Abstract
Purpose of Review
The goal of this review is to present a summary of the recent literature of a non-invasive brain stimulation (NIBS) to alleviate pain in people with chronic pain syndromes. This article reviews the current evidence for the use of transcranial direct current (tDCS) and repetitive transcranial magnetic stimulation (rTMS) to improve outcomes in chronic pain. Finally, we introduce the reader to novel stimulation methods that may improve therapeutic outcomes in chronic pain.
Recent Findings
While tDCS is approved for treatment of fibromyalgia in Canada and the European Union, no NIBS method is currently approved for chronic pain in the United States. Increasing sample sizes in randomized clinical trials (RCTs) seems the most efficient way to increase confidence in initial promising results. Trends at funding agencies reveal increased interest and support for NIBS such as recent Requests for Application from the National Institutes of Health. NIBS in conjunction with cognitive behavioral therapy and physical therapy may enhance outcomes in chronic pain. Novel stimulation methods, such as transcranial ultrasound stimulation, await rigorous study in chronic pain.
Keywords: non-invasive brain stimulation, repetitive transcranial magnetic stimulation, rTMS, chronic pain, transcranial direct current stimulation, tDCS
Summary
Excitatory NIBS targeting motor cortex or left dorsolateral prefrontal cortex has the greatest support for ameliorating pain in chronic pain patients, particularly in Chronic Overlapping Pain Conditions, such as fibromyalgia. Confidence in the efficacy of NIBS interventions is most negatively affected by RCTs with small sample sizes. Increased attention from funding agencies to the promise of NIBS and to the problem of small sample sizes in applied neuroscience is anticipated to improve confidence in these relatively side-effect free interventions.
Introduction
Epidural motor cortex stimulation: prelude to non-invasive neuromodulation for pain alleviation
Non-invasive brain stimulation (NIBS) protocols as adapted for intervention in chronic pain, arguably have their intellectual origins in invasive epidural motor cortex stimulation (EMCS) [1, 2]. Invasive methods requiring neurosurgery are reserved for patients presenting with intractable chronic pain with clear neuropathic signs and symptoms. While EMCS appears to be effective for central post stroke pain (CPSP), it seems ineffective for spinal cord injury pain (SCIP) leaving a vast gap in analgesic potential for many patients who may benefit from NIBS [3]. NIBS using transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS) or more recently reported methods such as transcranial alternating current stimulation (tACS) and low-intensity transcranial ultrasound stimulation (TUS) are promising avenues of research that may lead to future treatments for chronic pain of all etiologies [4-6]. However, the field of NIBS for chronic pain has been plagued by promising preliminary published reports of analgesic effects in a variety of chronic pain syndromes with little follow-up in appropriately powered, blinded, randomized and sham-controlled clinical trials [7]. Historically this was in part caused by the lack of funding and attention from major funding agencies such as the National Institutes of Health, which we note in recent years has improved substantially (Figure) [8].
Figure.
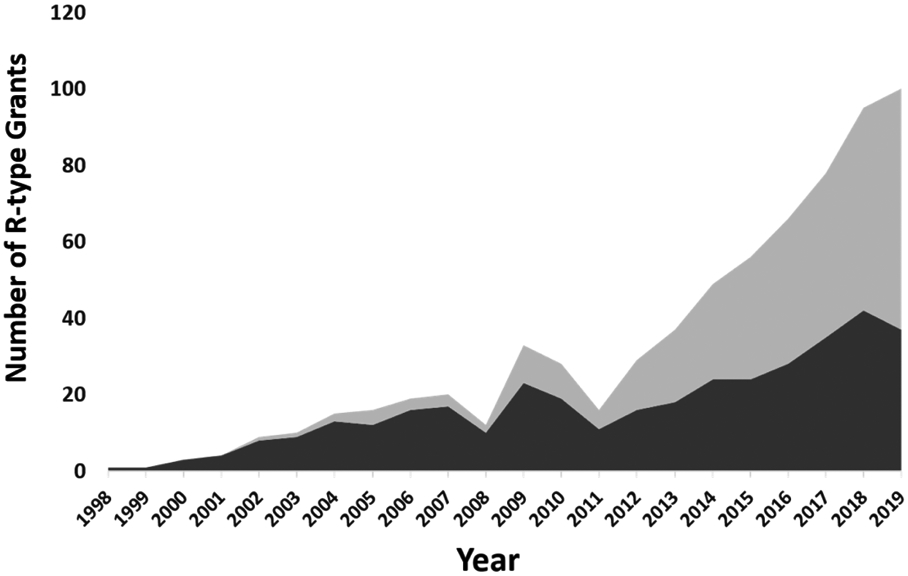
In dark grey, R-type grants mentioning rTMS in the public abstract. In light grey, R grants mentioning tDCS in the public abstract.
In neuropathic pain caused by a lesion of the central nervous system, for example in patients with central post-stroke pain (CPSP) and spinal cord injury-related pain (SCIP), medications including morphine and pregabalin frequently have limited or no effect on the patient’s pain [9-11]. In the early 1990s, Tsubokawa and colleagues pioneered EMCS for treatment of CPSP, and 9 of 12 patients experienced strongly positive long term pain relief from this invasive therapy [12]. EMCS is an invasive form of motor cortex neuromodulation, which requires neurosurgical implantation and has been reported to provide 40% to 50% pain amelioration in 45% of treated patients at one-year follow-up [13]. While early reports of pain alleviation by EMCS generally lacked periods of double blind testing, where the intracranial stimulator would be turned off or on by random determination, several later studies included double-blind test periods [14-17]. Of the four studies reporting double-blind testing, three showed generally positive pain ameliorating effects.
Short-term pain reduction produced by excitatory, high frequency M1 rTMS correlates with the clinical success of EMCS, suggesting these interventions may have similar mechanisms [18, 19]. Recent evidence in a case series of 12 patients demonstrated rTMS’s improved positive and negative predictive value for EMCS success when applied over a 4 session regime [20].
In an attempt to translate the pain alleviating effects of invasive EMCS to a wider patient population by using noninvasive techniques known to modulate cortical excitability, randomized controlled trials (RCTs) of both high frequency repetitive transcranial magnetic stimulation (rTMS) and anodal transcranial direct current stimulation (tDCS) were found to alleviate neuropathic pain [21-25]. An RCT comparing high frequency M1 rTMS to anodal M1 tDCS, and each to their own sham, found active rTMS superior to sham and active tDCS, but importantly found analgesic effects of M1 rTMS correlated positively to those of anodal M1 tDCS, suggesting individual patients respond similarly to tDCS and rTMS [26]. It is important to note that while typical courses of treatment with tDCS only reach levels of analgesia of clinical significance after at least three 20 minute once-daily sessions, temporary pain alleviation from EMCS and rTMS occurs after 10 to 20 minutes of stimulation, and these early effects predict clinical success [13, 22, 27, 28, 20]. We discuss recent studies using non-invasive neuromodulation methods for alleviation of pain perception in chronic pain syndromes.
Non-invasive Brain Stimulation: Analysis of the analgesic effect of M1-targeted rTMS in chronic pain patients
Since Lefaucheur and colleagues first reported analgesic effects of M1 rTMS in patients with neuropathic pain in hopes of using rTMS as a tool to predict efficacy of EMCS, several guidelines and meta-analyses investigating the potential analgesic effects of M1 rTMS have been published [29, 24, 7]. In general, the most frequently investigated M1 rTMS protocols generally aim to increase cortical excitability in the motor cortex. For example, the most recent Cochrane review update of NIBS for chronic pain listed that 32 published reports of 42 rTMS studies used high frequency (≥ 5 Hz) M1 rTMS, which is known to increase cortical motor excitability. In addition, a variety of sham methodologies were taken advantage of in this population of studies. Optimal sham stimulation, where somatosensory, visual and auditory perception of the verum (or true) coil is mimicked, is reported in only eight of 42 analyzed studies. Generally, suboptimal sham coils are used where only two perceptual aspects of the verum stimulation are mimicked; 14 of 42 sham coils mimicked only auditory and visual aspects. It is probable that in a crossover study there is no perfect sham for active rTMS given that sensations from verum stimulation arise from the scalp and subcutaneous tissues, as well as from the meninges and periosteum [30]. A sham coil with the ability to stimulate these targets would likely not be considered ethical. Furthermore, sham coils which stimulate the scalp may deliver neurophysiologically relevant energy which may produce neuromodulation. Therefore, for rTMS studies, sham blinding may always remain questionable.
