Abstract
Background
In 2012, we identified the dissemination of methicillin-resistant Staphylococcus aureus (MRSA) sequence type (ST)45 strain in 14 nursing homes in Taiwan and foreign nurse workers, a significant risk factor for MRSA carriage. We conducted this study to understand MRSA carriage and molecular characteristics among foreign workers recruited from Southeastern Asian countries.
Methods
A cross-sectional study involving a total of 1935 foreign workers—929 (arrival group) and 1006 (staying group)—from Vietnam (n = 843), Indonesia (n = 780), the Philippines (n = 239), and Thailand (n = 70) were conveniently recruited during upon-arrival and regular health examination in a regional hospital. A nasal swab was obtained from each participant for detection of MRSA.
Results
Overall, MRSA carriage rate was 2.72%, with 2.26% for arrival group and 3.18% for staying group, and 4.74% for Vietnamese, 1.28% for Indonesians, 1.26% for Filipino, and none for Thai workers. Pulsotype AK/ST45 (57%) and pulsotype AX/ST188 (14%) were the top 2 dominant clones for the arrival group, whereas pulsotype D/ST59 (41%) (an endemic community clone in Taiwan) and pulsotype AK/ST45 (19%) were predominant for the staying group. A significant decrease of pulsotype AK/ST45 from 57% to 19% (P = .007) and increase of pulsotype D/ST59 from 4.8% to 41% (P = .004) were found between the arrival and the staying groups.
Conclusions
Approximately 3% of foreign workers recruited from Southeastern Asian countries to Taiwan were colonized with MRSA, including the ST45 strain. However, the MRSA isolates from workers staying in Taiwan were mostly a locally endemic clone and genetically different from those identified from workers on arrival.
Keywords: carriage, foreign worker, methicillin-resistant Staphylococcus aureus, sequence type 45, Taiwan
Staphylococcus aureus is a common cause of skin and soft tissue infection. It can also cause more serious conditions such as myositis, bone and/or joint infection, pneumonia, endocarditis, and bacteremia as well as life-threatening infections such as septicemia, necrotizing fasciitis, and toxic shock syndrome. It is always a challenge to treat infections due to S aureus, especially methicillin-resistant S aureus (MRSA) and strains resistant to beta-lactams antibiotics.
Methicillin-resistant S aureus strains are classified as community-associated MRSA (CA-MRSA) and healthcare-associated MRSA (HA-MRSA) according to epidemiological and/or molecular characteristics, but distinctions between CA- and HA-MRSA became more difficult to make because the strain types with these designations were not limited to community or healthcare settings, respectively [1, 2]. Both CA- and HA-MRSA clones varied in different continents, countries, or even smaller regions [1, 2]. As we know, international travel has been shown to contribute to the spread of some transmissible diseases as well as multidrug-resistant clones of bacteria among different countries. In Taiwan, sequence type (ST)59 is the most prevalent CA-MRSA clone whereas ST239 and ST5 are the most common HA-MRSA clones in the past decade [2]. In our previous study conducted in 2012 [3], we identified the dissemination of ST45 strain, not a major CA- and HA-MRSA clone in Taiwan, among residents and health workers in 14 nursing homes island wide in Taiwan, and we also found that foreign nurse workers were a significant risk factor for MRSA carriage. Most foreign workers in Taiwan were recruited from Southeastern Asian countries, in which the prevalence as well as molecular characteristics of MRSA are scant. To trace the potential origin of MRSA ST45, we conducted this study to determine the nasal colonization rate, risk factors for carriage, and microbiologic characteristics of MRSA among foreign workers recruited to Taiwan.
MATERIALS AND METHODS
Subjects
A cross-sectional study, one-time point survey with 1935 foreign workers recruited was conducted in St. Paul’s Hospital, which is located in the city center of Taoyuan city, in the northern part of Taiwan. It is one of the designated hospitals in Taiwan that offers health examination for recruited foreign workers. Between February and July 2017, any foreign worker undergoing a health examination in the hospital was eligible for this study. There were 2 types of health examination for foreign workers who came to Taiwan: one was an upon-arrival health examination (arrival group), and the other was a regular health examination (staying group). It was stipulated in the law that any foreign workers coming to Taiwan were subjected to upon-arrival health examination and regular health examination at 6, 18, and 30 months after formal employment. The arrival time was calculated by subtracting the arrival date, which was an official record of the government, from the nasal sampling date. All of them came from 4 countries, namely, Vietnam, Indonesia, the Philippines, and Thailand. This study was approved by the Institutional Reviewing Board of Chang Gung Memorial Hospital (201509728B0), and informed consent was obtained from each participant.
