Abstract
Increased glucagon is a hallmark of diabetes and leads to worsening of the hyperglycemia, but the molecular mechanisms causing it are still unknown. We therefore investigated the possibility that microRNAs might be involved in the regulation of glucagon. Indeed, analysis of the glucagon 3′ untranslated region (UTR) revealed potential binding sites for miR-320a, and using luciferase reporter assays we found that miR-320a directly targets the 3′ UTRs of human and rodent glucagon. In addition, endogenous glucagon mRNA and protein expression as well as glucagon secretion were reduced in response to miR-320a overexpression, whereas inhibition of miR-320a upregulated glucagon expression. Interestingly, miR-320a expression was decreased by high glucose, and this was associated with an increase in glucagon expression in human islets and mouse αTC1-6 cells. Moreover, miR-320a overexpression completely blunted these effects. Importantly, miR-320a was also significantly downregulated in human islets of subjects with type 2 diabetes and this was accompanied by increased glucagon expression. Thus, our data suggest that glucose-induced downregulation of miR-320a may contribute to the paradoxical increase in glucagon observed in type 2 diabetes and reveal for the first time that glucagon expression is under the control by a microRNA providing novel insight into the abnormal regulation of glucagon in diabetes.
Keywords: glucagon, microRNA, diabetes, human islets, alpha cells
Hyperglucagonemia is a key characteristic of diabetes and, in fact, diabetes has been defined as a bihormonal disease characterized not only by insulin deficiency, but also by glucagon excess due to continuous glucagon secretion from alpha cells (1-3). Under physiological conditions, glucagon plays an important role as a counter-regulatory hormone to insulin in increasing plasma glucose levels by stimulating hepatic glucose production during the fasting state (4). Glucagon is also involved in energy metabolism, including the stimulation of thermogenesis in brown adipose tissue (5) and lipid metabolism through the activation of lipolysis and the inhibition of lipid synthesis (6). However, under diabetic conditions, hyperglycemia, inadequate insulin levels, and insulin resistance lead to paradoxically increased serum glucagon levels in the postprandial state and in the fasting state. This pathologic elevation of glucagon levels also contributes to a vicious cycle of persistently increased blood glucose levels and worsening of the diabetes (7, 8). Although reports about the potential cause of type 2 diabetes–associated hyperglucagonemia have started to emerge during recent years (9), the molecular mechanisms involved in the dysregulation of glucagon control in the context of diabetes are still unclear.
MicroRNAs are small noncoding RNAs that bind to the 3′ untranslated region (UTR) of target mRNAs in a sequence-specific manner (10-12) resulting in RNA silencing and/or post-transcriptional regulation of gene expression and thereby play important roles in cell biology including the function of pancreatic islets (11, 13, 14). Previous studies have shown that alterations in some microRNA levels can contribute to the development of diabetes via impaired pancreas development (14, 15), alterations in insulin levels (16-18) and beta cell apoptosis (19, 20) and efforts are currently underway to study the function of microRNAs as potential therapeutic targets for preventing the development of diabetes (21, 22). We therefore hypothesized that microRNAs may also play a role in the regulation of alpha cell glucagon production. Using multiple computational prediction programs, we identified miR-320a as the top putative microRNA predicted to bind to the human, mouse and rat glucagon 3′ UTR and in turn, glucagon to be the top predicted target of miR-320a.
The aim of the present study was therefore to investigate whether glucagon is indeed under the control of miR-320a and whether alterations in miR-320a might contribute to the pathologic elevation of glucagon during diabetes.
Materials and Methods
Tissue culture
Murine alpha TC1 (clone 6) (αTC1-6) cells (ATCC #CRL-2934, Manassas, VA) (23) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (#11966025, Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, 16.7 mM glucose, 1 mM sodium pyruvate, and 15 mM HEPES. HEK293 cells (ATCC #CRL-1573) (24) were grown in high glucose DMEM (#11965092, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin.
Human islets were obtained from the Integrated Islet Distribution Program (IIDP) and at least islets from 3 different donors were used per experiment and always islets from the same donor were used as a control. Detailed information about the islet preparations used is provided in Table 1 as recommended (25). Human islets isolated from donors with type 2 diabetes and from nondiabetic control donors were harvested immediately upon receipt. For experiments conducted in the context of high (25 mM) glucose, αTC1-6 cells and primary human islets were maintained overnight in 5 mM glucose DMEM or RPMI 1640 medium (Thermo Fisher Scientific) and then incubated for 24 hours in 25 mM glucose DMEM or RPMI 1640 medium using the miRNeasy Mini Kit (#217004, Qiagen, Valencia, CA) for RNA isolation and were examined for miR-320a and glucagon expression using quantitative real-time polymerase chain reaction (PCR).
Table 1.
