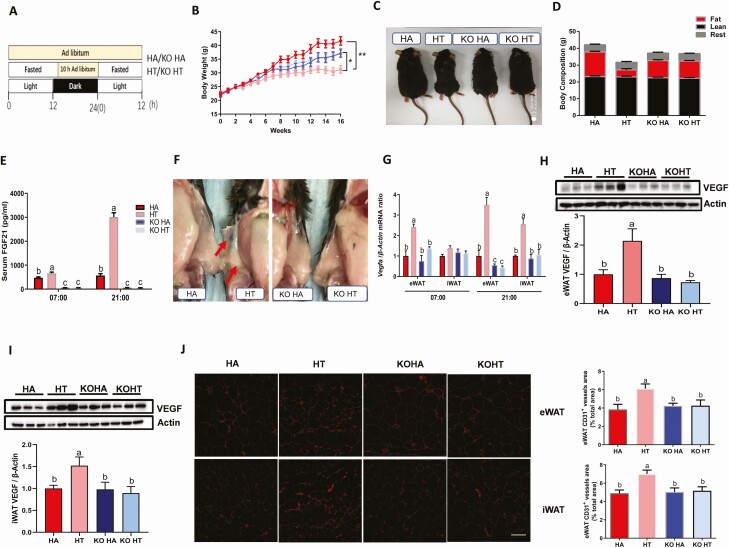
Figure 3.
Intermittent fasting induced adipose-VEGF expression and angiogenesis depend on liver FGF21 signaling. Mice were fed with HFD ad libitum or time-restricted access to food for 16 weeks. HA means WT mice eating a high-fat diet with ad libitum, HT means WT mice eating a high-fat diet with time-restricted access to food, KOHA means FGF21 LKO mice eating a high-fat diet with ad libitum, KOHT means FGF21 LKO mice eating a HFD with time-restricted access to food. (A) Schematic illustration of the experimental design. (B) Body weight. (C) Representative HT mice were remarkably leaner than the HA mice. (D) Body composition was evaluated by EchoMRI. (E) Serum FGF21 levels as determined by enzyme-linked immunosorbent assay. (F) A representative macroscopic image illustrating increased vascularization in iWAT of HT mice, compared to HA mice but not in FGF21 LKO mice. (G) Real-time quantitative PCR analysis for mRNA expression levels of Vegfa in eWAT and iWAT. Representative protein levels for VEGF in eWAT (G) and iWAT (I). (J) Immunofluorescence staining of CD31 (scale bar, 100 mm) in eWAT and iWAT, illustrating IF increased vascularization in WT mice but not in FGF21 LKO mice. Data are mean ± SEM; n = 6–8/group. Statistical significance was evaluated by the 2-way ANOVA test and the Tukey’s test for multiple comparisons to determine differences between each group. HA vs HT, *P < 0.05, **P < 0.01; labeled means without a common letter differ, P < 0.05.
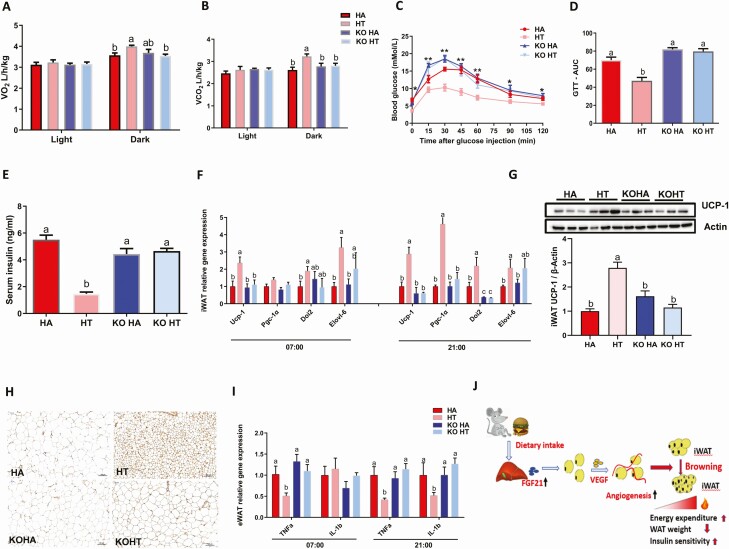
Figure 4.
