Abstract
SARS-CoV-2 is the virus that has caused the current coronavirus disease 2019 (COVID-19) pandemic. SARS-CoV-2 is characterized by significantly affecting the cardiovascular system of infected patients. In addition to the direct injuries caused by the virus, the subsequent cytokine storm – an overproduction of immune cells and their activating compounds – also causes damage to the heart. The development of anti-SARS-CoV-2 treatments is necessary to control the epidemic. Despite an explosive growth in research, a comprehensive review of up-to-date information is lacking. Herein, we summarize pivotal findings regarding the epidemiology, complications, and mechanisms of, and recent therapies for, COVID-19, with special focus on its cardiovascular impacts.
Keywords: Cardiovascular injuries, COVID-19, Cytokine storm, Therapies
1. EPIDEMIOLOGY OF CARDIOVASCULAR INVOLVEMENT OF COVID-19
In the first study of cardiovascular involvement of COVID-19, 32% of 41 patients diagnosed with COVID-19 had underlying disease, including cardiovascular disease (15%), hypertension (15%), and diabetes (20%).1 In another study which focused on 138 hospitalized patients with COVID-19, 64 patients (46.4%) had one or more comorbidities, including hypertension (31%), diabetes (10%), and cardiovascular disease (14.5%).2 These findings suggest that a large proportion of patients with underlying cardiovascular disease or cardiac risk factors may be more susceptible to SARS-CoV-2. In addition, patients with cardiovascular disease infected by SARS-CoV-2 may have a higher risk of adverse outcomes compared to those without cardiovascular disease. In a cohort of 191 patients with laboratory-confirmed COVID-19, 54 died and 137 survived, and those who died had higher rates of acute cardiac injury (59% vs. 1%), heart failure (52% vs. 12%) and coronary heart disease (24% vs. 1%) compared to those who survived.3 In a large series of 44672 patients diagnosed with COVID-19 in China, 4.2% of the patients had underlying coronary artery disease. Among them, there was a higher mortality rate (10.5%) than the overall rate of 2.3% in COVID-19 patients.4 Likewise, in another study of 187 patients hospitalized for COVID-19 in China, 28% of the patients had acute myocardial injury, resulting in significantly higher mortality compared to those free from cardiac complications (59.6% vs. 8.9%, p < 0.001).5 Taken together, mortality due to COVID-19 is strongly associated with cardiovascular disease. In the following sections, we discuss individually the main presentations of COVID-19-associated cardiovascular complications, including myocarditis, coronary artery disease/acute coronary syndrome, heart failure, and fatal arrhythmia.
2. COVID-19-ASSOCIATED CARDIOVASCULAR COMPLICATIONS
2.1 Myocarditis
Among patients diagnosed with SARS, MERS, and COVID-19, cytokine release syndrome (CRS), a phenomenon of hyper-inflammation that involves elevation of various cytokines, is believed to contribute to increased myocardial oxygen consumption, endothelial dysfunction, and suppressed cardiac function.6-8 Regarding myocarditis, viral infection has also been shown to be one of the most common causes. In a retrospective study of 150 patients diagnosed with COVID-19, cardiovascular disease was more prevalent in the patients who died, including 27 due to myocardial damage and circulatory failure.9 The patients who died had elevated levels of circulating troponin, myoglobin, ferritin, C-reactive protein (CRP), and interleukin-6 (IL-6). In another study, an increased infiltration of mononuclear cells into the myocardium was revealed by autopsy.10 Both studies demonstrate the possible underlying mechanism of hyper-activation of the immune response in COVID-19. An extremely robust cytokine storm has also been reported to be the core pathophysiological mechanism in fulminant myocarditis related to COVID-19.7,8 Crucial for the early identification and interventions for COVID-19 is the recognition of acute myocarditis as a possible complication associated with COVID-19.