Human islet preparations used in the study
| Islet prep | Origin | Islet isolation center | Unique identifier | Age (y) | Sex | BMI (kg/ m2) | HbA1c (%)/Blood glucose (mg/dL) | Hx of DM | Cause of death | Cold ischemia (hours) | Purity (%) | Viability (%) | Handpicked |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IIDP | Southern CA | ADF1197 | 43 | F | 29.5 | 5.7/192 | N | Stroke | N/A | 97 | 97 | Y |
| 2 | IIDP | U Miami | ADF2439 | 62 | F | 30.4 | 5.5/219.2 | Y | Stroke | 16 | 93 | 93 | Y |
| 3 | IIDP | Southern CA | ADGU064 | 30 | M | 35.9 | N/A/176.4 | N | Stroke | 12.5 | 90 | 95 | Y |
| 4 | IIDP | U Miami | ADGW466A | 39 | F | 24.8 | N/A/223.4 | N | Stroke | 16.5 | 95 | 95 | Y |
| 5 | IIDP | Southern CA | ADHR238B | 47 | M | 31 | 6.3/220.6 | N | Anoxia | N/A | 85 | 97 | Y |
| 6 | IIDP | Southern CA | ADHR295A | 62 | M | 35.9 | 7.4/189.8 | Y | Stroke | N/A | 63 | 63 | Y |
| 7 | IIDP | Sharp Lacy | ADHW192 | 61 | M | 28.4 | 5.2/162.6 | Y | Stroke | 12.5 | 95 | 95 | Y |
| 8 | IIDP | U Wisconsin | ADI3256 | 47 | F | 36.4 | 5.3 121.2 | N | Anoxia | N/A | 97 | 97 | Y |
| 9 | IIDP | Southern CA | ADIY035 | 15 | M | 24.6 | 5.1/122.2 | N | Head trauma | N/A | 97 | 97 | Y |
| 10 | IIDP | U Penn | AD12469 | 47 | M | 32.2 | 5.7/234.2 | Y | Stroke | N/A | 70 | 70 | Y |
| 11 | IIDP | U Penn | AD12469 | 47 | M | 32.2 | 5.7/234.2 | Y | Stroke | N/A | 70 | 70 | Y |
| 12 | IIDP | U Penn | AEC2011D | 65 | F | 42.6 | 5.9/174.4 | Y | Anoxia | 13.2 | 90 | 90 | Y |
| 13 | IIDP | Sharp Lacy | AEDM302 | 29 | M | 26.7 | N/A/155.6 | N | Anoxia | 9 | 85 | 85 | Y |
| 14 | IIDP | U Wisconsin | AEDT128 | 26 | F | 38.9 | N/A/188 | N | Stroke | 8.5 | 95 | 98 | Y |
| 15 | IIDP | Sharp Lacy | AEEM022 | 57 | M | 29.1 | N/A/158.8 | N | Stroke | 13 | 95 | 95 | Y |
| 16 | IIDP | Sharp Lacy | SAMN11642375 | 58 | M | 32.5 | 6.7/171.6 | Y | Stroke | 11 | 85 | 85 | Y |
| 17 | IIDP | Southern CA | SAMN11791244 | 47 | M | 36.1 | N/A/166.2 | N | Stroke | 6.5 | 95 | 95 | Y |
| 18 | IIDP | U Wisconsin | SAMN12227196 | 51 | M | 32.8 | N/A/96.8 | N | Cardiac arrest | 12 | 95 | 95 | Y |
| 19 | IIDP | U Miami | SAMN12274306 | 37 | M | 25.3 | N/A/192.8 | N | Anoxia | 15.2 | 80 | 80 | Y |
| 20 | IIDP | Sharp Lacy | SAMN16547514 | 60 | F | 28.3 | 4.6/N/A | N | Head trauma | 8.6 | 90 | 95 | Y |
Plasmid construction, transfection, and luciferase assays
The wild-type (WT) human, mouse and rat glucagon 3′ UTR regions containing miR-320a binding sites were amplified from genomic DNA using the primers listed in Table 2. To generate the glucagon 3′ UTRs with mutated miR-320a binding sites, mutations were introduced by PCR and primers listed in Table 2. PCR products were subcloned into the SpeІ and PmeІ sites of the pMIR-REPORT Luciferase vector (#AM5795, Thermo Fisher Scientific) yielding the WT and mutant glucagon 3′ UTR luciferase reporter plasmids. All plasmids were confirmed by sequencing. For luciferase assays, HEK293 cells were plated in 12-well plates and grown overnight to ~60% confluence. Cells were transfected with a glucagon 3′ UTR luciferase reporter plasmid together with the miRIDIAN microRNA Human hsa-miR-320a mimic (#C-300645-05-0002, GE Dharmacon, Lafayette, CO) or scrambled control oligos (#AM17111, Thermo Fisher Scientific) using DharmaFECT Duo transfection reagent (#T-2010, GE Dharmacon). To control for transfection efficiency, cells were cotransfected with pRL-TK (#E2231, Promega, Fitchburg, WI) control plasmid expressing renilla luciferase, and 48 hours after transfection firefly as well as renilla luciferase activity were determined using the Dual Luciferase Assay Kit (#E1910, Promega).
Table 2.