Liver-FGF21 is required for intermittent fasting-induced metabolic benefits. Mice were fed with HFD ad libitum or time-restricted access to food for 16 weeks. (A) O2 consumption (VO2), (B) CO2 production (VCO2). (C and D) GTT shows normal glucose tolerance in HT mice but not for FGF21 LKO mice. (E) Serum insulin levels. real-time quantitative PCR analysis for mRNA expression levels of browning related genes (F), UCP-1 protein level (G) and immunohistochemical staining (H) of UCP-1 in iWAT. (I) The expression of pro-inflammatory cytokines (Tnfα and IL1-β) in eWAT. (J) A working model of dietary intake regulating iWAT browning via FGF21 signaling. Data are mean ± SEM; n = 6–8/group. Statistical significance was evaluated by the 2-way ANOVA test and the Tukey’s test for multiple comparisons to determine differences between each group. *P < 0.05, **P < 0.01; labeled means without a common letter differ, P < 0.05.
Feeding schedule and diets
For IF, body weight-matched 8-week-old male C57BL/6J mice were fed with HFD (D12492; 60% kcal from fat) with unrestricted (ad libitum) or restricted access to food for 16 weeks. Under IF, mice were allowed access to food between 9:00 pm to 7:00 am. Food access was regulated by transferring mice daily between cages with food and water and cages with water only. As control for mouse handling, ad libitum fed mice were also transferred between feeding cages at the same time. Weekly food intake and body weight were measured.
For one-time fasting, body weight-matched 12-week-old male C57BL/6J mice were fed with standard diet (D12450J), and the mice were killed at the same time (9:00 am) after overnight fasting for 6 h, 12 h, and 24 h.
Metabolic profiling
Body composition was analyzed using the EchoMRI-100 machine (Echo Medical Systems, Houston, TX, USA), which determines fat and lean mass in conscious mice. Body metabolic rates were tested by indirect calorimetry in a comprehensive laboratory animal monitoring system (Columbus Instruments) for 2 days according to the manufacturer’s instructions. Light and feeding conditions were kept the same as in the regular cages.
Glucose tolerance test
Mice were fasted overnight. The next morning, mice fed with chow or a HFD were administered with D-glucose at the dose of 1 g/kg body weight, respectively, by intraperitoneal injection. Blood glucose levels were measured at 0, 15, 30, 45, 60, 90, and 120 min post injection with tail vein blood using glucose test strips (Roche Diagnostics).
Analysis of serum biochemistry and hormones
Mice were euthanized using carbon dioxide, followed by cervical dislocation. Serum was collected for further analysis. Serum free fatty acid and β-hydroxybutyrate levels were measured on an automatic biochemical analyzer (7020; Hitachi, Tokyo, Japan) with the respective analysis kits (Beijing Strong Biotechnologies, Beijing, China), according to the manufacturer’s instructions. Serum FGF21 (MF2100; R&D Systems; Bio-Techne, Minneapolis, MN, USA) and VEGF (KE10009; Proteintech Group, Rosemont, PA, USA) levels were measured with commercial enzyme-linked immunosorbent assay kits, according to the manufacturer’s instructions.
Isolation of adipocytes and stromal vascular cells from adipose tissue
Adipocytes and stromal vascular cells from adipose tissue were isolated as previously described (32). In brief, 1 eWAT or iWAT pad from 16-week-old male C57BL/6J mice was excised, weighed, and rinsed in isolation buffer. Fat pads were then cut into small pieces in isolation buffer supplemented with 1 mg/mL type I collagenase and digested at 37°C in shaking-water bath at 100 rpm for 45 min. Digested tissues were filtered through 400 mesh to get single-cell suspension. After centrifugation at 800 g, floating adipocytes and pellet cells were rinsed twice with isolation buffer. The adipocytes, pelleted stromal vascular cells, and the other fat pad were used for ribonucleic acid (RNA) extraction and determination of gene expression.