Recent evidence-based guidelines were published by a European commission which reviewed clinical rTMS studies for several chronic disorders including chronic pain disorders, movement disorders, stroke, epilepsy, tinnitus, depression and anxiety disorders [31]. Their general conclusions included an absence of efficacy of low frequency rTMS protocols in all chronic pain disorders evaluated, and significant efficacy of high frequency, excitatory, rTMS protocols in neuropathic pain and perhaps in some other chronic pain disorders studied. Specifically, they found evidence that 1) there is a suggestion that low frequency M1 rTMS contralateral to the side of pain is frequently ineffective in neuropathic pain, 2) there is a definite analgesic effect of high frequency M1 rTMS contralateral to the side of pain in chronic neuropathic pain, and 3) there is a suggested analgesic effect of M1 rTMS contralateral to the affected side in CRPS. This guideline did not find enough high-quality evidence to make any further evidence-based recommendations. At the time, they found a lack of high-quality evidence in non-neuropathic pain syndromes. The recent Cochrane review update was less charitable, finding the current evidence for a heterogenous small effect size favoring active high frequency M1 rTMS to be below the minimal level of clinical significance based on low quality evidence [7]. Factors that lowered the quality of evidence for clinical efficacy of high frequency M1 rTMS included suboptimal sham, small sample sizes in many studies, as well as suboptimal randomization and blinding procedures.
Recently published studies and clinical trials are more rigorous in design and have benefited from the past nearly 20 years of research. For example, a multi-center parallel RCT in 144 patients with intractable chronic neuropathic pain compared a 4 week, 20 once-daily stimulation session intervention using high frequency rTMS of M1 to an optimal sham [32]. Despite finding no significant difference between sham and verum rTMS in their primary outcome (mean visual analogue scale decreases), patients enrolled in the verum continuous weekly follow-up maintained lower pain intensity ratings compared to those experiencing sham stimulation. It is notable that this trial used a relatively low dose (500 pulses) of M1 rTMS, whereas more successful studies have used both higher frequency stimulation (20 Hz vs. 5 Hz) and a higher dose (2000 to 3000 pulses) [32]. Recently, a study has found verum neuronavigated M1 rTMS to be superior to sham or non-neuronavigated M1 rTMS consistent with clinical opinion in EMCS that accurate targeting of the painful somatotopic area of motor cortex is critical for clinically meaningful outcomes [33, 3]. Further supporting this contention is evidence reported by Shimizu and colleagues that conventional high frequency rTMS had no analgesic effect in patients with neuropathy of the lower limb, while 5 Hz M1 rTMS using a double cone coil capable of reaching the paracentral lobule was superior to both conventional and sham stimulation [34].
Non-invasive Brain Stimulation: Analysis of the analgesic effect of DLPFC-targeted rTMS in chronic pain patients and acute post-operative pain
High frequency rTMS of the left DLPFC is approved by the US Food and Drug Administration for the treatment of depression. and a recent study in patients with depression found that those depressives with widespread pain found rTMS of left DLPFC analgesic [35, 36]. More recently, studies of DLFPC rTMS have been conducted in chronic pain patients as well. These studies used high frequency rTMS targeting left DLPFC or low frequency rTMS targeted right DLPFC as an intervention [7]. Three studies from the same lab group have investigated high frequency left DLPFC rTMS to reduce post-surgical patient-controlled analgesia (PCA). While the first two studies found that treatment reduced use of PCA, the most recent study, with the largest number of subjects, found no effect of DLFPC rTMS on PCA use [37-39]. Additional studies have found analgesic and therapeutic effects of high frequency rTMS of left DLPFC in patients with central poststroke pain, chronic widespread pain (CWP), burning mouth syndrome and fibromyalgia [40-46]. While five published studies in fibromyalgia and CWP show positive effects on pain, fatigue and physical functioning, no study show improvement in all three domains and all studies have been conducted in sample sizes of 7 to 12 per group, which significantly increases the risk of false positive results [47]. Overall there is limited, but promising evidence of the analgesic effects of high frequency rTMS of the left DLPFC in both neuropathic and non-neuropathic chronic pain syndromes. Given variability of effects of neuromodulation depending on accuracy of targeting and small effect sizes relative to investigation of all-or-none phenomena, larger sample sizes and better targeting should be goals for future studies.
Non-invasive Brain Stimulation: Analysis of the analgesic effect of rTMS of other cortical targets in chronic pain patients
In chronic pain patients the effects of cortical stimulation of targets alternate to M1 and DLPFC have been reported. Four of these studies reported high quality evidence supporting the analgesic effects of cortical neurostimulation, including the second somatosensory cortex, vertex, and dorsal anterior cingulate cortex (ACC) [48-50]. Another study, in a smaller group of migraine patients showed supporting evidence of 1 Hz rTMS to the vertex for the treatment of chronic migraine [49]. An important caveat of NIBS studies that aim to target locations deep to the cortical surface is that all neural structures between the scalp and the target receive at least as much stimulation as the target. Additionally, the scalp sensations and muscular contractions produced by deep rTMS are much more intense than those produced when aiming to stimulate the cortical surface. Tzabazis and colleagues applied H-coil 10 Hz rTMS targeting the dorsal ACC and found superior analgesic effects in fibromyalgia patients when compared to sham stimulation, which were present on follow-up 4 weeks after the intervention [50]. A recently published study used deep rTMS to target the posterior insula and anterior cingulate cortex (ACC) in an attempt to alleviate chronic central neuropathic pain after stroke or spinal cord injury [51]. This study compared the effects of deep rTMS of the ACC or insula to sham deep rTMS of either target in 98 patients suffering from central neuropathic pain in a protocol of 5 once-daily sessions followed by 11 once-weekly stimulation sessions. Neuromodulation of neither target was superior to sham stimulation on measures of pain interference, pain dimensions, neuropathic pain symptoms, medication use or quality of life. However, ACC deep rTMS reduced anxiety symptoms during the 12-week treatment period whereas posterior insula rTMS reduced sensitivity to heat pain and warmth as indicated by elevated thresholds. Finally, since S2 plays a prominent role in pain processing, Lindholm and colleagues found that active high frequency S2 rTMS was superior to both M1/S1 stimulation and sham stimulation in alleviating neuropathic orofacial pain [48]. Future exploratory studies should seek to develop these novel cortical targets in order to optimize neurostimulation for long-term analgesia and larger studies are needed to replicate these results in order to support the analgesic action of non-invasive cortical neurostimulation.
Non-invasive Brain Stimulation: Analysis of the analgesic effect of M1-targeted tDCS in chronic pain patients
The exposure of neural tissue to electric fields set up by direct currents (DC) has been known for more than 50 years to produce long-term changes in the activity of neurons [52, 53]. The rediscovery of the potential clinical applications of this technique had to await the development of an objective way of measuring the effects of neuroplasticity in humans, namely development of the TMS coil to evoke motor-evoked potentials [54]. When neurons are exposed to a DC field, the area under the anodal electrode experiences a depolarization of the resting membrane potential and endogenous neural activity is increased in rate, ultimately augmenting the baseline excitability of that population of neurons [55, 56]. Under the cathodal electrode, the neural population experiences a hyperpolarization of the resting membrane potential and endogenous neural activity is decreased in rate, ultimately suppressing the baseline excitability of that population of neurons. This dichotomy of neuromodulatory responses is oversimplified for many reasons, including the variability of the geometry of in vivo neuronal elements such as axons, dendrites and cell bodies as well as the effects of past plasticity-inducing events [55, 57]. However, for our purposes the dichotomy of anodal tDCS enhancing cortical excitability and cathodal tDCS suppressing cortical excitability remains useful [54, 56].
From 2006 until 2017, 34 studies investigated the effects of M1 tDCS in chronic pain patients with various etiologies of chronic pain [7]. In a recently published set of guidelines commissioned by the European Chapter of the International Federation of Clinical Neurophysiology, an expert panel found enough supporting evidence to make a guideline recommendation for M1 anodal tDCS for neuropathic pain secondary to spinal cord injury and for fibromyalgia [6]. M1 anodal tDCS for neuropathic pain secondary to spinal cord injury, the expert commission found, is possibly effective. Further, the panel found sufficient evidence to support that M1 anodal tDCS is probably effective for treating pain in fibromyalgia as assessed by pain intensity reports and the fibromyalgia impact questionnaire. Six studies of pain alleviation from anodal M1 tDCS in fibromyalgia reported an analgesic effect when using at least five once-daily sessions of 1 or 2 mA delivered for 20 minutes compared to sham. However, one study found no pain alleviating effect after 10 consecutive once-daily 20 minutes sessions of 2 mA stimulation compared to sham [58-63]. Three studies have studied the effects of M1 anodal tDCS on PCA during postoperative recovery, either from total knee arthroplasty or lumbar spine surgery, and found pain to be less with active stimulation compared to sham and the total amount of drug during PCA to be lower in the post-surgery period [64-66]. The positive findings of analgesia mediated by anodal M1 tDCS in fibromyalgia and in post-operative pain provide strong preliminary evidence to support an analgesic effect superior to sham stimulation in both acute and chronic pain conditions. However, the evidence is not without limitations, and the size of the superiority effect of anodal tDCS over sham stimulation is often less than a ten or twenty percent reduction in patients’ self-reported pain intensity , which calls into question its clinical relevance [6].