Primers used in the study
| Primers | Sequences (5′-3′) |
|---|---|
| 18s qPCR 5′ primer | GCTTAATTTGACTCAACACGGGA |
| 18s qPCR 3′ primer | GCTATCAATCTGTCAATCCTGTCC |
| Human GCG 5′ primer | TGGCAACGTTCCCTTCAAGA |
| Human GCG 3′ primer | GATCACTGAGTGGGTCTGCC |
| Mouse Gcg 5′ primer | ATCTTGCCACCAGGGACTTC |
| Mouse Gcg 3′ primer | AAGTGACTGGCACGAGATGT |
| Mouse MafB 5′ primer | CCTACAAGGTCAAGTGCGAGAA |
| Mouse MafB 3′ primer | AGCCCGCCTCCCTGAA |
| Mouse Pax6 5′ primer | CCACTTCAACAGGACTCATTTCAC |
| Mouse Pax6 3′ primer | CACATGCTCTCTCCTTCTCTCTTTA |
| Mouse FoxA2 5′ primer | GGAGCCGTGAAGATGGAAG |
| Mouse FoxA2 3′ primer | TGTGTTCATGCCATTCATCC |
| Human GCG WT 3′UTR 5′ cloning primer-1 | ATAACTAGTAAGAGTCCAAAGGATGGAGGGA |
| Human GCG WT 3′UTR 3′ cloning primer-1 | ATAGTTTAAACAGAAAACAGGGGTGCTTGGA |
| Human GCG WT 3′UTR 5′ cloning primer-2 | ATAACTAGTCTATATCACTATTCAAGATC |
| Human GCG WT 3′UTR 3′ cloning primer-2 | ATAGTTTAAACAGACAATGGTAAAGAAGCTTGAA |
| Human GCG M1 3′UTR 5′ cloning primer | ATATTGATCTGGAAATATGAAAGTGC |
| Human GCG M1 3′UTR 3′ cloning primer | ATTTCCAGATCAATATTTTAGCAATATTTTG |
| Human GCG M2 3′UTR 5′ cloning primer | TACTTGATCTGCAATGTATCAAAGATAC |
| Human GCG M2 3′UTR 3′ cloning primer | ATTGCAGATCAAGTATTTTTAAGACAAAG |
| Mouse Gcg WT 3′UTR 5′ cloning primer-1/2 | ATAACTAGTGTATTTCACCATTCACAACCATCTT |
| Mouse Gcg WT 3′UTR 3′ cloning primer-1 | ATAGTTTAAACTATGGCCACCAGCTGAGAAA |
| Mouse Gcg WT 3′UTR 3′ cloning primer-2 | ATAGTTTAAACTTTATTTAAATCTATTCTTGATACATC |
| Mouse Gcg M 3′UTR 5′ cloning primer | CACTTGATCTGGTGATGTATCAAGAATAG |
| Mouse Gcg M 3′UTR 3′ cloning primer | ATCACCAGATCAAGTGCATTTATGGTAAAG |
| Rat Gcg WT 3′UTR 5′ cloning primer-1 | ATAACTAGTAACAATTCCTGCTTTTGTCCTCA |
| Rat Gcg WT 3′UTR 3′ cloning primer-1 | ATAGTTTAAACATGGTAGAGTACTGCACGGC |
| Rat Gcg WT 3′UTR 5′ cloning primer-2 | ATAACTAGTGAATATTTCACCATTCACAACC |
| Rat Gcg WT 3′UTR 3′ cloning primer-2 | ATAGTTTAAACTAATAACAAAGGATGAGATATTTA |
| Rat Gcg M 3′UTR 5′ cloning primer | CGCTTGATCTGGCAATGTATCAAGAATAG |
| Rat Gcg M 3′UTR 3′ cloning primer | ATTGCCAGATCAAGCGCATTTATGACAAAG |
MicroRNA transfection
To overexpress or knockdown miR-320a, αTC1-6 cells were plated in 6-well plates and grown overnight to ~60% confluence. Cells were maintained at 25 mM glucose and transfected with hsa-miR-320a miRIDIAN microRNA Mimic (GE Dharmacon, Lafayette, CO), hsa-miR-320a miRIDIAN microRNA Hairpin Inhibitor (#IH-300645-06-0002, GE Dharmacon) or corresponding control oligos at a final concentration of 25 nM using the DharmaFECT1 transfection reagent (#T-2001, GE Dharmacon).
Glucagon secretion and content assays
αTC1-6 cells were plated in 24-well plates, grown overnight to ~60% confluency and transfected with has-miR-320a miRIDIAN miRNA mimic or inhibitor or corresponding control oligos. Seventy-two hours after transfection, cells were preincubated in Krebs-ringer bicarbonate (KRB) buffer containing 5 mM glucose (135 mM NaCl, 3.6 mM KCl, 10 mM Hepes [pH 7.4], 5 mM NaHCO3, 0.5 mM NaH2PO4, 0.5 mM MgCl2, 1.5 mM CaCl2) for 1 hour and then in KRB buffer containing 25 mM glucose for an additional 2 hours. The supernatant was collected for assessment of glucagon secretion. Cells were rinsed and harvested for cellular glucagon content using acid–ethanol extraction buffer (75% v/v ethanol supplemented with 0.15 N HCl). Glucagon was measured using Mouse Glucagon ELISA Kit (Crystal Chem #81518, Elk Grove Village, IL) (26) by following the manufacturer’s instructions and normalized for protein by Pierce BCA protein assay (Thermo Fisher Scientific). Human islets were incubated overnight at 5 mM glucose, then 100 islets per sample were handpicked, mildly dispersed with 0.25% trypsin-EDTA and transfected with has-miR-320a miRIDIAN miRNA mimic or control oligos using DharmaFECT1. After 72 hours, islets were again incubated in KRB buffer and the supernatant was collected for assessment of glucagon secretion. Glucagon was measured using Human Glucagon ELISA Kit (Crystal Chem #81520, Elk Grove Village, IL) (27) by following the manufacturer’s instructions and normalized for protein by Pierce BCA protein assay (Thermo Fisher Scientific).
RNA isolation and quantitative real-time RT-PCR
Total RNA was isolated using miRNeasy Mini Kit (#217004, Qiagen, Valencia, CA) and converted to cDNA using Transcriptor First Strand cDNA Synthesis Kit (#04897030001, Roche Life Science, Indianapolis, IN). Quantitative real-time PCR was performed on a LightCycler 480 system (Roche) using SYBR-Green (#4309155, Thermo Fisher Scientific). MicroRNA-320a expression was quantified using a TaqMan microRNA Assay kit (#002277, Thermo Fisher Scientific) with TaqMan Universal PCR Master Mix (#4324018, Thermo Fisher Scientific). Gene and microRNA expression results were corrected for 18s and U6, respectively. The primers used for quantitative real-time PCR are listed in Table 2.