RNA extraction and gene expression analysis
RNA extraction and real-time polymerase chain reaction (PCR) were performed as previously reported (20). Briefly, RNA from adipose tissues were extracted by Trizol (15596018; Thermo Fisher Scientific) and purified using RNA mini-columns (RR037A; Takara Bio, Kusatsu, Japan). Reverse transcription and SYBR green quantitative PCR (RR820A; Takara Bio) were performed according to the manufacturer protocols. Target primer sequences are shown in Table 1.
Table 1.
List of primers used
| Name | Forward | Reverse |
|---|---|---|
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| Ucp-1 | GCCAAAGTCCGCCTTCAGAT | CAGTTTCGGCAATCCTTCTGTT |
| Fgf 21 | CTGCTGGGGGTCTACCAAG | CTGCGCCTACCACTGTTCC |
| Pgc-1α | TATGGAGTGACATAGAGTGTGCT | CCACTTCAATCCACCCAGAAAG |
| Dio2 | GCTTACGGGGTAGCCTTTGA | TGTAGGTTATAGCTGAAGGGGC |
| Elovl6 | TCACCTTGTCCCAGATCACTC | CTGAGGTACATGAGCGAGGAC |
| Vegfa | CTGTAACGATGAAGCCCTGGAG | TGGTGAGGTTTGATCCGCAT |
| TNFα | CAGCCTCTTCTCATTCCTGC | GGTCTGGGCCATAGAACTGA |
| IL1β | CTGGTGTGTGACGTTCCCATTA | CCGACAGCACGAGGCTTT |
Western blot analysis
Protein extraction and western blotting were performed as previously described (20). Sources of antibodies anti-VEGF (33) was purchased from Abcam (Cambridge, UK); anti-β-actin (34), from Cell Signaling Technology (Danvers, MA, USA); and UCP-1 (35), from Sigma-Aldrich.
Tissue histology and Immunohistochemistry
Adipose tissues were fixed with 4% paraformaldehyde in phosphate-buffered saline and then dehydrated, embedded in paraffin, and sectioned (5-μm thickness). Sections were stained with hematoxylin and eosin or incubated with UCP-1 (Sigma-Aldrich) or CD31 antibody (36) followed by 3,3-diaminobenzidine staining and examined under bright-field microscopy (Nikon 80i). Representative of 3–4 biological replicates for histological analyses are shown.
Statistics
Data were analyzed by unpaired t test between 2 independent groups. To analyze more than 2 groups, 1-way analysis of variance (ANOVA) was used, and Tukey’s test for multiple comparisons to determine differences between each group with Prism 6 software (GraphPad Software, La Jolla, CA, USA). For the experiments involving FGF21 LKO mice, data were analyzed by the 2-way ANOVA test and the Tukey’s test for multiple comparisons to determine differences between each group. Data are presented as means ± standard error of mean (SEM). Statistical significance was set at P < 0.05.
Results
Liver FGF21 and adipose-specific VEGF expression are induced in parallel during fasting
To explore the physiological roles of FGF21 and VEGF in fasting, we first investigated the dynamic changes of FGF21 and VEGF expressions in various types of adipose tissues (iWAT, BAT, and eWAT), the liver, and the circulation levels at various time periods after fasting. Consistent with a previous report (37), there was a modest decline in serum level of VEGF after 6 h of fasting (Fig. 1A). Interestingly, Vegfa expression levels in eWAT (Fig. 1B) and iWAT (Fig. 1C) increased as early as 6 h of fasting. However, the Vegfa messenger (mRNA) expression levels in the BAT (Fig. 1D), liver (Fig. 1E), and muscle (Fig. 1F), remained unchanged. Consistently, VEGF protein levels were also increased in eWAT and iWAT by fasting (Fig. 1G and 1H). In agreement with previous reports (22,38), the increase of circulating FGF21 (Fig. 1I) and liver Fgf21 expression occurred as early as 6 h of fasting (Fig. 1J), while Fgf21 expression in eWAT (Fig. 1K), iWAT (Fig. 1L), BAT (Fig. 1M) , and muscle (Fig. 1N) remained unaltered. In addition, food removal for 12 h was sufficient to increase the free fatty acid (Fig. 1O) and ketone bodies levels (Fig. 1P). These results suggested that there may be a close relationship between liver FGF21 and adipose VEGF expression and fasting led to a selective upregulation of VEGF in WAT.