2.2 Coronary artery disease and acute coronary syndrome
The reported prevalence of coronary artery disease in patients with COVID-19 ranges widely from around 4 to 25%.3,11 Several studies have reported that patients with both COVID-19 and underlying cardiovascular diseases may have a worse prognosis, more critical illness, and higher mortality.12-15 Although the exact mechanism has not been elucidated, the possible explanations include direct and indirect impacts on the cardiovascular system, such as SARS-CoV-2 infection per se, hypoxic injury, subsequent cytokine storm, coronary spasm, microthrombi, and an overactive immune system.16,17 These series of reactions may result in acute coronary syndrome among patients with COVID-19. First, patients with severe COVID-19 may develop a high fever or hypoxia due to pulmonary damage, and the body compensates with increased cardiac output. This compensation may lead to type 2 myocardial infarction. Second, SARS-CoV-2 infection may cause microthrombi due to coagulopathy and destabilize coronary artery plaques, resulting in obstructive coronary artery disease and even type 1 myocardial infarction.18 Despite the higher risks of myocardial infarction, some national statistics have shown a significant decrease in the catheterization activation for ST-elevation myocardial infarction (STEMI) during the COVID-19 pandemic, including a 40% drop in Spain and a 38% drop in the US.19,20 This phenomenon may be due to patients avoiding hospital visits due to the fear of getting COVID-19, and less daily activity due to social distancing and quarantine.19,20 Similar findings have been reported in Taiwan, with a 27% increase in the symptom onset-to-door time [142 (75 to 338) vs. 180 (84 to 460) min, p < 0.01] during the pandemic compared to the equivalent months in 2019.21 However, due to maintaining normal service through active and prompt public health measures, there were no significant differences in the average STEMI case number per hospital (27.3 ± 18.4 versus 26.0 ± 16.7, p = 0.27) and the door-to-device time [65 (50 to 81) versus 66 (52 to 81) min, p = 0.20] from the previous year.21
2.3 Heart failure
In a study in Wuhan, China, among 113 patients diagnosed with COVID-19, 72 and 41 developed acute myocardial injury and heart failure, respectively.22 Identified in multiple organs, including the heart, kidneys, lungs, and epithelium, angiotensin-converting enzyme 2 (ACE2) has been hypothesised to be involved in the mechanism of COVID-19 infection, as ACE2 is the key host cellular receptor of SARS-CoV-2.23 It has not yet been determined whether the use of ACE inhibitors (ACEIs) or angiotensin II type 1 receptor blockers (ARBs) can facilitate coronavirus entry by increasing the ACE2 expression in animals or humans, although angiotensin II type 1 receptor blockers have been shown to upregulate ACE2 in experimental animals.24 As ACE2 is a negative regulator of the renin-angiotensin system that also protects against heart failure and acute lung injury, it is a double-edged sword.24,25 It remains under debate in clinical practice whether to continue or replace the use of ACEIs/ angiotensin II receptor blockers in patients diagnosed with COVID-19.
2.4 Arrhythmias
The impact of COVID-19 on cardiac arrhythmias originates from the systemic inflammation it causes, which in turn induces myocardial injury and dysfunction.26 In a Chinese cohort study of 138 patients hospitalized with COVID-19, cardiac arrhythmia was noted in 16.7% of the patients. Among them, 36 (26.1%) were transferred to the intensive care unit owing to acute respiratory distress syndrome (ARDS; 61%), arrhythmias (44%), and shock (31%).2 The commonly observed hypoxia and electrolyte abnormalities also exacerbate cardiac arrhythmia in the acute phase of severe illness. Notably, a potential therapy for reducing the number of SARS-CoV-2 copies is currently hydroxychloroquine (HCQ).27 However, through blocking Kv11.1 (HERG), HCQ also causes QTc prolongation and may contribute to fatal arrhythmias.28 Recently, owing to the risk of abnormal heart rhythm, the Food and Drug Administration (FDA) announced caution against the use of HCQ or chloroquine for COVID-19 outside of the hospital setting.
3. PROPOSED MECHANISM OF SARS-COV-2-INDUCED MYOCARDIAL INJURY
During the epidemic, the pathophysiology of this viral illness has been studied widely, and more data are accumulating. Here, we focus on the possible pathophysiological mechanisms of COVID-19-related cardiac complications.
