Abstract
Silent circulation of polioviruses complicates the polio endgame by affecting the confidence with which we can certify successful eradication (i.e., the end of transmission everywhere) given a long enough period of time with active surveillance and no observed detections. The Global Polio Eradication Initiative continues to use three years without observing paralytic cases caused by wild poliovirus (WPV) infection as an indication of sufficient confidence that poliovirus circulation stopped (assuming good surveillance). Prior modeling demonstrated the complexities of real populations and the imperfect nature of real surveillance systems, and highlighted the need for modeling the specific last reservoirs of undetected circulation. We use a poliovirus transmission model developed for Borno and Yobe to characterize the probability of undetected poliovirus circulation once apparent die-out occurs (i.e., in the absence of epidemiological signals) for WPV serotypes 1 and 3. Specifically, we convert the model to a stochastic form that supports estimates of confidence about no circulation given the time since the last detected event and considering the quality of both immunization and surveillance activities for these states. We find high confidence of no WPV3 circulation, and increasing confidence of WPV1 circulation, which we anticipate will imply high confidence in the absence of any detected cases in mid-2020 so long as Borno and Yobe maintain similar or achieve improved conditions. Our results confirm that gaps in poliovirus surveillance or reaching elimination with borderline sufficient population immunity can substantially increase the time to reach a high confidence about no undetected poliovirus transmission.
Keywords: Polio, eradication, Nigeria, disease outbreaks, infection transmission modeling, surveillance, vaccination
Introduction
Recent modeling related to the global certification of wild poliovirus eradication and the impact of hard-to-reach subpopulations on the confidence about the absence of transmission included a review of the relevant literature and experience (Duintjer Tebbens, Kalkowska, & Thompson, 2019). Based on analysis of empirical field experience (Debanne & Rowland, 1998) and the results of theoretical modeling (Eichner & Dietz, 1996), the Global Polio Eradication Initiative (GPEI) determined that 3 years without observing paralytic cases caused by wild polioviruses (WPVs) in the presence of active acute flaccid paralysis (AFP) surveillance provided sufficient confidence that poliovirus circulation had stopped. Subsequent modeling demonstrated the importance of considering the assumptions that impact population immunity (i.e., vaccination strategies), serotype-specific paralysis-to-infection ratios (PIRs), and population-specific seasonal fluctuations in poliovirus transmissibility (Kalkowska, Duintjer Tebbens, & Thompson, 2012), all of which affect estimation of the duration and probability of undetected transmission of live polioviruses (LPVs) in real populations. Other work focused on the need to model conditions that correspond to the last global reservoirs of WPV transmission, including consideration of how the quality of information obtained from AFP surveillance and environmental surveillance (ES) affects characterization of the confidence about no circulation as a function of time without detected events (i.e., paralytic cases or positive sewage samples) (Kalkowska et al., 2015; Kalkowska, Duintjer Tebbens, Pallansch, & Thompson, 2019).
The confidence about no circulation in the context of not detecting any signals while actively looking represents an important factor in global decisions related to the cessation of oral poliovirus vaccine (OPV) use. High coverage with OPV provides the benefits of driving out WPVs, however, its use comes with some risks, including vaccine-associated paralytic polio (VAPP) and vaccine-derived poliovirus (VDPV) cases (Duintjer Tebbens et al., 2006; Thompson & Duintjer Tebbens, 2012, 2014). After global certification of an eradication of a serotype of WPV occurs, global cessation of use of that serotype of OPV can occur. The more quickly after WPV eradication that OPV cessation occurs, the greater the number of VAPP cases prevented. Thus reaching high confidence faster makes a difference. While we defer the selection of the actual threshold applied as sufficient confidence about no circulation to the global health leaders who make certification decisions, we recognize the important role that analysts play with respect to the characterization of the time required to reach different levels of confidence.
Northeast Nigeria represents an important epidemiological region (Kalkowska, Franka, et al., 2020), with the last globally-reported cases of WPV serotype 3 (WPV3) in Borno and Yobe in November 2012 (Kew et al., 2014), the last African region last reported cases of WPV serotype 1 (WPV1) cases in Borno in 2016 (Nnadi et al., 2017; World Health Organization, 2016), and ongoing challenges with circulating vaccine derived polioviruses (cVDPVs) of serotype 2 (cVDPV2s) in the same area (Etsano et al., 2016). Since Nigeria went for a nearly 2-year period with no reported cases from July 24, 2014 in Kano (with a reported case in April 19, 2014 in Yobe) until the detection of WPV1 transmission in Borno on July 4, 2016, the African Region previously began considering regional certification of all WPVs. An independent analysis published in 2015 that used different methods estimated very high confidence of no circulation of WPV1 in Nigeria at the end of 2015 in the absence of any reported cases by the end of 2015 (Famulare, 2015). Discovery of WPV1 in 2016 revealed significant gaps in surveillance and issues with inaccessibility associated with insurgency in the Borno and Yobe states of northeast Nigeria, which restricted polio program activities. The detection of cases also restarted the time since the last reported case that occurred in Borno and Nigeria to August 21, 2016. Now that 3 full years have passed since the detection of the last reported WPV1 case, we apply a previously developed differential equation based model (Kalkowska, Franka, et al., 2020) and stochastic approach (Kalkowska et al., 2015) to explore the confidence about no circulation of poliovirus transmission as a function of time without detected events for conditions relevant to Borno and Yobe. We use the model to consider the effect of different assumptions about the quality of AFP surveillance on the confidence about no circulation.