VEGF expression in adipocytes is induced by FGF21
We have showed that fasting dramatically induced VEGF expression in adipose tissue (Fig. 1G and 1H). To evaluate which kind of cells in WAT were involved in this process, primary adipocytes and stromal vascular cells were isolated from the iWAT and eWAT of fed or 24-h–fasted mice. Consistent with the previous results (Fig. 1G and 1H), fasting stimulated Vegfa expression in adipose tissues (Fig. 2A and 2B). Intriguingly, fasting only induced significant increase of Vegfa expression in adipocytes but not in stromal vascular cells neither in iWAT nor eWAT (Fig. 2A and 2B), indicating that adipocytes might be the producer of VEGF in response to fasting in vivo. To evaluate the effect of FGF21 on VEGF expression during fasting, wild-type and FGF21 LKO mice were fasting for 24 h. The mRNA levels of Vegfa in both iWAT and eWAT in wild-type mice were greater compared to FGF21 LKO mice after 24 h of fasting (Fig. 2C), which suggested that liver FGF21 play a role in fasted-induced WAT VEGF expression. To evaluate the direct effect of FGF21 on VEGF expression, mice were treated with a single bolus of recombinant mouse FGF21 (rmFGF21). Compared with the effects in control mice, rmFGF21 administration induced Vegfa mRNA expression levels after 3 h of rmFGF21 injection in both eWAT (Fig. 2D) and iWAT (Fig. 2E), but not in BAT (Fig. 2F). To our surprise, rmFGF21 injection increased the WAT VEGF expression levels as early as 1 h after injection (Fig. 2G and 2H), which suggested that FGF21 injection not only increase VEGF protein synthesis but also increase the protein stability.
Dietary intake induced WAT VEGF expression and angiogenesis requires liver FGF21 signaling
Liver-derived FGF21 is the main contributor to circulating FGF21 (20-22); thus, FGF21 LKO mice were used to examine the role of hepatic FGF21 in the regulation of WAT VEGF expression and angiogenesis induced by IF. Wild-type and FGF21 LKO mice on a HFD were fed ad libitum or subjected to IF for 16 weeks (Fig. 3A). Neither the genotype nor the feeding pattern affected food intake (data not shown). Intermittent fasting prevented body weight gain in WT mice but not in FGF21 LKO mice (Fig. 3B and 3C), which was accompanied by a reduction in fat composition in WT mice with time-restricted access to food (HT) and no effect was observed in FGF21 LKO mice (Fig. 3D). Since IF is a cyclic fast-refed pattern, serum and tissue samples were collected at 7:00 am when the IF mice were fed for 10 h and at 9:00 pm when the IF mice were fasted for 14 h. Intermittent fasting increased serum FGF21 concentrations at both time points in WT mice, and the increase in serum FGF21 was especially augmented during fasting, which did not occur in FGF21 LKO mice (Fig. 3E). Intermittent fasting significantly increased WAT vascularization in WT mice, but not in FGF21 LKO mice (Fig. 3F). Indeed, IF induced VEGF mRNA and protein expression in iWAT and eWAT especially at night (Fig. 3G–I), which was also abolished in FGF21 LKO mice. Immunocytochemical analysis epitopes for the vasculatures, CD31, demonstrated that IF increased vascularization in WT mice, but not in FGF21 LKO mice (Fig. 3J). Collectively, these data strongly suggest that secretion of FGF21 is critical for IF on facilitating WAT VEGF expression and vascularization.