3.1 Direct viral injury
SARS-CoV-2 infects cells through interactions between its spike protein (S) and the host cell surface, and its utilisation of ACE2 receptors on the host cell surface.29 As a class I viral envelope glycoprotein, S protein is responsible for receptor recognition followed by the process of fusion that allows the virus to enter.29 Another transmembrane protease, serine (TMPRSS), has been identified as facilitating cleavage of the S protein.30 The coexistence of ACE2 receptors and TMPRSS2 on the host cell surface enhances the entry and propagation of SARS-CoV-2.30,31 A ubiquitous pro-protein convertase, furin, is responsible for the cleavage of S protein, and plays a pivotal role in the signalling of fibrosis after myocardial injury.32 While further investigations are necessary, it may play an important role in the process of myocardial damage induced by SARS-CoV-2.
3.2 Up- and downregulation of ACE2
ACE2 initiates subsequent viral internalisation after binding to the SARS-CoV-2 S1 protein through endocytosis. ACE2 is commonly expressed on the cells of the lungs, heart, kidney, intestines, brain, and endothelium.23,31 ACE2 is regarded to be a protective factor against cardiovascular diseases, since it counterbalances the activity of angiotensin-converting enzyme (ACE).25 Also, ACE2 has been observed to protect against lung injury in patients with ARDS.33 The internalization of ACE2 during SARS-CoV-2 infection leads to the reduction of its expression and function in infected tissues.23 Subsequent elevation of angiotensin II levels have been associated with acute lung injury in cases with COVID-19.25 The loss of ACE2 by SARS-CoV-2 may therefore contribute to the exacerbation of cardiovascular disease through the com-pensatory elevation of angiotensin II levels in patients with cardiac dysfunction.25 Currently, the pros and cons of continuing ACEI/ARB use in patients at risk of SARS-CoV-2 infection is an intense debate. The major viewpoints are summarized as follows:
Pros of continuing ACEI/ARB therapy
1. It has been postulated that through binding to ACE2, SARS-CoV-2 may reduce the activity of residual ACE2 and contribute to an elevated level of angiotensin II.31 This then leads to pulmonary vasoconstriction, inflammation, and subsequent lung injury.25,31 ACEIs/ARBs may help mitigate some of these deleterious effects of angiotensin II upon an increased expression of ACE2.33
2. In support of this, animal studies have suggested that ACEIs/ARBs may be protective against serious lung complications in patients with COVID-19 infection, but to date there are no data for humans.33
3. Conversely, it has also been postulated that by slowing virus entry into cells and protecting against lung injury, increased levels of the soluble form of ACE2 may act as a competitive interceptor of SARS-CoV-2.34
4. There is no evidence to support an association with cardiovascular disease, hypertension, and diabetes, although these conditions may have a more severe clinical course upon infection with SARS-CoV-2.26 In addition, comorbid cardiovascular or renal disease may be exacerbated and result in higher mortality with abrupt discontinuation of ACEIs/ARBs in specific populations.24
Cons of continuing ACEI/ARB therapy
1. The evidence at present shows that ACEIs/ARBs play a protective role in the cardiovascular system, and that they can increase the expression and activity of ACE2 in the heart. However, the impact of ACEIs/ARBs on ACE2 in other organs remains unknown, especially regarding their influence on the expression level and activity of ACE2 in the lungs.31,35
2. SARS-CoV-2 uses the ACE2 receptor for entry into target cells. ACE2 is predominantly expressed by the epithelial cells of the lungs, intestines, kidneys, heart, and blood vessels.24 The expression of ACE2 has been reported to be substantially increased in patients treated with ACEIs/ARBs in animal studies.36,37 Some experts have speculated that the use of ACEIs/ARBs could potentially facilitate COVID-19 infection through the increased expression of ACE2.36
Currently, all guidelines recommend continuing ACEIs/ARBs in COVID-19 patients unless clinically contraindicated. Additional studies and prospective trials are urgently needed.33
3.3 Cytokine release syndrome