Methods
Using methods developed and demonstrated elsewhere (Eichner & Dietz, 1996; Kalkowska et al., 2015; Kalkowska et al., 2019; Kalkowska et al., 2012), we characterize the probability of no circulation as a function of the time since the last detected event specifically for Borno and Yobe in northeast Nigeria. We model LPV transmission within Borno and Yobe by characterizing individuals in the population using 8 immunity states further subdivided by a 5-stage process of waning of immunity to infection, a infections using a 20-stage poliovirus reversion process for both fecal-oral and oropharyngeal routes of transmission and a 6-stage infection process and (Kalkowska, Wassilak, Cochi, Pallansch, & Thompson, 2020). We divide the population into 11 age groups and assign these to 3 preferentially mixing age groups, which reflect the tendencies of individuals to mix more with other individuals of similar age (Kalkowska, Franka, et al., 2020). We model Borno and Yobe as one epidemiological block, but divide it into a general population, an under-vaccinated subpopulation, and two distinct isolated subpopulations. We assume the two distinct isolated populations are part of the under-vaccinated subpopulation prior to 2013, then they gradually become increasingly isolated in 2013 and 2014, become completely isolated from 2015 to early 2016, and then gradually become slightly more accessible after early 2016 with the implementation of special interventions (Kalkowska, Franka, et al., 2020). The model specifies demographic information, poliovirus transmissibility and seasonality, mixing among the four subpopulations, and the history of poliovirus vaccination, including both oral poliovirus vaccine (OPV) and inactivated poliovirus vaccine (IPV) use for routine immunization (RI) and supplemental immunization activities (SIAs) as previously described (Kalkowska, Franka, et al., 2020). The model specifically includes the limited immunization, surveillance, and population mixing assumptions related to the insurgency and insecurity that affected polio program performance in Borno and Yobe starting in 2013 (Kalkowska, Franka, et al., 2020). We consider the time since the last reported cases in our characterizations of confidence of no circulation given the time since that last reported case.
For the stochastic model, we focus only on WPV transmission (serotypes 1 and 3), and we consider two scenarios for WPV1 (Kalkowska, Franka, et al., 2020). Scenario 1 assumes indirect vaccination introductions into the isolated subpopulations that are sufficient to eliminate undetected WPV1 transmission after the outbreak detected in 2016 (Kalkowska, Franka, et al., 2020). Scenario 2 assumes indirect vaccination introductions into isolated subpopulations that are insufficient to eliminate undetected WPV1 transmission, but which results in sufficiently low transmission that appears consistent with the current lack of reported detection of WPV1 through the end of 2019. We run our existing deterministic poliovirus transmission model up to a chosen (serotype-specific) starting date using serotype-specific PIRs (i.e., 1/200 for WPV1 and 1/1000 for WPV3, see all details about the transmission model elsewhere (Kalkowska, Franka, et al., 2020)) and then transform the model into a discrete, stochastic model (Kalkowska et al., 2015).
Our approach of modeling transmission using our differential equation based transmission and OPV evolution model (Kalkowska, Wassilak, et al., 2020) differs from the much simpler theoretical transmission model with far fewer immunity states for which prior studies used the Gillespie algorithm to simulate events with variable time steps (Eichner & Dietz, 1996; Kalkowska et al., 2012). Our approach uses a stochastic compartmental model approach (Kalkowska et al., 2015; Kalkowska et al., 2019). We convert the deterministic differential equation-based model into a stochastic model by first rounding the fractional number of individuals for all stocks (i.e., immunity states by age group, waning state, etc.) from the deterministic model at the time of the transformation into integers. We draw a random uniform number for each stock to determine whether we will round the fractional number in that stock up or down to the nearest integer, and we perform a stochastic iteration using a fixed time step of 0.5 day. Thus, for each time step, we calculate all of the rates that change the current state of the model (i.e., transition rates, which we note differ from the viral transmission rate), and then for all of these transition rates, we draw a random number from the Poisson distribution with an expected value equal to the transition rate times the fixed time step. Using this approach, each random Poisson draw returns the expected number of individuals that follow the given transition rate, and we constrain the model such that the sum off all transitions cannot exceed the size of the stock. At each time step, we use random uniform numbers to determine whether each new infection event leads to a paralytic case, and we track the effective proportion of infectious individuals excreting the virus, which environmental surveillance could detect. We then run the stochastic model 1,000 times, resulting in distinct realizations of times when paralytic cases occur, which we use to obtain estimates of confidence about no circulation (Kalkowska et al., 2015). We use the same algorithms as others (Eichner & Dietz, 1996; Kalkowska et al., 2015; Kalkowska et al., 2019; Kalkowska et al., 2012) to characterize confidence about no circulation. Specifically, we focus on detected events (here polio cases detected by AFP surveillance) and the detected-event-free periods, to account for the information provided by the poliovirus surveillance. We describe the results using the following metrics (Kalkowska et al., 2015):
POE – “the probability of eradication defined as the fraction of stochastic iterations in which die-out occurs”
DEFP – “the detected-event-free period defined as the time in months since the last detected case (AFP) or positive isolate (environmental surveillance)”
CNC – “confidence about no circulation given the DEFP approximated as (1 - the number of DEFPs equal to t months with ongoing WPV circulation, divided by all DEFPs of t months)”
CNCx% – “the time when the confidence about no circulation exceeds x% (i.e., CNC95%, CNC99%)”
TUC – “the time of undetected circulation after the last detected-event (for those iterations in which extinction occurs)”
TUCx% – “the xth percentile of the TUC (i.e., TUC95%, TUC99%)”
We present the results of the confidence about no circulation as a function of time without detected events in the context of both perfect and imperfect surveillance. We use our best estimates of accessibility factors (Kalkowska, Franka, et al., 2020) as indicators of overall AFP surveillance quality to describe the variable probability of detecting polio AFP cases as a function of time. We consider different levels of accessibility factors for the general, under-vaccinated, and isolated subpopulations, and consider lower and upper bounds to reflect a range of possible limited AFP surveillance access levels in those subpopulations (Table 1). For WPV3, we focus on the CNC since the last reported case on November 10, 2012, for which we convert the model into a stochastic form on January 1, 2013. For WPV1, we focus on the CNC since the last reported case in August 21, 2016, for which we convert the model into a stochastic form on January 1, 2016. However, for comparison, we also consider the situation for WPV1 that existed between the last case in Yobe on April 19, 2014 (noting the last case in Nigeria on July 24, 2014) and July 4, 2016 (the first reported case in Borno in 2016), for which we convert the model into a stochastic form on January 1, 2014.