Statistical analysis
Student t-tests were used to calculate the significance of a difference between 2 groups. For data sets of more than 2 groups we performed 1-way analysis of variance calculations. A P-value of <0.05 was considered significant.
Results
MiR-320a directly targets and controls the glucagon 3′ UTR
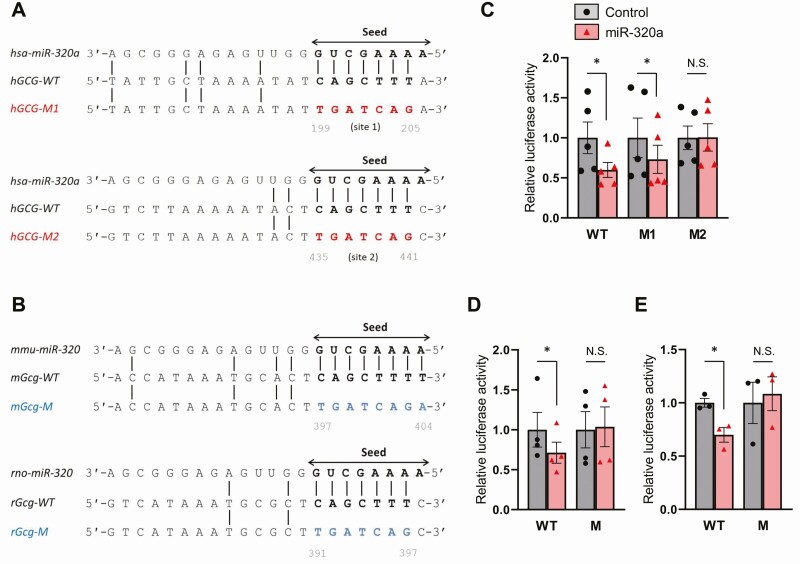
Sequence analysis of the 3′ UTR of the (pro)glucagon gene using microRNA prediction software programs (microRNA.org, microT-CDS [aka Diana tools], and mirDIP) led to the identification of two potential miR-320a seed sequence target sites in the human glucagon gene as well as one potential target site in the mouse and rat glucagon genes, respectively (Fig. 1A and 1B). In addition, glucagon was identified as the top target of miR-320a by miRDB.org. To address the question of whether miR-320a indeed targets the glucagon 3′ UTR, we performed luciferase reporter assays. To this end, we generated a WT human glucagon 3′ UTR luciferase reporter construct as well as two mutant constructs disrupting the first (hGCG-M1) or second (hGCG-M2) seed sequence binding sites (Fig. 1A). Interestingly, cotransfection experiments demonstrated that miR-320a significantly decreased luciferase activity through the WT as well as the hGCG-M1 construct with mutation of the first miR-320a binding site. However, this effect was completely blunted with mutation of the second binding site hGCG-M2 (Fig. 1C). These results revealed that miR-320a directly targets and regulates the 3′ UTR of human glucagon and identified the second putative target site as the actual, functional miR-320a binding site. To further determine the functionality of the putative miR-320a seed sequence target sites identified in the mouse and rat glucagon 3′ UTR, we also generated both mouse and rat WT glucagon 3′ UTR luciferase reporter constructs as well as mutant constructs disrupting the miR-320a binding sites (Fig. 1B). Again, while cotransfection with miR-320a led to significantly decreased luciferase activity through the mouse and rat WT construct, these effects were completely blunted when the miR-320a binding sites were mutated (Fig. 1D and 1E). These findings demonstrate that miR-320a directly targets the 3′ UTR of human as well as mouse and rat glucagon through specific seed sequence binding sites.
Figure 1.
miR-320a directly targets and controls the glucagon 3′ UTR. Alignment of the miR-320a seed sequence (arrow) with (A) 2 putative wild-type human glucagon 3′ UTR target sequences (hGCG-WT, bold) and the mutant target sequences (hGCG-M1 and -M2, red) and with (B) the putative wild-type mouse and rat glucagon 3′ UTR target sequences (mGcg-WT and rGcg-WT, bold) and mutated target sequences (mGcg-M and rGcg-M, blue). miR-320a–mediated effects on (C) human, (D) mouse, and (E) rat glucagon 3′ UTR luciferase reporter activity as assessed 48 hours after cotransfecting HEK293 cells with miR-320a mimic and the wild-type or mutant reporter plasmids. All data are shown as the mean ± standard error of the mean of at least 3 independent experiments, *P < 0.05. The triangles and circles represent individual measurements. N.S., not significant.