Regarding neuropathic pain secondary to spinal cord injury (SCIP), three studies reported an analgesic effect, superior to sham, of at least one 20 minute session of 2 mA anodal M1 tDCS, while three additional studies found no significant effect compared to sham stimulation [22, 67-70]. An additional three studies of neuropathic phantom limb pain found anodal M1 tDCS to be superior to sham in its analgesic effects [71-73]. One study of neuropathic radiculopathy found only a trend of an analgesic effect with M1 anodal tDCS. But notably, this tDCS effect correlated positively with the analgesic effects of high frequency M1 rTMS [26]. The evidence for superior analgesic effects of anodal M1 tDCS compared to sham stimulation in neuropathic pain, particularly of peripheral origin is more consistent compared to other chronic pain disorders. However, the strongest evidence relies on relatively few trials with small sample sizes (n < 25 per group) and therefore more work is needed to substantiate the analgesic effects of anodal M1 tDCS in neuropathic pain syndromes [47, 74, 75, 6].
Non-invasive Brain Stimulation: Analysis of the analgesic effect of DLPFC-targeted tDCS in chronic pain patients
The use of tDCS of the DLPFC has only been reported using left sided stimulation [6]. Three of these studies assessed the effects of left DLPFC tDCS in fibromyalgia, one of which found that verum stimulation reduced experimental pain sensitivity and increased heat pain tolerance, while only one of the other two studies found significant analgesic effects of stimulation on clinical pain [59, 62, 76]. While one study of post-operative pain and PCA usage found reduced PCA usage after left DLPFC tDCS compared to sham stimulation, another study in patients recovering from lumbar spine surgery found no significant difference between verum DLPFC and sham stimulation [77, 78]. More recently, post-surgical opioid use was found to be reduced more by left DLPFC than left M1 tDCS when applied at 2 mA in four 20-minute sessions after total knee arthroplasty [79].
Potential mechanisms of primary motor cortex neuromodulation for pain amelioration
M1 neuromodulation may affect multiple levels of the neuraxis to ameliorate pain. Motor cortex excitability is altered by acute and tonic noxious stimuli, and in chronic pain conditions including painful diabetic neuropathy, fibromyalgia and complex regional pain syndrome [80-87]. This modulation of cortical excitability by tonic or chronic nociceptive stimulation is remedied by pain alleviating neuromodulation [27, 88]. Among M1 neuromodulation’s neurophysiological effects is the modulation of thalamic activity [89-91]. Studies in animal pain models demonstrated that EMCS decreased nociceptive driven neural activity and BOLD response in S1 [92, 93]. Additionally, high frequency M1 rTMS causes reorganization of the S1 somatotopic map and reduction in the amplitude of painful laser evoked potentials, while anodal tDCS reduces BOLD responses to painful stimuli [94-96, 73, 97]. Together these findings suggest that S1 excitability is potentially mediated through M1 corticocortical pathways.
Research during the last two decades has begun to unravel the bidirectional influences between the function of the motor system and somatosensory system [80, 98]. Lasting plasticity in M1 and S1 can be evoked by repetitive patterned stimulation originating from corticocortical fibers arising in the opposite primary cortex (S1 to M1 as well as M1 to S1) both in humans and animal models [99-102]. Acute phasic cutaneous pain as well as tonic cutaneous and muscular pain suppresses motor cortex excitability in healthy subjects [80, 82, 86, 103]. Motor cortex oscillatory activity shows enhanced coherence during acute phasic pain, while voluntary movement preparation suppresses subjective pain intensity and evoked potentials elicited by painful laser stimuli [104-106]. Acute prolonged tonic pain impairs retention of motor training without impairing performance improvements during acquisition [107]. Interestingly, TMS studies have shown decreased inhibition in the form of reductions in cortical silent period (CSP) and short interval intracortical inhibition (SICI), GABAergic mediated measures in chronic pain syndromes such as complex regional pain syndrome and painful diabetic neuropathy, but also in fibromyalgia [27, 84, 85, 98, 108, 87, 109]. Previous healthy subject studies have shown that moderate prolonged tonic pain mediated by capsaicin suppresses MEPs, while enhancing SICI and CSP for the 60 to 80 minute duration of capsaicin mediated pain [82, 88, 110]. In fact, aberrant motor cortex excitability induced either by a prolonged tonic pain model in healthy subjects or by a chronic pain disorder in patients may be normalized by analgesic M1 neuromodulation [27, 111, 88, 112].
Descending pain modulatory network involvement in motor cortex neuromodulation
A series of studies by the Lyon group found patients with implanted EMCS had increased brain activity as measured by PET in areas including pgACC, dACC, medial thalamus, and periaqueductal grey (PAG), which correlated with magnitude of pain alleviation as well as enhanced functional connectivity between pgACC and PAG after 30 to 45 minutes of EMCS [90, 113, 114]. Further studies by this group found evidence of enhanced endogenous opioid release in anterior midcingulate cortex (aMCC) and PAG in response to EMCS, and that prestimulation opioid receptor availability positively predicted magnitude of pain alleviation [115, 116]. This pattern of responses in the ACC, medial thalamus and PAG has been found by other groups using pain alleviative EMCS in humans, and replicated in animal models of neuropathic pain and in tonic pain ameliorated by M1 tDCS [89, 117-121, 97]. Studies in healthy subjects and chronic pain patients have found evidence that high frequency rTMS targeting M1 is in part mediated by opioid- and NMDA-dependent mechanisms as well as evidence of β-endorphin release [122-124, 63]. Studies in patients and healthy controls have found variable neural responses and pain amelioration effects after excitatory, anodal transcranial direct current stimulation (M1-anodal tDCS) and excitatory M1 rTMS. Additionally, while neurophysiological responses show no clear direction of modification across studies, there is evidence of response modulation in pain-associated regions including PAG, ACC, somatomotor cortex, anterior and posterior insula, S2 cortex, dorsal medulla, and basal ganglia structures [125-130, 97]. Related evidence from rTMS studies targeting DLPFC, which has been shown to reverse the effects of prolonged tonic pain on motor cortex excitability, demonstrate a naloxone-sensitive effect that reduces activity in pgACC and PAG [88, 123, 131]. Furthermore, recent evidence in healthy subjects undergoing [11C]carfentanil PET after high frequency rTMS of the somatomotor cortex found evidence of release of endogenous opiates in the ACC and medial prefrontal cortex ipsilateral to rTMS and in operculoinsular structures contralateral to rTMS [132].
Animal studies of EMCS have demonstrated naloxone-sensitive anti-nociceptive effects in acute and chronic pain models [92, 118, 119, 133, 134]. Studies in animal pain models have demonstrated that reduced nociceptive-related defensive responses associated with EMCS is accompanied by decreases in spontaneous or evoked neural activity related to nociception in the spinal dorsal horn (SDH), pontine reticular formation, PAG and parafascicular nucleus, the centromedian, ventroposterolateral and posterior nuclei of the thalamus, as well as the somatosensory and prefrontal cortex [92, 93, 118-120, 134, 1, 135]. Further, anti-nociceptive effects were associated with increased nociceptive and basal neural activity in the ACC, basolateral and central nuclei of the amygdala, and PAG coupled with increased release of the inhibitory amino acids glycine and GABA in the PAG [118-120, 136]. The profile of neuronal activation by motor cortex stimulation in rodent studies supports the involvement of descending pain modulatory network, especially the ACC and PAG as well as modulation of SDH processing of noxious stimuli [119, 135].
Future promising directions in NIBS for chronic pain
In addition to the need for clinical trials with greater numbers of patients and better trial design, larger analgesic effects may be expected from alternative protocols using neuromodulatory methods as adjuncts to cognitive behavioral therapy or rehabilitation or from neuromodulation targeted to known neurophysiological abnormalities which accompany chronic pain. Recent studies have revealed important potential examples of alternative protocols and a novel method of neuromodulation. Increased attention from funding agencies such as the NIH, should spur future progress, particularly in response to RFAs from the BRAIN Initiative such as “Non-Invasive Neuromodulation - New Tools and Techniques for Spatiotemporal Precision (R01)" and “Non-Invasive Neuromodulation - Mechanisms and Dose/Response Relationships for Targeted CNS Effects (R01)."
Chronic pain conditions are accompanied by alterations in cortical excitability, but these alterations are thought to be caused by loss of inhibitory drive from the thalamus. The result is a phenomenon known as thalamocortical dysrhythmia (TCD) [137, 138]. The result of TCD is the well-known cortical reorganization that accompanies chronic pain as well as the lesser known reduction in alpha EEG power, oscillations in the 8 to 12 Hz range and enhancement of theta power [139-142]. Recent studies have shown this shift in peak alpha to occur not only in chronic pain patients, but to occur in response to tonic pain models and peak alpha to be predictive of individual sensitivity to such tonic pain models [142, 143]. Transcranial alternating current stimulation (tACS) at alpha frequencies, such as 10 Hz, at intensities like tDCS allows entrainment of cortical oscillations [144]. Recent studies have shown alpha tACS induced an increase in alpha EEG in chronic low back pain patients which was correlated and accompanied by a reduction in pain intensity [145]. In healthy subjects, evoked pain was reduced by alpha tACS only when the stimulus was of an uncertain intensity, perhaps reflecting enhanced threat or anxiety [4].