Sample Collection and Microbiologic Methods
A nasal swab was obtained from each participant with a sterile cotton-top swab for detection of MRSA. Each nasal swab was rubbed against both anterior nares of the participants, then the swab was placed into the transport medium (Venturi Transystem; Copan Innovation, Copan Diagnostics, Italy) immediately and sent to the laboratory of Chang Gung Memorial Hospital within 3 days for culture. Using streak plate method, swab samples were inoculated onto Trypticase soy agar with 5% sheep blood plates. The plates were incubated at 37°C overnight. To identify S aureus, morphology, Gram stain, and coagulase tests of strains grown were done on agar plates. To identify MRSA, cefoxitin disk was used by disk-diffusion method according to the recommendation of Clinical and Laboratory Standard Institutes [4]. Once MRSA was identified, molecular characteristics were determined.
Molecular Typing
Chromosomal deoxyribonucleic acid was extracted from MRSA isolates for molecular characterization. All of them were characterized by pulsed-field gel electrophoresis (PFGE) with SmaI digestion [5, 6], staphylococcal cassette chromosome (SCCmec) typing by multiplex polymerase chain reaction (PCR) [7], and detection of the presence of Panton-Valentine leukocidin (PVL) genes by PCR assay [8]. Certain isolates of representative pulsotypes were selected for further typing by multilocus sequence typing (MLST) [9] and spa gene typing [10]. We chose the isolates for MLST analysis according to the isolates’ number of individual pusotype (proportion) and at least 1 isolate for each pulsotype. For those untyped or undigested by SmaI on PFGE, we routinely performed MLST analysis.
Statistical Analysis
Analysis of variance was used for analysis of variation in MRSA carriage among foreign workers from 4 different countries. For continuous variables, Student’s t test was used. For categorical variables, the χ 2 test or Fisher’s exact test was used, as appropriate. Odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated. The definition of statistical significance was P < .05. Variables with P < .05 in the univariate analysis were considered for inclusion in the multivariate model. SPSS 22nd edition was used for analysis.
RESULTS
Baseline Characteristics
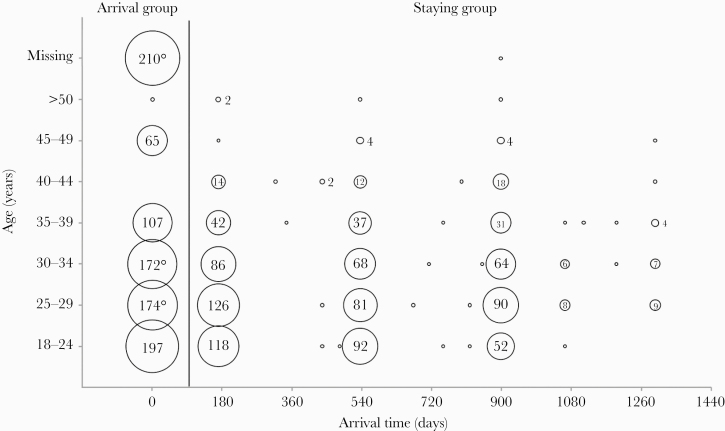
Between February and July 2017, a total of 1935 foreign workers recruited to Taiwan were sampled. Eight hundred forty-three people were from Vietnam, 780 from Indonesia, 239 from the Philippines, and 73 from Thailand. There were 929 people in the arrival group, 98.6% of whom arrived in Taiwan within ≤1 day (range, 0–32 days), and 1006 people in the staying group with a median of arrival time of 532 days (range, 152–1295 days). Distribution of age and arrival time of the recruited workers are illustrated in Figure 1. Other characteristics of the workers including age and gender are summarized in Table 1.
Figure 1.
Geographic distribution of foreign workers from Southeastern countries recruited to Taiwan stratified by age and arrival time. A total of 98.6% of workers in the arrival group (n = 929) arrived in Taiwan within ≤1 day. The arrival time of the staying group (n = 1006) was approximately 180, 540, and 900 days according to the government statute.
Table 1.
Distribution of Nasal Methicillin-Resistant Staphylococcus aureus Carriage Rate Among Foreign Workers Recruited to Taiwan Stratified by Gender, Age, and Countries
| Variables | Total (n = 1935) | MRSA (n = 53) | Non-MRSA (n = 1882) | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|---|---|
| Male | 951 (49.1%) | 28 (52.8%) | 923 (49.0%) | 1.164 | 0.674–2.011 | .676 |
| Age (mean ± SD) | 29.22 ± 6.5 | 28.49 ± 5.5 | 29.24 ± 6.5 | .069 | ||
| Vietnam | 843 (43.6%) | 40 (75.5%) | 803 (42.7%) | Indonesia 0.261 | 0.129–0.525 | <.001a |
| Philippines 0.255 | 0.078–0.832 | .013 | ||||
| Indonesia | 780 (40.3%) | 10 (18.9%) | 710 (40.9%) | Philippines 0.979 | 0.267–3.586 | 1.000 |
| Philippines | 239 (12.4%) | 3 (5.7%) | 236 (12.5%) | |||
| Thailand | 73 (3.8%) | 0 (0.0%) | 73 (3.9%) |
Abbreviations: CI, confidence interval; OR, odds ratio; MRSA, methicillin-resistant S aureus; SD, standard deviation.
a P < .05.