MiR-320a regulates endogenous glucagon mRNA levels and protein content
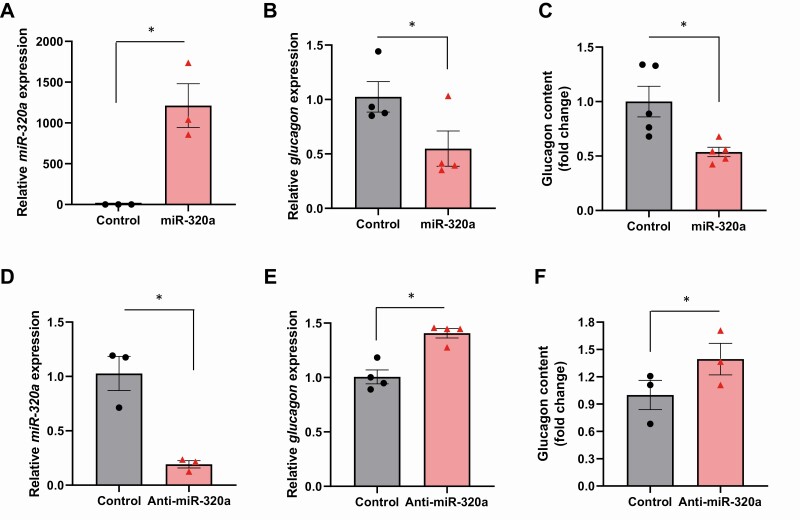
Next, we analyzed the effect of miR-320a on endogenous glucagon gene expression in mouse αTC1-6 cells. Transfection led to effective miR-320a overexpression (Fig. 2A) and, consistent with the results of the luciferase assays, resulted in a significant decrease in endogenous glucagon mRNA levels (Fig. 2B). To further investigate whether these changes in glucagon mRNA levels were also translated into altered protein levels, we examined the effect of miR-320a on glucagon content. Again, endogenous glucagon content was significantly decreased in response to miR-320a overexpression (Fig. 2C). We also achieved highly effective knockdown of miR-320a (Fig. 2D) and this in turn resulted in a significant increase in glucagon mRNA levels (Fig. 2E) as well as in glucagon content (Fig. 2F). These results demonstrate that miR-320a regulates alpha cell glucagon production.
Figure 2.
miR-320a regulates endogenous glucagon mRNA levels and protein content. Mouse αTC1-6 cells were transfected with miR-320a mimic or negative control for 72 hours. (A) Effective miR-320a overexpression was confirmed and (B) glucagon mRNA expression was assessed by qRT-PCR and (C) glucagon content determined by ELISA after acid–ethanol extraction. Effects of transfection with a miR-320a inhibitor (anti-miR-320a) or a negative control on (D) miR-320a knockdown, (E) glucagon mRNA expression and (F) glucagon protein content. All data are shown as the mean ± standard error of the mean of at least 3 independent experiments, *P < 0.05. The triangles and circles represent individual measurements.
MiR-320a modulates glucagon secretion
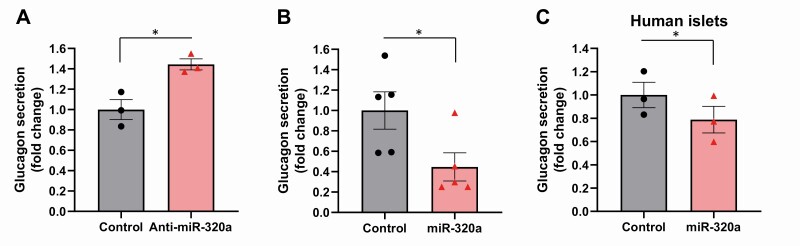
Next, we investigated whether these changes in glucagon mRNA and protein levels also resulted in altered glucagon secretion. Indeed, we found that miR-320a knockdown promoted glucagon secretion (Fig. 3A), whereas miR-320a overexpression led to a significant decrease in glucagon secretion from mouse αTC1-6 cells (Fig. 3B) as well as from human islets (Fig. 3C). These findings parallel the effects of miR-320a on glucagon mRNA and protein levels and suggest that any increase in glucagon content observed with miR-320a knockdown was truly due to increased expression rather than to any inhibition in glucagon secretion. They also support the notion that miR-320a has functional effects in terms of modulating glucagon.
Figure 3.
miR-320a modulates glucagon secretion. Glucagon secretion was assessed by ELISA in mouse αTC1-6 cells with (A) miR-320a knockdown or (B) miR-320a overexpression as well as in (C) nondiabetic primary human islets transfected with miR-320a. All data are shown as the mean ± standard error of the mean of at least 3 experiments, *P < 0.05. The triangles and circles represent individual measurements.
Glucose downregulates miR-320a expression leading to increased glucagon
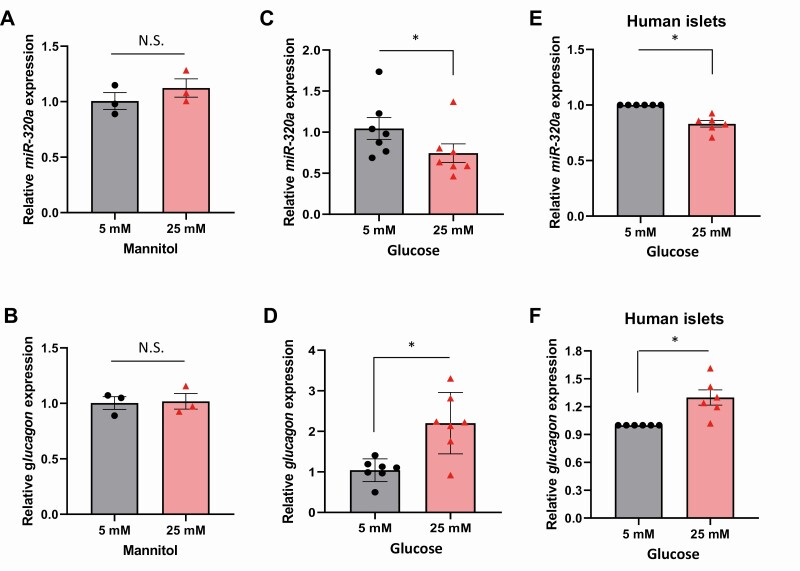
Glucagon is known to be elevated in diabetes (2, 3) and high glucose levels have been reported to play a role in this paradoxical induction of glucagon release and alpha cell glucagon mRNA levels (28-32). However, the exact molecular mechanisms mediating this phenomenon have remained elusive. Based on our discovery that miR-320a regulates glucagon levels, we hypothesized that this microRNA might also be involved in the glucose-induced glucagon elevation. In such a case, we would expect that high glucose levels would downregulate miR-320a expression, which in turn would release the glucagon 3′ UTR from inhibition by this microRNA. In fact, this is exactly what we observed. While incubation with mannitol had no effect on miR-320a (Fig. 4A) or glucagon expression (Fig. 4B) making any osmotic effects very unlikely, exposure of mouse αTC1-6 cells to 5 mM glucose (simulating euglycemia) and 25 mM glucose (simulating hyperglycemia) revealed significantly decreased miR-320a expression at high, 25 mM glucose compared with 5 mM glucose concentrations (Fig. 4C). In addition, this decrease in miR-320a expression was associated with a significant increase in glucagon expression (Fig. 4D). Of note, these effects of high glucose were not restricted to mouse αTC1-6 cells, but also observed in human islets (Fig. 4E and 4F).