Patients with COVID-19 have been found to have significantly elevated levels of vascular endothelial growth factor (VEGF), IL-6 and reduced E-cadherin.8,38 Increased VEGF and reduced E-cadherin expressions cause vascular permeability and leakage and contribute to the development of shock and ARDS.8 IL-6 is associated with the pathophysiology of respiratory failure and pneumonia, and has also been reported to result in mortality in critically ill patients.8,38 Characterized by elevated levels of cytokines, including IL-6 and other inflammatory cytokines, the so-called CRS has been a significant clinical finding in patients with SARS, MERS, and COVID-19.39 Both innate and cellular immune responses are activated via the secretion of IL-6 by activated monocytes, macrophages, and dendritic cells in response to SARS-CoV-2 infection.7 The subsequent signalling pathway involves the activation of cytotoxic T cells and the differentiation of B cells, as well as the activation of endothelial cells.7 Presenting as increased vascular permeability and leakage, hypotension, shock, and ARDS, the secretion of cytokines by these activated immune cells leads to a systemic cytokine storm.7,8
However, the exact mechanism of cytokine-induced myocardial injury is not well understood. Inflammatory cytokines such as tumour necrosis factor-alpha have been shown to induce cardiomyocyte death.40 Possible mechanisms include the induction of nitric oxide, the generation of oxygen free radicals, and the subsequent activation of apoptosis.41,42 Increased cardiovascular events have also been reported in other hyper-inflammatory syndromes such as CRS associated with chimeric antigen receptor (CAR)-T cell therapy.43 As disease severity increases due to the presence of these cytokines, these findings suggest the potential use of immunomodulatory agents in the management of COVID-19.
3.4 Aggravation of underlying cardiac diseases
According to a recent study in China, around 35% of patients with COVID-19 had cardiovascular morbidities, including hypertension, coronary heart disease, and cardiomyopathy.5 More than half of those with underlying cardiovascular diseases had an elevation of cardiac enzymes during the clinical course of the disease, indicating acute myocardial injury.5 Notably, patients who had cardiac injury also had the highest mortality rate. Therefore, in patients with cardiovascular morbidities, aggressive management is mandatory to avoid the high risk of mortality during the clinical course of infection.
3.5 Drug-induced cardiotoxicity
Including anti-viral regimens and immunomodulatory regimens, the updated treatments for COVID-19 are summarized in section 5. Nevertheless, there are still concerns over drug-related heart injury. QT-interval prolongation has been observed with the use of HCQ, azithromycin, and protease inhibitors.27,44 Furthermore, when treating patients with COVID-19, potential drug-drug interactions between novel anti-viral drugs and cardiovascular medications should be reviewed carefully.
In conclusion, complex interactions among the virus, host factors, and pathophysiology of the disease are possible biological mechanisms of cardiac injury in COVID-19, and understanding these mechanisms is pivotal to the development of possible curative medications and the management of cardiac injury in patients with COVID-19.
4. DETECTION AND MANAGEMENT OF CARDIOVASCULAR COMPLICATIONS IN COVID-19
Myocardial injury in patients with COVID-19 may result from systemic inflammation, myocarditis, stress cardiomyopathy, hypoxia, and ischemic damage.16,17 COVID-19 patients with concurrent myocardial injury mainly develop the symptoms and signs of SARS-CoV-2 infection, including fever, cough and breathlessness, while the cardiac symptoms of myocardial injury, such as chest pain and arrhythmia, only present in some patients.45 Therefore, how to early detect myocardial injury and prevent cardiovascular complications is crucial. Apart from a prudent history taking and physical examination, electrocardiogram changes may enable clinicians to detect cardiac injury early and prevent cardiovascular complications when treating patients with COVID-19. New-onset T wave depression or inversion, ST-segment depression or elevation, and Q wave occurrence may imply myocardial injury.46 In particular, clinicians may need to differentiate between myocardial infarction and other causes of myocardial injury when the ST segment elevates. Moreover, with the prolongation of QT or corrected QTc on electrocardiogram, QT-prolonging therapies such as hydroquinone, chloroquine, and azithromycin, should be carefully assessed and prescribed.