Overall, S aureus, MRSA, and methicillin-sensitive S aureus carriage rates of the workers were 18.9%, 2.74%, 16.1%, respectively. The MRSA carriage rate was 2.26% for arrival group and 3.18% for staying group. The rate was 4.74% for Vietnamese (5.20% for arrival and 4.53% for staying), 1.28% for Indonesians (0.91% for arrival and 2.14% for staying), 1.26% for the Philippines (2.60% for arrival and 0.62% for staying), and none for Thai workers. A total of 53 MRSA isolates were found in the subjects: 21 from the arrival group and 32 from the staying group. The MRSA carriage rate of Vietnamese was significantly higher than Indonesians (OR = 0.261; 95% CI, 0.129–0.525; P < .001) and Filipinos (OR = 0.255; 95.0% CI, 0.078–0.832; P = .013). Potential risk factors including gender and age showed no significant influence on MRSA carriage rate (Tables 1 and 2).
Table 2.
Nasal Staphylococcus aureus Carriage Rate Among Foreign Workers Recruited to Taiwan, 2017
| Country/Timing of Arrival | No. Subjects | S aureus No. (%) | MRSA No. (%) | MSSA No. (%) | P Value of MRSA Carriage |
|---|---|---|---|---|---|
| Total | 1935 | 365 (18.9) | 53 (2.74) | 312 (16.1) | |
| Arrival group | 929 | 176 (18.9) | 21 (2.26) | 155 (16.7) | .265a |
| Staying group | 1006 | 189 (18.8) | 32 (3.18) | 157 (15.6) | |
| Vietnam | 843 | 141 (16.7) | 40 (4.74) | 101 (12.0) | |
| Arrival group | 269 | 39 (14.5) | 14 (5.20) | 25 (9.29) | .729a |
| Staying group | 574 | 102 (17.8) | 26 (4.53) | 76 (13.2) | |
| Indonesia | 780 | 159 (20.4) | 10 (1.28) | 149 (19.1) | |
| Arrival group | 547 | 114 (20.9) | 5 (0.91) | 109 (19.2) | .176a |
| Staying group | 233 | 45 (19.2) | 5 (2.14) | 40 (17.1) | |
| Philippines | 239 | 54 (22.6) | 3 (1.26) | 51 (21.3) | |
| Arrival group | 77 | 20 (26.0) | 2 (2.60) | 18 (23.4) | .244a |
| Staying group | 162 | 34 (21.0) | 1 (0.62) | 33 (20.4) | |
| Thailand | 73 | 11 (15.1) | 0 | 11 (15.1) | - |
| Arrival group | 36 | 3 (8.33) | 0 | 3 (8.33) | |
| Staying group | 37 | 8 (21.6) | 0 | 8 (21.6) |
Abbreviations: MRSA, methicillin-resistant S aureus; MSSA, methicillin-sensitive S aureus.
aObtained using Fisher’s exact test, 2-tailed.
Molecular Characterization of Methicillin-Resistant Staphylococcus aureus Isolates
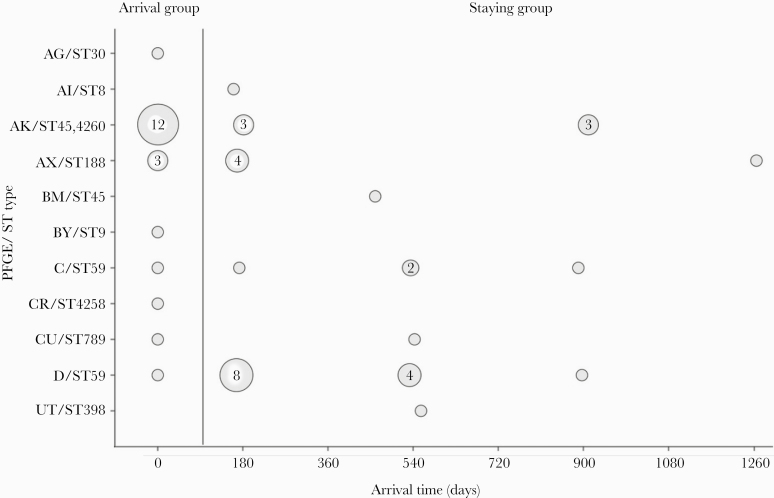
Of the 53 MRSA isolates, 10 pulsotypes were identified and 1 isolate was untypeable by PFGE. Seventeen isolates were characterized by MLST and 29 isolates were characterized by spa typing. The detailed molecular characteristics of all 53 isolates stratified by pulsotypes are shown in Table 3. Pulsotype AK/ST45 (57%) and pulsotype AX/ST188 (14%) were the top 2 dominant clones for arrival group. In staying group, 13 (41%) isolates were characterized as pulsotype D/ST59, 6 (19%) were characterized as pulsotype AK/ST45, 5 (16%) were characterized as pulsotype AX/ST188, and 4 (13%) were characterized as pulsotype C/ST59. Pulsotype BY/ST9 and pulsotype AG/ST30 were only identified in the arrival group, and pulsotype BM/ST45, pulsotype AI/ST8/SCC IV/PVL-positive (USA300), and ST398/t034/SCC VT/PVL-negative were specific in the staying group.