Figure 4.
Glucose downregulates miR-320a expression leading to increased glucagon. To rule out any potential osmotic effects, αTC1-6 cells were exposed to mannitol (5 or 25 mM) for 24 hours and (A) miR-320a and (B) glucagon expression was assessed. Mouse αTC1-6 cells were incubated for 24 hours at low (5 mM) or high (25 mM) glucose and (C) miR-320a and (D) glucagon expression was assessed by qRT-PCR. Primary human islets were incubated for 24 hours at low (5 mM) or high (25 mM) glucose and (E) miR-320a and (F) glucagon expression was assessed by qRT-PCR. All data are shown as the mean ± standard error of the mean; n = 7 independent experiments and human islets from 6 individual donors each serving as its own control, *P < 0.05. The triangles and circles represent individual measurements. N.S., not significant.
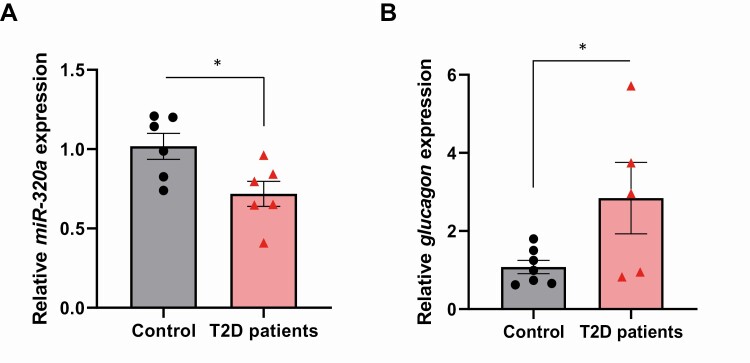
MiR-320a is downregulated and glucagon is upregulated in human islets of donors with type 2 diabetes
Next, to determine whether these processes also occur in human type 2 diabetes, we analyzed miR-320a and glucagon expression in human islets of type 2 diabetic donors. Interestingly, miR-320a expression was significantly decreased (Fig. 5A) whereas glucagon expression was significantly increased in diabetic compared with nondiabetic control human islets (Fig. 5B). These results are consistent with the findings in isolated human islets and mouse αTC1-6 cells exposed in vitro to high glucose and suggest that the observed decrease in miR-320a expression may contribute to the pathological increase in glucagon observed in type 2 diabetes.
Figure 5.
miR-320a is downregulated and glucagon is upregulated in human islets of donors with type 2 diabetes. (A) miR-320a expression and (B) glucagon expression in primary human islets from donors with type 2 diabetes compared with islets of nondiabetic control subjects. All data are shown as the mean ± standard error of the mean of at least 5 individual donors per group, *P < 0.05. The triangles and circles represent individual measurements.
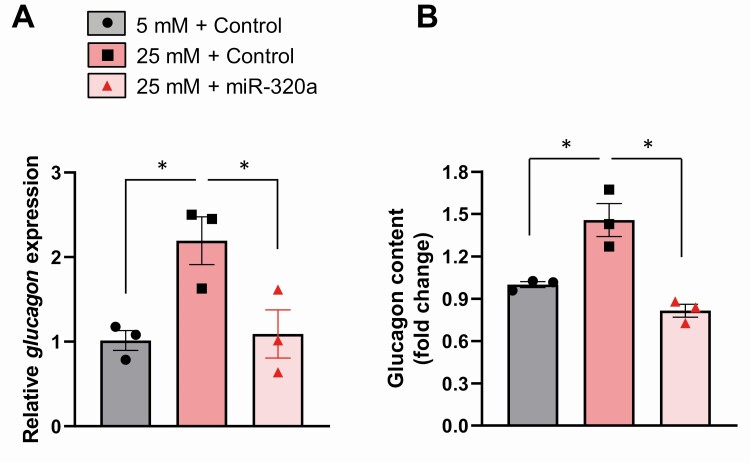
MiR-320a prevents glucose-induced glucagon production
To further assess the role of miR-320a downregulation in the observed glucose-induced elevation of glucagon, we overexpressed miR-320a to determine whether this could inhibit the glucose effect. Indeed, while glucagon expression was again increased more than 2-fold in response to high glucose concentrations in alpha cells overexpressing scrambled control oligonucleotides, overexpression of miR-320a completely blunted the glucose effect on glucagon expression (Fig. 6A) and glucagon content (Fig. 6B). These results support the notion that miR-320a controls glucagon production and that glucose-induced downregulation of miR-320a mediates, at least in part, the resulting increase in glucagon.