Table 1:
List of model inputs for the AFP surveillance component:
| Year | Isolated (C) | Isolated (N) | Under-vaccinated | General |
|---|---|---|---|---|
| 2013 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2014 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2015 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2016 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2017 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2018 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2019 + | 1.00 | 1.00 | 1.00 | 1.00 |
| Year | Isolated (C) | Isolated (N) | Under-vaccinated | General |
| 2013 | 0.05 | 0.05 | 0.05 | 1.00 |
| 2014 | 0.05 | 0.05 | 0.05 | 1.00 |
| 2015 | 0.00 | 0.00 | 0.05 | 1.00 |
| 2016 | 0.00 | 0.00 | 0.05 | 1.00 |
| 2017 | 0.00 | 0.00 | 0.05 | 1.00 |
| 2018 | 0.00 | 0.00 | 0.05 | 1.00 |
| 2019 + | 0.00 | 0.00 | 0.05 | 1.00 |
| Year | Isolated (C) | Isolated (N) | Under-vaccinated | General |
| 2013 | 0.89 | 0.89 | 0.89 | 1.00 |
| 2014 | 0.73 | 0.73 | 0.73 | 1.00 |
| 2015 | 0.00 | 0.00 | 0.44 | 1.00 |
| 2016 | 0.00 | 0.00 | 0.60 | 1.00 |
| 2017 | 0.00 | 0.00 | 0.69 | 1.00 |
| 2018 | 0.00 | 0.00 | 0.73 | 1.00 |
| 2019 + | 0.00 | 0.00 | 0.77 | 1.00 |
| Year | Isolated (C) | Isolated (N) | Under-vaccinated | General |
| 2013 | 0.95 | 0.95 | 0.95 | 1.00 |
| 2014 | 0.95 | 0.95 | 0.95 | 1.00 |
| 2015 | 0.00 | 0.00 | 0.95 | 1.00 |
| 2016 | 0.00 | 0.00 | 0.95 | 1.00 |
| 2017 | 0.00 | 0.00 | 0.95 | 1.00 |
| 2018 | 0.00 | 0.00 | 0.95 | 1.00 |
| 2019 + | 0.00 | 0.00 | 0.95 | 1.00 |
Abbreviations: AFP, acute flaccid paralysis; C, central; N, north.
Results
We present the confidence about no circulation as a function of time without detected events, and show black horizontal lines at the top that indicate the 99% (small dots) and 95% (larger dots) confidence levels for reference. Figs. 1, 2, and 3 show colored lines that represent the results for the indicated serotype and scenario (i.e., red for WPV1 scenario 1, dark red for WPV1 scenario 2, and blue for WPV3).
Figure 1: Confidence about no circulation in Borno and Yobe as a function of the detected-event free period (DEFP) assuming perfect AFP surveillance, and reference lines provided to indicate 95% and 99% confidence for WPV1* (two scenarios) and WPV3.
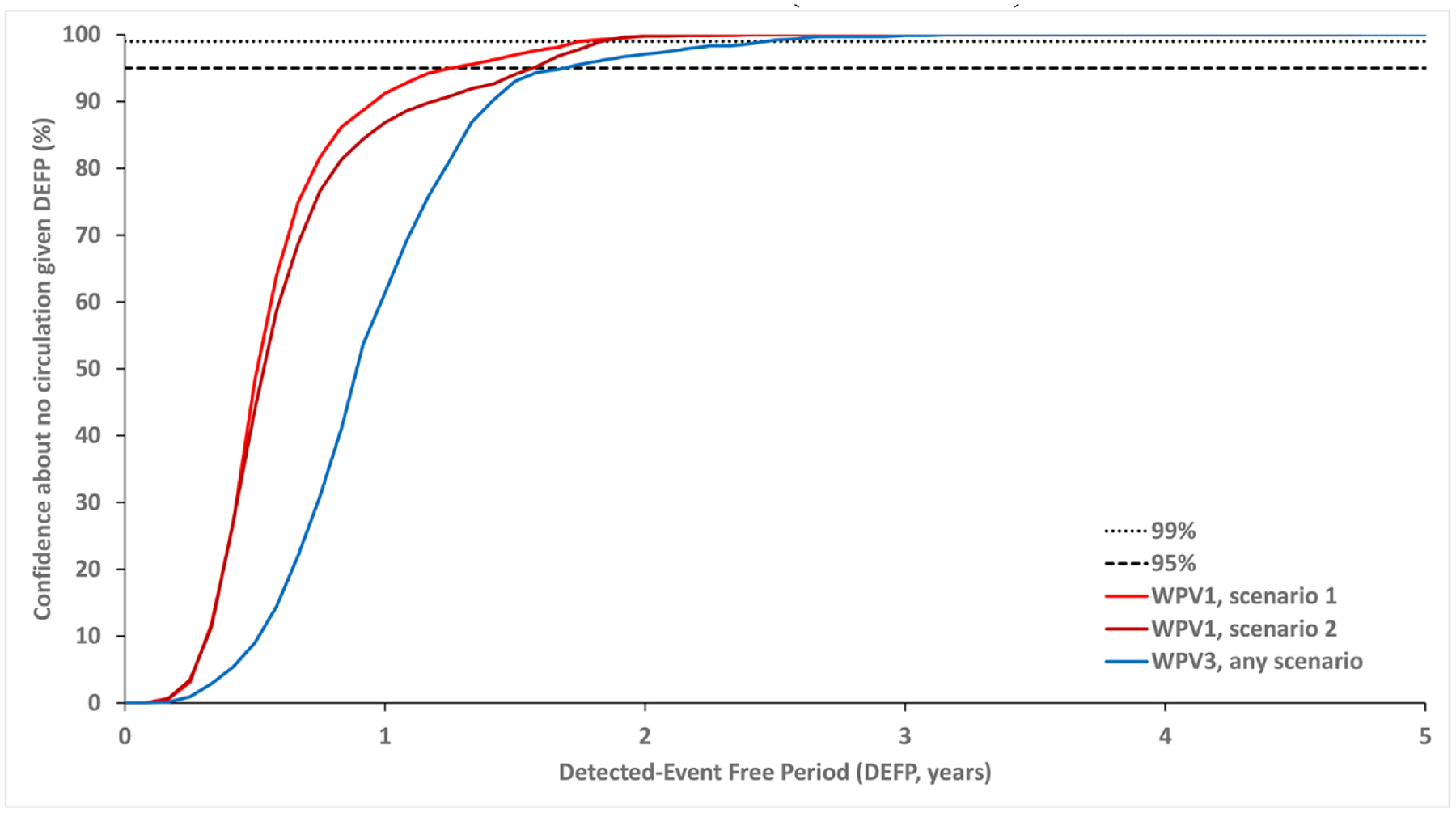
* Scenario 1 assumes sufficient indirect vaccination introductions into the isolated subpopulations to eliminate undetected WPV1 transmission after the outbreak detected in 2016 and Scenario 2 assumes insufficient indirect vaccination introductions into isolated subpopulations to eliminate undetected WPV1 transmission through the end of 2019.