Troponin testing may provide a tool to evaluate cardiac injury and prognosis for COVID-19 patients with cardiovascular complications, especially for those with more severe illness.47 Nevertheless, troponin elevation in hospitalized COVID-19 patients has commonly been observed,15 which may mask the manifestations of acute coronary syndrome. When clinicians face this situation, the troponin peak can be helpful in the differential diagnosis, because myocardial infarction usually leads to a higher troponin elevation than non-coronary myocardial injury in COVID-19 patients.46 If the clinical presentation highly indicates myocardial infarction, a prompt and prudent assessment is essential, and the subsequent treatment strategy, i.e., medical reperfusion or intervention, depends on the local healthcare capacity and policy during the COVID-19 pandemic. Furthermore, except for myocardial infarction, given a moderate or progressive elevation in troponin, the patients may also be experiencing myocarditis, stress cardiomyopathy, and severe cytokine storm.3 Further biomarkers including D-dimer, ferritin, and lactate dehydrogenase may help to assess the current condition.3,27 In addition, in patients with suspected heart failure, a natriuretic peptide level [B-type natriuretic peptide (BNP) or N-terminal proBNP] can assist in the diagnosis of heart failure and provide evidence for guide-directed medical therapy.
5. UPDATED THERAPIES FOR COVID-19
No drugs have yet passed US FDA screening for the treatment of COVID-19; however, "Solidarity", an international clinical trial launched by the World Health Organisation, has attempted to identify drugs with therapeutic potential to treat COVID-19.48 A process called "repurposing" or "repositioning" is intended to rapidly evaluate the potential efficacy of thousands of existing anti-viral and anti-inflammatory agents which have not yet been approved for the treatment of COVID-19.48,49 Several candidate drugs are currently being screened and are under investigation, and mainly include anti-viral regimens (e.g. remdesivir, atazanavir, ritonavir/lopinavir, favipiravir, etc.) and immunomodulatory regimens (e.g., chloroquine/HCQ, tocilizumab, interferon-β1, etc.).49-53 The potential anti-SARS-CoV-2 regimens and ongoing trials are summarized below.
5.1 Anti-viral drugs
Remdesivir is viewed as being one of the most potentially effective regimens, as it reduces SARS-CoV-2 replication and its binding to the active site on RNA polymerase.54 It has been approved for the treatment of Ebola, respiratory syncytial virus infection, Junin virus infection, Lassa fever, Nipah virus infection, Hendra virus infection, and coronaviruses (including MERS and SARS).55 Currently, several clinical trials have been launched to evaluate the effect of remdesivir on patients with moderate or severe COVID-19 compared with standard care, and according to a recent case report, a patient recovered after remdesivir treatment.56 In a preliminary published report of 53 patients who received at least one dose of remdesivir, 36 patients (68%) showed improved oxygen-support, 25 patients (47%) were discharged, and seven patients (13%) died.54 The major weakness of this study was its lack of placebo and its non-randomised design, despite a relatively high incidence of clinical improvements in COVID-19 patients receiving remdesivir. Recently released, a preliminary report conducted mainly in the United States and Europe has shown that remdesivir is superior to placebo in shortening the time to recovery in patients hospitalized with COVID-19 and evidence of lower respiratory tract infection.56 In contrast, another randomized, double-blind, placebo-controlled study conducted in China indicated that remdesivir was not associated with statistically significant clinical benefits.57 The major adverse effects of remdesivir have been reported to be liver and renal toxicity, while cardiotoxicity is rare.58
Ritonavir/lopinavir has been approved for the treatment of HIV infection.49,52 An important enzyme that cleaves a long protein chain during the assembly of new viruses, ritonavir/lopinavir may act through the inhibition of protease and also be capable of binding SARS-CoV-2 3C-like proteinase and consequently suppressing its replication.52 In the first randomized and open-label trial conducted in China for treatment with ritonavir/lopinavir, 199 patients diagnosed with COVID-19 exhibited no differences compared to standard care with regards to clinical improvements and 28-day mortality.52 In addition, the percentage of patients with detectable viral RNA at various time points was similar. Currently, there are several ongoing trials investigating the anti-SARS-CoV-2 effects of ritonavir/lopinavir. Likewise, the adverse effects of ritonavir/lopinavir mainly include liver toxicity, pancreatitis and neurotoxicity.58
Favipiravir, which competitively inhibits RNA-dependent RNA polymerase, has been approved for the treatment of influenza.59,60 Little preclinical support has been established, although one report showed a shorter viral clearance duration and improved chest imaging in patients diagnosed with COVID-19 compared to controls.61 Additionally, ivermectin, an anti-parasitic regimen, has also been identified as an inhibitor of the interaction between the human HIV integrase protein and the importin α/β 1 heterodimer.62,63 Notably, studies of SARS-CoV proteins have revealed the potential role of importin α/β 1 in infection.63 Although an in vitro study indicated the ability of iver-mectin to reduce SARS-CoV2 by 5000-fold, clinical evidence is currently lacking.