Figure 6.
miR-320a prevents glucose-induced glucagon production. αTC1-6 cells were transfected with miR-320a mimic or negative control for 48 hours and then incubated for 24 h in 5 or 25 mM glucose prior to assessment of (A) glucagon mRNA expression and (B) glucagon protein content. All data are shown as the mean ± standard error of the mean of 3 independent experiments, *P < 0.05. The triangles and circles represent individual measurements.
Discussion
In summary, our studies demonstrates for the first time that glucagon gene expression is under the control of a microRNA, miR-320a, and reveal that glucose-induced downregulation of miR-320a plays a role in glucagon production in the context of high glucose and human type 2 diabetes.
Hyperglucagonemia is one of the major factors, aside from relative insulin deficiency and insulin resistance that exacerbate fasting as well as postprandial hyperglycemia in diabetes (2, 3, 7). Although this glucagon excess in the context of diabetes has been reported decades ago by several studies (8, 9), the molecular mechanisms causing this abnormal regulation of glucagon production have remained unclear. The results of our current study suggest that the glucose-induced downregulation of miR-320a, resulting in disinhibition of glucagon production may be involved. Consistent with this notion, we demonstrated that glucagon is a direct target of miR-320a and that miR-320a downregulates endogenous glucagon mRNA and protein levels and results in decreased glucagon secretion. Moreover, we found that miR-320a expression was decreased under high glucose conditions, which is consistent with previous reports that miR-320a is regulated by glucose concentrations (33, 34). In addition, glucose-mediated downregulation of miR-320a was associated with an increase in glucagon production and this increase in glucagon was completely blunted by miR-320a overexpression.
While physiologically glucagon levels are reduced by glucose levels in the normoglycemic range (35), several studies have previously reported that hyperglycemic glucose concentration not only diminish this reduction (36, 37), but even paradoxically stimulate alpha cell glucagon production (28, 29, 31). Our results now suggest that this glucagon production under high glucose conditions is controlled by a specific microRNA, miR-320a. The findings are consistent with the notion of microRNAs leading to mRNA degradation upon binding to the 3′ UTR of target genes and our identification of specific and well conserved miR-320a binding sites in the 3′ UTR of human and rodent glucagon. In addition, we confirmed the functionality of this targeting by luciferase assays and assessment of endogenous glucagon mRNA expression and alpha cell glucagon protein content in response to miR-320a gain and loss of function. Importantly, we also found that altered miR-320a expression translated into modulation of glucagon secretion. Of note, our findings were not restricted to rodent alpha cells, but were also observed in primary human islets. This is in alignment with the well conserved nature of miR-320a across species and the proposed alpha cell intrinsic regulation conferred by this microRNA.
In addition, we found that miR-320a expression was significantly decreased in islets of subjects with type 2 diabetes compared with nondiabetic control subjects and this was associated with a dramatic elevation in glucagon expression suggesting that miR-320a may be involved in the glucagon excess of diabetes. Interestingly, this idea is supported by previous reports of miR-320a being involved in the pathophysiology of several metabolic diseases and the development of diabetes and its complications (33, 34, 38-41). Recent studies using microRNA expression profiling have shown that plasma miR-320 expression levels are also decreased in people with diabetes (39), consistent with our observation of decreased islet miR-320a expression in the context of high glucose and diabetes.
Taken together, the results of our study provide new insight into the molecular mechanisms involved in glucagon production especially in the context of high glucose concentrations and human type 2 diabetes and reveal for the first time that glucagon is controlled by miR-320a. Modulating miR-320a may therefore become a potential novel therapeutic approach to inhibit the pathological increase in glucagon in the context of diabetes.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health grant R01DK078752 to A.S.
Author Contributions: S.J. conducted the experiments, analyzed the data and drafted the manuscript. G.X. and G.J. helped with the human islet experiments and study design and analysis. J.C. was responsible for recording and handpicking all the human islets. A.S. supervised the study design and analysis and revised the manuscript. All authors read and approved the paper.