Several studies have evaluated the effects of NIBS combined with other therapies such mirror therapy for phantom limb pain, aerobic exercise for fibromyalgia and peripheral electrical stimulation for chronic low back pain [146, 147, 73]. Finding the optimal combinations of NIBS and complementary or traditional pain therapies will take several elaborate and sophisticated RCTs.
While electrical stimulation of the brain is a logical extension of the electrical properties of nervous tissue, recently it has been reported that transcranial ultrasound at sub-lesional intensities can lead to the modulation of neurons in both animal models as well as humans [5]. Low intensity transcranial ultrasound (TUS) has shown promising analgesic effects and improvement of mood in chronic pain patients [148]. No additional studies have reported effects of low intensity TUS in chronic pain populations.
Conclusion
The last 5 years of research activity on noninvasive brain stimulation (NIBS) has yielded promising results in the treatment of chronic pain. Studies investigating the potential mechanisms underlying the analgesic effects of NIBS has shown that both endogenous opioid releasing regions of the brain and modifications of somatomotor plasticity that accompany chronic pain syndromes are involved in the initial and lasting analgesic actions of NIBS. An exciting decade lays ahead where novel stimulation methods and modalities such as tACS and TUS should be expected to contribute more flexibility to the NIBS armamentarium. Future improvements in clinical trial protocols for devices, conduct and reporting will be necessary to further refine the precision of trial results and interpretation. Increased support and attention from funding agencies such as the NIH would encourage larger and more mechanism driven clinical trials. Much work remains, but recent developments inspire more interest in the field of NIBS for chronic pain.
ACKNOWLEDGMENTS
TJM and FAL acknowledge funding from the Johns Hopkins Neurosurgical Pain Research Institute and NIH grant R01-NS107602 (to FAL). All authors declare no conflicts of interest.
Footnotes
Conflict of Interest
Timothy Meeker, Rithvic Jupudi, Frederik Lenz and Joel Greenspan declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Reference List
Papers of particular interest, published recently, have been highlighted as:
• Of importance
• Of major importance
- 1.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin Electrophysiol. 1991; 14( 1): 131–4. doi : 10.1111/j.1540-8159.1991.tb04058.x. [DOI] [PubMed] [Google Scholar]
- 2.Lefaucheur JP. Cortical neurostimulation for neuropathic pain: state of the art and perspectives. Pain. 2016;157 Suppl 1:S81–9. doi: 10.1097/j.pain.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 3.Kurt E, Henssen D, Steegers M, Staal M, Beese U, Maarrawi J et al. Motor Cortex Stimulation in Patients Suffering from Chronic Neuropathic Pain: Summary of Expert Meeting and Premeeting Questionnaire, Combined with Literature Review. World Neurosurg. 2017;108:254–63. doi: 10.1016/j.wneu.2017.08.168. [DOI] [PubMed] [Google Scholar]
- 4.Arendsen LJ, Hugh-Jones S, Lloyd DM. Transcranial Alternating Current Stimulation at Alpha Frequency Reduces Pain When the Intensity of Pain is Uncertain. Journal of Pain. 2018;19(7):807–18. doi: 10.1016/j.jpain.2018.02.014.*-This is the first study to show significant pain alleviating effects of transcranial alternating current stimulation (tACS) targeting the somatosensory cortex. The effect on acute phasic pain was specific to stimuli that were unexpected to the participant.
- 5.Wang P, Zhang J, Yu J, Smith C, Feng W. Brain Modulatory Effects by Low-Intensity Transcranial Ultrasound Stimulation (TUS): A Systematic Review on Both Animal and Human Studies. Frontiers in Neuroscience. 2019; 13(696). doi: 10.3389/fnins.2019.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology. 2017;128(1):56–92. doi: 10.1016/j.clinph.2016.10.087.*-A thorough review of current evidence-based guidelines on the use of transcranial direct current stimulation for depression, chronic pain, Parkinson's Disease, motor stroke, multiple sclerosis, tinnitus, and schizophrenia.
- 7.O'Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database of Systematic Reviews. 2018;4(4):CD008208. doi: 10.1002/14651858.CD008208.pub5.*-The latest Cochrane review of non-invasive brain stimulation for chronic pain reviews includes 94 randomized controlled trials, assesses the quality of each published trial, and makes overall judgements of efficacy of interventions.
- 8.Bikson M, Brunoni AR, Charvet LE, Clark VP, Cohen LG, Deng ZD et al. Rigor and reproducibility in research with transcranial electrical stimulation: An NIMH-sponsored workshop. Brain Stimul. 2018; 11 (3):465–80. doi: 10.1016/j.brs.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JS, Bashford G, Murphy TK, Martin A, Dror V, Cheung R. Safety and efficacy of pregabalin in patients with central post-stroke pain. Pain. 2011; 152(5): 1018–23. doi: 10.1016/j.pain.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurology. 2009;8(9):857–68. doi: 10.1016/s1474-4422(09)70176-0. [DOI] [PubMed] [Google Scholar]
- 11.Siniscalchi A, Gallelli L, De Sarro G, Malferrari G, Santangelo E. Antiepileptic drugs for central post-stroke pain management. Pharmacol Res. 2012;65(2):171–5. doi: 10.1016/j.phrs.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien). 1991;52:137–9. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- 13.Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2009;110(2):251–6. doi: 10.3171/2008.6.17602. [DOI] [PubMed] [Google Scholar]
- 14.Drouot X, Nguyen JP, Peschanski M, Lefaucheur JP. The antalgic efficacy of chronic motor cortex stimulation is related to sensory changes in the painful zone. Brain. 2002;125:1660–4. doi: 10.1093/brain/awf161. [DOI] [PubMed] [Google Scholar]
- 15.Lefaucheur JP, Drouot X, Cunin P, Bruckert R, Lepetit H, Creange A et al. Motor cortex stimulation for the treatment of refractory peripheral neuropathic pain. Brain. 2009;132(Pt 6):1463–71. doi: 10.1093/brain/awp035. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen JP, Velasco F, Brugieres P, Velasco M, Keravel Y, Boleaga B et al. Treatment of chronic neuropathic pain by motor cortex stimulation: results of a bicentric controlled crossover trial. Brain Stimul. 2008;1(2):89–96. doi: 10.1016/j.brs.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Radic JA, Beauprie I, Chiasson P, Kiss ZH, Brownstone RM. Motor Cortex Stimulation for Neuropathic Pain: A Randomized Cross-over Trial. Can J Neurol Sci. 2015;42(6):401–9. doi: 10.1017/cjn.2015.292. [DOI] [PubMed] [Google Scholar]
- 18.Hosomi K, Saitoh Y, Kishima H, Oshino S, Hirata M, Tani N et al. Electrical stimulation of primary motor cortex within the central sulcus for intractable neuropathic pain. Clin Neurophysiol. 2008;119(5):993–1001. doi: 10.1016/j.clinph.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Andre-Obadia N, Mertens P, Lelekov-Boissard T, Afif A, Magnin M, Garcia-Larrea L. Is Life Better After Motor Cortex Stimulation for Pain Control? Results at Long-Term and their Prediction by Preoperative rTMS. Pain Physician. 2014;17(1):53–62. [PubMed] [Google Scholar]
- 20.Pommier B, Quesada C, Fauchon C, Nuti C, Vassal F, Peyron R. Added value of multiple versus single sessions of repetitive transcranial magnetic stimulation in predicting motor cortex stimulation efficacy for refractory neuropathic pain. Journal of Neurosurgery. 2019;130(5):1750–61. doi: 10.3171/2017.12.Jns171333.*This study showed the predictive value of multiple high frequency rTMS treatments for effective analgesic outcome in implanted epidural motor cortex stimulation. It was found increasing the number of trials from 1 to 4 gave the predictive effects of high frequency rTMS for analgesic efficacy of EMCS 100% positive predictive value.