5.2 Immunomodulatory regimens
HCQ, an antimalarial and anti-autoimmune agent, has been found to inhibit the replication of SARS-CoV by interfering with the glycosylation of its cellular receptor, ACE2.27 Various degrees of the therapeutic effect of HCQ in the treatment of COVID-19 have been shown in small-scale studies.27,53 Recently, a small open-label, non-randomized clinical trial from France demonstrated the positive effect of HCQ in combination with azithromycin.27 Upon release of this finding, the US FDA issued an emergency use authorization for HCQ in treating COVID-19, but recently announced caution against its use outside of the hospital setting due to the risk of arrhythmia. In contrast, Boulware et al. reported that HCQ failed to result in benefits on post-exposure prophylaxis of COVID-19.64 Up to June 2020, there have been almost 300 COVID-19 trials with HCQ registered in ClinicalTrials.gov. Upcoming reports will help to elucidate the true anti-COVID-19 effects of HCQ, but in the meantime HCQ-induced cardiotoxicity should not be ignored.58
An anti-human IL-6 receptor monoclonal antibody, tocilizumab, has been approved for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis.65 Specific blockade of IL-6-regulated signalling pathways may be a promising approach to attenuating inflammation-associated damage, given that the rapid development of ARDS in COVID-19 may mainly be due to the cytokine storm as opposed to SARS-CoV2 itself.39,65 A recent study showed that among 15 patients diagnosed with COVID-19, tocilizumab combined with methylprednisolone countered the increase in CRP and IL-6, but this result was insufficient to support its therapeutic effects.66
6. CONCLUSIONS
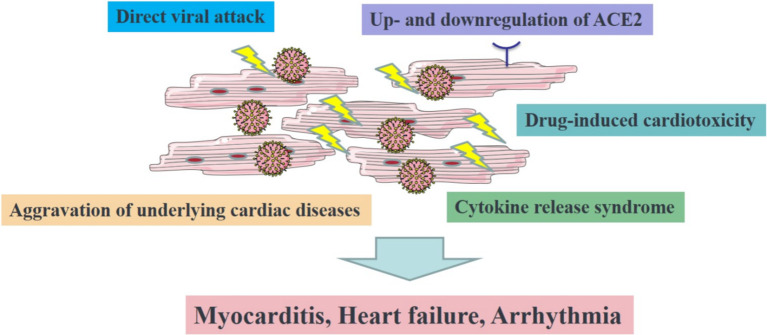
SARS-CoV-2 may either trigger new cardiac pathologies or exacerbate underlying cardiovascular diseases due to its high inflammatory burden. In Figure 1, we summarize the current state of knowledge about the possible mechanisms and complications of SARS-CoV-2-induced myocardial damage. Careful evaluation and management of the possible cardiovascular complications of the aforementioned drugs is crucial, given that patients affected by this disease commonly have cardiovascular comorbidities. It is also reasonable to expect, with the continued efforts of physicians and scientists, the development of more optimal therapies to treat SARS-CoV2 while attenuating the risk of cardiovascular complications.
Figure 1.
The illustration of the proposed mechanism of COVID-19-induced cardiovascular injuries. ACE2, angiotensin-converting enzyme 2.
CONTRIBUTORS
All authors contributed equally to the searches, design and writing of the manuscript.
PATIENT CONSENT FOR PUBLICATION
Not required.