Abbreviations: AFP, acute flaccid paralysis; DEFP, detected-event-free period; WPV(1,3), wild poliovirus (serotypes 1,3).
Figure 2: Confidence about no circulation in Borno and Yobe as a function of the detected-event free period (DEFP) assuming different estimates of less than perfect AFP surveillance, with perfect AFP surveillance, and reference lines provided to indicate 95% and 99% confidence for WPV1* (two scenarios) and WPV3.
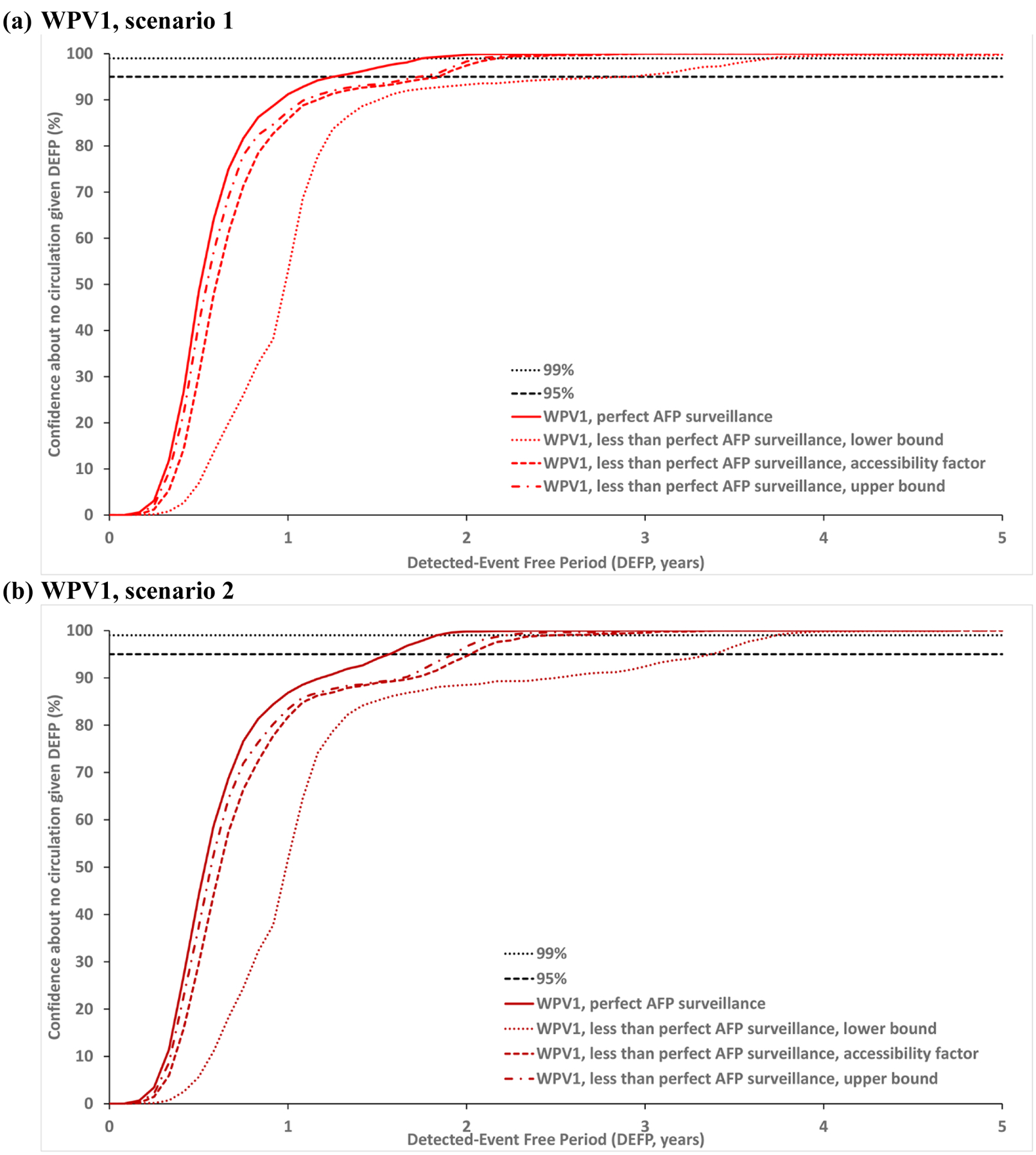
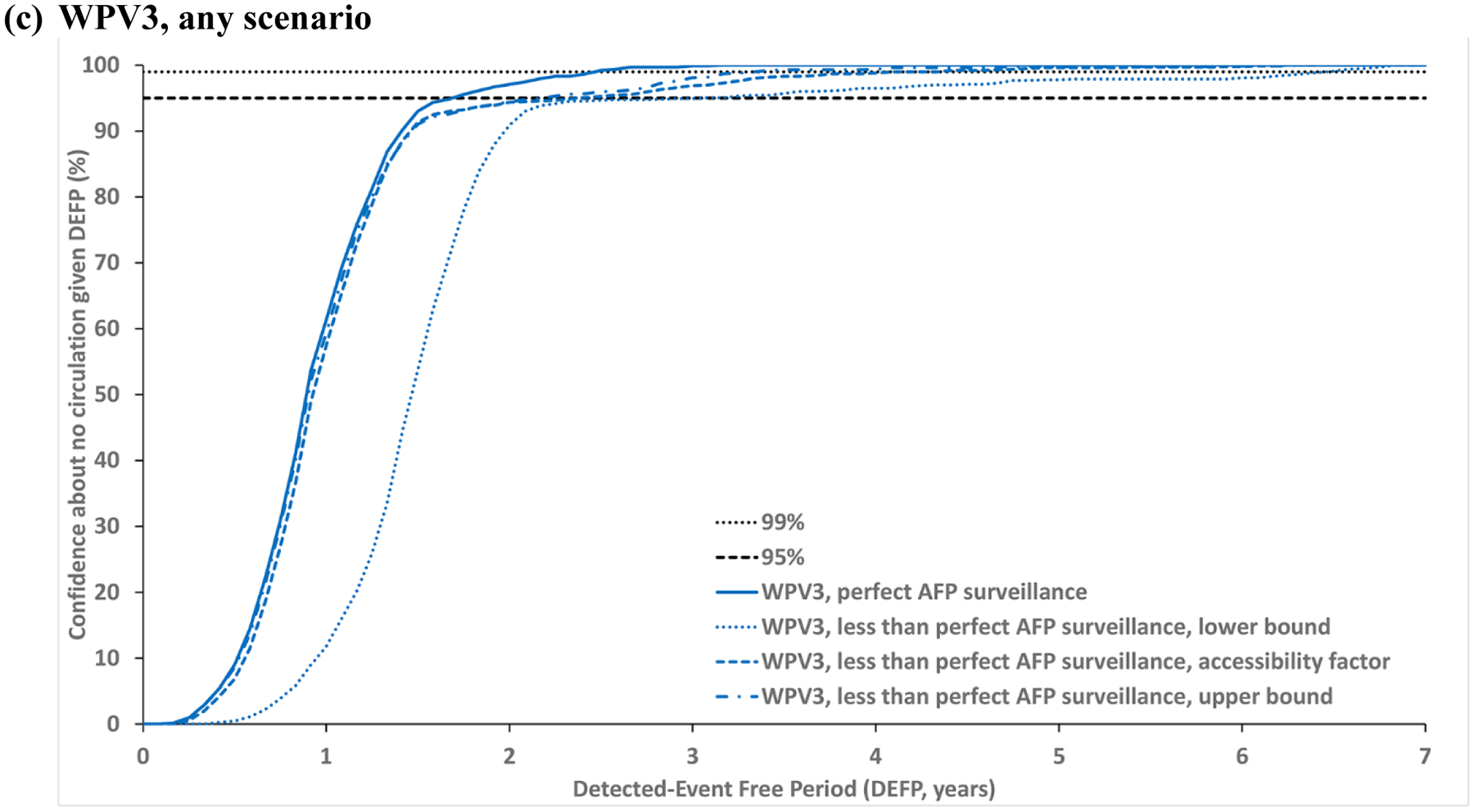
* Scenario 1 assumes sufficient indirect vaccination introductions into the isolated subpopulations to eliminate undetected WPV1 transmission after the outbreak detected in 2016; Scenario 2 assumes insufficient indirect vaccination introductions into isolated subpopulations to eliminate undetected WPV1 transmission through the end of 2019.
Abbreviations: AFP, acute flaccid paralysis; DEFP, detected-event-free period, WPV(1,3), wild poliovirus (serotypes 1, 3).
Figure 3: Confidence about no circulation in Borno and Yobe as a function of the detected-event free period (DEFP) assuming different estimates of less than perfect AFP surveillance, with perfect AFP surveillance, and reference lines provided to indicate 95% and 99% confidence for WPV1* (two scenarios) and WPV3. WPV1 scenario 1* with the stochastic model start date: January 1, 2014.