Table 3.
Molecular Characteristics of 53 Methicillin-Resistant Staphylococcus aureus Isolates, Categorized by Pulsed-Field Gel Electrophoresis Pattern
| PFGE Pattern | No. of Isolate (%) | SCCmec Type | PVL-Positive | MLST Type | spa Type |
|---|---|---|---|---|---|
| AG | 1 (2) | IV | 0 | 30 | t019 |
| AI | 1 (2) | IV | 1 | 8 | t008 |
| AK | 16 (30) 2 (4) |
IV | 0 | 45 4260 |
t026, t779, t133 |
| AX | 8 (15) | IV | 0 | 188 | t189 |
| BM | 1 (2) | V | 0 | 45 | t1081 |
| BY | 1 (2) | XII | 0 | 9 | t899 |
| C | 5 (9) | IV | 0 | 59 | t441, t437 |
| CR | 1 (2) | IV | 0 | 4258 | t941 |
| CU | 2 (4) | V | 0 | 789 | t091 |
| D | 14 (26) | VT | 12 | 59 | t437, t441 |
| UT | 1 (2) | VT | 0 | 398 | t034 |
Abbreviations: MLST, multilocus sequence type; PFGE, pulsed-field gel electrophoresis; PVL, Panton-Valentine leukocidin; SCCmec, staphylococcal cassette chromosome mec; UT, untypeable.
Genotypes of MRSA isolates between the arrival group and the staying group showed significant difference. Among the staying group, there was a significantly lower prevalence of AK/ST45 (19% vs 57%) and a higher prevalence of pulsotype D/ST59 (41% vs 4.8%) compared with the arrival group (P = .007 and P = .004, respectively).
No other potential risk factors, including gender and country, illustrated significant differences in the molecular characteristics of MRSA isolates. A comparison of the major clones in different countries and arrival time are shown in Table 4, and the distribution of pulsotypes and arrival time are summarized in Figure 2. All 14 of the isolates characterized as ST59/pulsotype D/SCCmec VT and the isolate characterized as ST8/SCCmec IV were PVL-positive.
Table 4.
Comparison of the Major Clones Distribution of MRSA Isolates From Foreign Workers Recruited to Taiwan, 2017, Stratified by Timing of Samplings
| Country/Timing of Arrival | ST45/Pulsotype AK No. (%) |
ST188/Pulsotype AX No. (%) |
ST59/Pulsotype C No. (%) |
ST59/Pulsotype D No. (%) |
Others No. (%) |
|---|---|---|---|---|---|
| Vietnam (n = 40) | 14 (35) | 8 (20) | 4 (10) | 13 (33) | 1 (3) |
| Arrival group (n = 14) | 9 (64) | 3 (21) | 0 (0) | 1 (7) | 1 (7) |
| Staying group (n = 26) | 5 (19) | 5 (19) | 4 (15) | 12 (46) | 0 (0) |
| Indonesia (n = 10) | 3 (30) | 0 (0) | 1 (10) | 1 (10) | 5 (50) |
| Arrival group (n = 5) | 2 (40) | 0 (0) | 1 (20) | 0 (0) | 2 (40) |
| Staying group (n = 5) | 1 (20) | 0 (0) | 0 (0) | 1 (20) | 3 (60) |
| Philippines (n = 3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (100) |
| Arrival group (n = 2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (100) |
| Staying group (n = 1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Total (n = 53) | 18 (34) | 8 (15) | 5 (9) | 14 (26) | 8 (15) |
| Arrival group (n = 21) | 12 (57)a | 3 (14) | 1 (5) | 1 (5)a | 4 (19) |
| Staying group (n = 32) | 6 (19)a | 5 (16) | 4 (13) | 13 (41a) | 4 (13) |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus.
aSignificant difference between arrival group and staying group (P < .01).
Figure 2.
Distribution of pulsotypes/sequence type of 53 methicillin-resistant Staphylococcus aureus isolates from foreign workers recruited to Taiwan stratified by timing of arrival. PFGE, pulsed-field gel electrophoresis; ST, sequence type.