FINDING
This work was supported by Chi-Mei Medical Center.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1106. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novel Coronavirus Pneumonia Emergency Response Epidemiology T. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. [Google Scholar]
- 5.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tay MZ, Poh CM, Renia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore BJB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 9.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiology. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 12.Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiology. 2020;5:751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 13.He X, Lai J, Cheng J, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E011. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 14.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby P, Loscalzo J, Ridker PM, et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. 2018;72:2071–2081. doi: 10.1016/j.jacc.2018.08.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez-Leora O, Cid-Álvarezd B, Ojedae S. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en España. REC Interv Cardiol. 2020;2:82–89. [Google Scholar]
- 21.Li YH, Huang WC, Hwang JJ. No reduction of ST-segment elevation myocardial infarction admission in Taiwan during coronavirus pandemic. Am J Cardiol. 2020;131:133–134. doi: 10.1016/j.amjcard.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 25.Nunes-Silva A, Rocha GC, Magalhaes DM, et al. Physical exercise and ACE2-angiotensin-(1-7)-mas receptor axis of the renin angiotensin system. Protein Pept Lett. 2017;24:809–816. doi: 10.2174/0929866524666170728151401. [DOI] [PubMed] [Google Scholar]
- 26.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cubeddu LX. Drug-induced inhibition and trafficking disruption of ion channels: pathogenesis of QT abnormalities and drug-induced fatal arrhythmias. Curr Cardiol Rev. 2016;12:141–154. doi: 10.2174/1573403X12666160301120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia S, Liu M, Wang C, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heurich A, Hofmann-Winkler H, Gierer S, et al. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coutard B, Valle C, de Lamballerie X, et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batlle D, Wysocki J, Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin Sci (Lond) 2020;134:543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 35.Glowacka I, Bertram S, Herzog P, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vuille-dit-Bille RN, Camargo SM, Emmenegger L, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693–705. doi: 10.1007/s00726-014-1889-6. [DOI] [PubMed] [Google Scholar]
- 38.Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B, Li M, Zhou Z, et al. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krown KA, Page MT, Nguyen C, et al. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–2865. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ing DJ, Zang J, Dzau VJ, et al. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ Res. 1999;84:21–33. doi: 10.1161/01.res.84.1.21. [DOI] [PubMed] [Google Scholar]
- 42.Jarrah AA, Schwarskopf M, Wang ER, et al. SDF-1 induces TNF-mediated apoptosis in cardiac myocytes. Apoptosis. 2018;23:79–91. doi: 10.1007/s10495-017-1438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh AK CD, Guha A, Mackenzie S, et al. CAR T cell therapy–related cardiovascular outcomes and management: systemic disease or direct cardiotoxicity? JACC: CardioOncology. 2020;2:97–109. doi: 10.1016/j.jaccao.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soliman EZ, Lundgren JD, Roediger MP, et al. Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS. 2011;25:367–377. doi: 10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. "Solidarity" clinical trial for COVID-19 treatments. 2020;https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments [Google Scholar]
- 49.Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rismanbaf A. Potential treatments for COVID-19; a narrative literature review. Arch Acad Emerg Med. 2020;8:e29. [PMC free article] [PubMed] [Google Scholar]
- 52.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colson P, Rolain JM, Lagier JC, et al. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu WL, Toh HS, Liao CT, Chang WT. A double-edged sword-cardiovascular concerns of potential anti-COVID-19 drugs. Cardiovasc Drugs Ther. 2020:1–10. doi: 10.1007/s10557-020-07024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Various combination of protease inhibitors, oseltamivir, favipiravir, and hydroxychloroquine for treatment of COVID-19: a randomized control trial (THDMS-COVID-19). 2020 [Google Scholar]
- 60.Qingxian Caia MY, Liu DJ, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clinical study for safety and efficacy of favipiravir in the treatment of novel coronavirus pneumonia (COVID-19). 2020 [Google Scholar]
- 62.Caly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagstaff KM, Sivakumaran H, Heaton SM, et al. Ivermectin is a specific inhibitor of importin alpha/beta-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang C, Wu Z, Li JW, et al. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo P, Liu Y, Qiu L, et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]