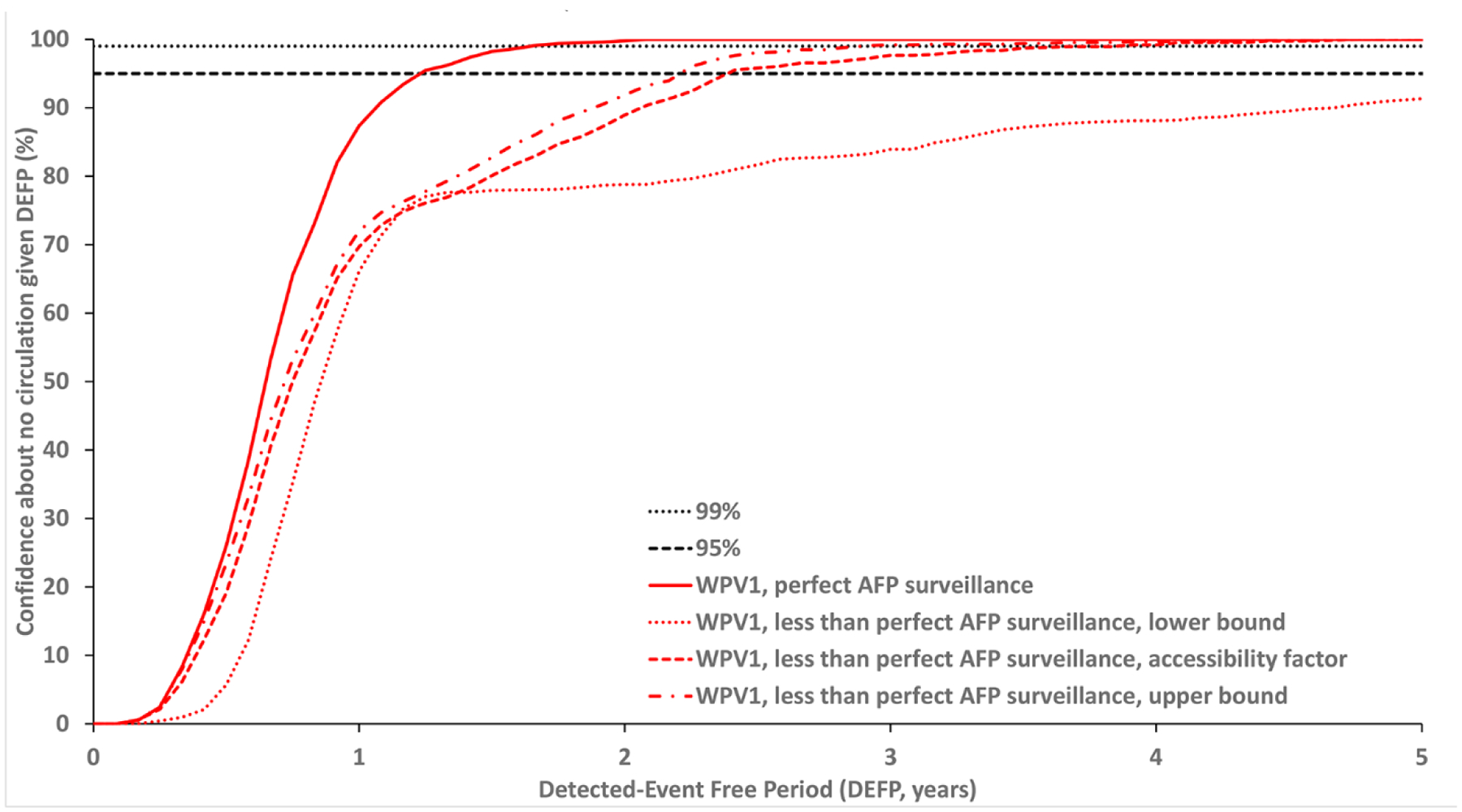
* Scenario 1 assumes sufficient indirect vaccination introductions into the isolated subpopulations to eliminate undetected WPV1 transmission after the outbreak detected in 2016.
Abbreviations: AFP, acute flaccid paralysis; DEFP, detected-event-free period, WPV1, serotype 1 wild poliovirus.
Fig. 1 shows the impact of differences between serotypes and the impact of vaccination penetration into the isolated subpopulations in the context of perfect surveillance information. Specifically, the relatively lower PIR of WPV3 (compared to WPV1) leads to longer times between paralytic cases (all else equal), and therefore implies longer times required to reach the same target level of confidence about no undetected circulation for WPV3 (compared to WPV1). Lower indirect vaccination introductions into the isolated subpopulations in scenario 2 (compared to scenario 1) affect the level of poliovirus transmission resulting in a higher number of stochastic iterations with longer times between paralytic cases, implying a need to wait longer to reach a specific level of confidence.
Fig. 2 compares the impact of less than perfect AFP surveillance of varied quality in the under-vaccinated and isolated subpopulations with the corresponding colors (as listed above). Each dotted line presents results using the lower bound estimates, each short-dashed line shows the results using the assumed accessibility factor, and each dotted-dashed line shows the results using the upper bound estimates for the probability of detecting a case by AFP surveillance (Table 1). Fig. 2 shows that even with perfect surveillance in the general population, poor AFP surveillance quality in the under-vaccinated and isolated subpopulations may result in up to almost two years more time required to reach a CNC95% compared to perfect AFP. Fig. 2a shows the results for WPV1 scenario 1 relevant to the last case in 2016 and Fig. 2b shows the results for WPV1 scenario 2 relevant to the last case in 2016. Fig. 2c shows the results for WPV3 relative to the last case in 2012, which leads to a longer x-axis.
Table 2 reports the POE, CNC95%, CNC99%, TUC95% and TUC99% estimates assuming perfect surveillance compared to three levels of imperfect AFP surveillance quality. The model suggests time periods of 1.33–1.58 or 1.75 years without paralytic cases required to achieve 95% confidence in the interruption of transmission caused by WPV1 or WPV3, respectively, in the context of perfect AFP surveillance. However, given less than perfect AFP surveillance, the CNC95% increases by up to 22 months for WPV1 and up to 16 months for WPV3.
Table 2:
Expected detected-event free period (DEFP) required for 95% and 99% confidence about no circulation (CNCx%) and time of undetected circulation between the last paralytic case and die-out (TUCx%) in Borno and Yobe assuming perfect and imperfect surveillance (based on 1,000 iterations) for WPV1* (two scenarios) and WPV3.