Antibiotics Susceptibility
The detailed antibiotic susceptible rates of the 53 MRSA isolates stratified by SCCmec types are shown in Supplementary Table 1. All isolates were susceptible to vancomycin, linezolid, and teicoplanin and resistant to penicillin. The resistant rate was 70% for erythromycin, 57% for clindamycin, and 28% for ciprofloxacin.
Whole-Genome Sequencing of Methicillin-Resistant Staphylococcus aureus ST59 Isolates
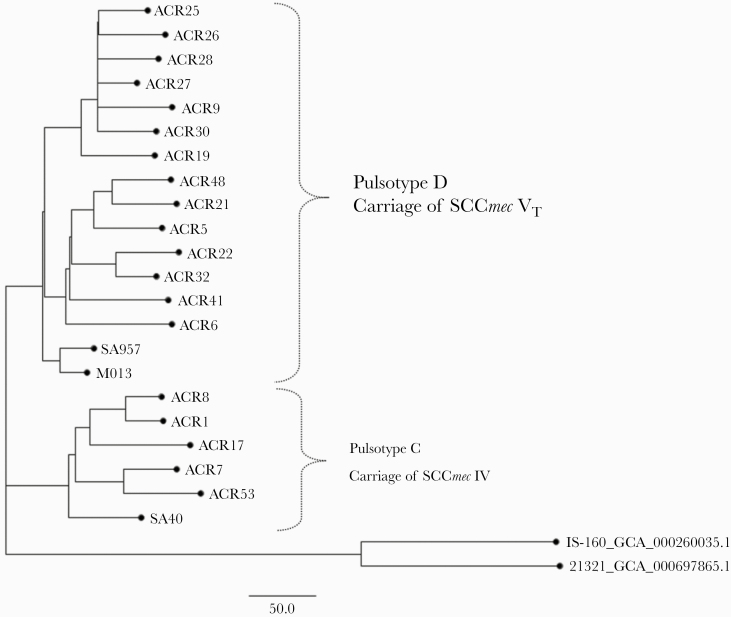
To delineate the genetic relatedness between the ST59 isolates identified from these foreign workers and those identified from native Taiwanese, whole-genome sequencing (WGS) analysis was performed for all 19 ST59 isolates identified in this study. Of the 19 ST59 isolates (named as ACR strains), 2 isolates (pulsotype C/SCC IV for 1 isolate, strain name as ACR 17; pulsotype D/SCC VT for 1 isolate, strain name as ACR 19) were identified from arrival group, and the other 17 isolates (pulsotype C for 4 isolates and pulsotype D for 13 isolates) were from the staying group. The methods for WGS were described previously [11, 12] and are provided briefly in the Supplementary Material. Five MRSA isolates of ST59 were included as reference, which included 3 clinical MRSA ST59 strains (SA957, SA40, and M013) with completed WGS data identified from Taiwan and 2 clinical MRSA ST59 strains (IS-160 and 21321) with draft genome assemblies identified from the United States. Accession numbers in the National Center for Biotechnology Information (NCBI) for the reference strains are SA40 (NC_022443.1), SA957 (NC_022442.1), M013 (NC_016928.2), IS-160 (GCA_000260035.1), and 21321 (GCA_000697865.1). The phylogenetic tree indicated that the ST59 isolates from Southeast Asian workers were phylogenetically close to the isolates from Taiwan but more genetically distant from the isolates from the United States (Figure 3).
Figure 3.
Whole-genome sequencing-based phylogenetic analysis of methicillin-resistant Staphylococcus aureus (MRSA) sequence type (ST)59 isolates of pulsotypes C and D identified from the workers recruited from Southeast Asian countries to Taiwan. Five MRSA isolates of ST59 were included as reference, which included 3 clinical MRSA ST59 strains (SA957, SA40, and M013) identified from Taiwan and 2 clinical MRSA ST59 strains (IS-160 and 21321) identified from the United States. SCCmec, staphylococcal cassette chromosome
DISCUSSION
Results from this study showed that the overall nasal MRSA carriage rate among foreign workers recruited to Taiwan was 2.74%: 2.26% for on-arrival subjects (ranged from none in Thailand subjects to 5.2% in Vietnamese subjects) and 3.18% for staying subjects (ranged from none in Thailand subjects to 4.53% in Vietnamese subjects). Among the colonizing isolates from the on-arrival subjects, ST45/pulsotype AK (57%) and ST188/pulsotype AX were the 2 most common clones, whereas ST59/pulsotype D (41%) and ST45/pulsotype AK (19%) were most commonly seen among those from staying subjects.