Glossary
Abbreviations
- αTC1-6
murine alpha TC1
- DMEM
Dulbecco’s modified Eagle’s medium
- IIDP
Integrated Islet Distribution Program
- PCR
polymerase chain reaction
- UTR
untranslated region
- WT
wild type
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Campbell JE, Drucker DJ. Islet α cells and glucagon–critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11(6):329-338. [DOI] [PubMed] [Google Scholar]
- 2. Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;1(7897):14-16. [DOI] [PubMed] [Google Scholar]
- 3. Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism. 1978;27(11):1691-1709. [DOI] [PubMed] [Google Scholar]
- 4. Unger RH, Dobbs RE, Orci L. Insulin, glucagon, and somatostatin secretion in the regulation of metabolism. Annu Rev Physiol. 1978;40:307-343. [DOI] [PubMed] [Google Scholar]
- 5. Kinoshita K, Ozaki N, Takagi Y, Murata Y, Oshida Y, Hayashi Y. Glucagon is essential for adaptive thermogenesis in brown adipose tissue. Endocrinology. 2014;155(9):3484-3492. [DOI] [PubMed] [Google Scholar]
- 6. Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. 2010;6(12):689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122(1):4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. 2012;153(3):1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369(4):362-372. [DOI] [PubMed] [Google Scholar]
- 10. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522-531. [DOI] [PubMed] [Google Scholar]
- 11. Guay C, Regazzi R. Role of islet microRNAs in diabetes: which model for which question? Diabetologia. 2015;58(3): 456-463. [DOI] [PubMed] [Google Scholar]
- 12. Esguerra JLS, Nagao M, Ofori JK, Wendt A, Eliasson L. MicroRNAs in islet hormone secretion. Diabetes Obes Metab. 2018;20(Suppl 2):11-19. [DOI] [PubMed] [Google Scholar]
- 13. Fernandez-Valverde SL, Taft RJ, Mattick JS. MicroRNAs in β-cell biology, insulin resistance, diabetes and its complications. Diabetes. 2011;60(7):1825-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Filios SR, Shalev A. β-cell MicroRNAs: small but powerful. Diabetes. 2015;64(11):3631-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joglekar MV, Parekh VS, Mehta S, Bhonde RR, Hardikar AA. MicroRNA profiling of developing and regenerating pancreas reveal post-transcriptional regulation of neurogenin3. Dev Biol. 2007;311(2):603-612. [DOI] [PubMed] [Google Scholar]
- 16. Poy MN, Eliasson L, Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432(7014):226-230. [DOI] [PubMed] [Google Scholar]
- 17. Xu G, Chen J, Jing G, Shalev A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat Med. 2013;19(9):1141-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jo S, Chen J, Xu G, Grayson TB, Thielen LA, Shalev A. miR-204 controls glucagon-like peptide 1 receptor expression and agonist function. Diabetes. 2018;67(2):256-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Filios SR, Xu G, Chen J, Hong K, Jing G, Shalev A. MicroRNA-200 is induced by thioredoxin-interacting protein and regulates Zeb1 protein signaling and beta cell apoptosis. J Biol Chem. 2014;289(52):36275-36283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu G, Chen J, Jing G, Grayson TB, Shalev A. miR-204 targets PERK and regulates UPR signaling and β-cell apoptosis. Mol Endocrinol. 2016;30(8):917-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Regazzi R. MicroRNAs as therapeutic targets for the treatment of diabetes mellitus and its complications. Expert Opin Ther Targets. 2018;22(2):153-160. [DOI] [PubMed] [Google Scholar]
- 22. Ji C, Guo X. The clinical potential of circulating microRNAs in obesity. Nat Rev Endocrinol. 2019;15(12):731-743. [DOI] [PubMed] [Google Scholar]
- 23. RRID:CVCL_B036.
- 24. RRID:CVCL_0045.
- 25. Hart NJ, Powers AC. Use of human islets to understand islet biology and diabetes: progress, challenges and suggestions. Diabetologia. 2019;62(2):212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. RRID:AB_2811007.
- 27. RRID:AB_2884901.
- 28. Dumonteil E, Ritz-Laser B, Magnan C, Grigorescu I, Ktorza A, Philippe J. Chronic exposure to high glucose concentrations increases proglucagon messenger ribonucleic acid levels and glucagon release from InR1G9 cells. Endocrinology. 1999;140(10):4644-4650. [DOI] [PubMed] [Google Scholar]
- 29. McGirr R, Ejbick CE, Carter DE, et al. Glucose dependence of the regulated secretory pathway in alphaTC1-6 cells. Endocrinology. 2005;146(10):4514-4523. [DOI] [PubMed] [Google Scholar]
- 30. Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, Gromada J. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Endocrinology. 2005;146(11):4861-4870. [DOI] [PubMed] [Google Scholar]
- 31. Salehi A, Vieira E, Gylfe E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes. 2006;55(8):2318-2323. [DOI] [PubMed] [Google Scholar]
- 32. Katsura T, Kawamori D, Aida E, Matsuoka TA, Shimomura I. Glucotoxicity induces abnormal glucagon secretion through impaired insulin signaling in InR1G cells. PLoS One. 2017;12(4):e0176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng B, Chakrabarti S. miR-320 Regulates Glucose-Induced Gene Expression in Diabetes. ISRN Endocrinol. 2012;2012:549875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao J, Ailifeire M, Wang C, et al. miR-320/VEGFA axis affects high glucose-induced metabolic memory during human umbilical vein endothelial cell dysfunction in diabetes pathology. Microvasc Res. 2020;127:103913. [DOI] [PubMed] [Google Scholar]
- 35. Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84-116. [DOI] [PubMed] [Google Scholar]
- 36. Vieira E, Salehi A, Gylfe E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia. 2007;50(2):370-379. [DOI] [PubMed] [Google Scholar]
- 37. Le Marchand SJ, Piston DW. Glucose suppression of glucagon secretion: metabolic and calcium responses from alpha-cells in intact mouse pancreatic islets. J Biol Chem. 2010;285(19):14389-14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ling HY, Ou HS, Feng SD, et al. CHANGES IN microRNA (miR) profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes. Clin Exp Pharmacol Physiol. 2009;36(9):e32-e39. [DOI] [PubMed] [Google Scholar]
- 39. Zampetaki A, Kiechl S, Drozdov I, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107(6):810-817. [DOI] [PubMed] [Google Scholar]
- 40. Tang H, Lee M, Sharpe O, et al. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J. 2012;26(11):4710-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lopes MB, Freitas RC, Hirata MH, et al. mRNA-miRNA integrative analysis of diabetes-induced cardiomyopathy in rats. Front Biosci (Schol Ed). 2017;9:194-229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.