| Virus | WPV1 (PIR = 1/200) | WPV3 (PIR = 1/1000) | ||||||
|---|---|---|---|---|---|---|---|---|
| POE | 100% | 100% | ||||||
| Metric | CNCx% | TUCx% | CNCx% | TUCx% | ||||
| x% | 95% | 99% | 95% | 99% | 95% | 99% | 95% | 99% |
| DEFP values assuming perfect AFP surveillance | ||||||||
| Scenario 1 | 1.33 | 1.83 | 0.97 | 1.38 | 1.75 | 2.50 | 1.41 | 1.76 |
| Scenario 2 | 1.58 | 1.92 | 0.98 | 1.34 | ||||
| DEFP values assuming less than perfect AFP surveillance, lower bound | ||||||||
| Scenario 1 | 3.00 | 3.75 | 2.75 | 3.69 | 3.08 | 6.50 | 2.21 | 6.49 |
| Scenario 2 | 3.42 | 3.75 | 3.35 | 3.72 | ||||
| DEFP values assuming less than perfect AFP surveillance, accessibility factor | ||||||||
| Scenario 1 | 1.92 | 2.25 | 1.14 | 1.58 | 2.42 | 4.42 | 1.52 | 3.99 |
| Scenario 2 | 2.08 | 2.50 | 1.11 | 1.69 | ||||
| DEFP values assuming less than perfect AFP surveillance, upper bound | ||||||||
| Scenario 1 | 1.75 | 2.08 | 1.07 | 1.58 | 2.25 | 3.42 | 1.48 | 3.18 |
| Scenario 2 | 2.00 | 2.33 | 1.05 | 1.46 | ||||
Scenario 1 assumes sufficient indirect vaccination introductions into the isolated subpopulations to eliminate undetected WPV1 transmission after the outbreak detected in 2016; Scenario 2 assumes insufficient indirect vaccination introductions into isolated subpopulations to eliminate undetected WPV1 transmission through the end of 2019.
Abbreviations: AFP, acute flaccid paralysis; CNCx%, DEFP at which the confidence about no circulation exceeds x%; DEFP, detected-event-free period; PIR, paralysis-to-infection ratio; POE, probability of eradication; TUCx%, the xth percentile of the distribution of the times of undetected circulation after the last detected-event (for those iterations in which extinction occurs); WPV(1,3), wild poliovirus (serotypes 1,3).
Fig. 3 shows the confidence for WPV1 starting the stochastic simulation in 2014 to explore the 2014–2016 period in which Nigeria reported no cases. Specifically, the dates between the last reported cases (provided above) imply a DEFP for Nigeria (the entire country) of 1.95 years and a DEFP of 2.18 years for Borno and Yobe. The conditions in Fig. 3 (for 2014–2016) imply different levels of population immunity and different qualities of surveillance compared to the conditions shown in Fig. 2a (for 2016 on). Fig. 3 shows the impact of the increasing isolation of the isolated subpopulations and lower accessibility factors for surveillance, such that confidence of no circulation for the accessibility factors curve does not reach 95% until 2.42 years for Fig. 3 (compared to 1.92 years for Fig. 2a). The results in Fig. 3 reflects stochastic variation, with the isolation that occurred in 2015 leading to continuous low-level circulation and an outbreak in 2016 in 19.5% of stochastic iterations. These results imply a nearly 1 in 5 chance of the 2016 outbreak occurring given the population immunity, mixing and access conditions, and the stochastic nature of virus transmission at the time. The combination of low-level transmission and low quality of AFP surveillance leads to longer DEFPs and therefore longer times required to reach the high confidence about no undetected circulation.
Discussion
To certify an area as WPV free, current policy requires at least 3 years of no paralytic polio cases detected by the AFP surveillance system (Duintjer Tebbens et al., 2019). Consistent with our previous analyses (Kalkowska et al., 2015; Kalkowska et al., 2019), depending on the vaccination levels and therefore population immunity, serotype, and the quality of the surveillance, a 3-year period without detection may or may not be sufficient to reach high confidence (e.g., 95% or higher) about the absence of circulation. The example of Borno and Yobe shows the impact of imperfect information from AFP surveillance, which highlights the need to focus on the under-vaccinated and isolated populations to reach a high level of confidence about no poliovirus circulation. Our experience modeling poliovirus transmission suggests the need to further increase vaccination quality penetrating isolated populations and surveillance quality to achieve a high confidence in WPV1 elimination.
Although the lower PIR of WPV3 (compared to WPV1) combined with problematic surveillance quality may lead to substantial increases in the length of DEFPs, with now over 7 years since the last reported WPV3 case (Kew et al., 2014), we estimate over 99% confidence about no WPV3 circulation. This analysis strongly supports the global certification of WPV3 eradication, which occurred in October 2019 (World Health Organization, 2019).
Similar to our previous modeling work, this analysis comes with several limitations (Kalkowska et al., 2015; Kalkowska et al., 2019). Model results reflect the assumptions that go into them, and uncertainties exist about how well the model assumptions represent the actual inaccessibility that occurred. The wide range for lower and upper bounds of AFP surveillance quality around the accessibility factors demonstrate our uncertainty about those estimates. Similarly, we chose the accessibility factors of 0 for AFP surveillance in isolated subpopulations and 1 for AFP surveillance in the general population across all surveillance options. However, we cannot be sure that efforts to increase visibility in inaccessible areas helps with poliovirus detections, or that AFP surveillance in the general population would never miss any cases. Despite the limitations, we believe this analysis provides useful insights by exploring a range of possibilities. We emphasize that the lack of reported WPV1 cases may indicate no poliovirus transmission, but could also result from substantial gaps in surveillance, and therefore to a false sense of security about the absence of poliovirus transmission. However, ongoing detection by the same surveillance system of serotype 2 circulating vaccine-derived polioviruses (cVDPV2s) provides confidence that the surveillance system is performing as intended. Thus, despite its limitations, our analyses suggest increasing confidence that WPV1 died out in Borno and Yobe, which represent the last known reservoirs in Nigeria. Recently, the publication of results of an ad hoc environmental sampling “sweep” conducted in Borno and Yobe in 2017 that did not detect any WPVs or cVDPVs (Hamisu et al., 2019) provided further support for our results. However, we could not map the sampling locations or explicitly model the results with respect to how the sweep may or may not represent our abstract characterization of the inaccessible areas. One challenge with negative environmental surveillance comes from the inability to determine whether the results convey absence of evidence or evidence of absence. In this case, our model is limited by its inability to fully consider all of the environmental surveillance information, which in part reflects our inability to evaluate the quality of the information.