Liver FGF21 is required for dietary intake mediated metabolic benefits
To assess energy expenditure, a comprehensive laboratory animal monitoring system was used. The oxygen consumption (Fig. 4A) and carbon dioxide production (Fig. 4B) were not changed during the light cycle but were increased by IF in the WT mice only during the dark phase, but this effect was not observed in FGF21 LKO mice (Fig. 4A and 4B). The glucose tolerance test (Fig. 4C and 4D) and serum insulin levels (Fig. 4E) demonstrated that IF ameliorated HFD-induced insulin resistance in WT mice but not FGF21 LKO mice. FGF21 liver specific knock-out also abolished IF-induced expression of browning genes, such as Ucp-1, Pgc-1a, Dio2, and Elovl6 in iWAT both at 7:00 am and 9:00 pm (Fig. 4F). Moreover, loss of liver FGF21 blocked the IF-driven induction of iWAT UCP-1 protein expression, as revealed by western blotting and immunohistochemistry analysis (Fig. 4G and 4H). Consistent with these changes, oxygen consumption (VO2) remains unchanged, indicating that IF did not increased energy expenditure in FGF21 LKO mice (Fig. 4A). To explore whether IF was associated with changes in WAT inflammation, the inflammation related gene expression levels were performed. The expression of M1 macrophage polarization marker genes, including Tnfα, and IL1β, were decreased by IF in WT mice but not in FGF21 LKO mice (Fig. 4I). These data suggested that IF regulate the inflammation in WAT through FGF21 signaling. Collectively, these data suggested that enhanced secretion of FGF21 is critical for IF induced alleviation of the inflammation and HDF-induced insulin resistance.
Discussion
Obesity and the associated multiple comorbidities represent one of the most serious health issues affecting one third of global adults (39). Improvements in adipose tissue angiogenesis offer a potential therapeutic avenue for metabolic diseases (2). The present study showed that a drastic effect of 1-time and intermittent fasting on WAT angiogenesis, which promoted metabolic flexibility in adipose tissues and prevented obesity associated metabolic disorder. Furthermore, we found that fasting induced a selective and drastic elevation in VEGF levels in WAT via liver FGF21. The fasting-induced Vegfa expression occurred predominantly in mature adipocytes, but not in the stromal vascular fraction in eWAT and iWAT. Long-term IF increased WAT angiogenesis and iWAT browning, which improved insulin resistance via liver FGF21. In summary, these data suggest that FGF21 is a potent regulator of WAT VEGF levels and emphasize the link between the dietary patterns and adipose angiogenesis.
Adipose tissue is essential for energy homeostasis and metabolic health and is considered to be a main target for the treatment of obesity and diabetes (2,40). In the present study, based on the EchoMRI™ analysis showed IF specifically, reduced fat mass without any effect on lean body mass (Fig. 3D). To support the metabolic homeostasis, the plasticity of the adipose tissues is crucial for maintaining appropriate energy balance (2). Adipose tissues, especially BAT, had a high level of vessel density (6). Adipose vasculature has multiple functions that is required for energy homeostasis, such as oxygen and nutrient supply, and transports of metabolic factors. Adipocyte overexpressing VEGF triggered angiogenesis and contributed to resistance of HFD-induced obesity (13,14). The present work revealed that 1-time fasting and cyclical intermittent fasting induced a selective and drastic elevation of VEGF expression levels in WAT and increased the WAT angiogenesis and thermogenesis in intermittent fasting (Figs. 1G and H and 3F-J). In mammals, the liver is the peripheral integrator of nutrient availability and energy needs of the organism (41,42); therefore, liver-derived factors hold great promise as targets for WAT VEGF expression and obesity-related metabolic disorder.