- 21.Antal A, Terney D, Kuhnl S, Paulus W. Anodal Transcranial Direct Current Stimulation of the Motor Cortex Ameliorates Chronic Pain and Reduces Short Intracortical Inhibition. Journal of Pain and Symptom Management. 2010;39(5):890–903. doi: 10.1016/j.jpainsymman.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122(1-2):197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76(6):833–8. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport. 2001;12(13):2963–5. doi: 10.1097/00001756-200109170-00041. [DOI] [PubMed] [Google Scholar]
- 25.Leung A, Donohue M, Xu RH, Lee R, Lefaucheur JP, Khedr EM et al. rTMS for Suppressing Neuropathic Pain: A Meta-Analysis. Journal of Pain. 2009;10(12):1205–16. doi: 10.1016/j.jpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Attal N, Ayache SS, Ciampi De Andrade D, Mhalla A, Baudic S, Jazat F et al. Repetitive transcranial magnetic stimulation and transcranial direct-current stimulation in neuropathic pain due to radiculopathy: a randomized sham-controlled comparative study. Pain. 2016;157(6):1224–31. doi: 10.1097/j.pain.0000000000000510.**A double-blind randomized crossover trial comparing anodal tDCS to 10 Hz rTMS in 35 patients with neuropathic lower back pain from radiculopathy. This study demonstrates that while 3 consecutive once-daily sessions of rTMS is superior to the same number of tDCS sessions the analgesic effects of each intervention are positively correlated.
- 27.Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology. 2006;67(9):1568–74. doi: 10.1212/01.wnl.0000242731.10074.3c. [DOI] [PubMed] [Google Scholar]
- 28.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993;78(3):393–401. doi: 10.3171/jns.1993.78.3.0393. [DOI] [PubMed] [Google Scholar]
- 29.Cruccu G, Garcia-Larrea L, Hansson P, Keindl M, Lefaucheur JP, Paulus W et al. EAN guidelines on central neurostimulation therapy in chronic pain conditions. European Journal of Neurology. 2016;23(10):1489–99. doi: 10.1111/ene.13103. [DOI] [PubMed] [Google Scholar]
- 30.Borckardt JJ, Smith AR, Hutcheson K, Johnson K, Nahas Z, Anderson B et al. Reducing pain and unpleasantness during repetitive transcranial magnetic stimulation. Journal of Ect. 2006;22(4):259–64. doi: 10.1097/01.yct.0000244248.40662.9a. [DOI] [PubMed] [Google Scholar]
- 31.Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125(11):2150–206. doi: 10.1016/j.clinph.2014.05.021.*-A thorough review of current evidence-based guidelines on the use of repetitive transcranial magnetic stimulation for depression, chronic pain, Parkinson's Disease, motor stroke, multiple sclerosis, tinnitus, and anxiety disorders.
- 32.Hosomi K, Sugiyama K, Nakamura Y, Shimokawa T, Oshino S, Goto Y et al. A randomized controlled trial of five daily sessions and continuous trial of four weekly sessions of repetitive transcranial magnetic stimulation for neuropathic pain. Pain. 2019. doi: 10.1097/j.pain.0000000000001712.**-Multi center study involving 144 patients with chronic neuropathic pain randomized to either 5 Hz rTMS or sham rTMS demonstrating no significant effect of 5 once daily sessions on pain intensity. However, participants who continued in an open-label extension to the trial achieved significant analgesia. Authors discuss possible reasons for failure to replicate previous findings and reasoned that higher frequency and greater dose (2000 versus 500 pulses) may prove more analgesic.
- 33.Ayache SS, Ahdab R, Chalah MA, Farhat WH, Mylius V, Goujon C et al. Analgesic effects of navigated motor cortex rTMS in patients with chronic neuropathic pain. Eur J Pain. 2016;20(9):1413–22. doi: 10.1002/ejp.864. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu T, Hosomi K, Maruo T, Goto Y, Yokoe M, Kageyama Y et al. Efficacy of deep rTMS for neuropathic pain in the lower limb: a randomized, double-blind crossover trial of an H-coil and figure-8 coil. J Neurosurg. 2017;127(5):1172–80. doi: 10.3171/2016.9.JNS16815. [DOI] [PubMed] [Google Scholar]
- 35.Avery DH, Holtzheimer PE, Fawaz F, Russo J, Neumaier J, Dunner D et al. Transcranial magnetic stimulation reduces pain in patients with major depression: A sham-controlled study. Biological Psychiatry. 2007;61(8):187S–8S. [DOI] [PubMed] [Google Scholar]
- 36.George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Current Opinion in Psychiatry. 2013;26(1):13–8. doi: 10.1097/YCO.0b013e32835ab46d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borckardt JJ, Reeves ST, Kotlowski P, Abernathy JH, Field LC, Dong L et al. Fast Left Prefrontal rTMS Reduces Post-Gastric Bypass Surgery Pain: Findings From a Large-Scale, Double-Blind, Sham-Controlled Clinical Trial. Brain Stimulation. 2014;7(1):42–8. doi: 10.1016/j.brs.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Borckardt JJ, Reeves ST, Weinstein M, Smith AR, Shelley N, Kozel A et al. Significant analgesic effects of one session of postoperative left prefrontal cortex repetitive transcranial magnetic stimulation: A replication study. Brain Stimulation. 2008;1(2):122–7. doi: 10.1016/j.brs.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR et al. Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology. 2006;105(3):557–62. doi: 10.1097/00000542-200609000-00020. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira RAA, de Andrade DC, Mendonca M, Barros R, Luvisoto T, Myczkowski ML et al. Repetitive Transcranial Magnetic Stimulation of the Left Premotor/Dorsolateral Prefrontal Cortex Does Not Have Analgesic Effect on Central Poststroke Pain. Journal of Pain. 2014;15(12):1271–81. doi: 10.1016/j.jpain.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Short EB, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves ST et al. Ten sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: A randomized, controlled pilot study. Pain. 2011;152(11):2477–84. doi: 10.1016/j.pain.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umezaki Y, Badran BW, DeVries WH, Moss J, Gonzales T, George MS. The Efficacy of Daily Prefrontal Repetitive Transcranial Magnetic Stimulation (rTMS) for Burning Mouth Syndrome (BMS): A Randomized Controlled Single-blind Study. Brain Stimul. 2016;9(2):234–42 doi: 10.1016/j.brs.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgibbon BM, Hoy KE, Knox LA, Guymer EK, Littlejohn G, Elliot D et al. Evidence for the improvement of fatigue in fibromyalgia: A 4-week left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation randomized-controlled trial. Eur J Pain. 2018;22(7):1255–67. doi: 10.1002/ejp.1213. [DOI] [PubMed] [Google Scholar]
- 44.Altas EU, Askin A, Besiroglu L, Tosun A. Is high-frequency repetitive transcranial magnetic stimulation of the left primary motor cortex superior to the stimulation of the left dorsolateral prefrontal cortex in fibromyalgia syndrome? Somatosens Mot Res. 2019;36(1):56–62. doi: 10.1080/08990220.2019.1587400. [DOI] [PubMed] [Google Scholar]
- 45.Cheng CM, Wang SJ, Su TP, Chen MH, Hsieh JC, Ho ST et al. Analgesic effects of repetitive transcranial magnetic stimulation on modified 2010 criteria-diagnosed fibromyalgia: Pilot study. Psychiatry and Clinical Neurosciences. 2019;73(4):187–93. doi: 10.1111/pcn.12812. [DOI] [PubMed] [Google Scholar]
- 46.Avery DH, Zarkowski P, Krashin D, Rho WK, Wajdik C, Joesch JM et al. Transcranial magnetic stimulation in the treatment of chronic widespread pain: a randomized controlled study. J ECT. 2015;31(1):57–66. doi: 10.1097/YCT.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–76. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 48.Lindholm P, Lamusuo S, Taiminen T, Pesonen U, Lahti A, Virtanen A et al. Right secondary somatosensory cortex-a promising novel target for the treatment of drug-resistant neuropathic orofacial pain with repetitive transcranial magnetic stimulation. Pain. 2015;156(7):1276–83. doi: 10.1097/j.pain.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 49.Teepker M, Hotzel J, Timmesfeld N, Reis J, Mylius V, Haag A et al. Low-frequency rTMS of the vertex in the prophylactic treatment of migraine. Cephalalgia. 2010;30(2):137–44. doi: 10.1111/j.1468-2982.2009.01911.x. [DOI] [PubMed] [Google Scholar]
- 50.Tzabazis A, Aparici CM, Rowbotham MC, Schneider MB, Etkin A, Yeomans DC. Shaped magnetic field pulses by multi-coil repetitive transcranial magnetic stimulation (rTMS) differentially modulate anterior cingulate cortex responses and pain in volunteers and fibromyalgia patients. Mol Pain. 2013;9:33. doi: 10.1186/1744-8069-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galhardoni GR, da Silva VA, Garcia-Larrea L, Dale C, Baptista AF, Barbosa LM et al. Insular and anterior cingulate cortex deep stimulation for central neuropathic pain Disassembling the percept of pain. Neurology. 2019;92(18):E2165–E75. doi: 10.1212/wnl.0000000000007396.*-Deep rTMS randomized controlled trial targeting the posterior insula or anterior cingulate cortex demonstrated no significant analgesic effect on neuropathic pain. However, authors found the right posterior insular cortex deep rTMS made patients less sensitive to heat pain. On the other hand, deep rTMS of ACC reduced symptoms of anxiety in the patient populations.