As demonstrated in this work, the different nature of the WPV serotypes can lead to different times required to reach the same level of confidence, with WPV1 reaching higher confidence with less time since the last reported case in the context of perfect surveillance. However, this work also demonstrates that the quality of the surveillance matters substantially with respect to confidence about no circulation. These results suggest that certification of a region (or the globe) as free of a WPV could in theory require more or less time that 3 years since the last case, depending on the conditions that exist in the last remaining reservoirs.
The risk of undetected poliovirus circulation and the confidence about no circulation in the absence of observed cases will depend on population immunity and the quality of surveillance. Given the long time between cases, we show high confidence of no WPV3 circulation, and increasing confidence of WPV1 circulation, which we anticipate will imply high confidence in the absence of any detected cases prospectively so long as Nigeria maintains similar or achieves further improved conditions prospectively. We hope that this analysis may prove useful as the World Health Organization (WHO) African region considers regional indigenous WPV1 certification and thus regional certification of eradication of all indigenous WPVs.
Acknowledgments
This publication was supported by Cooperative Agreement Number 5NU2RGH001913-04-00 funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. The authors thank Abdullahi Walla Hamisu, Stephanie Kovacs, and Corey Peak for helpful comments on this manuscript.
References
- Debanne SM, & Rowland DY (1998). Statistical certification of eradication of poliomyelitis in the Americas. Mathematical Biosciences, 150(1), 83–103. [DOI] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Kalkowska DA, & Thompson KM (2019). Global certification of wild poliovirus eradication: insights from modelling hard-to-reach subpopulations and confidence about the absence of transmission. BMJ Open, 9(1), e023938. doi: 10.1136/bmjopen-2018-023938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, … Thompson KM (2006). Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Analysis, 26(6), 1471–1505. [DOI] [PubMed] [Google Scholar]
- Eichner M, & Dietz K (1996). Eradication of poliomyelitis: when can one be sure that polio virus transmission has been terminated? American Journal of Epidemiology, 143(8), 816–822. [DOI] [PubMed] [Google Scholar]
- Etsano A, Damisa E, Shuaib F, Nganda GW, Enemaku O, Usman S, … Wiesen E (2016). Environmental isolation of circulating vaccine-derived poliovirus after interruption of wild poliovirus transmission - Nigeria, 2016. Morbidity and Mortality Weekly Report, 65(30), 770–773. doi: 10.15585/mmwr.mm6530a4 [DOI] [PubMed] [Google Scholar]
- Famulare M (2015). Has wild poliovirus been eliminated from Nigeria? PLoS One, 10(8), e0135765. doi: 10.1371/journal.pone.0135765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamisu AW, Onyemelukwe GC, Gerald SE, Hassan IA, Sunday A, Idowu A, … Shuaib F (2019). Environmental surveillance sweep, Nigeria’s experience (March-April 2017). African Journal of Environment and Natural Science Research, 2(3), 29–39. [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Pallansch MA, Cochi SL, Wassilak SGF, & Thompson KM (2015). Modeling undetected live poliovirus circulation after apparent interruption of transmission: Implications for surveillance and vaccination. BMC Infectious Diseases, 15, 66. doi: 10.1186/s12879-015-0791-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, Pallansch MA, & Thompson KM (2019). Modeling undetected live poliovirus circulation after apparent interruption of transmission: Pakistan and Afghanistan. Risk Analysis, 39(2), 402–413, on-line October 408, 2018. doi: 10.1111/risa.13214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Duintjer Tebbens RJ, & Thompson KM (2012). The probability of undetected wild poliovirus circulation after apparent global interruption of transmission. American Journal of Epidemiology, 175(9), 936–949. [DOI] [PubMed] [Google Scholar]
- Kalkowska DA, Franka R, Higgins J, Kovacs SD, Forbi JC, Wassilak SGF, … Thompson KM (2020). Modeling poliovirus transmission in Borno and Yobe, northeast Nigeria. Risk Analysis, online April 29, doi: 10.1111/risa.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkowska DA, Wassilak SGF, Cochi SL, Pallansch MA, & Thompson KM (2020). Global transmission of live polioviruses: Updated integrated dynamic modeling of the polio endgame. Risk Analysis, online January 22, doi: 10.1111/risa.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew OM, Cochi SL, Jafari HS, Wassilak SG, Mast EE, Diop OM, … Centers for Disease Control and Prevention (CDC). (2014). Possible eradication of wild poliovirus type 3--worldwide, 2012. Morbidity and Mortality Weekly Report, 63(45), 1031–1033. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25393222 [PMC free article] [PubMed] [Google Scholar]
- Nnadi C, Damisa E, Esapa L, Braka F, Waziri N, Siddique A, … Adamu U (2017). Continued endemic wild poliovirus transmission in security-compromised areas - Nigeria, 2016. MMWR. Morbidity and mortality weekly report, 66(7), 190–193. doi: 10.15585/mmwr.mm6607a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2012). Current polio global eradication and control policy options: perspectives from modeling and prerequisites for oral poliovirus vaccine cessation. Expert Review of Vaccines, 11(4), 449–459. doi: 10.1586/erv.11.195 [DOI] [PubMed] [Google Scholar]
- Thompson KM, & Duintjer Tebbens RJ (2014). National choices related to inactivated poliovirus vaccine, innovation, and the end game of global polio eradication. Expert Review of Vaccines, 13(2), 221–234. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2016). First wild poliovirus cases in Nigeria since July 2014. Retrieved from http://polioeradication.org/news-post/first-wild-poliovirus-cases-in-nigeria-since-july-2014/
- World Health Organization. (2019). Two out of three wild poliovirus strains eradicated. Retrieved from https://www.who.int/news-room/feature-stories/detail/two-out-of-three-wild-poliovirus-strains-eradicated