Most researchers have focused on the role of a single tissue metabolism with particular interest in the adipose tissue to investigate the molecular mechanism of the beneficial effects of intermittent and periodic fasting on energy homeostasis (43-45). However, this feeding pattern induces a series of metabolic changes that might require interorgan crosstalk. In most of the mammalian species, the liver is the primary peripheral organ that senses circulating nutrient availability and is important for fasting, feeding, digestion, and metabolic balance (41,46). Liver-derived FGF21 is considered as a starvation hormone that could be rapidly induced in the liver by fasting (25,26), and, in turn, FGF21 acted as a critical endocrine signal to regulate the systemic energy metabolism (31,47). In the present study, liver Fgf21 expression and circulating FGF21 concentrations underwent coincident changes with WAT VEGF levels (Fig. 1G-I), confirming the close relationship between serum FGF21 levels and WAT VEGF expression. Interestingly, fasting increased VEGF expression in adipocytes but not in stromal vascular fraction in eWAT and iWAT (Fig. 2A and 2B), where there is high expression levels of FGF21 receptor Klothoβ and fibroblast growth factor receptor-1 (48). In the present study, we showed that a single bolus of recombinant mouse FGF21 increased VEGF levels in WAT (Fig. 2G and 2H). It is worth noting that FGF21 injection increased the VEGF protein expression before the changes of mRNA levels, which suggested that FGF21 not only increase VEGF protein synthesis but also increase the protein stability through unknown mechanisms.
The high thermogenic activity of adipose tissues requires a particularly high rate of blood perfusion both to supply O2 and substrates and to export metabolic factors (2). VEGF has long been associated with increased vascular permeability and improves the access of metabolic substrates to tissues (49). VEGF is highly expressed in adipose tissue (6, 7) and overexpression of VEGF in adipocytes increases iWAT browning (4). In the present study, we showed that both acute fasting and IF induced substantial increase in VEGF expression in WAT, enhanced oxygen consumption during the night in IF, which was consistent with the upregulated UCP-1 expression in iWAT. Periodic fasting resulted in benefits ranging from the prevention to the enhanced treatment of diseases (5,50). Consistent with previous investigations (5,43,45), the results of the present study revealed that IF significantly reduced HFD-induced body weight and adipose tissue gain, as well as improved glucose clearance (Fig. 3B-D and 4C-E). Recently, the beneficial effects of IF on adipose thermogenesis and M2 macrophage activation were reported to be dependent on adipose VEGF (37). Indeed, we found that both IF and acute fasting induced substantial increase in VEGF expression and the anti-inflammation related genes in adipose tissue in vivo (Fig. 3F-I and 4I). Interestingly, the effects of IF on VEGF expression and anti-inflammation were abolished when liver FGF21 was absent, suggested that the beneficial effects of IF on adipose tissues metabolism are mainly attributed to FGF21 signaling. In the present study, loss of liver FGF21 abolished the effects of IF on reducing body weight (Fig. 3B) and browning of adipose tissue (Fig. 4F), indicating the critical role of FGF21 in mediating liver-adipose crosstalk during IF. However, the body weight and adipose tissue weight of FGF21 LKO mice were greater than the WT mice in the HT group (Fig. 3B), and the reason for that need further investigation.
For the first time, our present study demonstrated that a drastic effect of 1-time and intermittent fasting induced a selective and drastic elevation in VEGF levels in WAT. In support of those findings, IF-induced WAT angiogenesis was abolished in FGF21 LKO mice during IF (Fig. 3F and 3J), suggesting that liver FGF21 is an important meditator in this process. However, it was unclear in the present study that the browning induced by FGF21 was attributed to its direct effect on adipocytes or its ability to induce VEGF-mediated browning. It will be interesting to examine the implication of FGF21-mediated metabolic benefits in this process. On the other hand, FGF21-induced VEGF expression may improve adipocyte access to metabolic substrates, which, in turn, promotes beige adipocyte development.
In conclusion, the present study showed a drastic effect of intermittent fasting on WAT angiogenesis, which promoted the metabolic flexibility of adipose tissues and prevented obesity-associated metabolic disorder (Fig. 4J). Furthermore, we found that fasting induced a selective and drastic elevation in VEGF levels in WAT. Long-term IF regulated WAT angiogenesis, iWAT browning, insulin resistance, and inflammation via liver FGF21. Based on the results in the present study, the interorgan FGF21 signaling induced WAT angiogenesis by VEGF could be a novel potential therapeutic target in combination with obesity-related metabolic disorders.