- 52.Bindman LJ, Lippold OC, Redfearn JW. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced bypolarizing currents. Nature. 1962;196:584–5. doi: 10.1038/196584a0. [DOI] [PubMed] [Google Scholar]
- 53.Purpura DP, Shofer RJ, Musgrave FS. Cortical intracellular potentials during augmenting and recruiting responses. II. Patterns of synaptic activities in pyramidal and nonpyramidal tract neurons. J Neurophysiol. 1964;27:133–51. doi: 10.1152/jn.1964.27.2.133. [DOI] [PubMed] [Google Scholar]
- 54.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527 Pt 3:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson MP, Rahman A, Lafon B, Kronberg G, Ling D, Parra LC et al. Animal models of transcranial direct current stimulation: Methods and mechanisms. Clin Neurophysiol. 2016;127(11):3425–54. doi: 10.1016/j.clinph.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127(2):1031–48. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588(Pt 13):2291–304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain. 2015;156(1):62–71. doi: 10.1016/j.pain.0000000000000006.**-In this study 48 fibromyalgia patients were randomized to receive either verum or sham left M1 tDCS of 2 mA for 20 minutes during 5 once daily sessions. Those who received active stimulation had significant improvements in pain intensity and fibromyalgia related disability compared to those receiving sham stimulation. Authors note the small superior effects compared to sham were possibly not clinically significant.
- 59.Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54(12):3988–98. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 60.Jales Junior LH, Costa MdDL, Jales Neto LH, Ribeiro JPM, Freitas WJSdN, Teixeira MJ. Transcranial direct current stimulation in fibromyalgia: effects on pain and quality of life evaluated clinically and by brain perfusion scintigraphy. Revista Dor. 2015;16(1). doi: 10.5935/1806-0013.20150008. [DOI] [Google Scholar]
- 61.Riberto M, Marcon Alfieri F, Monteiro de Benedetto Pacheco K, Dini Leite V, Nemoto Kaihami H, Fregni F et al. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J. 2011;5:45–50. doi: 10.2174/1874312901105010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2(3):353–61. [PMC free article] [PubMed] [Google Scholar]
- 63.Khedr EM, Omran EAH, Ismail NM, El-Hammady DH, Goma SH, Kotb H et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimul. 2017;10(5):893–901. doi: 10.1016/j.brs.2017.06.006.*-This randomized controlled trial in fibromyalgia patients demonstrated a significant analgesic, antidepressive and anxiolytic effect of 10 once-daily sessions of anodal left M1 at 2 mA for 20 minutes compared to sham stimulation. Serum beta-endorphin levels changes post-anodal stimulation were negatively correlated with changes in symptom ratings.
- 64.Borckardt JJ, Reeves ST, Robinson SM, May JT, Epperson TI, Gunselman RJ et al. Transcranial Direct Current Stimulation (tCS) Reduces Postsurgical Opioid Consumption in Total Knee Arthroplasty (TKA). Clinical Journal of Pain. 2013;29(11):925–8. doi: 10.1097/AJP.0b013e31827e32be. [DOI] [PubMed] [Google Scholar]
- 65.Glaser J, Reeves ST, Stoll WD, Epperson TI, Hilbert M, Madan A et al. Motor/Prefrontal Transcranial Direct Current Stimulation (tDCS) Following Lumbar Surgery Reduces Postoperative Analgesia Use. Spine. 2016;41(10):835–9. doi: 10.1097/brs.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 66.Khedr EM, Sharkawy ESA, Attia AMA, Ibrahim Osman NM, Sayed ZM. Role of transcranial direct current stimulation on reduction of postsurgical opioid consumption and pain in total knee arthroplasty: Double randomized clinical trial. Eur J Pain. 2017;21(8):1355–65. doi: 10.1002/ejp.1034. [DOI] [PubMed] [Google Scholar]
- 67.Jensen MP, Sherlin LH, Askew RL, Fregni F, Witkop G, Gianas A et al. Effects of non-pharmacological pain treatments on brain states. Clin Neurophysiol. 2013;124(10):2016–24. doi: 10.1016/j.clinph.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ngernyam N, Jensen MP, Arayawichanon P, Auvichayapat N, Tiamkao S, Janjarasjitt S et al. The effects of transcranial direct current stimulation in patients with neuropathic pain from spinal cord injury. Clin Neurophysiol. 2015;126(2):382–90. doi: 10.1016/j.clinph.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 69.Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 2010;133(9):2565–77. doi: 10.1093/brain/awq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoon EJ, Kim YK, Kim HR, Kim SE, Lee Y, Shin HI. Transcranial Direct Current Stimulation to Lessen Neuropathic Pain After Spinal Cord Injury: A Mechanistic PET Study. Neurorehabilitation and Neural Repair. 2014;28(3):250–9. doi: 10.1177/1545968313507632. [DOI] [PubMed] [Google Scholar]
- 71.Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain. 2013;154(8):1274–80. doi: 10.1016/j.pain.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 72.Bolognini N, Spandri V, Ferraro F, Salmaggi A, Molinari AC, Fregni F et al. Immediate and Sustained Effects of 5-Day Transcranial Direct Current Stimulation of the Motor Cortex in Phantom Limb Pain. J Pain. 2015;16(7):657–65. doi: 10.1016/j.jpain.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Kikkert S, Mezue M, O'Shea J, Henderson Slater D, Johansen-Berg H, Tracey I et al. Neural basis of induced phantom limb pain relief. Ann Neurol. 2019;85(1):59–73. doi: 10.1002/ana.25371.**This study combined anodal M1 tDCS with physical therapy to reduce phantom limb pain (PLP) and revealed underlying neural correlates of maladaptive plasticity leading to PLP. PLP relief correlated with activity change during stimulation in the insula and second somatosensory cortex.
- 74.Ioannidis JP, Hozo I, Djulbegovic B. Optimal type I and type II error pairs when the available sample size is fixed. J Clin Epidemiol. 2013;66(8):903–10.e2. doi: 10.1016/j.jclinepi.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol. 2011;64(12):1283–93. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Silva AF, Zortea M, Carvalho S, Leite J, Torres IL, Fregni F et al. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: randomized clinical trial. Sci Rep. 2017;7(1):135. doi: 10.1038/s41598-017-00185-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borckardt JJ, Romagnuolo J, Reeves ST, Madan A, Frohman H, Beam W et al. Feasibility, safety, and effectiveness of transcranial direct current stimulation for decreasing post-ERCP pain: a randomized, sham-controlled, pilot study. Gastrointest Endosc. 2011;73(6):1158–64. doi: 10.1016/j.gie.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 78.Dubois PE, Ossemann M, de Fays K, De Bue P, Gourdin M, Jamart J et al. Postoperative analgesic effect of transcranial direct current stimulation in lumbar spine surgery: a randomized control trial. Clin J Pain. 2013;29(8):696–701. doi: 10.1097/AJP.0b013e31826fb302. [DOI] [PubMed] [Google Scholar]
- 79.Borckardt JJ, Reeves ST, Milliken C, Carter B, Epperson TI, Gunselman RJ et al. Prefrontal versus motor cortex transcranial direct current stimulation (tDCS) effects on post-surgical opioid use. Brain Stimul. 2017;10(6):1096–101. doi: 10.1016/j.brs.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burns E, Chipchase LS, Schabrun SM. Primary sensory and motor cortex function in response to acute muscle pain: A systematic review and meta-analysis. Eur J Pain. 2016;20(8):1203–13. doi: 10.1002/ejp.859. [DOI] [PubMed] [Google Scholar]
- 81.Duerden EG, Albanese MC. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp. 2013;34(1):109–49. doi: 10.1002/hbm.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farina S, Valeriani M, Rosso T, Aglioti S, Tamburin S, Fiaschi A et al. Transient inhibition of the human motor cortex by capsaicin-induced pain. A study with transcranial magnetic stimulation. Neuroscience Letters. 2001;314(1-2):97–101. doi: 10.1016/s0304-3940(01)02297-2. [DOI] [PubMed] [Google Scholar]
- 83.Frot M, Magnin M, Mauguiere F, Garcia-Larrea L. Cortical representation of pain in primary sensory-motor areas (S1/M1)--a study using intracortical recordings in humans. Hum Brain Mapp. 2013;34(10):2655–68. doi: 10.1002/hbm.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwenkreis P, Janssen F, Rommel O, Pleger B, Volker B, Hosbach I et al. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology. 2003;61(4):515–9. doi: 10.1212/wnl.61.4.515. [DOI] [PubMed] [Google Scholar]
- 85.Turgut N, Altun BU. Cortical disinhibition in diabetic patients with neuropathic pain. Acta Neurol Scand. 2009;120(6):383–8. doi: 10.1111/j.1600-0404.2009.01235.x. [DOI] [PubMed] [Google Scholar]
- 86.Valeriani M, Restuccia D, Di Lazzaro V, Oliviero A, Profice P, Le Pera D et al. Inhibition of the human primary motor area by painful heat stimulation of the skin. Clinical Neurophysiology. 1999;110(8):1475–80. doi: 10.1016/s1388-2457(99)00075-9. [DOI] [PubMed] [Google Scholar]
- 87.Mhalla A, de Andrade DC, Baudic S, Perrot S, Bouhassira D. Alteration of cortical excitability in patients with fibromyalgia. Pain. 2010;149(3):495–500. doi: 10.1016/j.pain.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Experimental Brain Research. 2010;203(1):31–8. doi: 10.1007/s00221-010-2206-6. [DOI] [PubMed] [Google Scholar]
- 89.Kishima H, Saitoh Y, Osaki Y, Nishimura H, Kato A, Hatazawa J et al. Motor cortex stimulation in patients with deafferentation pain: activation of the posterior insula and thalamus. J Neurosurg. 2007;107(1):43–8. doi: 10.3171/JNS-07/07/0043. [DOI] [PubMed] [Google Scholar]
- 90.Peyron R, Faillenot I, Mertens P, Laurent B, Garcia-Larrea L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. Neuroimage. 2007;34(1):310–21. doi: 10.1016/j.neuroimage.2006.08.037.*-This study included intractable neuropathic pain patients who received epidural motor cortex stimulation and underwent positron emission tomography. The results show stimulation associated increases in neural metabolic activity in areas of the brain related to opioid-related analgesia, such as the periaqueductal gray, anterior cingulate cortex and other regions of the frontal cortex.