The MRSA carriage rate of on-arrival workers from Vietnam (5.20%), Indonesia (0.91%), the Philippines (2.60%), and Thailand (0%) may also represent a rough estimate of nasal MRSA carriage among the previously healthy adults in the respective country because the specimens of these people were almost all tested within 1 day of arrival. Few studies regarding the prevalence and molecular epidemiology of nasal MRSA carriage in these Southeast Asian countries were available. Selected previous publications [13–18] on prevalence of MRSA carriage rates in these 4 Southeast Asian countries are summarized in Table 5. Van Nguyen et al [13] reported MRSA nasopharyngeal carriage rate of 7.9% among 1016 healthy individuals in the general population in northern Vietnam. The nasal MRSA carriage rate in Indonesia was reported to be 0.8% [19] among 384 patients for elective surgery, and only 1 of 3995 individuals in 2 cities had MRSA carriage in another report [15]. The MRSA carriage rate among the previously healthy Thailand population was 0% [16] among 128 preclinical medical students in 2012, 1% among 200 university students in 2009–2010 [17], and 2.3% [18] among 217 individuals in the pediatric population in 2010–2011. There have been no data for MRSA carriage among the healthy general population reported from the Philippines.
Table 5.
Selected Publications on Prevalence of MRSA Carriage Rate Among Previously Healthy Subjects in Southeastern Asian Countries
| Country | Study Period | Study Subjects and Number | Age | MRSA No. (%) | Reference |
|---|---|---|---|---|---|
| Vietnam | February to June 2012 | 1016 healthy subjects from urban area of Dong Da and rural area of Ba Vi | All ages are included median: in the age group 30–59 years | 80 (7.9%) | Van Nguyen et al [11] |
| Indonesia | April to September 2015 | 384 Elective surgery patients in Cipto Mangunkusumo Hospital | Median = 46 years | 3 (0.78%) | Nelwan et al [12] |
| July to October 2001 in Surabaya and January to May 2002 in Semarang | 3995 people (patients and their relatives) in 2 cities: Semarang and Surabaya, Indonesia | Not mentioned | 1 (0.025%) | Severin et al [13] | |
| Thailand | March 2012 | 128 preclinical medical students | Mean age of 20.9 ± 0.9 years | 0 (0%) | Treesirichod et al [14] |
| October 2009 to September 2010 | 200 healthy students | 19–25 years old | 2 (1%) | Kitti et al [15] | |
| 2010–2011 | 217 healthy children in 3 primary schools | 3–12 years old | 5 (2.3%) | Tangchaisuriya et al [16] |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus.
Previous studies conducted in Taiwan showed that the nasal MRSA carriage rate was 3.8% for 502 adult patients visiting emergency rooms [20], 3.8% for 3098 adults receiving health examinations [21], and 2.2% for 322 medical students [22]. Compared with the local prevalence, the general population in Indonesia, the Philippines, and Thailand seemed to have a lower nasal MRSA carriage rate, whereas those in Vietnam had a higher nasal MRSA carriage rate. In this study the workers recruited from Vietnam had a significantly higher nasal MRSA carriage rate than those from the other 3 countries. However, no significant difference was found for MRSA carriage in terms of gender and age in this study, consistent with previous reports [23, 24].
Molecular characterizations of the MRSA isolates in the present survey showed that ST45/pulsotype AK and ST188/pulsotype AX were dominant among on-arrival workers, whereas, unexpectedly, pulsotype D/ST59 (41%) and pulsotype C/ST59 (12%) became common among the staying workers. A significant difference of pulsotype AK/ST45 and pulsotype D/ST59 carriage may indicate transmission and/or acquisition of certain MRSA strains. The strains of pulsotype C and D/ST59 in the staying workers were long recognized as the main CA-MRSA clone in Taiwan [2, 25], whereas ST45 was reported to be 1 of the 3 common strains among the S aureus isolates from both community and clinical settings in Vietnam in a recent study [26]. Natural loss and acquisition of S aureus had been described in previous studies [27, 28]. Miller et al [27] found that 30% of their study participants acquired new SCCmec type and 68% subsequently lost their first recognized spa-type. These may infer that some foreign workers carried MRSA ST45 strain on arrival but lost this strain carriage later and acquired the local endemic strain of ST59. We further performed WGS analysis of these ST59 isolates and demonstrated that the ST59 isolates from Southeast Asian workers were phylogenetically close to the isolates from Taiwan but more genetically distant from the isolates from the United States. These findings suggest that when people go to a foreign country and stay there for a period time, they might acquire a new MRSA strain colonization that is endemic in that country.
In the present study, MRSA ST45, the targeted clone of this study, was indeed found to be the major clone of MRSA identified from the on-arrival workers recruited as we expected; however, the pulsotype of these isolates was AK, which was different from that (pulsotype BM) previously identified and circulating in nursing homes in Taiwan [3]. Only 1 isolate of pulsotype, BM/ST45, was identified from a Filipino, who stayed in Taiwan for more than 6 months, and the occupation of this worker was not obtainable. The issue of whether the pulsotype BM/ST45 strain circulating in Taiwan was transmitted from the foreign workers needs further studies. The MRSA ST45 strain was initially reported in Berlin, and it spread through Europe and Asia [29–31]. Studies also showed increasing prevalence of MRSA ST45 in long-term care facilities in Germany, Hong Kong, China, and Taiwan [3, 32–34]. In Taiwan, ST45 was first identified during an outbreak investigation in 2006 and reported in 2011 [35]. Whole-genome sequencing study to compare these strains are ongoing.