Acknowledgments
The authors wish to thank the laboratory staff for their ongoing assistance and Prof. Jingyuan Xiong, West China School of Public Health, Sichuan University, for helping to analyze body composition. The authors would also like to thank Dr. Paul Dyce, Department of Animal Science, Auburn University for his careful editing of the manuscript.
Financial Support: This study was supported by National Key R&D Program of China (Grant 2018YFD0501005), National Natural Science Foundation of China, PR China (Grant 31772616) and the Higher Education Discipline Innovation Project of China (D17015). The funding sources had no role in study design, collection, analysis, and interpretation of data, writing of the report, or in the decision to submit the paper for publication.
Author Contributions: DW, YZ, and BF designed the research; DW, LH, YZ, and BF wrote the paper; LH, YZ, and JL performed the research and analyzed the data; XMJ and DDJ contributed to the analysis and manuscript preparation; SYX, LY, ZFF, TL, and LQC contributed to constructive discussions. DW had primary responsibility for final content. All authors read and approved the final manuscript.
Glossary
Abbreviations
- BAT
brown adipose tissue
- Dio2
iodothyronine deiodinase 2
- Elovl6
ELOVL family member 6, elongation of very long chain fatty acids
- eWAT
epididymal white adipose tissue
- FGF21
fibroblast growth factor 21
- FGF21 LKO
fibroblast growth factor 21 liver-specific knockout
- GTT
glucose tolerance test
- HFD
high-fat diet
- IF
intermittent fasting
- Il1-β
interleukin 1β
- iWAT
inguinal white adipose tissue
- Pgc1α
peroxisome proliferator activated receptor gamma coactivator 1-alpha
- Tnfα
tumor necrosis factor α
- UCP-1
uncoupling protein 1
- VEGF
vascular endothelial growth factor
- WAT
white adipose tissues
Additional Information
Disclosure Summary: The authors have no conflicts of interest to report.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References. The data that support the findings of this study are available from De Wu or Yong Zhuo upon reasonable request.
References
- 1. Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126(1):126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cao Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013;18(4):478-489. [DOI] [PubMed] [Google Scholar]
- 3. Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoon-Ki S, Kyung-Oh D, Joe Eun S, et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17(1):61-72. [DOI] [PubMed] [Google Scholar]
- 5. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212-1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brakenhielm E, Cao Y. Angiogenesis in adipose tissue. Methods Mol Biol. 2008;456:65-81. [DOI] [PubMed] [Google Scholar]
- 7. Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2(59):re1. [DOI] [PubMed] [Google Scholar]
- 8. Abdullahi A, Jeschke MG. White adipose tissue browning: a double-edged sword. Trends Endocrinol Metab. 2016;27(8):542-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279(2):H772-H778. [DOI] [PubMed] [Google Scholar]
- 10. Kraus RM, Stallings HW 3rd, Yeager RC, Gavin TP. Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. J Appl Physiol (1985). 2004;96(4):1445-1450. [DOI] [PubMed] [Google Scholar]
- 11. Sun K, Kusminski CM, Luby-Phelps K, et al. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Mol Metab. 2014;3(4):474-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xue Y, Petrovic N, Cao R, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9(1):99-109. [DOI] [PubMed] [Google Scholar]
- 13. Wu LE, Meoli CC, Mangiafico SP, et al. Systemic VEGF-A neutralization ameliorates diet-induced metabolic dysfunction. Diabetes. 2014;63(8):2656-2667. [DOI] [PubMed] [Google Scholar]
- 14. Park J, Kim M, Sun K, An YA, Gu X, Scherer PE. VEGF-A-expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4-independent metabolic improvements. Diabetes. 2017;66(6):1479-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim M, An YA, Bang HS, et al. VEGF-A-expressing adipose tissue shows rapid beiging, enhanced survival after transplantation. Diabetes. 2018; 67(Supplement1):279-LB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elias I, Franckhauser S, Bosch F. New insights into adipose tissue VEGF-A actions in the control of obesity and insulin resistance. Adipocyte. 2013;2(2):109-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guridi M, Tintignac LA, Lin S, Kupr B, Castets P, Rüegg MA. Activation of mTORC1 in skeletal muscle regulates whole-body metabolism through FGF21. Sci Signal. 2015;8(402):ra113. [DOI] [PubMed] [Google Scholar]