- 91.Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58(1):26–33. doi: 10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chiou RJ, Lee HY, Chang CW, Lin KH, Kuo CC. Epidural motor cortex stimulation suppresses somatosensory evoked potentials in the primary somatosensory cortex of the rat. Brain Res. 2012;1463:42–50. doi: 10.1016/j.brainres.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 93.Jiang L, Ji Y, Voulalas PJ, Keaser M, Xu S, Gullapalli RP et al. Motor cortex stimulation suppresses cortical responses to noxious hindpaw stimulation after spinal cord lesion in rats. Brain Stimul. 2014;7(2):182–9. doi: 10.1016/j.brs.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lefaucheur JP, Jarry G, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS reduces acute pain provoked by laser stimulation in patients with chronic neuropathic pain. Clin Neurophysiol. 2010;121(6):895–901. doi: 10.1016/j.clinph.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 95.de Tommaso M, Brighina F, Fierro B, Francesco VD, Santostasi R, Sciruicchio V et al. Effects of high-frequency repetitive transcranial magnetic stimulation of primary motor cortex on laser-evoked potentials in migraine. J Headache Pain. 2010; 11(6):505–12. doi: 10.1007/s10194-010-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Houze B, Bradley C, Magnin M, Garcia-Larrea L. Changes in sensory hand representation and pain thresholds induced by motor cortex stimulation in humans. Cereb Cortex. 2013;23(11):2667–76. doi: 10.1093/cercor/bhs255. [DOI] [PubMed] [Google Scholar]
- 97.Meeker TJ, Keaser ML, Khan SA, Gullapalli RP, Seminowicz DA, Greenspan JD. Non-invasive Motor Cortex Neuromodulation Reduces Secondary Hyperalgesia and Enhances Activation of the Descending Pain Modulatory Network. Front Neurosci. 2019;13:467. doi: 10.3389/fnins.2019.00467.*-This randomized controlled mechanism-based trial in healthy subjects demonstrated that left anodal M1 tDCS of 1 mA for 20 minutes produced an accelerated recovery of capsaicin-induced hyperalgesia compared to sham or cathodal tDCS. Further event-related functional magnetic resonance imaging studies in the same healthy subjects demonstrated enhanced BOLD response to mechanical pain in the anterior cingulate cortex and periaqueductal gray and reduced activity in left S1.
- 98.Parker RS, Lewis GN, Rice DA, McNair PJ. Is Motor Cortical Excitability Altered in People with Chronic Pain? A Systematic Review and Meta-Analysis. Brain Stimul. 2016;9(4):488–500. doi: 10.1016/j.brs.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 99.Enomoto H, Ugawa Y, Hanajima R, Yuasa K, Mochizuki H, Terao Y et al. Decreased sensory cortical excitability after 1 Hz rTMS over the ipsilateral primary motor cortex. Clin Neurophysiol. 2001;112(11):2154–8. doi: 10.1016/s1388-2457(01)00667-8. [DOI] [PubMed] [Google Scholar]
- 100.Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation in the motor cortex. Science. 1989;245(4924):1385–7. doi: 10.1126/science.2551038. [DOI] [PubMed] [Google Scholar]
- 101.Sakamoto T, Porter LL, Asanuma H. Long-lasting potentiation of synaptic potentials in the motor cortex produced by stimulation of the sensory cortex in the cat: a basis of motor learning. Brain Res. 1987;413(2):360–4. doi: 10.1016/0006-8993(87)91029-8. [DOI] [PubMed] [Google Scholar]
- 102.Vidoni ED, Acerra NE, Dao E, Meehan SK, Boyd LA. Role of the primary somatosensory cortex in motor learning: An rTMS study. Neurobiol Learn Mem. 2010;93(4):532–9. doi: 10.1016/j.nlm.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 103.Raij TT, Forss N, Stancak A, Hari R. Modulation of motor-cortex oscillatory activity by painful Adelta- and C-fiber stimuli. Neuroimage. 2004;23(2):569–73. doi: 10.1016/j.neuroimage.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 104.Le Pera D, Brancucci A, De Armas L, Del Percio C, Miliucci R, Babiloni C et al. Inhibitory effect of voluntary movement preparation on cutaneous heat pain and laser-evoked potentials. Eur J Neurosci. 2007;25(6):1900–7. doi: 10.1111/j.1460-9568.2007.05389.x. [DOI] [PubMed] [Google Scholar]
- 105.Stancak A, Johnstone J, Fallon N. Effects of motor response expectancy on cortical processing of noxious laser stimuli. Behav Brain Res. 2012;227(1):215–23. doi: 10.1016/j.bbr.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 106.Stancak A, Raij TT, Pohja M, Forss N, Hari R. Oscillatory motor cortex-muscle coupling during painful laser and nonpainful tactile stimulation. Neuroimage. 2005;26(3):793–800. doi: 10.1016/j.neuroimage.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 107.Bouffard J, Bouyer LJ, Roy JS, Mercier C. Tonic pain experienced during locomotor training impairs retention despite normal performance during acquisition. J Neurosci. 2014;34(28):9190–5. doi: 10.1523/JNEUROSCI.5303-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krause P, Foerderreuther S, Straube A. Effects of conditioning peripheral repetitive magnetic stimulation in patients with complex regional pain syndrome. Neurol Res. 2005;27(4):412–7. doi: 10.1179/016164105X17224. [DOI] [PubMed] [Google Scholar]
- 109.Salerno A, Thomas E, Olive P, Blotman F, Picot MC, Georgesco M. Motor cortical dysfunction disclosed by single and double magnetic stimulation in patients with fibromyalgia. Clin Neurophysiol. 2000;111(6):994–1001. doi: 10.1016/s1388-2457(00)00267-4. [DOI] [PubMed] [Google Scholar]
- 110.Cheong JY, Yoon TS, Lee SJ. Evaluations of inhibitory effect on the motor cortex by cutaneous pain via application of capsaicin. Electromyogr Clin Neurophysiol. 2003;43(4):203–10. [PubMed] [Google Scholar]
- 111.Schabrun SM, Jones E, Cancino ELE, Hodges PW. Targeting Chronic Recurrent Low Back Pain From the Top-down and the Bottom-up: A Combined Transcranial Direct Current Stimulation and Peripheral Electrical Stimulation Intervention. Brain Stimulation. 2014;7(3):451–9. doi: 10.1016/j.brs.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 112.Antal A, Terney D, Kuhnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manage. 2010;39(5):890–903. doi: 10.1016/j.jpainsymman.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 113.Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83(2):259–73. doi: 10.1016/s0304-3959(99)00114-1. [DOI] [PubMed] [Google Scholar]
- 114.Peyron R, Garcia-Larrea L, Deiber MP, Cinotti L, Convers P, Sindou M et al. Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain. 1995;62(3):275–86. doi: 10.1016/0304-3959(94)00211-v. [DOI] [PubMed] [Google Scholar]
- 115.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M et al. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain. 2013;154(11):2563–8. doi: 10.1016/j.pain.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 116.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology. 2007;69(9):827–34. doi: 10.1212/01.wnl.0000269783.86997.37.*-This study included intractable neuropathic pain patients who received epidural motor cortex stimulation and underwent positron emission tomography with radioligands for opioid receptors. The results show stimulation associated decreases in opioid receptor binding, indicating endogenous opioid release, in areas of the brain such as the periaqueductal gray, anterior cingulate cortex and other regions of the frontal cortex which correlated with analgesic magnitude.