CONCLUSIONS
This study had several limitations. First, the nasal carriage rate of the respective countries may represent an estimate but not a complete picture of the true prevalence of MRSA carriage rate because of the selection bias. The group of foreign workers may represent a healthy portion of the whole population (healthy worker effect) of the respective countries to fulfill recruitment regulation to Taiwan. Second, from each study subject, samplings for MRSA detection were obtained only from 1 site (nares), so some MRSA-colonizing patients might be undetected, and the carriage may be underestimated. Third, this is a convenient sample of subjects in 1 hospital instead of random recruitment of foreign workers in several hospitals in Taiwan, which may affect the validity of the study. However, our study obtained preliminary data of MRSA prevalence among foreign workers, and further research may be needed across more hospitals. Fourth, a longitudinal study is needed to investigate whether there is a reciprocal transmission of MRSA ST45 and ST188 strains from foreign workers to the local people and the local ST59 strain to the foreign workers staying longer than 5 months in Taiwan.
In conclusion, approximately 3% of foreign workers recruited from Southeast Asian countries to Taiwan were colonized with MRSA, including the ST45 strain. However, the MRSA strains identified from the workers who stayed in Taiwan for more than 5 months were mostly endemic clones in Taiwan and were genetically different from those identified from the workers on arrival.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank all of the participants for their donations of the nasal specimens.
Authors’ contributions. K.-H. C., W.-C. C., and W.-K. W. obtained the nasal specimens, laboratory works, analyzed and interpreted the patient data, and prepared the first draft of manuscript. C.-H. C. communicated with and enrolled study subjects. C.-J. C. helped analyzed and interpret the data. Y.-C. H. designed the study, applied the grants, provided the laboratory works, analyzed and interpreted the patient data, and wrote the manuscript. All authors read and approved the final manuscript.
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. The study was funded by grants from the Ministry of Science and Technology, Executive Yuan, Taiwan (MOST 105-2314-B-182A-139 and MOST 107-2813-C-182A-009B) and a grant from Medical Foundation in Memory of Dr. Deh-Lin Cheng.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 2010; 375:1557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect 2014; 20:605–23. [DOI] [PubMed] [Google Scholar]
- 3. Tsao FY, Kou HW, Huang YC. Dissemination of methicillin-resistant Staphylococcus aureus sequence type 45 among nursing home residents and staff in Taiwan. Clin Microbiol Infect 2015; 21:451–8. [DOI] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 20st Informational Supplement. CLSI document M100-S20. Wayne, PA: CLSI; 2010. [Google Scholar]
- 5. Lin SY, Lin NY, Huang YY, et al. Methicillin-resistant Staphylococcus aureus nasal carriage and infection among patients with diabetic foot ulcer. J Microbiol Immunol Infect 2020; 53:292–9. [DOI] [PubMed] [Google Scholar]
- 6. Pan HH, Huang YC, Chen CJ, et al. Prevalence of and risk factors for nasal methicillin-resistant Staphylococcus aureus colonization among children in central Taiwan. J Microbiol Immunol Infect 2019; 52:45–53. [DOI] [PubMed] [Google Scholar]
- 7. Kondo Y, Ito T, Ma XX, et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother 2007; 51:264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lina G, Piémont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 1999; 29:1128–32. [DOI] [PubMed] [Google Scholar]
- 9. Enright MC, Day NP, Davies CE, et al. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 2000; 38:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harmsen D, Claus H, Witte W, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 2003; 41:5442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19:455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Croucher NJ, Page AJ, Connor TR, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 2015; 43:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Nguyen K, Zhang T, Thi Vu BN, et al. Staphylococcus aureus nasopharyngeal carriage in rural and urban northern Vietnam. Trans R Soc Trop Med Hyg 2014; 108:783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nelwan EJ, Sinto R, Subekti D, et al. Screening of methicillin-resistant Staphylococcus aureus nasal colonization among elective surgery patients in referral hospital in Indonesia. BMC Res Notes 2018; 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Severin JA, Lestari ES, Kuntaman K, et al. ; Antimicrobial Resistance in Indonesia, Prevalence and Prevention Study Group Unusually high prevalence of Panton-Valentine leukocidin genes among methicillin-sensitive Staphylococcus aureus strains carried in the Indonesian population. J Clin Microbiol 2008; 46:1989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Treesirichod A, Hantagool S, Prommalikit O. Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus among medical students at the HRH Princess Maha Chakri Sirindhorn Medical Center, Thailand: a follow-up study. J Infect Public Health 2014; 7:205–9. [DOI] [PubMed] [Google Scholar]