- 19. Ruan CC, Kong LR, Chen XH, et al. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab. 2018;28(3):476-489.e5. [DOI] [PubMed] [Google Scholar]
- 20. Hua L, Zhuo Y, Jiang D, et al. Identification of hepatic fibroblast growth factor 21 as a mediator in 17β-estradiol-induced white adipose tissue browning. Faseb J. 2018;32(10):5602-5611. [DOI] [PubMed] [Google Scholar]
- 21. Zhuo Y, Hua L, Feng B, et al. Fibroblast growth factor 21 coordinates adiponectin to mediate the beneficial effects of low-protein diet on primordial follicle reserve. Ebiomedicine. 2019;41:623-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Solon-Biet SM, Cogger VC, Pulpitel T, et al. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 2016;24(4):555-565. [DOI] [PubMed] [Google Scholar]
- 23. Laeger T, Albarado DC, Burke SJ, et al. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 2016;16(3):707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med. 2011;17(7-8):736-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Markan KR, Naber MC, Ameka MK, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63(12):4057-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dutchak PA, Katafuchi T, Bookout AL, et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148(3):556-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hua L, Feng B, Huang L, et al. Time-restricted feeding improves the reproductive function of female mice via liver fibroblast growth factor 21. Clin Transl Med. 2020;10(6):e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fisher FM, Kleiner S, Douris N, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26(3):271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19(2):302-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li H, Wu G, Fang Q, et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat Commun. 2018;9(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Owen BM, Ding X, Morgan DA, et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014;20(4):670-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng B, Jiao P, Helou Y, et al. Mitogen-activated protein kinase phosphatase 3 (MKP-3)-deficient mice are resistant to diet-induced obesity. Diabetes. 2014;63(9):2924-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. RRID; AB_299738, https://antibodyregistry.org/search.php?q=AB_299738
- 34. RRID; AB_2242334, https://antibodyregistry.org/search.php?q=AB_2242334
- 35. RRID; AB_11182298, https://antibodyregistry.org/search.php?q=AB_11182298
- 36. RRID; AB_2739310, https://antibodyregistry.org/search.php?q=AB_2739310
- 37. Kim KH, Kim YH, Son JE, et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res. 2017;27(11):1309-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78:223-241. [DOI] [PubMed] [Google Scholar]
- 39. Font-Burgada J, Sun B, Karin M. Obesity and cancer: the oil that feeds the flame. Cell Metab. 2016;23(1):48-62. [DOI] [PubMed] [Google Scholar]
- 40. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Della Torre S, Rando G, Meda C, et al. Amino acid-dependent activation of liver estrogen receptor alpha integrates metabolic and reproductive functions via IGF-1. Cell Metab. 2011;13(2):205-214. [DOI] [PubMed] [Google Scholar]
- 42. Maida A, Zota A, Sjøberg KA, et al. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J Clin Invest. 2016;126(9):3263-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marosi K, Moehl K, Navas-Enamorado I, et al. Metabolic and molecular framework for the enhancement of endurance by intermittent food deprivation. Faseb J. 2018;32(7):3844-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kinouchi K, Magnan C, Ceglia N, et al. Fasting imparts a switch to alternative daily pathways in liver and muscle. Cell Rep. 2018;25(12):3299-3314.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kliewer SA, Mangelsdorf DJ. A dozen years of discovery: insights into the physiology and pharmacology of FGF21. Cell Metab. 2019;29(2):246-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ding X, Boney-Montoya J, Owen BM, et al. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16(3):387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Langlet F, Levin BE, Luquet S, et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013;17(4):607-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017;37:371-393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References. The data that support the findings of this study are available from De Wu or Yong Zhuo upon reasonable request.