- 117.Hsieh JC, Meyerson BA, Ingvar M. PET study on central processing of pain in trigeminal neuropathy. Eur J Pain. 1999;3(1):51–65. doi: 10.1053/eujp.1998.0101. [DOI] [PubMed] [Google Scholar]
- 118.Kudo K, Takahashi T, Suzuki S. The Changes of c-Fos Expression by Motor Cortex Stimulation in the Deafferentation Pain Model. Neurologia medico-chirurgica. 2014;54(7):537–44. doi: 10.2176/nmc.oa.2013-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pagano RL, Assis DV, Clara JA, Alves AS, Dale CS, Teixeira MJ et al. Transdural motor cortex stimulation reverses neuropathic pain in rats: a profile of neuronal activation. Eur J Pain. 2011;15(3):268 e1–14. doi: 10.1016/j.ejpain.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 120.Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain. 2012;153(12):2359–69. doi: 10.1016/j.pain.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 121.Saitoh Y, Osaki Y, Nishimura H, Hirano S, Kato A, Hashikawa K et al. Increased regional cerebral blood flow in the contralateral thalamus after successful motor cortex stimulation in a patient with poststroke pain. J Neurosurg. 2004;100(5):935–9. doi: 10.3171/jns.2004.100.5.0935. [DOI] [PubMed] [Google Scholar]
- 122.Ahmed MA, Mohamed SA, Sayed D. Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol Res. 2011;33(9):953–8. doi: 10.1179/1743132811Y.0000000045. [DOI] [PubMed] [Google Scholar]
- 123.Ciampi de Andrade D, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: The role of endogenous opioids. Pain. 2011;152(2):320–6. doi: 10.1016/j.pain.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 124.Ciampi de Andrade D, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Repetitive transcranial magnetic stimulation induced analgesia depends on N-methyl-D-aspartate glutamate receptors. Pain. 2014;155(3):598–605. doi: 10.1016/j.pain.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 125.Foerster BR, Nascimento TD, DeBoer M, Bender MA, Rice IC, Truong DQ et al. Excitatory and Inhibitory Brain Metabolites as Targets of Motor Cortex Transcranial Direct Current Stimulation Therapy and Predictors of Its Efficacy in Fibromyalgia. Arthritis & Rheumatology. 2015;67(2):576–81. doi: 10.1002/art.38945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoon EJ, Kim YK, Kim HR, Kim SE, Lee Y, Shin HI. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: a mechanistic PET study. Neurorehabil Neural Repair. 2014;28(3):250–9. doi: 10.1177/1545968313507632. [DOI] [PubMed] [Google Scholar]
- 127.DosSantos MF, Martikainen IK, Nascimento TD, Love TM, DeBoer MD, Schambra HM et al. Building up analgesia in humans via the endogenous mu-opioid system by combining placebo and active tDCS: a preliminary report. PLoS One. 2014;9(7):e102350. doi: 10.1371/journal.pone.0102350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ihle K, Rodriguez-Raecke R, Luedtke K, May A. tDCS modulates cortical nociceptive processing but has little to no impact on pain perception. Pain. 2014;155(10):2080–7. doi: 10.1016/j.pain.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 129.Tamura Y, Okabe S, Ohnishi T, D NS, Arai N, Mochio S et al. Effects of 1-Hz repetitive transcranial magnetic stimulation on acute pain induced by capsaicin. Pain. 2004;107(1-2):107–15. doi: 10.1016/j.pain.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 130.Ohn SH, Chang WH, Park CH, Kim ST, Lee JI, Pascual-Leone A et al. Neural correlates of the antinociceptive effects of repetitive transcranial magnetic stimulation on central pain after stroke. Neurorehabil Neural Repair. 2012;26(4):344–52. doi: 10.1177/1545968311423110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR et al. Naloxone-reversible modulation of pain circuitry by left prefrontal rTMS. Neuropsychopharmacology. 2013;38(7):1189–97. doi: 10.1038/npp.2013.13.*-In healthy subjects investigators showed that a single session of high frequency left dorsolateral prefrontal cortex rTMS reduced radioligand binding of opiates indicating endogenous opioid release as measured by PET. Active stimulation reduced sensitivity of skin sensitized by capsaicin and these effects were reversed by the opioid receptor antagonist naloxone.
- 132.Lamusuo S, Hirvonen J, Lindholm P, Martikainen IK, Hagelberg N, Parkkola R et al. Neurotransmitters behind pain relief with transcranial magnetic stimulation - positron emission tomography evidence for release of endogenous opioids. Eur J Pain. 2017;21(9):1505–15. doi: 10.1002/ejp.1052.*-Study of a single session of high frequency left M1 rTMS in healthy subjects during PET to interrogate opioid and dopamine receptor binding showed that opioid release, but not dopamine release was mediated by active stimulation. Opioid release was most prominent in the anterior cingulate cortex and anterior insula.
- 133.Fonoff ET, Dale CS, Pagano RL, Paccola CC, Ballester G, Teixeira MJ et al. Antinociception induced by epidural motor cortex stimulation in naive conscious rats is mediated by the opioid system. Behav Brain Res. 2009;196(1):63–70. doi: 10.1016/j.bbr.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 134.Kim J, Ryu SB, Lee SE, Shin J, Jung HH, Kim SJ et al. Motor cortex stimulation and neuropathic pain: how does motor cortex stimulation affect pain-signaling pathways? J Neurosurg. 2016;124(3):866–76. doi: 10.3171/2015.1.JNS14891. [DOI] [PubMed] [Google Scholar]
- 135.Senapati AK, Huntington PJ, Peng YB. Spinal dorsal horn neuron response to mechanical stimuli is decreased by electrical stimulation of the primary motor cortex. Brain Res. 2005;1036(1-2):173–9. doi: 10.1016/j.brainres.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 136.de Andrade EM, Martinez RCR, Pagano RL, Lopes PSS, Auada AVV, Gouveia FV et al. Neurochemical effects of motor cortex stimulation in the periaqueductal gray during neuropathic pain. J Neurosurg. 2019:1–13. doi: 10.3171/2018.7.JNS173239. [DOI] [PubMed] [Google Scholar]
- 137.Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96(26):15222–7. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Walton KD, Dubois M, Llinas RR. Abnormal thalamocortical activity in patients with Complex Regional Pain Syndrome (CRPS) type I. Pain. 2010;150(1):41–51. doi: 10.1016/j.pain.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 139.Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. Journal of Neuroscience. 2001;21(10):3609–18. doi: 10.1523/jneurosci.21-10-03609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gustin SM, Schwarz A, Birbaumer N, Sines N, Schmidt AC, Veit R et al. NMDA-receptor antagonist and morphine decrease CRPS-pain and cerebral pain representation. Pain. 2010;151(1):69–76. doi: 10.1016/j.pain.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 141.Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with neurogenic pain. Neuroimage. 2008;39(4):1910–7. doi: 10.1016/j.neuroimage.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 142.Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129(Pt 1):55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- 143.Furman AJ, Meeker TJ, Rietschel JC, Yoo S, Muthulingam J, Prokhorenko M et al. Cerebral peak alpha frequency predicts individual differences in pain sensitivity. Neuroimage. 2018;167:203–10. doi: 10.1016/j.neuroimage.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 144.Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. 2014;24(3):333–9. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 145.Ahn S, Prim JH, Alexander ML, McCulloch KL, Frohlich F. Identifying and Engaging Neuronal Oscillations by Transcranial Alternating Current Stimulation in Patients With Chronic Low Back Pain: A Randomized, Crossover, Double-Blind, Sham-Controlled Pilot Study. J Pain. 2019;20(3):277 e1–e11. doi: 10.1016/j.jpain.2018.09.004.**-A recent randomized controlled trial in patients with chronic low back pain used modeling of current flow during transcranial alternating current stimulation to target the somatosensory cortex. This is the first study to show an analgesic effect of tACS at 10 Hz on pain in chronic pain patients.
- 146.Hazime FA, Baptista AF, de Freitas DG, Monteiro RL, Maretto RL, Hasue RH et al. Treating low back pain with combined cerebral and peripheral electrical stimulation: A randomized, double-blind, factorial clinical trial. Eur J Pain. 2017;21(7):1132–43. doi: 10.1002/ejp.1037. [DOI] [PubMed] [Google Scholar]
- 147.Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial Direct Current Stimulation Combined with Aerobic Exercise to Optimize Analgesic Responses in Fibromyalgia: A Randomized Placebo-Controlled Clinical Trial. Frontiers in Human Neuroscience. 2016;10:68. doi: 10.3389/fnhum.2016.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hameroff S, Trakas M, Duffield C, Annabi E, Gerace MB, Boyle P et al. Transcranial ultrasound (TUS) effects on mental states: a pilot study. Brain Stimul. 2013;6(3):409–15. doi: 10.1016/j.brs.2012.05.002.*-First study using transcranial ultrasound in chronic pain patients demonstrates significant effects on mood and trend of an effect on pain intensity.