- 17. Kitti T, Boonyonying K, Sitthisak S. Prevalence of methicillin-resistant Staphylococcus aureus among university students in Thailand. Southeast Asian J Trop Med Public Health 2011; 42:1498–504. [PubMed] [Google Scholar]
- 18. Tangchaisuriya U, Yotpanya W, Kitti T, Sitthisak S. Distribution among Thai children of methicillin-resistant Staphylococcus aureus lacking cna, fnbA and icaAD. Southeast Asian J Trop Med Public Health 2014; 45:149–56. [PubMed] [Google Scholar]
- 19. Kuntaman K, Hadi U, Setiawan F, et al. Prevalence of methicillin-resistant Staphylococcus aurues from nose and throat of patients on admission to medical wards of DR Soetomo Hospital, Surabaya, Indonesia. Southeast Asian J Trop Medi Public Health 2016; 47:66–70. [PubMed] [Google Scholar]
- 20. Lu SY, Chang FY, Cheng CC, et al. Methicillin-resistant Staphylococcus aureus nasal colonization among adult patients visiting emergency department in a medical center in Taiwan. PLoS One 2011; 6:e18620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang JT, Liao CH, Fang CT, et al. Prevalence of and risk factors for colonization by methicillin-resistant Staphylococcus aureus among adults in community settings in Taiwan. J Clin Microbiol 2009; 47:2957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen CS, Chen CY, Huang YC. Nasal carriage rate and molecular epidemiology of methicillin-resistant Staphylococcus aureus among medical students at a Taiwanese university. Int J Infect Dis 2012; 16:e799–803. [DOI] [PubMed] [Google Scholar]
- 23. Halablab MA, Hijazi SM, Fawzi MA, Araj GF. Staphylococcus aureus nasal carriage rate and associated risk factors in individuals in the community. Epidemiol Infect 2010; 138:702–6. [DOI] [PubMed] [Google Scholar]
- 24. Mainous AG 3rd, Hueston WJ, Everett CJ, Diaz VA. Nasal carriage of Staphylococcus aureus and methicillin-resistant S aureus in the United States, 2001–2002. Ann Fam Med 2006; 4:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chuang YY, Huang YC. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Asia. Lancet Infect Dis 2013; 13:698–708. [DOI] [PubMed] [Google Scholar]
- 26. Ngoc Thi Vu B, A JJ, Aardema M, et al. Population structure of colonizing and invasive Staphylococcus aureus strains in Northern Vietnam. J Med Microbiol 2016; 65:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller RR, Walker AS, Godwin H, et al. Dynamics of acquisition and loss of carriage of Staphylococcus aureus strains in the community: the effect of clonal complex. J Infect 2014; 68:426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bloemendaal AL, Fluit AC, Jansen WT, et al. Colonization with multiple Staphylococcus aureus strains among patients in European intensive care units. Infect Control Hosp Epidemiol 2009; 30:918–20. [DOI] [PubMed] [Google Scholar]
- 29. Witte W. Antibiotic resistance in gram-positive bacteria: epidemiological aspects. J Antimicrob Chemother 1999; 44(Suppl A):1–9. [DOI] [PubMed] [Google Scholar]
- 30. Enright MC, Robinson DA, Randle G, et al. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci U S A 2002; 99:7687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teo J, Tan TY, Hon PY, et al. ; Network for Antimicrobial Resistance Surveillance (Singapore) ST22 and ST239 MRSA duopoly in Singaporean hospitals: 2006-2010. Epidemiol Infect 2013; 141:153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luk S, Ho AY, Ng TK, et al. Prevalence, prediction, and clonality of methicillin-resistant Staphylococcus aureus carriage at admission to medical units in Hong Kong, China. Infect Control Hosp Epidemiol 2014; 35:42–8. [DOI] [PubMed] [Google Scholar]
- 33. Ghebremedhin B, König W, König B. Heterogeneity of methicillin-resistant Staphylococcus aureus strains at a German university hospital during a 1-year period. Eur J Clin Microbiol Infect Dis 2005; 24:388–98. [DOI] [PubMed] [Google Scholar]
- 34. Ho PL, Chow KH, Lo PY, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus associated with spread of the ST45 lineage in Hong Kong. Diagn Microbiol Infect Dis 2009; 64:131–7. [DOI] [PubMed] [Google Scholar]
- 35. Lee YT, Lin DB, Wang WY, et al. First identification of methicillin-resistant Staphylococcus aureus MLST types ST5 and ST45 and SCCmec types IV and Vt by multiplex PCR during an outbreak in a respiratory care ward in central Taiwan. Diagn Microbiol Infect Dis 2011; 70:175–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.